Abstract
A bone-pancreas endocrine loop has been identified recently that involves insulin secreted from β-cells in the pancreas stimulating insulin receptors in osteoblasts, leading to osteoblastic differentiation and increased secretion of osteocalcin (Ocn), a bone-derived hormone that regulates insulin secretion in β-cells. The identity of the Ocn-sensing receptor in β-cells is a missing component of this endocrine loop. The abnormalities in glucose homeostasis in Gprc6a null mice suggests that this pertussis toxin–sensitive G protein– coupled receptor is a candidate for mediating the effects of Ocn on insulin secretion in the pancreas. In support of this possibility, we found that transfection of non-Gprc6a-expressing HEK-293 cells with a full-length Gprc6a cDNA imparted a dose-dependent response to Ocn (5 to 60 ng/mL), as measured by PKD1 and ERK phosphorylation. In addition, Gprc6a is highly expressed in mouse pancreatic tissue and in the mouse TC-6 pancreatic β-cell line. Ocn also stimulated ERK activity in TC-6 pancreatic β-cells. Finally, intraperitoneal injection of Ocn stimulated ERK activity in the pancreas and increased serum insulin levels in wild-type mice, but these responses were markedly attenuated in Gprc6a−/− mice. These findings suggest that GPRC6A is a candidate for mediating the response to Ocn in the bone-pancreas endocrine loop regulating insulin signaling.
Keywords: GPRC6A, OSTEOCALCIN RECEPTOR, GPCR, INSULIN, PANCREATIC β CELLS
Introduction
Osteocalcin (Ocn), also known as bone γ-carboxyglutamic acid–containing protein (BGLAP), is a multifunctional protein secreted exclusively by osteoblasts. Ocn undergoes vitamin K–dependent carboxylation on three Gla residues that allows calcium binding and sequestration to bone mineral surfaces, where it facilitates attachment of osteoclasts and precursor myelomonocytes leading to bone resorption. A small portion of osteocalcin remains undercarboxylated and is secreted into the circulation.(1) Recently, uncarboxylated Ocn has been implicated in a bone-pancreas endocrine loop through which insulin signaling in the osteoblasts stimulates osteocalcin production, which, in turn, regulates insulin sensitivity and pancreatic insulin secretion.(2–4) The molecular mechanism whereby Ocn regulates pancreatic insulin secretion is not known.
GPRC6A is orphan receptor belonging to the C family of GPCRs, which consists of eight metabotropic glutamate receptors (mGluR1–8), two γ-aminobutyric acid receptors (GABAβR1/2), three taste receptors (T1R1, T1R2, and T1R3), the calcium-sensing receptor CaSR, and five other orphan receptors (RAIG1, GPRC5B–D, and GABAβ).(5–10) GPRC6A is widely expressed and senses amino acids and extracellular calcium.(11–13) We also have shown that Ocn in the presence of extracellular calcium significantly enhanced SRE-luciferase activity in HEK-293 cells that stably expressed Gprc6a.(11) The physiologic functions of GPRC6A are not clear, but Gprc6a null mice have a complex phenotype involving multiple organ systems, including osteopenia, hepatic steatosis, hyperglycemia, glucose intolerance, and insulin resistance.(11,14) These metabolic abnormalities suggest that GPRC6A might participate in the bone-pancreas endocrine loop by mediating the response of circulating osteoclacin.
To test this possibility that GPRC6A is the putative Ocn receptor, we employed a heterologous cell system expressing Gprc6a, pancreatic β-cells, and Gprc6a null mice to assess the role of GPRC6A in mediating the responses of Ocn in vitro and in vivo. Our findings suggest that GPRC6A is a physiologically relevant Ocn-sensing receptor.
Materials And Methods
Cell culture, reagents, and antibodies
Human embryonic kidney (HEK-293) and mouse pancreatic β-cell TC-6 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% PBS (Gibco Life Technologies, Inc., Rockville, MD, USA) in humidified 5% CO2 at 37°C. Calcium chloride, l-arginine, and U73122 were purchased from Sigma Chemical Company (St Louis, MO, USA). Bovine serum albumin (faction V) was obtained from Roche Applied Science (Indianapolis, IN, USA). Osteocalcin recombinant protein with GST (human full-length protein) was purchased from Novus USA (Littleton, CO, USA). Ro31-8220 was obtained from Calbiochem (La Jolla, CA, USA). The Phospho-PKD/PKCmu (Ser744/748) antibody was purchase from Cell Signaling Technology (Beverly, MA, USA). Insulin (mouse) ultrasensitive ELISA kit was obtained from ALPCO Immunoassays (Salem, NH, USA).
RT-PCR
Total RNA was isolated from TC-6 cells and mouse pancreas with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed using two-step RNA PCR (Perkin-Elmer, Waltham, MA, USA). The primers for mouse GPRC6A consisted of mGPRC6A.F189: CGGGATccagacgaccacaaatccag and mGPRC6A.R539: CCAAGCTTgattcataactcacctgt, and for the housekeeping gene control G3PDH gene consisted of G3PDH.F143: gaccccttcattgacctcaactaca and G3PDH.R1050: ggtcttactccttggaggccatgt.
Measurement of total and phospho-ERK by Western blot
HEK-293, HEK-293 transfected with Gprc6a, and TC-6 cells (2 × 105 cells/well) were cultured in triplicate in 6-well plates in DMEM supplemented with 10% fetal bovine serum and 1% PBS for 48 hours followed by overnight incubation in DMEM/F12 containing 0.1% BSA to achieve quiescence. Quiescent cells were treated with various concentrations of GPRC6A ligands, including calcium, arginine, and osteocalcin (Ocn) for 5 minutes at 37°C. ERK activation will be assessed by immunoblotting using anti-phospho-ERK1/2 MAP kinase antibody (Cell Signaling Technology) corrected for the total amount of ERK using an anti-ERK1/2 MAP kinase antibody (Cell Signaling Technology).
Mouse models
Mice were maintained and used in accordance with recommendations described previously (National Research Council, 1985; Guide for the Care and Use of Laboratory Animals, DHHS Publication NIH 86-23; Institute on Laboratory Animal Resources, Rockville, MD, USA) and following guidelines established by the University of Tennessee Health Science Center Institutional Animal Care and Use Committee. The Gprc6a-deficient mouse model was created and genotyped as described previously.(14) Ocn (1 or 3 µg/kg) or PBS vehicle was injected into the intraperitoneal cavity of wild-type (Gprc6a+/+) and Gprc6a−/− mice, and the pancreas was harvested after 20 minutes for assessment of ERK phosphorylation and 4 hours for serum collection.(15,16)
Statistics
We evaluated differences between groups by one-way analysis of variance. All values are expressed as means ± SEM. All computations were performed using the Statgraphic statistical graphics system (STSC, Inc., Rockville, MD, USA).
Results
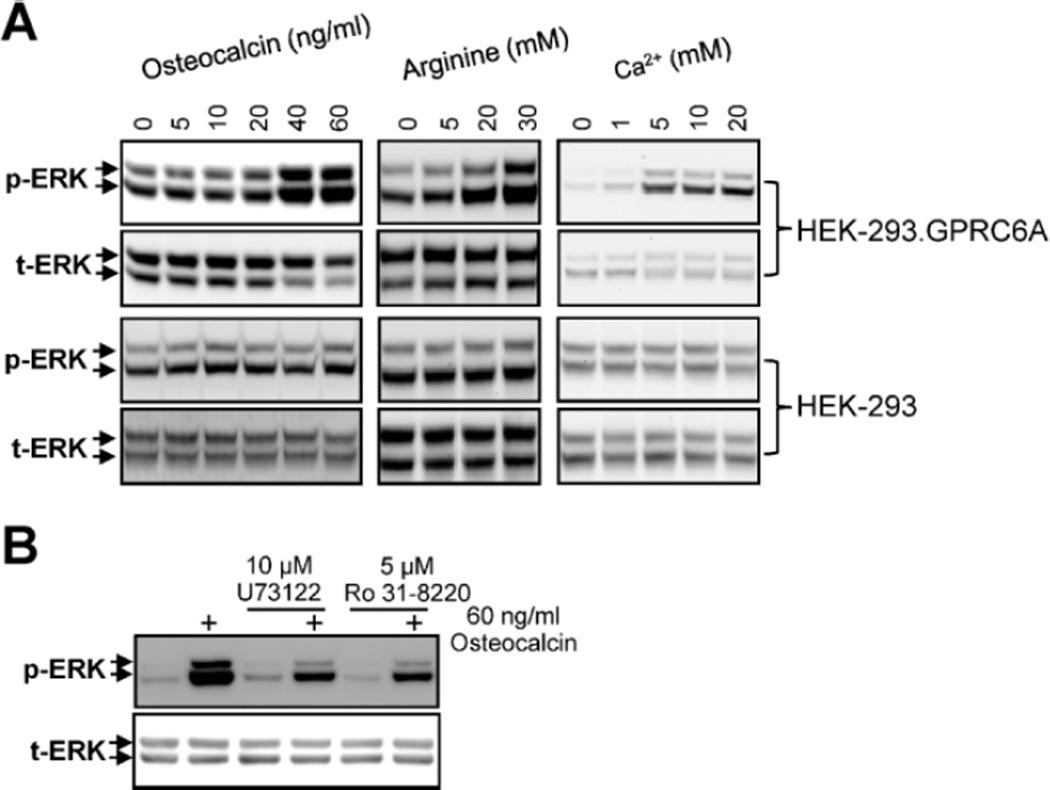
First, we confirmed that Ocn activates GPRC6A.(11,17) Recombinant human Ocn (5 to 60 ng/mL) resulted in a dose-dependent stimulation of ERK activity in HEK-293 cells overexpressing Gprc6a but not in untransfected controls (Fig. 1A). The response of GPRC6A to Ocn was similar to that of known GPRC6A ligands,(11,17) calcium (1 to 20 mM), and arginine (5 to 30 mM). The putative Ocn receptor is pertussis toxin–sensitive and is coupled with phospholipase C (PLC) and protein kinase C (PKC).(18) We found that recombinant human Ocn activation of phospho-ERK in Gprc6a-transfected HEK-293 cells was significantly blocked by U73122 (PI-PLC inhibitor) and Ro31-8220 (PKC inhibitor; Fig. 1B).
Fig. 1.
Evidence for GPRC6A sensing of Ocn in HEK-293 cells transfected with Gprc6a. (A) Dose-dependent effects of recombinant human Ocn, l-arginine, and calcium to stimulate GPRC6A. Oc, l-arginine, and calcium stimulated ERK activation in HEK-293 cells transfected with Gprc6a cDNA but had no effect in nontransfected HEK-293 cells. (B) Involvement of PLC and PKC in GPRC6A signaling. Ocn-stimulated activation of phospho-ERK in Gprc6a-expressing HEK-293 cells was inhibited by the PLC inhibitor U73122 (10 µM) and the PKC inhibitor Ro31-8220 (5 µM). Neither U73122 nor Ro31-8200 alone exhibited effects on phospho-ERK in unstimulated HEK-293 cells stably transfected with Gprc6a. Representative blots are shown, and the results were verified in at least three independent experiments.
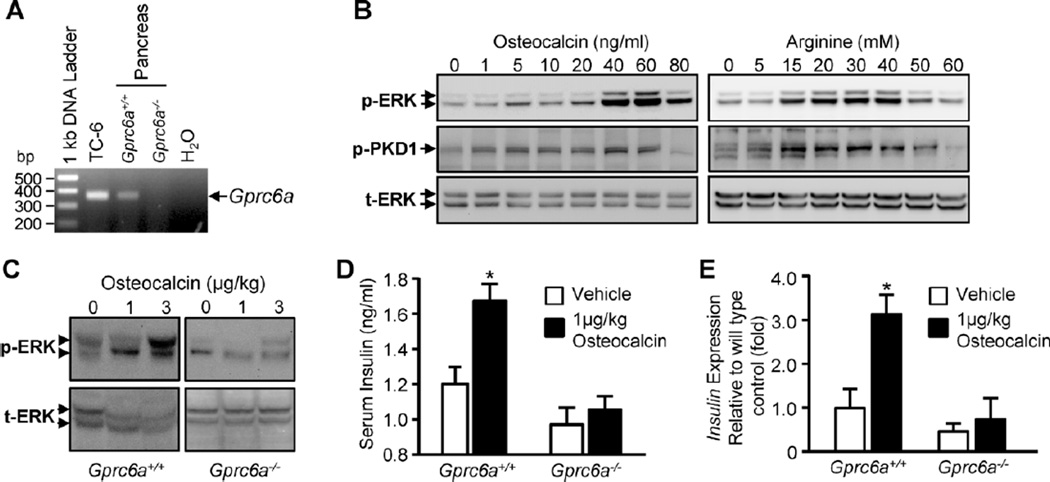
Next, we found that Gprc6a transcripts were present in TC-6 cells and in the pancreas from wild-type mice but not in the pancreas from Gprc6a−/− mice (Fig. 2A). The gene products from TC-6 cells and pancreas were identified as mouse Gprc6a cDNA by DNA sequence analysis. We found that the addition of recombinant human Ocn, as well as arginine and calcium, resulted in a dose-dependent increased phospho-ERK in TC-6 cells. Recombinant human Ocn (5 to 60 ng/mL) and arginine (5 to 60 mM) also resulted in a dose-dependent stimulation of PKD1 activity in TC-6 cells (Fig. 2B).
Fig. 2.
In vitro and in vivo effects of Ocn on TC-6 mouse β cells and pancreatic tissues in wild-type and Gprc6a null mice. (A) RT-PCR analysis showing that Gprc6a message is expressed in TC-6 cells and mouse pancreas but is not present in the pancreas from Gprc6a−/− mice. (B) Effects of Ocn, l-arginine, and calcium on pancreatic β-cell signaling. TC-6 cells expressing endogenous Gprc6a exhibited a dose-dependent stimulation of ERK activation in response to the addition of recombinant human Ocn, l-arginine, and calcium to the culture medium. (C, D) Gprc6a deficiency attenuates the pancreatic response to systemic Ocn administration. (C) ERK phosphorylation. Recombinant human Ocn (1 or 3 µg/kg) or PBS vehicle was injected intraperitoneally into wild-type or Gprc6a−/− male mice. Western blot analysis of ERK phosphorylation in pancreatic tissue assessed 20 minutes after injection of Ocn shows loss of Ocn-mediated ERK activation in the pancreas obtained from Gprc6a null mice but markedly stimulated ERK activity in the pancreas of wild-type mice. Representative blots are shown, and the results were verified in at least three independent experiments. (D) Serum insulin level. Recombinant human Ocn 1 µg/kg or PBS vehicle was injected intraperitoneally into wild-type (n = 5 each group) or Gprc6a−/− male mice (n = 4 each group). The serum was collected 20 minutes after injection of Ocn. Serum insulin level was measured by insulin (mouse) ultrasensitive ELISA kit (ALPCO Immunoassays) according to the manufacturer’s instructions. (E) Pancreatic insulin expression. Recombinant human Ocn 1 µg/kg or PBS vehicle was injected intraperitoneally into wild-type (n = 4 each group) or Gprc6a−/− male mice (n = 4 each group). Pancreas were collected 4 hours after injection of Ocn.
To establish a linkage between GPRC6A receptor activation and pancreatic response in vivo, we examined the impact of loss of Gprc6a on the capacity of Ocn to stimulate phospho-ERK activity in pancreas isolated from wild-type and Gprc6a−/− mice after the intraperitoneal administration of recombinant human Ocn. We found that Ocn treatment at a dose of 1 and 3 µg/kg stimulated ERK activity in pancreas of wild-type mice, but this response was markedly attenuated in Gprc6a−/− mice (Fig. 2C). Next, we assessed the ability of Ocn to stimulate insulin secretion. For these studies, we used modifications of a previous report that showed that glutathione S-transferase (GST) in recombinant proteins did not exaggerate or regulate insulin secretion.(19) We found that the administration of 1 µg/kg of recombinant human Ocn resulted in a 40% increase in serum insulin in wild-type mice, and this response was reduced fivefold in Gprc6a−/− mice (Fig. 2D). We also found that the recombinant human Ocn stimulated insulin expression (approximately threefold increase) in wild-type mice, but the Ocn failed to stimulate insulin expression significantly in Gprc6a−/− mice (Fig. 2E).
Discussion
Our results show that GPRC6A is a receptor for osteocalcin. First, in vitro studies in heterologous cell culture models demonstrated the Ocn-sensing functions of GPRC6A.(11) Second, we also found that TC-6 pancreatic β-cells and mouse pancreas express Gprc6a, and TC-6 cells exhibit a signaling response to Ocn as well as GPRC6A ligands, arginine, and calcium. Third, use of biochemical inhibitors suggest that GPRC6A activates PLC, PKC, PKD1, and Ras/Raf/MEK/ERK pathways, which are implicated in insulin secretion in pancreatic β-cells,(20) as well as Ocn regulation of osteoblast function.(18) Fourth, the in vivo relevance of GPRC6A regulation of pancreatic responses was demonstrated by the attenuated effect of Ocn administration pancreatic ERK activity, serum insulin level, and pancreatic insulin expression in Gprc6a−/− mice. Taken together, these data provide the first evidence that GPRC6A is a biologically relevant Ocn-sensing GPCR and is a candidate for mediating the β-cell response in the bone-pancreas endocrine loop.
Additional studies will be needed to confirm that the effects of Ocn are directly and solely mediated by GPRC6A in β-cells in the pancreas and potentially other tissues, such as adipocytes. Since loss of Gprc6a results in multiple systemic abnormalities, the conditional deletion of Gprc6a in specific sites will be needed to establish the tissue-specific functions of this receptor, such as its effects on insulin secretion and sensitivity. Indeed, our findings do not exclude the existence of other molecular targets or other signal-transduction pathways that also may mediate the actions of Ocn. However, it is intriguing to speculate that GPRC6A, given its ability to sense both nutrient-derived factors, such as calcium and amino acids, and osteoclacin, not only may participate in the bone-pancreas hormone loop regulating insulin secretion and sensitivity but also may be broadly involved in the integrative physiology of multiple tissues. Indeed, GPRC6A may represent an anabolic receptor that responds to a variety of nutritional and hormonal signals to mediate the metabolic cooperation between bone, muscle, liver, and β-cell pancreatic functions to regulate bone, muscle, and fat mass in synchrony with glucose and energy metabolism.
Acknowledgments
LDQ serves as a consultant for Amgen, Shire, Cytochroma, Novartis, and Osteometrics and receives research support from Amgen, Genzyme, Servier, and VasoGenix.
This work was supported by funds from NIH R01-AR37308 (to LD Q).
Footnotes
Disclosures
All the other authors state that they have no conflicts of interest.
References
- 1.Plantalech L, Guillaumont M, Vergnaud P, Leclercq M, Delmas PD. Impairment of gamma carboxylation of circulating osteocalcin (bone gla protein) in elderly women. J Bone Miner Res. 1991;6:1211–1216. doi: 10.1002/jbmr.5650061111. [DOI] [PubMed] [Google Scholar]
- 2.Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin-receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–219. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca2+ -sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 6.Mohler H, Fritschy JM. GABAB receptors make it to the top--as dimers. Trends Pharmacol Sci. 1999;20:87–89. doi: 10.1016/s0165-6147(99)01323-1. [DOI] [PubMed] [Google Scholar]
- 7.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 8.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 9.Robbins MJ, Michalovich D, Hill J, et al. Molecular cloning and characterization of two novel retinoic acid-inducible orphan G-protein-coupled receptors (GPRC5B and GPRC5C) Genomics. 2000;67:8–18. doi: 10.1006/geno.2000.6226. [DOI] [PubMed] [Google Scholar]
- 10.Brauner-Osborne H, Krogsgaard-Larsen P. Sequence and expression pattern of a novel human orphan G-protein-coupled receptor, GPRC5B, a family C receptor with a short amino-terminal domain. Genomics. 2000;65:121–128. doi: 10.1006/geno.2000.6164. [DOI] [PubMed] [Google Scholar]
- 11.Pi M, Faber P, Ekema G, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem. 2005;93:383–391. doi: 10.1111/j.1471-4159.2005.03025.x. [DOI] [PubMed] [Google Scholar]
- 13.Wellendorph P, Brauner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Pi M, Chen L, Huang MZ, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE. 2008;3:e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 16.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Brauner-Osborne H. Deorphanization of GPRC6A: a promiscuous Lalpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- 18.Bodine PV, Komm BS. Evidence that conditionally immortalized human osteoblasts express an osteocalcin receptor. Bone. 1999;25:535–543. doi: 10.1016/s8756-3282(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 19.Torii S, Zhao S, Yi Z, Takeuchi T, Izumi T. Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1a. Mol Cell Biol. 2002;22:5518–5526. doi: 10.1128/MCB.22.15.5518-5526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumara G, Formentini I, Collins S, et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]