Abstract
For potential applications in nano-mediated radiofrequency cancer hyperthermia, the nanomaterial under investigation must increase the heating of any aqueous solution in which it is suspended when exposed to radiofrequency electric fields. This should also be true for a broad range of solution conductivities, especially those that artificially mimic the ionic environment of biological systems. Herein we demonstrate enhanced heating of biologically relevant aqueous solutions using kosmotropes and a hexamalonoserinolamide fullerene.
The use of radiofrequency (RF) electric fields for non-invasively inducing hyperthermia has been investigated as a treatment modality for cancer.1–7 RF exposure has been shown to dilate the constricted blood vessel network of tumours, allowing the delivery of imaging agents and chemotherapeutics deep into a tumor.8–9 Due to electronic properties, limited in vivo cytotoxicity, and ease of altering their surface functionality, gold nanoparticles (AuNPs) were initially considered as the particle of choice for investigating targeted nano-mediated RF cancer hyperthermia. It was hypothesized that AuNPs functionalized with cancer targeting surface moieties would allow for high intratumoral accumulation and therefore localized heating due to their metallic, electronic properties. This has been met with some success both in vitro and in vivo10–11 but has been the subject of intense debate in regards to the origins of heat production.
Initial accounts of a size-dependent Joule heating mechanism underpinning the excessively large, observed high heating rates from as-purchased AuNPs solutions were quickly refuted.12 The background heating of the ionic buffer solutions, which the AuNPs were suspended in, were shown to account for the observed heating rates.13–16 It was also shown that the geometry and shape of the holder for which the samples are suspended in also plays a major role in heat production, in NaCl solutions; possibly more so in fact than the dielectric nature of the sample itself.17
Extending this field, it was shown that AuNPs could in fact produce heat when exposed to a RF field provided that certain criteria were met; such as the use of non-aggregated, fully suspended AuNPs with diameters less than 10 nm of concentrations at least 500 mg/L. Note, the electric-field strength should also be significantly high (~90 kV/m).18–19
Recent work investigating the heating behaviours of highly enriched metallic and semiconducting single-walled carbon nanotubes also observed heating phenomenon that correlated strongly with the conductivity of the sample suspension; becoming overshadowed or diminished for samples with biologically relevant conductivities.20 These experimental findings extended the initial theoretical works of Hanson et al. 21–22 and introduced the alignment phenomenon of bundled carbon nanotubes. Reviews can be found in the literature.23–24
From the evidence reported to date, it is clear that these nanomaterials cannot enhance heating when placed in highly conductive aqueous environments, such as those that mimic biological systems. Although it cannot be said that nano-induced heating of biologically relevant solutions such as phosphate-buffered saline and blood directly translates to their effective use as a hyperthermia agent in vitro or in vivo, it does provide a plausible framework for possible nanoparticle design and synthesis strategies.
Numerous RF hyperthermia studies have been performed at 13.56 MHz, at which peak heating for aqueous media occurs at a conductivity of 0.060 S/m. Figure 1 illustrates this peak heating behavior and the effects of addition of conductive material, such as salts or nanoparticles. Blood and body fluids are present on the far right of the curve with conductivities of 1.1 and 1.5 S/m, respectively, at 13.56 MHz.25 Intra- and extracellular environments of cells have similarly high ion content. Thus, increasing the heating rate of biologically relevant solutions under RF energy requires diluting existing ionic content, a task not readily performable nor tolerated in vivo.
Figure 1.
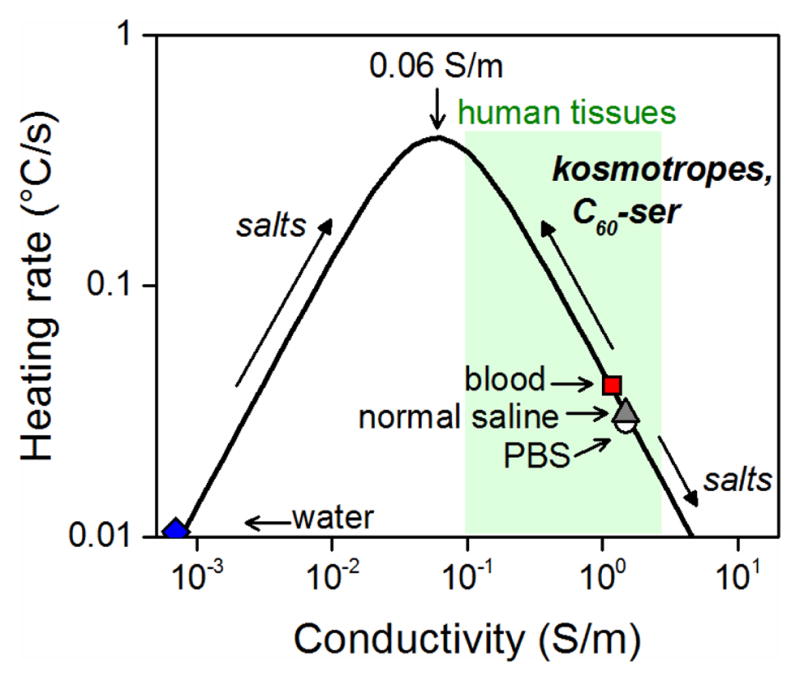
The aqueous RF heating curve at 13.56 MHz. The peak heating conductivity and location of materials of interest on the curve are indicated and arrows indicate the shift resulting from addition of various materials. Note that maximum heating rate is dependent on the geometry20 of the material and the strength of the applied RF field.
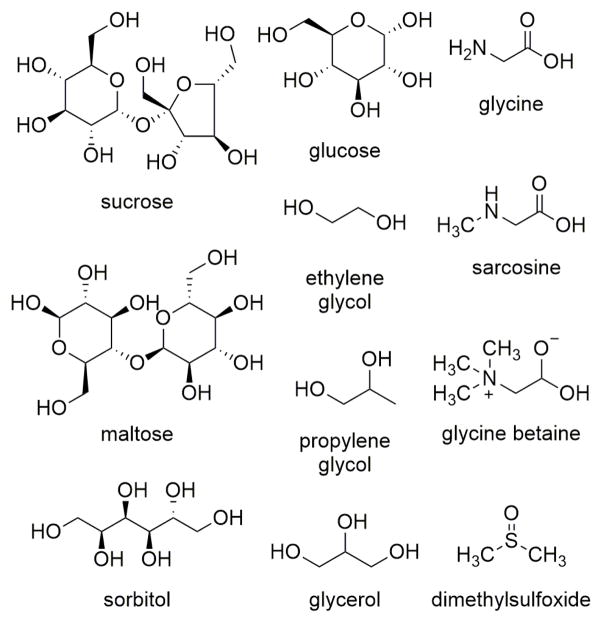
An alternative approach presented here is to introduce neutral, water-soluble materials called kosmotropes that, when added to an ionic aqueous solution, serve to decrease conductivity and thus increase the RF heating rate of the solution. Kosmotropic (“structure-forming”) materials preferentially interact with water solvent molecules and form hydrogen bonds to water that are stronger than the water-water hydrogen bonds, thus stabilizing the water network.26 The structures of kosmotropic materials used in this study are shown in Figure 2. Glycerol (GLY), propylene glycol (PG), ethylene glycol, and methanol are examples of kosmotropes that are used as anti-freeze agents; in nature, sugars such as trehalose and sucrose act as cryoprotective agents when present in high concentrations in flora and fauna. Biotechnologists commonly use dimethylsulfoxide (DMSO) as their cryoprotectant agent of choice to reduce cellular damage by ice when freezing cells for long-term storage. Marine life, such as seagrasses, use a wide variety of kosmotropic materials, such as sugars, sugar alcohols, and zwitterions to maintain osmotic balance in the rapidly changing salinity of seawater.27 Quaternary ammonium compounds called betaines, also in high concentrations, allow bacteria to survive in highly ionic environments. For example, glycine betaine is used by E. coli to counteract the effects of low pH and high urea concentrations in the urinary tract.28
Figure 2.
Structures of kosmotropes used in this study.
In stabilizing the water network, these materials can also reduce ion mobility, a property we exploit to reduce the conductivity of highly ionic solutions, thereby increasing their heating rate. To our knowledge, this is the first time that the RF heating rates of aqueous solutions have been modulated in this way. We also identify a water-soluble fullerene as the first nanomaterial that could be used for bulk RF heating enhancement of biological systems.
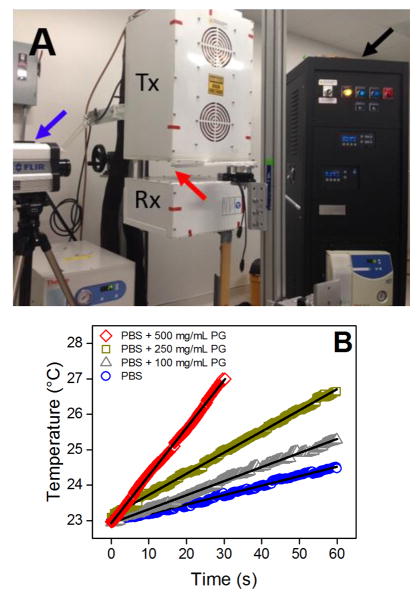
Kosmotropes (Fig. 2) were individually dissolved at 100, 250, and 500 mg/mL in phosphate buffered saline (PBS), a biologically iso-osmolar solution with similar conductivity (1.15 S/m) to blood and biological systems. To maintain equivalent ionic concentrations in all samples, solutions were prepared and appropriately diluted from concentrated stock PBS (10x PBS containing 90 g/L NaCl, 1.44 g/L KH2PO4, and 7.95 g/L Na2HPO4). Samples were loaded into a non-conducting 1.3 mL quartz cuvette and exposed to a 13.56 MHz electric field at 100 W (Fig. 3A). Temperature was monitored using an IR camera and RF heating rates were obtained by applying linear regressions to the sample temperature plots (Fig. 3B). Conductivity at 13.56 MHz was obtained from permittivity measurements over the frequency range 10 MHz-3 GHz obtained using an impedance analyzer connected to a dielectric probe.
Figure 3.
(A) A radiofrequency (13.56 MHz) generator (black arrow) was used to generate a focused, high-voltage electric field between the transmitting (Tx) and receiving (Rx) heads. An infrared camera (blue arrow) was used to record the temperature of the sample (red arrow). (B) Temperature plots for PBS alone and with 100, 250, or 500 mg/mL propylene glycol. Least-squares linear regressions (solid lines) are used to calculate RF heating rates.
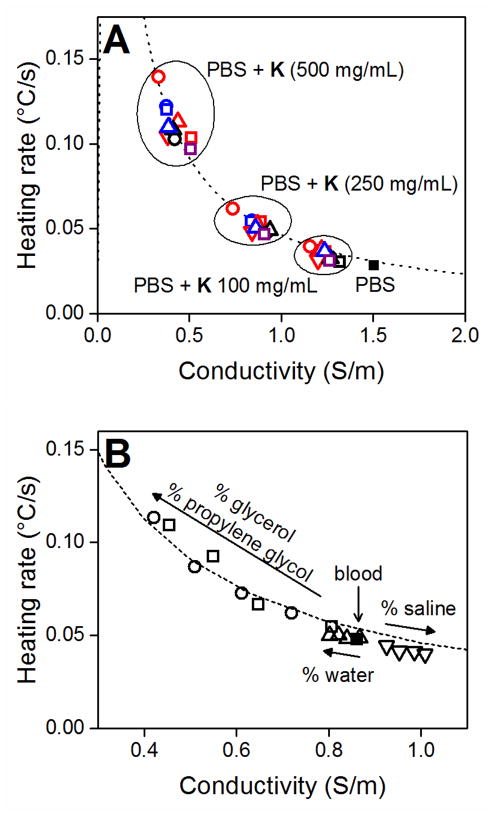
In a similar study, various additives were added to porcine blood containing 2–3% ethylenediamine tetraacetic acid (EDTA) to prevent coagulation. These included PG, GLY, pure water and normal saline at concentrations of 1–20% by weight. Addition of PG and GLY resulted in a decrease in blood conductivity and increase in heating rate (Fig. 4B). A significant increase in heating rate (p < 0.005) was observed starting at 5% PG and 10% GLY. In contrast, addition of hypertonic saline (1.49 S/m, 0.031 °C/s) resulted in opposite trends in conductivity and heating although the changes were not statistically significant. Addition of pure water had similar, though reduced, effects to those of PG and GLY on blood. Although these three additives all dilute the ionic content of blood and are nonconductive, only PG and GLY significantly modulate conductivity and heating. This difference in conductivity attenuation is attributed to the water-structuring properties of these materials.
Figure 4.
(A) Kosmotropes (K) enhance the heating rate of PBS (■) by reducing its conductivity. Materials added to PBS at the indicated concentrations include sugar alcohols (red; ethylene glycol - □, propylene glycol - ○, glycerol - △, sorbitol - ▽), sugars (blue; maltose - □, glucose - ○, sucrose - △), amines (black; glycine - □, sarcosine - ○, glycine betaine - △), and dimethylsulfoxide (purple, □). (B) The conductivity and heating rate of blood (■) are modulated in a concentration dependent manner by addition of propylene glycol (□), glycerol (○), water (△) and hypertonic saline (○). Arrows indicate increasing additive content (5, 10, 15, 20%). Dashed lines indicate general aqueous heating curve. All heating rates and conductivity values reported are averages of three replicates. Error bars are omitted for clarity but are generally smaller than the symbols.
Figure 4B demonstrates that it is possible to move along the heating curve, that is, to modulate the heating rate of a material, by using different additives. In this case, the initial or unaltered conductivity and heating rate of blood are 0.86 S/m and 0.045 °C/s, respectively. The addition of GLY and PG shifts blood to the left of the curve towards greater heating rates. Although to a much lesser degree, the same is true for water and the reciprocal effect is observed for hypertonic saline. Hypertonic saline reduces the conductivity of blood, consequently reducing the heating rate.
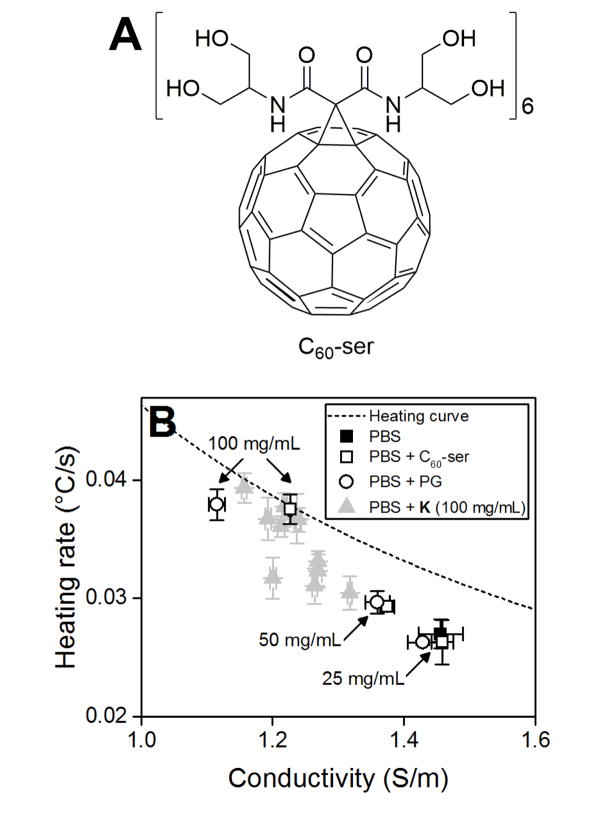
Nanoparticles could be functionalized to have water-structuring activity like the kosmotropes presented above. In these regards, hexamalonoserinolamide fullerene (C60[C(COSer)2]6, Ser = 2-amino-1,3-propanediol) or C60-ser (Fig. 5A) was prepared according to the literature procedures.29–30 C60-ser is a neutral, water-soluble fullerene that reduces the conductivity of PBS and enhances its heating rate accordingly like the kosmotropes described above (Fig. 5B). To our knowledge, this is the first time a nanoparticle has been used to enhance the bulk heating rate of a highly conductive, biologically relevant system.
Figure 5.
(A) Structure of C60-ser. (B) C60-ser (□), shown in comparison to propylene glycol (○) and the kosmotropes at 100 mg/mL from Fig. 4A (△), enhances the heating rate of PBS by reducing its conductivity. Dashed line indicates general aqueous heating curve. All heating rates reported are averages of three replicates with error bars indicating SD. Note that error bars may be smaller than symbols.
The conductivity of most biological ionic solutions exceeds the peak RF heating conductivity, and precludes the addition of salts or polar materials to increase RF heating. At this low frequency, the absence of high heat producing phenomena, such as surface plasmon resonance, calls for an alternative heating enhancement method. Here we showed that addition of kosmotropes reduces the conductivity of biological systems and increases their heating rate accordingly. The addition of kosmotropes and nanomaterials to these solutions will also affect their viscosity properties and, indeed, this physical variable and its role in influencing the heating properties of solutions warrants further investigation.
Most of the substances tested here for RF heating enhancement are widely available, inexpensive, and are generally considered safe, making them highly attractive for clinical use, although their delivery in high concentrations is potentially a significant obstacle. Such high concentrations could be achieved by percutaneous arterial injection into the local tumour vasculature, similar to the well-known and clinically available technique of arterial chemo-embolization. Further in vitro and in vivo work is required however to validate the use of the aforementioned kosmotropes and nanomaterials in modulating the heating behaviour of cells, tissues, and tumours.
Supplementary Material
Acknowledgments
N.C.L. was supported by the National Science Foundation Graduate Research Fellowship Program (1450681). S.A.C. is supported by the Kanzius Research Foundation and NIH Physical Sciences in Oncology (U54CA143837). S.J.C. is supported by EP5/6/9 NIH Physical Sciences in Oncology (U54CA143837). J.C.H is supported by Baylor College of Medicine Oncology Scholars (T32CA174647). A.R.B. is supported by the Welsh Government Sêr Cymru Programme and the Robert A. Welch Foundation (C-0002). L.J.W. is also supported by the Welch Foundation (C-0627). C60-ser was prepared and generously provided by Yuri Mackeyev.
Footnotes
Electronic Supplementary Information (ESI) available: For experimental methods, see DOI: 10.1039/x0xx00000x
References
- 1.Perez CA, Emami B. Radiol Clin North Am. 1989;27:525–542. [PubMed] [Google Scholar]
- 2.Hiraoka M, Mitsumori M, Hiroi N, Ohno S, Tanaka Y, Kotsuka Y, Sugimachi K. IEEE Trans Microw Theory Techn. 2000;48:1789–1799. [Google Scholar]
- 3.van der Zee J, González González D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Lancet. 2000;355:1119–1125. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 4.Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. Int J Hyperthermia. 2001;17:97–105. doi: 10.1080/02656730010001333. [DOI] [PubMed] [Google Scholar]
- 5.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. J Clin Oncol. 2005;23:3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 6.Vasanthan A, Mitsumori M, Park JH, Zhi-Fan Z, Yu-Bin Z, Oliynychenko P, Tatsuzaki H, Tanaka Y, Hiraoka M. Int J Radiat Oncol. 2005;61:145–153. doi: 10.1016/j.ijrobp.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 7.Mitsumori M, Zeng ZF, Oliynychenko P, Park JH, Choi IB, Tatsuzaki H, Tanaka Y, Hiraoka M. Int J Clin Oncol. 2007;12:192–198. doi: 10.1007/s10147-006-0647-5. [DOI] [PubMed] [Google Scholar]
- 8.Corr SJ, Shamsudeen S, Vergara LA, Ho JCS, Ware MJ, Keshishian V, Yokoi K, Savage DJ, Meraz IM, Kaluarachchi W, Cisneros BT, Raoof M, Nguyen DT, Zhang Y, Wilson LJ, Summers H, Rees P, Curley SA, Serda RE. PLoS ONE. 2015;10:e0136382. doi: 10.1371/journal.pone.0136382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapin NA, Krzykawska-Serda M, Ware MJ, Curley SA, Corr SJ. Cancer Nanotechnology. 2016;7:5. doi: 10.1186/s12645-016-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raoof M, Corr SJ, Kalaurachchi WD, Massey KL, Briggs K, Zhu C, Cheney MA, Wilson LJ, Curley SA. Nanomedicine: Nanotechnology, Biology, and Medicine. 2012;8:1096–1105. doi: 10.1016/j.nano.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raoof M, Corr SJ, Zhu C, Cisneros BT, Kalaurachchi WD, Phounsavath S, Wilson LJ, Curley SA. Nanomedicine: Nanotechnology, Biology, and Medicine. 2014;10:1121–1130. doi: 10.1016/j.nano.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran CH, Wainerdi SM, Cherukuri TK, Kittrell C, Wiley BJ, Nicholas NW, Curley SA, Kanzius JS, Cherukuri P. Nano Res. 2009;2:400–405. [Google Scholar]
- 13.Li D, Jung YS, Tan S, Kim HK, Chory E, Geller DA. J Colloid Interface Sci. 2011;358:47–53. doi: 10.1016/j.jcis.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 14.Kim HK, Hanson GW, Geller DA. Science. 2013;340:441–442. doi: 10.1126/science.1237303. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Chen HJ, Chen X, Alfadhl Y, Yu J, Wen D. J Appl Phys. 2014;115:094903. [Google Scholar]
- 16.Liu X, Chen H-J, Chen X, Parini C, Wen D. Nanoscale. 2012;4:3945–3953. doi: 10.1039/c2nr30166k. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Jung YS, Kim HK, Chen J, Geller DA, Shuba MV, Maksimenko SA, Patch S, Forati E, Hanson GW. IEEE Trans Biomed Eng. 2012;59(12):3468–3474. doi: 10.1109/TBME.2012.2219049. [DOI] [PubMed] [Google Scholar]
- 18.Corr SJ, Raoof M, Mackeyev Y, Phounsavath S, Cheney MA, Cisneros BT, Shur M, Gozin M, McNally PJ, Wilson LJ, Curley SA. Journal of Physical Chemistry C. 2012;116:24380–24389. doi: 10.1021/jp309053z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogdanov A, Jr, Gupta S, Koshkina N, Corr SJ, Zhang S, Curley SA, Han G. Bioconjugate Chem. 2014;26(1):39–50. doi: 10.1021/bc5005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corr SJ, Raoof M, Cisneros BT, Orbaek AW, Cheney MA, Law JJ, Lara NC, Barron AR, Wilson LJ, Curley SA. Nano Res. 2015 [Google Scholar]
- 21.Hanson GW, Patch SK. J Appl Phys. 2009;106:054309. [Google Scholar]
- 22.Hanson GW, Monreal RC, Apell SP. J Appl Phys. 2011;109:124306. [Google Scholar]
- 23.Liu X, Chen H-J, Chen X, Alfadhl Y, Yu J, Wen D. J Appl Phys Rev. 2015;2:011103. [Google Scholar]
- 24.Collins CB, McCoy RS, Ackerson BJ, Collins GJ, Ackerson CJ. Nanoscale. 2014;6:8459–8472. doi: 10.1039/c4nr00464g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabriel S, Lau RW, Gabriel C. Phys Med Biol. 1996;41:2271–2293. doi: 10.1088/0031-9155/41/11/003. [DOI] [PubMed] [Google Scholar]
- 26.Galinski EA, Stein M, Amendt B, Kinder M. Comp Biochem Physiol A. 1997;117:357–365. [Google Scholar]
- 27.Touchette BW. J Exp Mar Biol Ecol. 2007;350:194–215. [Google Scholar]
- 28.Chambers ST, Peddie BA, Randall K, Lever M. Int J Antimicrob Ag. 1999;11:293–296. doi: 10.1016/s0924-8579(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 29.Wharton T, Wilson LJ. Bioorg Med Chem. 2002;10:3545–3554. doi: 10.1016/s0968-0896(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 30.Cerar J, Pompe M, Gucek M, Cerkovnik J, Skerjanc J. J Chromatogr A. 2007;1169:86–94. doi: 10.1016/j.chroma.2007.08.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.