Summary
There are emerging data that the skeleton is connected to systemic biological functions through the release of two osteoblast-/osteocyte-derived hormones, fibroblastic growth factor 23 (FGF23) and undercarboxylated osteocalcin (Ocn). FGF23 is important in the regulation of phosphate and vitamin D metabolism, whereas Ocn participates in endocrine networks, coordinating bone and fat mass, energy metabolism, and sex hormone production. Bone remodeling and mineralization per se, along with the hormones leptin, insulin, glucocorticoids, PTH, and 1,25(OH)2D, regulate the release of FGF23 and Ocn, leading to complex cross-talk and coordination between endocrine networks previously thought to be distinct. These pathways are particularly important in chronic kidney disease, in which both FGF23 and Ocn are increased. Although these hormones initially serve an adaptive role, with progressive loss of renal function they show maladaptive effects, particularly on the cardiovascular system, through multiple mechanisms, including possible cross-talk with the renin angiotensin system. The complex interconnections between the various endocrine networks in chronic kidney disease may account for the difficulty in treating the uremic state.
Keywords: 1,25(OH)2D; bone; kidney; calcium; osteoblasts (Obs); osteocytes; cyp27b1; cyp24; Klotho; FGF23; PTH
Morbidity and mortality are unacceptably high in the end-stage renal disease (ESRD) population despite efforts to increase dialysis efficiency, improve nutrition, treat anemia, correct the abnormalities of mineral metabolism, and block the renin angiotensin system (RAS).1 The reasons for the limited therapeutic success in ESRD are not clear, but may be owing to the difficulty in fully restoring physiological homeostasis and/or a failure to recognize key factors contributing to the pathophysiology of the uremic state. Morbidity and mortality in ESRD, however, potentially are reversible, as shown by the ability of successful renal transplantation to significantly reduce cardiovascular disease progression.2
Recently, clinical epidemiologic studies have attributed the increased mortality in chronic kidney disease (CKD) to nontraditional risks factors.3 Indeed, strong associations exist between disorders of mineral metabolism and poor outcomes in ESRD, including correlations between increased mortality and increases of serum concentrations of phosphate and parathyroid hormone (PTH).4 More recently, the bone-derived hormones, fibroblastic growth factor 23 (FGF23), which regulates phosphate and vitamin D metabolism, and uncarboxylated osteocalcin (Ocn), which regulates glucose homeostasis and energy metabolism, have been identified as additional risk factors for increased mortality.5 In addition, treatment with vitamin D analogues and phosphate binders are purported to improve survival in CKD.6,7 At present, however, direct roles of any of these factors in mediating the adverse outcomes in CKD have not been established by prospective controlled trials.
We typically think of endocrine networks as self-contained feedback or feed-forward loops. We are at the early stages of understanding the greater complexity of the endocrine pathways affected by CKD, and in this review we explore potential connections between the pathways regulating bone and mineral metabolism, energy metabolism and glucose homeostasis, and the (RAS).
PTH–VITAMIN D AXIS
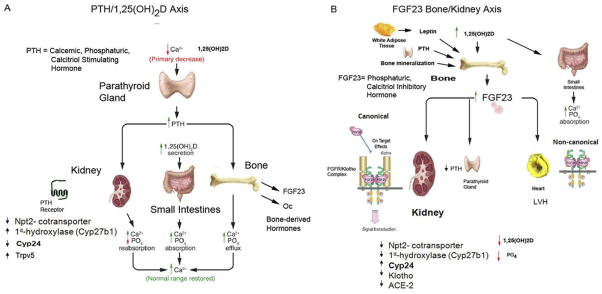
The PTH–vitamin D endocrine loop consists of the following. First, PTH, a calcemic and phosphaturic hormone, is secreted by the parathyroid glands (PTGs). Second, PTH activates PTH receptor (PTHR1) in kidney and bone. PTHR1 in proximal tubular cells mediates PTH stimulation of Cyp27b1 expression to increase the production of 1,25(OH)2D and inhibition of Npt2a and Npt2c brush-border membrane insertion to inhibit phosphate transport. In the distal tubule it mediates PTH stimulation of calcium reabsorption through TRPV5, and PTHR1 in osteoblasts mediates PTH stimulation of bone remodeling and calcium and phosphate efflux. Third, 1,25(OH)2D, whose production is stimulated by PTH, and which targets the vitamin D receptor (VDR)/retinoic acid receptor complex, is present in multiple tissues. In the gastrointestinal tract, 1,25(OH)2D, increases calcium and phosphate absorption, in osteoblasts it enhances bone resorption and inhibits bone mineralization, and in PTG it suppresses PTH gene transcription.8 Next is the calcium-sensing receptor (CaSR), a G-protein–coupled receptor located predominantly in the PTG and kidney distal tubule. PTH secretion is stimulated by changes in serum calcium through activation of the CaSR in parathyroid chief cells. Loss of CaSR function in the kidney and excess PTH leads to hypocalciuria.
The net effect and primary physiological function of PTH is to increase calcium absorption from the gastrointestinal track and efflux from bone while limiting renal excretion, thereby maintaining serum calcium levels in a narrow range (Fig. 1A). The phosphaturic actions of PTH are secondary and permit increments in serum calcium to occur without concomitant increases of serum phosphate that would occur from 1,25(OH)2D-mediated increases in phosphate absorption.
Figure 1.
Endocrine networks involved in the pathogenesis of CKD mineral and bone disease. (A) PTH–vitamin D axis. PTH secreted by the parathyroid glands under control of the CaSR directly targets PTHR1 in bone and kidney to stimulate, respectively, calcium efflux from bone, renal conservation of calcium and production of 1,25(OH)2D, the latter targets VDR in intestines to increase calcium absorption as well as in other tissues to regulate multiple cellular functions. PTH also has phosphaturic actions on the kidney and in certain settings also stimulates FGF23 and Ocn secretion from bone. (B) FGF23–bone-kidney axis. FGF23 is secreted by osteoblasts/osteocytes in bone under control of multiple factors, including 1,25(OH)2D, leptin, glucocorticoids, PTH, and bone mineralization/turnover. Circulating FGF23 targets FGFR/α-Klotho complexes located in the kidney, pituitary, thymus, and parathyroid glands. The principal biological actions of FGF23 are to suppress circulating 1,25(OH)2D levels, owing to combined actions to decrease Cyp27b1-mediated synthesis and possibly stimulation of Cyp24-mediated degradation of this active vitamin D sterol, and to inhibit the renal reabsorption of phosphate leading to phosphaturia. FGF23 also may suppress PTH secretion. In addition, direct effects of FGF23 on FGFR pathways in the absence of the co-receptor α-Klotho have been proposed.
ENDOCRINE FUNCTIONS OF BONE
Bone is a metabolically active tissue that provides structural support and undergoes constant renewal through the process of bone remodeling. Indeed, both PTH and 1,25(OH)2D target PTHR1 and VDR:retinoic acid receptor complex in osteoblasts and osteocytes to regulate osteoblast-mediated bone formation and osteoclast-mediated bone resorption.9 In addition, calcium and phosphate are in equilibrium with extracellular fluids,10 and efflux and influx of these ions are under both physiochemical and hormonal control.
Bone is also an endocrine organ that releases two hormones, FGF23 and undercarboxylated Ocn,11–13 which are involved in complex endocrine networks regulating phosphate and vitamin D metabolism, energy metabolism, and sexual reproduction.8
FGF23 BONE–KIDNEY AXIS
FGF23 is an approximately 32-kDa protein with an N-terminal FGF homology domain and a novel 71–amino acid C-terminus14 that is a member of the hormone-like FGF genes (ie, FGF-19, FGF-21, and FGF23), which differ from the classic autocrine/paracrine ligands by their ability to diffuse from tissues into the circulation. The C-terminus14 allows FGF23 to activate FGF receptors independent of heparin, through trimeric complex formation with FGF receptors15,16 and α-Klotho (Kl), a transmembrane β-glucuronidase.15,17 FGF23 is expressed predominately in osteoblasts and osteocytes in bone (Fig. 1B).18–20 The physiological targets of FGF23 are organs that co-express FGF receptor (FGFR)/Kl complexes,15,17 including the kidney, parathyroid gland, pituitary gland, and choroid plexus.15 The principal biological actions of FGF23 in the kidney are to inhibit phosphate reabsorption by decreasing Na-dependent co-transporters and to suppress 1,25(OH)2D levels by inhibiting Cyp27b1 (or 1 α-hydroxylase, which converts 25[OH]D to 1,25[OH]2D) and by stimulating the catabolism of 1,25(OH)2D by activating the 24-hydroxylase (Cyp24).21–24
Alterations of circulating FGF23 are both physiologically and clinically important. Increases of circulating FGF23 concentrations cause hereditary and acquired hypophosphatemic disorders,22,24–27 whereas reductions in circulating FGF23 concentrations cause familial tumoral calcinosis.19,21,28–32 FGF23 has essential biological functions because ablation of FGF23 is lethal in the early postnatal period owing to hyperphosphatemia and excessive 1,25(OH)2D production. FGF23 is involved in several endocrine feedback loops.8
FGF23–1,25(OH)2D Endocrine Loop
There is strong evidence that FGF23 acts as a counter-regulatory factor for 1,25(OH)2D33 (Fig. 2), that is, 1,25-(OH)2D stimulates FGF23 production by bone through VDR-dependent mechanisms and increased circulating FGF23 suppresses 1,25-(OH)2D production in the kidney.33,34 The physiological role of FGF23 may be to prevent vitamin D toxicity. PTH and FGF23 have different effects on 1,25(OH)2D production (ie, increased by PTH and decreased by FGF23) and 1,25(OH)2D has opposite effects on these hormones (ie, suppresses PTH and stimulates FGF23).
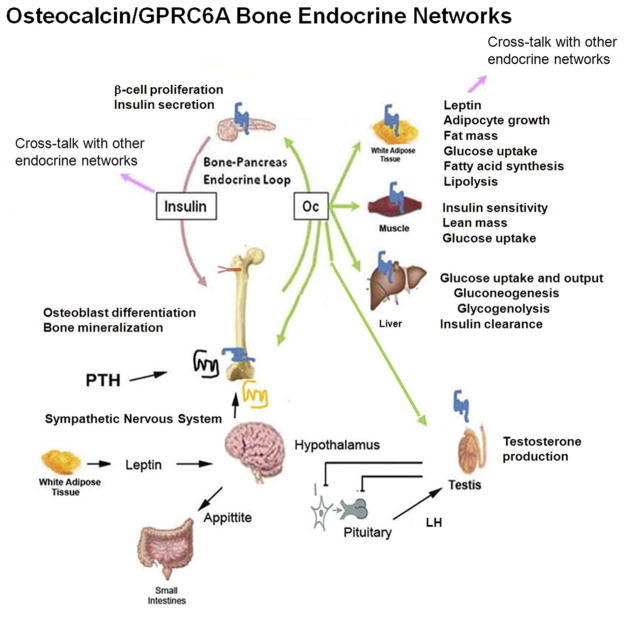
Figure 2.
Osteocalcin (Ocn)–GPRC6A bone endocrine networks. A complex network is depicted whereby undercarboxylated Ocn is released from bone in response to osteoclast-mediated bone resorption. Circulating Ocn targets a nutrient G-protein–coupled receptor, with high homology to CaSR, which is present in multiple organs, including pancreatic β-cells, adipocytes, skeletal muscle, hepatocytes, osteoblasts, Leydig cells in testes and prostate, as well as other tissues and cell types. Experimental evidence in the mouse suggests that Ocn is involved in regulating insulin secretion and insulin sensitivity as well as sexual reproduction through control of testosterone production in Leydig cells. Insulin may have a feed-forward loop to stimulate Ocn release from bone. Ocn regulation of testosterone may coordinate skeletal growth and sex hormone production during skeletal growth. The clinical significance of these pathways in human beings remains to be established. LH, luteinizing hormone.
FGF23 Regulation of Phosphate Homeostasis?
Although PTH is the calcemic hormone that is tightly regulated by extracellular calcium, FGF23, which also is called phosphatonin because of its effects to cause hypophosphatemia, is not tightly coupled to serum phosphate in an analogous feedback loop. Indeed, despite a positive correlation between serum phosphate levels and increases in FGF23 levels in ESRD,35 phosphate restriction and/or loading has minimal and/or delayed effects on FGF23 levels in both animal models and clinical settings, both in the presence of normal and impaired renal function.36–40 At present, direct evidence that phosphate regulates FGF23 gene transcription is lacking.33 Phosphate effects on FGF23 might be mediated indirectly by bone mineralization, which could account for the delay in the effect of phosphate on FGF23 expression. Indeed, Phex, Dmp1, and ENPP1 mutations, which cause hereditary hypophosphatemic rickets, are regulators of both bone mineralization and FGF23 production.41 Current evidence supports the presence of a connection between inhibition of extracellular matrix mineralization and stimulation of FGF23 gene transcription in osteoblasts/osteocytes, through poorly defined alterations in the matrix milieu that activate FGFR1-dependent FGF23 gene transcription.42 The linkage between bone mineralization and FGF23 secretion creates a bone– kidney axis that may function to balance bone phosphate uptake or release with renal phosphate handling.33,34,43 In CKD, impaired coordination of renal phosphate handling to match bone mineralization may promote vascular calcifications.19,44–46 Defining the relationship between FGF23 and bone remodeling in CKD may help define the optimal level of bone remodeling.
PTH–FGF23 Endocrine Loop?
PTH, at least under some circumstances, stimulates FGF23 expression in bone, and FGF23 also directly may suppress PTH production by the PTG.47 Both the efferent and afferent limbs of this parathyroid– bone endocrine network, however, are context-dependent.
Indeed, FGF23 effects on PTGs are controversial.48,49 On the one hand, FGF23 targets FGFR/Kl complexes in PTGs50 and FGF23 directly suppresses PTH messenger RNA expression in vitro and decreases serum PTH in vivo.47 However, FGF23 does not prevent the development of hyperparathyroidism (HPT) in any clinical circumstance and there is a strong association between increased FGF23 levels and the severity of HPT in CKD and other disorders,15 suggesting that FGF23 may promote the development of HPT.15,48,49 Recent studies have indicated that resistance to FGF23 develops in uremic PTGs owing to down-regulation of α-Klotho and FGFR expression,51,52 which may explain this paradox.
Effects of PTH to regulate FGF23 expression in bone are also variable. Several studies have indicated that activation of PTH-dependent pathways stimulate FGF23 secretion by bone. Activating mutations of the PTH1R and Guanine nucleotide-binding protein, alpha-stimulating polypeptide (GNAS) genes in human beings, overexpression of a constitutively active PTH receptor in osteocytes in mice, and continuous exposure to high levels of PTH in animal models and clinical settings, result in increased FGF23 expression.15,48,49,53–56 PTH–cyclin D1 transgenic mice with primary HPT have increased FGF23 levels that decrease after PTX.54 In addition, PTX reduces FGF23 levels in CKD.56,58 In vitro studies have shown a direct regulation of FGF23 expression by PTH in osteoblasts,55,57,59 through mechanisms involving sclerostin regulation of Wnt signaling. FGF23 could be increased indirectly by PTH stimulation of 1,25(OH)2D.60
In other studies, however, PTH is inhibitory or fails to stimulate FGF23. PTH did not directly stimulate FGF23 production in osteoblasts in vitro33 or in normal mice.61 Also, FGF23 is not increased in patients with primary HPT.62 PTH stimulation of FGF23 also has been shown to be dependent on calcium and vitamin D status. In VDR−/− mice, FGF23 is undetectable despite increases of PTH.63 The ability of PTH to regulate FGF23 also is modified by whether PTH induces a net anabolic or catabolic effect on bone (ie, excessive bone resorption induced by continuous PTH administration, results in increased FGF23, whereas the administration of intermittent PTH that leads to a net increase in bone formation results in reductions in FGF2355,61). Acute administration of PTH even has been shown to actually suppress FGF23 in normal adults.64 Further studies are needed to understand the context-dependent regulation of FGF23 by PTH.
OCN/G-PROTEIN COUPLED RECEPTOR C6A BONE ENDOCRINE NETWORKS
The skeleton also releases the osteoblast-derived hormone Ocn into the circulation to regulate multiple metabolic processes through activation of its receptor, G-protein coupled receptor C6a (GPRC6A), which is present in multiple organs (Fig. 2). Ocn is an osteoblast-specific protein and a major noncollagenous protein in the extra-cellular matrix. Glutamic acid residues in Ocn undergo post-translational γ-carboxylation into γ-carboxyglutamic acid (Gla), which enhances the affinity of Ocn for hydroxyapatite; whereas undercarboxylated Ocn is released from mineralized bone matrix in response to bone resorption and acts as a circulating hormone that targets GPRC6A. Detailed saturation binding kinetics have not been performed of Ocn and GPRC6a, but based on functional data it is presumed that the undercarboxylated form of Ocn has higher affinity for the receptor.
GPRC6A is a G-protein–coupled receptor that is formed by the fusion of a periplasmic nutrient Venus fly trap motif and a classic seven-transmembrane domain. This fusion of the Venus fly trap motif and seven-transmembrane domain creates the structural basis for both independent biological and pharmacologic actions of orthosteric ligands and allosteric modulators with different affinities and efficacies, but also for cooperative interactions between the orthosteric and allosteric ligand binding sites. Consequently, GPRC6A can sense basic L amino-acid, various cations, Ocn, and testosterone.
GPRC6A is highly expressed in β-cells and in other tissues, including brain, white adipose tissue, skeletal muscle, liver, and bone. The promiscuous expression and multiligand specificity of GPRC6A provides a means to integrate seemingly disparate biological processes, ranging from insulin secretion, energy metabolism, sexual reproduction, hypothalamic-pituitary function, bone formation, and prostate cancer. GPRC6A has been shown to directly regulate signaling in osteoblasts,65 prostate cells,66 Leydig cells,67,68 and pancreatic β-cells.69 Ocn and GPRC6A are involved in several endocrine loops.
Bone–Pancreas Loop
Ocn and GPRC6A are involved in a bone–pancreas endocrine loop. This endocrine network involves insulin activation of insulin receptors (IRs) in osteoblasts to increase Ocn secretion and bioactivity; in turn, circulating undercarboxylated Ocn activates GPRC6A in β-cells12,69 to regulate insulin secretion and other β-cell functions.70 The existence of this bone–pancreas network is supported by several findings in mice. First, osteoblast-specific deletion of the IR results in loss of insulin-mediated release of bioactive Ocn from bone.12 Insulin stimulates Ocn bioactivity by inhibiting osteoprotegerin, which favors bone resorption, and a local acidic pH that decarboxylates Ocn. Second, ablation of Ocn (Ocn−/−) leads to glucose intolerance in mice and compound heterozygous mutant IRob-cko+/−/Ocn+/− mice display glucose intolerance similar to IRob-cko mice or Ocn−/− mice.67 Third, genetically modified mice with an increase in uncarboxylated Ocn are protected from type 2 diabetes and obesity71; and the administration of recombinant Ocn to mice stimulates β-cell functions, including an increase in β-cell mass (proliferation) and insulin secretion.67,69 The result is a feed-forward loop, in which insulin signaling in osteoblasts promotes its own secretion by activating Ocn.
Although speculative, it has been proposed that this feed-forward loop may be regulated negatively by leptin. Leptin, a hormone produced by white fat, targets the hypothalamus to suppress appetite and regulates bone metabolism and β-cell function indirectly via activation of the sympathetic nervous system (which inhibits Ocn and insulin secretion).72 In addition to promoting β-cell proliferation and insulin expression and secretion, Ocn increases insulin sensitivity and energy expenditure, possibly through activation of GPRC6A expressed in muscle, fat, and liver.
BONE AND ANABOLIC STEROID ENDOCRINE NETWORK
Recombinant Ocn also regulates testosterone production by Leydig cells in the testes and male fertility through GPRC6A-dependent mechanisms.11 Ocn’s relevance in this endocrine loop is confounded by the fact that GPRC6A also mediates the nongenomic effects of testosterone, as evidence by the loss of the rapid signaling responses to testosterone in Gprc6a−/− mice.73 Testosterone also regulates insulin secretion in β-cells74 through activation of GPRC6A.73 Thus, GPRC6A may account, at least in part, for testosterone’s known role in glucose homeostasis, as well as its salutary effects on obesity and lipid metabolism.69,73,75–77 Thus, testosterone may regulate both insulin sensitivity and insulin secretion, at least in part through activation of GPRC6A.78 Because insulin suppresses testicular steroid production,78 theoretically a positive feedback loop also may exist linking testosterone production by Leydig cells with testosterone stimulation of insulin release from the pancreas. In addition, testosterone also regulates luteinizing hormone (LH) secretion, also possibly through GPRC6A. Testosterone and Ocn activation of GPRC6A to regulate LH and testosterone secretion, respectively, may provide a linkage between skeletal growth and alterations in endocrine functions during puberty in males.79
Consistent with the existence of even more complex endocrine networks, the complete absence of GPRC6A in Gprc6a−/− mice results in multiple metabolic abnormalities, some explained by the bone–pancreas axis, such as obesity, glucose intolerance, hepatic steatosis, and insulin resistance, and others explained by the bone–testes axis, such as decreased circulating testosterone, IGF1, and insulin, and increased estradiol, LH, and growth hormone, and still others implicating effects on the kidney (ie, hyperphosphatemia) and bone (ie, osteopenia). Thus, GPRC6A, as the biologically relevant receptor for Ocn, not only defines a molecular mechanism for linking bone metabolism with metabolic regulation of β-cells and sexual reproduction, but as a common receptor mediating the effects of testosterone and dietary factors also involved in multiple endocrine networks integrating the functions of pancreas, muscle, liver, fat, testes, bone, and the hypothalamic–pituitary axis with alterations in both environmental and endogenous ligands.
LINKAGE BETWEEN Ocn AND FGF-23 ENDOCRINE NETWORKS?
FGF23 and Ocn may be interconnected through leptin, the adipocyte-derived hormone that regulates appetite.80 Leptin directly stimulates FGF23 expression and there is evidence that leptin inhibits insulin secretion indirectly through suppression of Ocn release from bone by actions of leptin to activate the sympathetic nervous system.72 Leptin, an adipocyte-specific molecule that targets the hypothalamus to limit appetite and increase energy expenditure, also acts centrally to reduce bone mass through a complex signaling involving serotonin and stimulation of the sympathetic nervous system acting through the β2 adrenergic receptor. Leptin inhibits bone mass accrual and appetite. The leptin receptor belongs to the class I cytokine receptor superfamily and possesses strong homology to the signal-transducing subunits of the interleukin-6 receptor.
It has been proposed that leptin, through the suppression of Ocn, acts as a physiological break on the insulin-induced Ocn feed-forward loop regulating energy metabolism.80 Leptin directly stimulates FGF23 synthesis in bone cells.81 Thus, leptin regulation of FGF23 and Ocn are related inversely. Because cellular uptake of phosphate is necessary for energy use in peripheral tissues, leptin suppression of insulin would decrease insulin-mediated phosphate uptake into cells and leptin stimulation of FGF23 would lead to an increase in renal phosphate excretion. The differential regulation of FGF23 and Ocn-mediated insulin secretion theoretically would allow renal phosphate handling to be coordinated with cellular uptake of phosphate. This possible endocrine network requires experimental validation. However, anorexia is a common complication in patients with CKD. Serum leptin concentrations are increased in patients with CKD82 and uremic serum stimulates the release of leptin from adipocytes.83 The insulin-responsive serum glucocorticoid-regulated kinase 3 (Sgk3) pathway also regulates FGF23 expression in bone, as evidenced by reduced FGF23 levels in Sgk3 knockout mice.84,85 In addition, there is a significant inverse correlation between FGF23 and fasting insulin level, insulin resistance index,86 and metabolic syndrome.87 FGF23 also may be linked to energy metabolism via its suppression of α-Kl expression,88,89 which can function as a circulating hormone affecting insulin90 and Wnt signaling.91 A complex network may exist in which insulin regulates FGF23 through activation of insulin receptor (InsR) in osteoblasts, which secrete FGF23 to regulate kidney retention of phosphate proportionate to the increased use of phosphate in peripheral tissues in postprandial states of nutrient excess. FGF23 and Ocn also may be linked through alterations in bone remodeling and mineralization.
CLINICAL IMPLICATIONS OF FGF-23 IN CKD
Altered Understanding of the Pathogenesis and Treatment of Secondary Hyperparathyroidism in CKD
The physiological relevance of the FGF23 endocrine network in human beings is well established by the effects of high and low FGF23 levels to cause, respectively, hereditary hypophosphatemic and hyperphosphatemic disorders. The clinical relevance of FGF23 in the setting of normal renal function is to maintain 1,25(OH)2D and phosphate homeostasis, as noted earlier. In CKD, FGF23 shows both adaptive and maladaptive functions that depend on the degree of renal impairment and the levels of FGF23. Cross-sectional studies in human beings show early FGF23 increased in CKD in proportion to reduced glomerular filtration rate92 and greater increases in ESRD.35,92,93 FGF23 levels correlate with the degree of hyperphosphatemia35,93 and predict refractory HPT.52 Before the discovery of FGF23, secondary HPT in CKD was thought to be caused by a decline in Cyp27b1-mediated production of 1,25(OH)2D by the proximal tubule owing to loss of renal mass.94 There are emerging data to support the hypothesis that increased FGF23 is the initial event leading to subsequent reductions in 1,25(OH)2D and increases of PTH in CKD. In this conceptual framework, CKD does not represent a true vitamin D– deficient state; rather FGF23–mediated suppression of circulating 1,25(OH)2D levels is an adaptive response, which protects against hyperphosphatemia through a reduction of 1,25(OH)2D’s effects on gastrointestinal phosphate absorption and by increasing PTH, which acts in concert with FGF23 to stimulate phosphaturia. Thus, FGF23 may be an early biomarker for earlier interventions.
In addition, treatment approaches to prevent the increases of FGF23 may become the initial therapeutic focus. Treatment with paracalcitol further increases FGF23 in ESRD.95 Because calcitriol analogues increase FGF23, calcitriol-sparing therapies may be warranted, such as combined low-dose paracalcitol and calcimimetics, which decreases FGF23 levels in ESRD patients.96,97 Alternatively, if FGF23–stimulated catabolism of 1,25(OH)2D is an important mechanism for reduced 1,25(OH)2D levels, there may be a role for Cyp24 inhibitors in the management of CKD mineral and bone disease.
With regard to bone, there is a debate regarding whether FGF23 has direct effects, or whether the bone changes are caused by PTH. The absence of α-Klotho in bone and other studies showing correction of defective mineralization in FGF23 null mice by ablation of 1-α hydroxylase suggests that these changes are secondary. However, increased FGF23 is associated with fracture healing, suggesting that it is a marker of osteoblast activity.98 If so, FGF23 may be a marker of bone turnover in secondary HPT in ESRD, and FGF23 may be a bio-marker for bone remodeling. Further studies are needed to define the relationship between bone turnover and circulating FGF23 levels in CKD.
Role of FGF-23 in Increased Mortality in CKD
Finally, the increase in FGF23, which initially is a positive adaptive response for maintenance of phosphate balance at the expense of suppressing 1,25(OH)2D production in early CKD, becomes maladaptive with more advanced CKD. Indeed, epidemiologic studies in both ESRD and CKD have found that increased circulating FGF23 levels is a strong independent risk factor for both renal failure progression and cardiovascular mortality, independent of serum phosphate levels.99 Increased circulating FGF23 concentrations also are associated with progression of renal disease100 and left ventricular hypertrophy, fat mass, and dyslipidemia in elderly patients.87 This positive correlation between FGF23 and mortality also is found in the general population with coronary artery disease.5 At present, it is not certain if these untoward effects associated with increases of FGF23 in CKD are caused FGF23–mediated toxicity or represent epiphenomena of the uremic state.
Currently, the evidence for FGF23 having a causative role in increased cardiovascular morbidity is indirect, but supported by multiple associative studies and several competing, but biologically plausible, hypotheses for mechanisms underlying the adverse effects of FGF23. One possibility is that increased cardiovascular mortality associated with FGF23 is mediated indirectly by FGF23 suppression of 1,25(OH)2D and effects of this functional vitamin D–deficient state. The effects of low 1,25(OH)2D on the cardiovascular system is thought to be mediated by activation of the RAS in CKD, leading to hypertension, left ventricular hypertrophy, and increased cardiovascular mortality (Fig. 3). Involvement of the RAS is plausible because of activation of the renal RAS in CKD and the beneficial effects of inhibition of RAS in protection against progressive CKD.
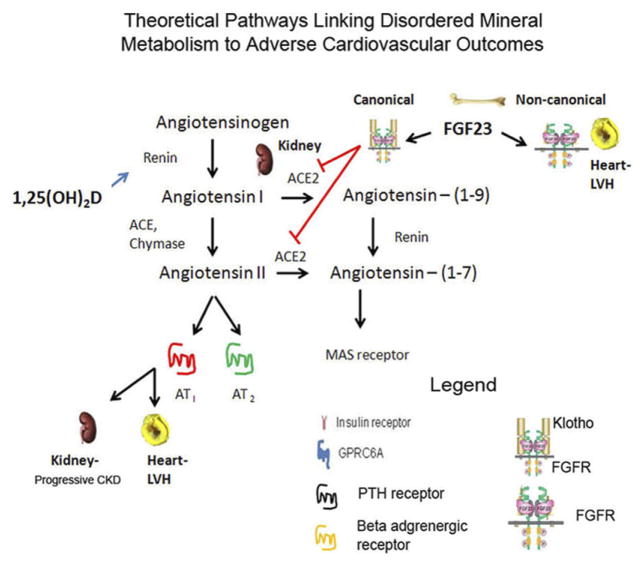
Figure 3.
The RAS. Angiotensin II is generated in the kidney by renin-mediated conversion of angiotensinogen to angiotensin followed by ACE conversion of Ang I to Ang II, which activates AT1 and AT2 receptors in target tissues. The RAS is activated in chronic kidney disease and contributes to increased cardiovascular mortality and renal failure progression. RAS is linked to the PTH–vitamin D endocrine network in CKD via effects of reduced 1,25(OH)2D to suppress renin and FGF23 effects to suppress ACE2, but leading to activation of RAS. LVH, left ventricular hypertrophy.
Angiotensin II is generated in the kidney by a cascade initiated by renin conversion of angiotensinogen to angiotensin I in the macula densa followed by angiotensin-converting enzyme (ACE) conversion of Ang I to Ang II, which is the ligand for both AT1 and AT2 receptors. ACE inhibitors target ACE, leading to reductions in Ang II and reduced stimulation of both AT1 and AT2 receptors. In experimental models of chronic kidney disease the RAS is up-regulated.101 Experimental studies in animals and clinical trials have shown a beneficial effect of ACE inhibitors and angiotensin-receptor blockers on control of hypertension, suppression of proteinuria, and the progression of kidney disease. Ang II is degraded to ANG-(1-7) by ACE2, which is also highly expressed in the kidney.
This vitamin D–RAS hypothesis is supported by the known effects of 1,25(OH)2D to suppress renin and the effects of vitamin D deficiency to activate the renin angiotensin system.102 Moreover, low 25(OH)D and 1,25(OH)2D in patients with CKD are associated with increased all-cause mortality and more rapid progression of kidney disease, and use of active vitamin D analogues to treat secondary hyperparathyroidism in CKD is associated with improved survival. However, these associative studies do not prove a cause and effect. Indeed, additional data challenge the salutary effects of active vitamin D therapy in ESRD. In this regard, active vitamin D therapy increases serum phosphate as a result of the effects to enhance phosphate transport and increases serum FGF23 levels as a result of the actions of active vitamin D analogues to stimulate FGF23 gene transcriptions. Both the resulting hyperphosphatemia and increased FGF23 are independent risk factors for increased mortality in CKD. Most importantly, the Paricalcitol Capsule Benefits in Renal Failure–Induced Cardiac Morbidity (PRIMO) study, a randomized double-blinded trial of paricalcitol on left ventricular hypertrophy in CKD, failed to identify a significant effect of active vitamin D treatment on cardiovascular end points.103
Another possibility is that FGF23 contributes to the increased cardiovascular morality in CKD. Multiple observational studies show a strong association between increases in circulating FGF23 concentrations and increased mortality in CKD, as well as in the general population.99 Whether FGF23–associated mortality represents causation, and, if so, by what mechanisms,104 is an area of active investigation.
There are two, not mutually exclusive, mechanisms whereby FGF23 may impact cardiovascular disease. First, there is evidence that FGF23 may regulate the RAS via suppression of ACE2 expression in the kidney. In experimental models of FGF23 excess α-Klotho expression is decreased and there is evidence for direct effects of FGF23 to suppress α-Klotho message expression through activation of FGFR/α-Klotho complexes (canonical pathway) in the distal tubule. ACE2 is an important counter-regulatory enzyme in the intrarenal RAS that may protect against renal injury by reducing kidney levels of ANG II.105 In a remnant kidney model, a high-phosphate diet further increased activation of the RAS in association with high FGF23 and low ACE2 expression.101 In mice, the genetic ablation of ACE2 leads to glomerulosclerosis, but additional information is needed regarding the relevance of ACE2 and ANG II degradation in CKD in human beings. Consistent with an effect of FGF23 on the cardiovascular system, X-linked hypophosphatemia (XLH) patients with increased FGF23 levels have a higher prevalence of left ventricular hypertrophy.106 Second, there are data that high levels of FGF23 may directly stimulate myocardial cells, leading to hypertrophy.107 This effect was observed in isolated neonatal cardiomyocytes that lack expression of α-Klotho, and only at concentrations greater than 10 ng/mL, a concentration that exceeds the normal circulating levels of FGF23 (0.05 ng/mL) by 200-fold. Thus, extremely high FGF23 levels directly activate FGFRs via noncanonical, α-Klotho–independent pathways. Understanding the cause-and-effect relationship between FGF23 and adverse outcomes and the mechanism of this effect is of critical importance because activation of the RAS may be treated by inhibitors of this pathway whereas off-target effects of FGF23 may require specific inhibition of the FGF23 signaling pathway, either through blocking antibodies or inhibition of FGF23 production or receptor activation.
There is also a competing hypothesis that activation of RAS inhibits α-Klotho expression in the kidney, which in turn leads to secondary increments in FGF23 in CKD.108 FGF23 and 1,25(OH)2D, however, suppress and stimulate, respectively, α-Klotho expression through direct actions on the distal tubule. Thus, the reductions in α-Klotho expression in CKD may be secondary to primary increases of FGF23 or FGF23–mediated reductions in 1,25(OH)2D, rather than in response to end-organ resistance to FGF23 caused by renal disease. Further studies are needed to understand the mechanisms of increased circulating FGF23 in CKD.
UNCERTAIN CLINICAL SIGNIFICANCE OF Onc-GPRC6A ENDOCRINE NETWORK IN CKD
The clinical significance of the Ocn endocrine networks is less certain and, at present, no mutations of Ocn have been reported to cause diseases in human beings. Polymorphisms in the Ocn receptor, GPRC6A, are associated with osteopenia in human beings13 and the GPRC6a locus is associated with increased prostate cancer risk in Asian males.109 Genome-wide association studies show that GPRC6A is a genetic locus highly associated with C-reactive protein levels,110 a heritable marker of chronic inflammation that is associated strongly with diabetes mellitus111 and cardiovascular diseases.112,113
Based on our knowledge of the function of GPRC6A derived from mouse genetic studies, increased uncarboxylated Ocn, and activation of GPRC6A, which is present in multiple organs, would be predicted to result in increased insulin secretion, improved insulin sensitivity, increased serum testosterone, and decreased fat mass.114 As noted earlier, Ocn stimulates insulin secretion and β-cell proliferation via GPRC6A, is lower in patients with diabetes, who have a low-turnover bone disease, and is a biomarker of insulin resistance in human beings.67,71,115,116 Ocn is correlated inversely with body mass index, fasting glucose and insulin, triglycerides, and leptin, and correlated positively with adiponectin.117 Administration of PTH increases and alendronate decreases Ocn levels and changes in Ocn are associated with changes in body weight, fat mass, and adiponectin concentrations, consistent with a role for Ocn in the skeletal regulation of energy metabolism.118 The Ocn endocrine axis may be particularly relevant to skeletal growth during male puberty. During rapid skeletal growth, increments in testosterone levels initiated by alterations in the hypothalamic–pituitary axis may be augmented further by increasing Ocn as a result of skeletal growth, thereby increasing bone size in males.79
Ocn is increased in CKD, likely owing to increased bone resorption, but its contribution to abnormalities in energy metabolism in CKD is not certain. Vitamin K status also determines the carboxylation of Ocn. Because CKD often is associated with vitamin K deficiency,119 this could contribute to increased uncarboxylated Ocn. CKD is associated with impaired glucose-mediated insulin secretion, increased insulin resistance, and decreased testosterone levels,120 alterations that are opposite from those predicted by increased Ocn and activation of GPRC6A. Thus, there currently is little evidence for activation of the Ocn–GPRC6A endocrine network in CKD. Serum Ocn, however, is associated positively with circulating adiponectin levels in patients with CKD121 and further studies are needed to understand the Ocn–GPRC6A endocrine networks in CKD. Impaired bone resorption results in a decrease in the uncarboxylated form of Ocn and glucose intolerance in both mice and human beings. Conversely, the ability of a high-fat diet to induce glucose intolerance in mice is attenuated by RANK ligand (RANKL)-mediated increases in bone resorption and release of bioactive Ocn. Recent studies have indicated that increased circulating concentrations of uncarboxylated Ocn are associated with lower mortality rates in an outpatient population with coronary artery disease.5 Thus, increments in Ocn in CKD may yet prove to have an important role in regulating energy metabolism.
SYSTEMS BIOLOGY APPROACH?
The depiction of endocrine pathways as bidirectional connections between a few endocrine pathways (Figs. 1–3) oversimplifies the actual complexity of these networks in CKD. The complexity of the biological regulatory systems and the new knowledge of genomics and proteomics necessitate a new way of thinking and testing of hypotheses regarding the physiology and pathophysiology of CKD. Even with the existing knowledge, more complex networks can be identified by computational models, such as the Ingenuity Pathway Analysis software program (Redwood City, CA). Ingenuity Pathway Analysis, performed on the genes depicted in Figures 1 through 3, showed that multiple interconnections exist between the PTH, vitamin D, FGF23, RAS, and Ocn endocrine networks (Fig. 4). The top networks identified by this analysis are drug metabolism, lipid metabolism, and molecular transport. Despite the complexity of the networks shown in Figure 4, even more data will be generated regarding genomic polymorphisms and differences in gene expression profiles, as well as proteomic, kinomics, and phospho-proteomic analysis of individual tissues in CKD to create a detailed integrative model of the uremic state. Although less practical in understanding physiological cause-and-effect relationships, the development of more robust computational models will provide more informative insights into the pathogenesis of CKD and lead to more rational, multisystem approaches to manage this disorder. The limited understanding of the multiple connections between pathways and the fact that treatments are directed at only some components of these networks may explain why current therapeutic interventions have not lead to improved survival in the ESRD population.
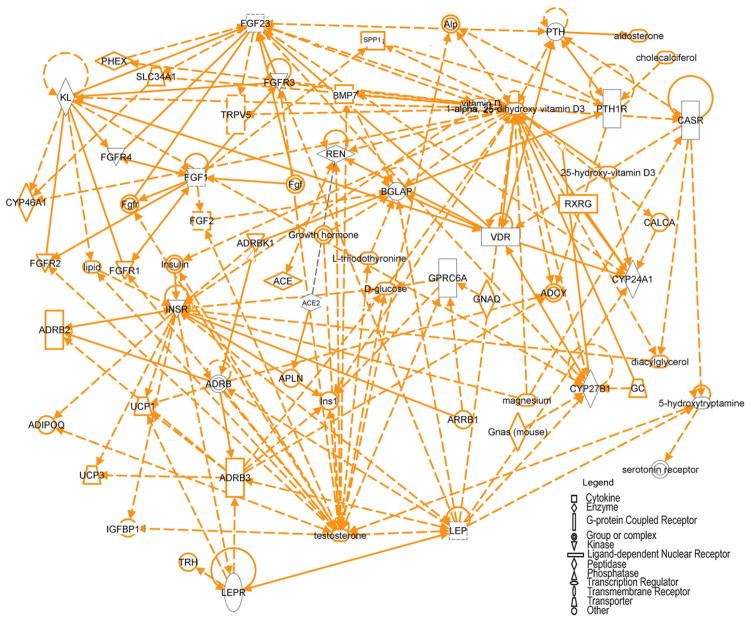
Figure 4.
Ingenuity pathway analysis of endocrine networks depicted in Figures 1 through 3. The Ingenuity Pathway Analysis systems identified multiple connections between these endocrine networks. Gene products are represented as nodes and biological relationships between two nodes are represented as a line. Continuous lines indicate direct interactions, and dashed lines represent indirect connections. Shapes of nodes symbolize functional classes of gene products. Notable genes identified in the network include ACE2, β-adrenergic receptor (ADRB), bone gamma-carboxylgutamic acid protein (BGLAP), Ocn, CASR, CYP24A1, CYP27B1, FGF23, FGFR, GPRC6A, insulin receptor (INSR), KL, leptin (LEP), leptin receptor (LEPR), PTH, PTH1R, renin (REN), vitamin D receptor (VDR), SPP1, TRPV5, uncoupling protein (UCP), phosphate regulating endopeptidase (Phex), alkaline phosphatase (Alp), glucocorticoid (GC).
Footnotes
Financial disclosure and conflict of interest statements: none.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, Reed A, Kaplan B. Kidney transplantation halts cardiovascular disease progression in patients with end-stage renal disease. Am J Transplant. 2004;4:1662–8. doi: 10.1111/j.1600-6143.2004.00573.x. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl. 2010;117:S10–21. doi: 10.1038/ki.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–37. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- 5.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–8. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–56. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 7.Di Iorio B, Bellasi A, Russo D. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7:487–93. doi: 10.2215/CJN.03820411. [DOI] [PubMed] [Google Scholar]
- 8.Quarles LD. The bone and beyond:‘Dem bones’ are made for more than walking. Nat Med. 2011;17:428–30. doi: 10.1038/nm0411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goltzman D, Miao D, Panda DK, Hendy GN. Effects of calcium and of the Vitamin D system on skeletal and calcium homeostasis: lessons from genetic models. J Steroid Biochem Mol Biol. 2004;89–90:485–9. doi: 10.1016/j.jsbmb.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 10.Messer HH, Yuen SY, Copp DH. Net uptake and release of calcium and phosphate by bone in vitro: effects of medium calcium and phosphate concentrations. Calcif Tissue Res. 1975;19:1–7. doi: 10.1007/BF02563985. [DOI] [PubMed] [Google Scholar]
- 11.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26:677–80. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 13.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–3. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–8. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 15.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–56. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–26. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 20.Stubbs J, Liu S, Quarles LD. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin Dial. 2007;20:302–8. doi: 10.1111/j.1525-139X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 21.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–86. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 24.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–95. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 25.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–79. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 26.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–94. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 27.Fukumoto S, Yamashita T. Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr Opin Nephrol Hypertens. 2002;11:385–9. doi: 10.1097/00041552-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–90. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 29.Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, et al. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005;146:3883–91. doi: 10.1210/en.2005-0431. [DOI] [PubMed] [Google Scholar]
- 30.Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, et al. Genetic ablation of vitamin d activation pathway reverses biochemical and skeletal anomalies in fgf-23-null animals. Am J Pathol. 2006;169:2161–70. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–32. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato K, Jeanneau C, Tarp MA, Benet-Pages A, Lorenz-Depiereux B, Bennett EP, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–7. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–15. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 34.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–42. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 35.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–34. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 36.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–91. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–91. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:257–64. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagano N, Miyata S, Abe M, Kobayashi N, Wakita S, Yamashita T, et al. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006;69:531–7. doi: 10.1038/sj.ki.5000020. [DOI] [PubMed] [Google Scholar]
- 40.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, et al. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–9. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Tang W, Fang J, Ren J, Li H, Xiao Z, et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–18. doi: 10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92:131–55. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quarles LD. Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest. 2003;112:642–6. doi: 10.1172/JCI19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, et al. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–19. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD. Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol. 2007;192:261–7. doi: 10.1677/joe.1.07059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 49.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–9. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, Zhai G, Kato BS, Hart DJ, Hunter D, Spector TD, et al. Association between KLOTHO gene and hand osteoarthritis in a female Caucasian population. Osteoarthritis Cartilage. 2007;15:624–9. doi: 10.1016/j.joca.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–8. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 52.Komaba H, Fukagawa M. FGF23-parathyroid interaction: implications in chronic kidney disease. Kidney Int. 2010;77:292–8. doi: 10.1038/ki.2009.466. [DOI] [PubMed] [Google Scholar]
- 53.Brown WW, Juppner H, Langman CB, Price H, Farrow EG, White KE, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–8. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 55.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–9. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 56.Sato T, Tominaga Y, Ueki T, Goto N, Matsuoka S, Katayama A, et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:481–7. [PubMed] [Google Scholar]
- 57.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–43. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, et al. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154:93–9. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 59.Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res. 2011;26:1035–46. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saji F, Shiizaki K, Shimada S, Okada T, Kunimoto K, Sakaguchi T, et al. Regulation of fibroblast growth factor 23 production in bone in uremic rats. Nephron Physiol. 2009;111:59–66. doi: 10.1159/000210389. [DOI] [PubMed] [Google Scholar]
- 61.Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology. 2009;150:4835–45. doi: 10.1210/en.2009-0472. [DOI] [PubMed] [Google Scholar]
- 62.Tebben PJ, Singh RJ, Clarke BL, Kumar R. Fibroblast growth factor 23, parathyroid hormone, and 1alpha,25-dihydroxyvita-min D in surgically treated primary hyperparathyroidism. Mayo Clin Proc. 2004;79:1508–13. doi: 10.4065/79.12.1508. [DOI] [PubMed] [Google Scholar]
- 63.Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36:971–7. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Gutierrez OM, Smith KT, Barchi-Chung A, Patel NM, Isakova T, Wolf M. (1–34) parathyroid hormone infusion acutely lowers fibroblast growth factor 23 concentrations in adult volunteers. Clin J Am Soc Nephrol. 7:139–45. doi: 10.2215/CJN.06240611. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pi M, Zhang L, Lei SF, Huang MZ, Zhu W, Zhang J, et al. Impaired osteoblast function in GPRC6A null mice. J Bone Miner Res. 2010;25:1092–102. doi: 10.1359/jbmr.091037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pi M, Quarles LD. GPRC6A regulates prostate cancer progression. Prostate. 2012;72:399–409. doi: 10.1002/pros.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–3. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bodine PV, Komm BS. Evidence that conditionally immortalized human osteoblasts express an osteocalcin receptor. Bone. 1999;25:535–43. doi: 10.1016/s8756-3282(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 71.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–75. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–42. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pi M, Parrill AL, Quarles LD. GPRC6A mediates the nongenomic effects of steroids. J Biol Chem. 2010;285:39953–64. doi: 10.1074/jbc.M110.158063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grillo ML, Jacobus AP, Scalco R, Amaral F, Rodrigues DO, Loss ES, et al. Testosterone rapidly stimulates insulin release from isolated pancreatic islets through a nongenomic dependent mechanism. Horm Metab Res. 2005;37:662–5. doi: 10.1055/s-2005-870575. [DOI] [PubMed] [Google Scholar]
- 75.Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–9. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasschaert J, Malaisse WJ. Expression of the calcium-sensing receptor in pancreatic islet B-cells. Biochem Biophys Res Commun. 1999;264:615–8. doi: 10.1006/bbrc.1999.1577. [DOI] [PubMed] [Google Scholar]
- 77.Ozbek M, Erdogan M, Karadeniz M, Cetinkalp S, Ozgen AG, Saygili F, et al. Evaluation of beta cell dysfunction by mixed meal tolerance test and oral L-arginine in patients with newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2009;117:573–6. doi: 10.1055/s-0029-1234087. [DOI] [PubMed] [Google Scholar]
- 78.Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, Elahi D, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005;90:2636–41. doi: 10.1210/jc.2004-2190. [DOI] [PubMed] [Google Scholar]
- 79.Kirmani S, Atkinson EJ, Melton LJ, 3rd, Riggs BL, Amin S, Khosla S. Relationship of testosterone and osteocalcin levels during growth. J Bone Miner Res. 2011;26:2212–6. doi: 10.1002/jbmr.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–20. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25:1711–23. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 82.Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH. Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest. 2005;115:1659–65. doi: 10.1172/JCI22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalbacher E, Koppe L, Zarrouki B, Pillon NJ, Fouque D, Soulage CO. Human uremic plasma and not urea induces exuberant secretion of leptin in 3T3-L1 adipocytes. J Ren Nutr. 2011;21:72–5. doi: 10.1053/j.jrn.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Bhandaru M, Kempe DS, Rotte A, Capuano P, Pathare G, Sopjani M, et al. Decreased bone density and increased phosphaturia in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase 3. Kidney Int. 2011;80:61–7. doi: 10.1038/ki.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11:147–60. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wojcik M, Dolezal-Oltarzewska K, Janus D, Drozdz D, Sztefko K, Starzyk JB. FGF23 contributes to insulin sensitivity in obese adolescents–preliminary results. Clin Endocrinol (Oxf) 2012;77:537–40. doi: 10.1111/j.1365-2265.2011.04299.x. [DOI] [PubMed] [Google Scholar]
- 87.Mirza MA, Alsio J, Hammarstedt A, Erben RG, Michaelsson K, Tivesten A, et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31:219–27. doi: 10.1161/ATVBAHA.110.214619. [DOI] [PubMed] [Google Scholar]
- 88.Li H, Martin AC, David V, Quarles LD. Compound deletion of FGFR3 and FGFR4 partially rescues the hyp mouse phenotype. Am J Physiol Endocrinol Metab. 2011;300:E508–17. doi: 10.1152/ajpendo.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 90.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 91.Kuro-o M. Klotho. Pflugers Arch. 2010;459:333–43. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 92.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 93.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, et al. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65:1943–6. doi: 10.1111/j.1523-1755.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 94.Craver L, Marco MP, Martinez I, Rue M, Borras M, Martin ML, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1–5—achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–6. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 95.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Chole-calciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–61. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wetmore JB, Quarles LD. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nat Clin Pract Nephrol. 2009;5:24–33. doi: 10.1038/ncpneph0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wetmore JB, Liu S, Krebill R, Menard R, Quarles LD. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5:110–6. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goebel S, Lienau J, Rammoser U, Seefried L, Wintgens KF, Seufert J, et al. FGF23 is a putative marker for bone healing and regeneration. J Orthop Res. 2009;27:1141–6. doi: 10.1002/jor.20857. [DOI] [PubMed] [Google Scholar]
- 99.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol. 2007;18:2600–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 101.Eraranta A, Riutta A, Fan M, Koskela J, Tikkanen I, Lakkisto P, et al. Dietary phosphate binding and loading alter kidney angiotensin-converting enzyme mRNA and protein content in 5/6 nephrectomized rats. Am J Nephrol. 2012;35:401–8. doi: 10.1159/000337942. [DOI] [PubMed] [Google Scholar]
- 102.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–84. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 104.Stubbs JR, Quarles LD. Fibroblast growth factor 23: uremic toxin or innocent bystander in chronic kidney disease? Nephrol News Issues. 2009;23:33–4. 36–7. [PubMed] [Google Scholar]
- 105.Dilauro M, Zimpelmann J, Robertson SJ, Genest D, Burns KD. Effect of ACE2 and angiotensin-(1–7) in a mouse model of early chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1523–32. doi: 10.1152/ajprenal.00426.2009. [DOI] [PubMed] [Google Scholar]
- 106.Nehgme R, Fahey JT, Smith C, Carpenter TO. Cardiovascular abnormalities in patients with X-linked hypophosphatemia. J Clin Endocrinol Metab. 1997;82:2450–4. doi: 10.1210/jcem.82.8.4181. [DOI] [PubMed] [Google Scholar]
- 107.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, et al. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011;26:800–13. doi: 10.1093/ndt/gfq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takata R, Akamatsu S, Kubo M, Takahashi A, Hosono N, Kawaguchi T, et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42:751–4. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 110.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–8. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56:872–8. doi: 10.2337/db06-0922. [DOI] [PubMed] [Google Scholar]
- 112.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 113.Hwang YC, Jeong IK, Ahn KJ, Chung HY. Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level. Osteoporos Int. 2012;23:1337–42. doi: 10.1007/s00198-011-1679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3:e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, et al. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J Clin Endocrinol Metab. 2011;96:E1092–9. doi: 10.1210/jc.2010-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827–32. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schafer AL, Sellmeyer DE, Schwartz AV, Rosen CJ, Vittinghoff E, Palermo L, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in post-menopausal women with osteoporosis (the PaTH study) J Clin Endocrinol Metab. 2011;96:E1982–9. doi: 10.1210/jc.2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schlieper G, Westenfeld R, Kruger T, Cranenburg EC, Magde-leyns EJ, Brandenburg VM, et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011;22:387–95. doi: 10.1681/ASN.2010040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. 1981;67:563–8. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bacchetta J, Boutroy S, Guebre-Egziabher F, Juillard L, Drai J, Pelletier S, et al. The relationship between adipokines, osteocalcin and bone quality in chronic kidney disease. Nephrol Dial Transplant. 2009;24:3120–5. doi: 10.1093/ndt/gfp262. [DOI] [PubMed] [Google Scholar]