Abstract
OBJECTIVE
This study evaluated the feasibility, safety, and efficacy of day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes under free-living conditions.
RESEARCH DESIGN AND METHODS
In an open-label randomized crossover study, 12 suboptimally controlled adolescents on insulin pump therapy (mean ± SD age 14.6 ± 3.1 years; HbA1c 69 ± 8 mmol/mol [8.5 ± 0.7%]; duration of diabetes 7.8 ± 3.5 years) underwent two 21-day periods in which hybrid closed-loop insulin delivery was compared with sensor-augmented insulin pump therapy in random order. During the closed-loop intervention, a model predictive algorithm automatically directed insulin delivery between meals and overnight. Participants used a bolus calculator to administer prandial boluses.
RESULTS
The proportion of time that sensor glucose was in the target range (3.9–10 mmol/L; primary end point) was increased during the closed-loop intervention compared with sensor-augmented insulin pump therapy by 18.8 ± 9.8 percentage points (mean ± SD; P < 0.001), the mean sensor glucose level was reduced by 1.8 ± 1.3 mmol/L (P = 0.001), and the time spent above target was reduced by 19.3 ± 11.3 percentage points (P < 0.001). The time spent with sensor glucose levels below 3.9 mmol/L was low and comparable between interventions (median difference 0.4 [interquartile range −2.2 to 1.3] percentage points; P = 0.33). Improved glucose control during closed-loop was associated with increased variability of basal insulin delivery (P < 0.001) and an increase in the total daily insulin dose (53.5 [39.5–72.1] vs. 51.5 [37.6–64.3] units/day; P = 0.006). Participants expressed positive attitudes and experience with the closed-loop system.
CONCLUSIONS
Free-living home use of day-and-night closed-loop in suboptimally controlled adolescents with type 1 diabetes is safe, feasible, and improves glucose control without increasing the risk of hypoglycemia. Larger and longer studies are warranted.
Introduction
Most adolescents and young adults with type 1 diabetes are poorly controlled (1–3), accelerating the onset of early micro- and macrovascular complications (4,5). Reduced therapy adherence linked to psychosocial and physiological changes in adolescence is contributory (6), because omissions of prandial insulin boluses are frequent (7), and acceptance of insulin pump therapy and continuous glucose monitoring systems is lower (8–10). Threshold-suspend and predictive low glucose management insulin pump therapy may alleviate the burden of hypoglycemia (11,12) but do not address the issue of hyperglycemia, the major challenge of diabetes management in adolescence.
The artificial pancreas (closed-loop systems) modulates insulin delivery below and above preset insulin pump delivery in response to real-time sensor glucose levels and can potentially reduce hypo- and hyperglycemia. After evaluations in children and adolescents in laboratory settings (13–15) and diabetes camps (16–18), the first at-home studies of up to 3-month applications of overnight closed-loop have demonstrated improved glucose control and reduced the burden of hypoglycemia (19–21). However, randomized outpatient trials evaluating day-and-night closed-loop insulin delivery in adolescents are limited to a maximum of a 1-week follow-up (16,18,22).
Here, we present results of a 21-day-long day-and-night closed-loop trial in young people aged 10 to 18 years with suboptimally controlled type 1 diabetes during free-living settings. We hypothesized that prolonged use of a 24/7 hybrid single hormone closed-loop system without remote monitoring or close supervision would be feasible, safe, and improve glycemic control compared with sensor-augmented insulin pump therapy.
Research Design and Methods
Study Management and Regulatory Approvals
The study received approval from the local independent research ethics committee and the U.K. competent authority (Medicines & Health Products Regulatory Agency). An independent data safety and monitoring board oversaw the study.
Participants
Study participants were identified from pediatric diabetes clinics at Addenbrooke’s Hospital (Cambridge, U.K.) and University College London Hospital (London, U.K.). Key inclusion criteria were age 10–18 years, diagnosis of type 1 diabetes, treatment with insulin pump therapy for at least 3 months, willingness to perform at least four fingerstick glucose measurements per day, and HbA1c ≤11% (≤97 mmol/mol). Exclusion criteria included established nephropathy, neuropathy, or proliferative retinopathy, total daily insulin dose ≥2.0 units/kg or <10 units/day, significant hypoglycemia unawareness, more than one incident of severe hypoglycemia within 6 months before enrollment, more than one episode of diabetic ketoacidosis within 12 months before enrollment, pregnancy, and breast-feeding. Participants aged ≥16 years and parents or guardians of participants aged <16 years signed informed consent, and written assent was obtained from minors before study-related activities.
Study Design and Procedures
The study adopted an open-label, randomized, two-period crossover design comparing automated closed-loop insulin delivery with sensor-augmented pump therapy in free-living home settings (Supplementary Fig. 1). Study intervention periods lasted 3 weeks each, with a 1- to 4-week washout period.
At enrollment, blood samples were taken for analysis of HbA1c. Random C-peptide levels were measured with concomitant plasma glucose >4 mmol/L. At the start of the run-in phase, participants received training regarding the use of the study pump (DANA Diabecare R; SOOIL, Seoul, South Korea) and the study real-time continuous glucose monitoring system (FreeStyle Navigator II; Abbott Diabetes Care, Alameda, CA) which are off-the-shelf devices and do not offer low glucose suspend functionality. The pump administered rapid-acting insulin analog aspart (Novo Nordisk, Bagsvaerd, Denmark) or lispro (Eli Lilly, Indianapolis, IN). Participants used a standard bolus calculator for all meals throughout the study.
At the end of the 1- to 2-week run-in period, compliance in the use of study pump and continuous glucose monitoring were assessed. Participants with at least 5 days’ worth of continuous glucose monitoring data were randomly assigned to receive 3 weeks of automated closed-loop insulin delivery, followed by sensor-augmented pump therapy, or vice versa. Permuted block randomization was applied and assignment was unblinded.
The two intervention periods were separated by a 1- to 4-week washout period during which the participants could continue using the study insulin pump. Continuous glucose monitoring was discontinued during washout.
On the first day of the closed-loop period, participants attended the clinical research facility. This 2- to 3-h visit included training on initiation and discontinuation of the closed-loop system, switching between closed-loop and usual pump therapy, meal bolus procedure, and the use of study devices during exercise. Competency on the use of closed-loop system was assessed. After discharge, participants continued the study intervention for the next 21 days under free-living settings in their home and school environment. The participants were free to consume meals of their choice. No restrictions were imposed on traveling. We encouraged participants to continue closed-loop use during exercise and to announce physical activity to the algorithm. However, participants were advised to discontinue closed-loop insulin delivery and follow their usual insulin pump therapy for activities such as diving or contact sports. At the end of the closed-loop intervention, participants completed a feedback questionnaire to assess user-friendliness and satisfaction with study devices. Participants were not remotely monitored or supervised.
The number of planned contacts with the study team was identical during the two study periods. The study pump and the study real-time continuous glucose monitoring device were used during both study periods. Participants were advised to calibrate the continuous glucose monitoring device according to the manufacturer’s instructions, but were free to decide on alarm settings for the continuous glucose monitoring device. All participants were provided with a 24-h telephone helpline to contact the study team in the event of study-related issues. All helpline contacts resulting in an immediate action by research staff (i.e., device replacement, adverse event reporting) were documented.
Closed-Loop System
The FlorenceD2A closed-loop system (University of Cambridge, Cambridge, U.K.) (23) comprised a model predictive control algorithm (version 0.3.41, University of Cambridge) residing on a Galaxy S4 smartphone (Samsung, Seoul, South Korea), which communicated wirelessly with continuous glucose monitoring receiver through a purpose-made translator unit (Triteq, Hungerford, U.K.) (Supplementary Fig. 2). Every 12 min, the control algorithm calculated an insulin infusion rate, which was set on the study insulin pump. This trial applied a hybrid closed-loop approach in which participants were required to count carbohydrates and use a standard bolus calculator for premeal boluses according to usual practice. The bolus calculations provided by the study pump’s built-in bolus calculator took into account carbohydrate content of meals, insulin on board, and entered capillary blood glucose readings. The control algorithm was initialized using preprogrammed basal insulin delivery downloaded from the study pump. In addition, information about the participant's weight and total daily insulin dose were entered at setup. During closed-loop operation, the algorithm adapted itself to the particular participant. The apparent total daily dose was modified according to sensor glucose levels achieved during closed-loop on previous days. In the current version of the algorithm, this learning capability was made more responsive, and enhanced adaptability was further supported by adaptation to varied insulin needs during the daytime and overnight periods. The treat-to-target control algorithm aimed to achieve glucose levels between 5.8 mmol/L and 7.3 mmol/L and adjusted the actual target glucose level depending on fasting versus postprandial status and the accuracy of model-based glucose predictions.
Safety Precautions During Closed-Loop
Participants were trained to perform a calibration check before breakfast and the evening meal. If the sensor glucose was above the fingerstick glucose by >3.0 mmol/L, the continuous glucose monitoring device was recalibrated. These instructions resulted from an in silico evaluation of hypoglycemia and hyperglycemia risk (24) using the validated Cambridge simulator (25).
If sensor glucose became unavailable or in case of other failures, preprogrammed insulin delivery automatically restarted within 30–60 min. This limited the risk of insulin under- and overdelivery (24). Safety rules limited the maximum insulin infusion and suspended insulin delivery if glucose was ≤4.3 mmol/L or when sensor glucose was rapidly decreasing.
Assays
C-peptide measurements were performed using chemiluminescence immunoassay (IV2-004; Invitron, Monmouth, U.K), with an interassay variation 7.8%, 4.3%, and 6.7% at 268 pmol/L, 990 pmol/L, and 1,862 pmol/L, respectively. Analytical sensitivity for the C-peptide assay was 5 pmol/L. HbA1c was measured centrally using ion exchange high-performance liquid chromatography (G8 HPLC Analyzer; Tosoh Bioscience, South San Francisco, CA), with interassay coefficients of variation (CVs) of 1.3% at 31.2 mmol/mol and 0.8% at 80.5 mmol/mol.
Questionnaire
The evaluation of participant-reported outcomes included a trial experience questionnaire completed by participants at the conclusion of the closed-loop phase. The questionnaire was composed of seven questions, four of which were closed questions. The three open questions requested comments and suggestions from participants regarding 1) what they liked about the closed-loop system, 2) what they did not like about the system, and 3) what additional features they would like to have in a closed-loop system.
Study Outcomes
The primary efficacy outcome was the proportion of time when glucose was in the target range (3.9–10.0 mmol/L) during the 21-day study periods as recorded by continuous glucose monitoring. Secondary outcomes included mean sensor glucose concentrations, glucose variability, time spent at glucose levels <3.9 mmol/L (hypoglycemia) and >10.0 mmol/L (hyperglycemia), and insulin delivery. Secondary outcomes were calculated over 24 h, daytime and overnight periods; daytime was classified between 8:00 a.m. and midnight, and night-time between midnight and 8:00 a.m. Glucose variability was assessed by the SD and the CV of sensor glucose. Hypoglycemia burden was assessed by calculating the glucose sensor area under the curve of <3.5 mmol/L.
Statistical Analysis
The investigators agreed on the statistical analysis plan in advance. All analyses were done on an intention-to-treat basis. Efficacy and safety data from all randomized participants were included in the analyses. The respective values obtained during the 21-day randomized interventions were compared using a least-square regression model. Sensor glucose and insulin outcomes were adjusted for period effect. Rank normal transformation analyses were used for highly skewed end points. Outcomes are presented as mean ± SD for normally distributed values or as median (interquartile range) for nonnormally distributed values. Outcomes were calculated using GStat 2.2 software (University of Cambridge). Analysis was done using SPSS 21 software (IBM Software, Hampshire, U.K.). A 5% significance level was used to declare statistical significance. All P values are two-sided.
Results
Participants
We approached 17 subjects, of whom 12 gave consent/assent and completed the study (7 males; age 14.6 ± 3.1 years; diabetes duration 7.8 ± 3.5 years; HbA1c 8.5 ± 0.7% [69 ± 8 mmol/mol]). Duration of insulin pump therapy duration was 5.5 ± 2.6 years, and total daily insulin dose was 0.82 ± 0.18 units/kg. All subjects were C-peptide negative except for two participants with levels of 61 and 262 pmol/L (Supplementary Table 1 and Supplementary Fig. 3).
Day-and-Night Glucose Control and Insulin Delivery
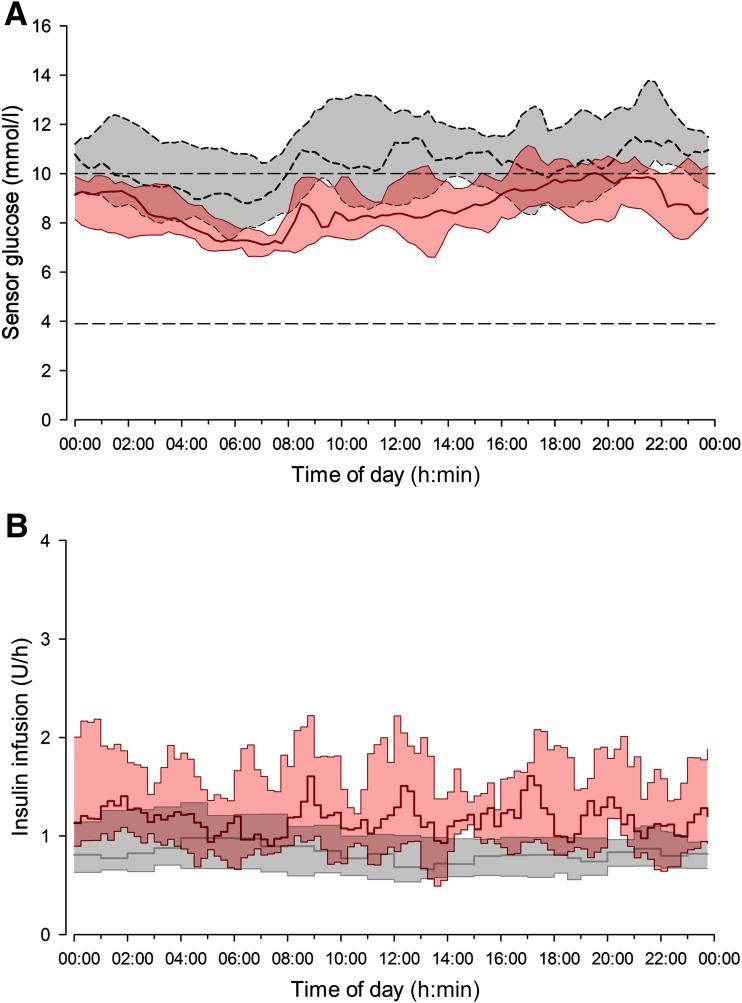
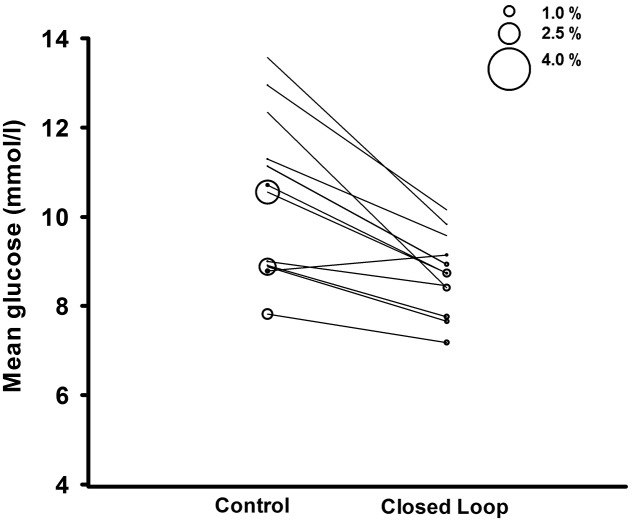
Primary and secondary end points are summarized in Table 1, and 24-h sensor glucose and insulin delivery profiles are shown in Fig. 1. The proportion of time that sensor glucose was in the target glucose range of 3.9 to 10.0 mmol/L (primary end point), was increased during closed-loop delivery compared with control period (P < 0.001) (Table 1). The mean glucose level was significantly lower with closed-loop use (P = 0.001) (Fig. 2) as was the time spent above the target glucose range (P < 0.001). The proportion of time spent with sensor readings in hypoglycemia (below 3.9 mmol/L and 2.8 mmol/L) (Fig. 2) and the area under the curve when sensor glucose was less than 3.5 mmol/L were low and comparable during the study interventions.
Table 1.
Comparison of glucose control and insulin delivery during closed-loop and control period over 21 days
| Closed loop (n = 12) | Control (n = 12) | Paired difference* | P | |
|---|---|---|---|---|
| Day-and-night glucose control | ||||
| Time spent at glucose level (%) | ||||
| 3.9 to 10.0 mmol/L† | 66.6 ± 7.9 | 47.7 ± 14.4 | 18.8 ± 9.8 | <0.001 |
| >10.0 mmol/L | 29.7 ± 9.2 | 49.1 ± 16.5 | −19.3 ± 11.3 | <0.001 |
| >16.7 mmol/L | 5.1 (0.8–5.6) | 8.0 (1.9–17.4) | −3.6 (−11.9 to −0.65) | <0.001 |
| <3.9 mmol/L | 4.3 (1.4–5.2) | 2.4 (0.3–5.7) | 0.4 (−2.2 to 1.3) | 0.33 |
| <2.8 mmol/L | 0.3 (0.0–0.5) | 0.1 (0.0–0.7) | −0.1 (−0.4 to 0.2) | 0.49 |
| AUCday <3.5 mmol/L (mmol/L × min)‡ | 11.1 (1.2–17.4) | 2.7 (0.2–20.4) | 0.0 (−10.5 to 6.8) | 0.21 |
| Mean glucose (mmol/L) | 8.7 ± 0.9 | 10.5 ± 1.8 | −1.8 ± 1.3 | 0.001 |
| Within day SD of glucose (mmol/L) | 3.7 ± 0.7 | 4.2 ± 0.8 | −0.5 ± 0.7 | 0.013 |
| CV of glucose within day (%) | 40.5 (38.1–47.7) | 38.3 (36.7–43.7) | 1.2 (−2.6 to 6.7) | 0.18 |
| CV of glucose between days (%) | 19.0 (13.8–23.7) | 17.4 (14.9–24.0) | −0.5 (−3.9 to 6.0) | 0.94 |
| Day-and-night insulin delivery | ||||
| Total daily insulin (units/day) | 53.5 (39.5–72.1) | 51.5 (37.6–64.3) | 4.5 (1.6–6.5) | 0.006 |
| Total bolus insulin (units/day) | 28.3 (16.7–32.6) | 29.4 (23.6–37.6) | −4.4 (−8.1 to −1.4) | 0.009 |
| Total basal insulin (units/day) | 25.8 (23.0–41.2) | 19.9 (14.8–26.3) | 7.6 (3.8–14.4) | 0.001 |
| SD of basal insulin delivery (units/h) | 1.2 (1.0–1.9) | 0.3 (0.1–0.3) | 1.1 (0.8–1.6) | <0.001 |
| CV of basal insulin delivery | 106.7 (97.2–111.1) | 23.9 (12.8–35.2) | 79.2 (77.1–91.3) | <0.001 |
| Bolus administrations (n/day) | 4.9 (4.8–6.3) | 6.3 (5.1–7.6) | −1.1 (−1.5 to −0.2) | 0.015 |
Data are presented as mean ± SD or as median (interquartile range). P values are adjusted for period effect.
*Closed-loop minus control. A positive value indicates the value was higher on the closed-loop compared with control.
†Primary end point.
‡AUCday indicates glucose area under curve <3.5 mmol/L per day.
Figure 1.
Median (interquartile range) of sensor glucose (A) and insulin delivery (B) during closed-loop (solid red line and red shaded area) and control period (dashed black line and gray shaded area) from midnight to midnight. The glucose range of 3.9 to 10.0 mmol/L is denoted by horizontal dashed lines (A).
Figure 2.
Individual values of mean sensor glucose during day-and-night closed-loop study. The size of the bubble indicates the proportion of time spent with low glucose below 2.8 mmol/L.
Glucose variability, measured as the standard deviation and the CV of sensor glucose level within 24 h and between days, did not differ between study periods. A higher percentage of time when glucose was in target range and lower mean glucose levels were achieved by closed-loop through increased variability of basal insulin delivery (P < 0.001) (Fig. 1) and slightly higher total daily insulin dose (P = 0.006). Basal insulin delivery during closed-loop was significantly higher than during control intervention (P = 0.001). Overall bolus insulin requirements during closed-loop were significantly lower (P = 0.009), as was the number of overall daily bolus administrations (P = 0.015). Compared with control, fewer correction boluses were observed during closed loop (0.2 [0.1–0.4] vs. 0.9 [0.1–1.4] per day, P = 0.015) but not meal boluses (4.8 [4.6–6.1] vs. 5.8 [4.1 to 7.0], P = 0.48).
Daytime and Overnight Glucose Control and Insulin Delivery
Secondary outcomes calculated for daytime (8:00 a.m. to midnight) and overnight periods (midnight to 8:00 a.m.) are reported in Table 2. The daytime (P = 0.002) and overnight (P = 0.002) mean glucose levels were significantly lower during closed-loop use (P = 0.002). The proportion of time that the glucose level was within the wide target range (3.9–10.0 mmol/L) and overnight target range (3.9–8.0 mmol/L) were higher during closed-loop compared with control (P < 0.001), without a difference in total daytime and overnight insulin amount. The percentage of time spent with sensor readings below target range did not differ between the two interventions during daytime and overnight.
Table 2.
Daytime and night-time glucose control and insulin delivery during closed-loop and control period
| Closed-loop (n = 12) | Control (n = 12) | Paired difference* | P | ||
|---|---|---|---|---|---|
| Daytime (from 08:01 to 23:59) | |||||
| Time spent at glucose level (%) | |||||
| 3.9 to 10.0 mmol/L | 62.9 ± 8.9 | 45.7 ± 13.7 | 17.1 ± 12.2 | 0.001 | |
| >10.0 mmol/L | 33.0 ± 10.7 | 51.8 ± 15.7 | −18.7 ± 13.7 | 0.001 | |
| <3.9 mmol/L | 4.2 (1.0–6.5) | 1.2 (0.3–3.9) | 0.3 (−0.8 to 4.1) | 0.15 | |
| AUCday <3.5 mmol/L (mmol/L × min)† | 11.2 (0.9–17.0) | 2.0 (0.2–12.3) | −0.4 (−5.0 to 12.8) | 0.26 | |
| Mean glucose (mmol/L) | 9.0 ± 1.0 | 10.8 ± 1.9 | −1.8 ± 1.5 | 0.002 | |
| Within day SD of glucose (mmol/L) | 3.9 ± 0.8 | 4.3 ± 0.9 | −0.4 ± 0.9 | 0.10 | |
| CV of glucose within day (%) | 42.8 (37.9–49.8) | 39.0 (36.0–42.6) | 3.0 (−3.7 to 8.7) | 0.20 | |
| CV of glucose between days (%) | 19.2 (17.4–25.6) | 21.6 (16.5–23.1) | −2.4 (−5.8 to 3.5) | 0.86 | |
| Daytime insulin delivery (units) | 42.7 (31.2–53.6) | 42.8 (30.9–48.4) | 3.5 (0.0–6.3) | 0.24 | |
| Night-time (from 00:00 to 08:00) | |||||
| Time spent at glucose level (%) | |||||
| 3.9 to 8.0 mmol/L | 54.4 ± 13.8 | 33.4 ± 16.3 | 20.9 ± 12.7 | <0.001 | |
| >8.0 mmol/L | 42.8 ± 14.0 | 62.0 ± 19.4 | −19.3 ± 14.5 | 0.001 | |
| <3.9 mmol/L | 2.5 (1.1–4.2) | 3.9 (0.3–7.2) | −1.3 (−4.9 to 1.4) | 0.70 | |
| AUCday <3.5 mmol/L (mmol/L × min)† | 5.3 (1.6–19.7) | 4.7 (0.0–21.8) | 1.2 (−20.0 to 5.9) | 0.56 | |
| Mean glucose (mmol/L) | 8.2 ± 1.1 | 9.8 ± 2.0 | −1.6 ± 1.4 | 0.002 | |
| Within night SD of glucose (mmol/L) | 3.1 ± 0.9 | 3.8 ± 0.7 | −0.7 ± 0.7 | 0.008 | |
| CV of glucose within night (%) | 37.3 ± 6.8 | 39.3 ± 7.3 | −2.0 ± 9.9 | 0.53 | |
| CV of glucose between nights (%) | 26.7 ± 8.5 | 30.9 ± 6.4 | −4.2 ± 10.1) | 0.20 | |
| Overnight insulin delivery (units) | 11.5 (9.5–17.3) | 11.0 (8.5–15.0) | 0.6 (−0.5 to 3.5) | 0.18 |
Data are presented as mean ± SD or as median (interquartile range).
*Closed-loop minus control. A positive value indicates the value was higher on the closed-loop compared with control.
†AUCday indicates glucose area under curve below 3.5 mmol/L per day.
Adverse Events
No serious adverse events or severe hypoglycemic episodes were observed during either study period. Three adverse events were documented, none of which was related to study devices or study procedures. One participants during the control intervention measured elevated urine ketone levels associated with hyperglycemia. This event was attributed to a viral infection and was self-managed. One participant during the closed-loop period and another participant during the control period required oral antibiotic treatment because of respiratory tract infections without metabolic deterioration.
Utility Analysis
Availability of sensor glucose data were 95% (91–98%) during closed-loop and higher than 90% (73–96%) recorded during control period (P = 0.036). Closed-loop was operational over 82% (76–88%) of time. Overall frequency of recorded helpline contacts (device replacement, adverse event reporting) was higher during closed-loop: The study pump had to be replaced on three occasions (once during run-in and twice during the control period), two translator modules were replaced during the closed-loop period, and the research staff had to reset the closed-loop system on eight occasions.
Questionnaire
All 12 participants completed the questionnaire. Results of the four closed questions are shown in Supplementary Fig. 4. All 12 participants (100%) were confident with the closed-loop system regulating their blood glucose and insulin delivery. Ten participants (83.3%) stated that using the closed-loop system, they spent less time to manage their diabetes, and two (16.7%) were unsure about this statement. The majority of the participants (91.7% [11 of 12]) expressed fewer concerns about their glucose control while using closed-loop. Improved sleep was indicated by 75% (9 of 12) of participants, whereas 8.3% (1 of 12) slept worse, and 16.7% (2 of 12) were unsure about the effect of the closed-loop system on their sleep.
Key positive themes of the closed-loop system as described by participants in the free-text section of the questionnaire were improved glucose control, a relief of diabetes management, and specific features of the closed-loop handset allowing remote meal bolusing and data review. Key negative themes were the number and size of devices, the necessity to carry around the equipment all the time, the continuous glucose monitor and pump alarms, connectivity and continuous glucose monitor calibration issues. According to participants, future closed-loop systems should be smaller, ideally integrating all different devices into a single device. Sensor life should be longer, and additional features to facilitate carbohydrate estimation should be implemented.
Conclusions
To our knowledge, this is the longest randomized controlled trial investigating day-and-night application of closed-loop insulin delivery in adolescents with type 1 diabetes during free-living conditions. We demonstrate the feasibility and benefits of prolonged use of single hormone 24/7 closed-loop. It increased the time when glucose was in the target range by 19 percentage points while reducing mean glucose by 1.8 mmol/L. Although more insulin was delivered during closed-loop in these suboptimally controlled adolescents, improvements were achieved without increasing the risk of hypoglycemia.
The current study extends findings from our previous home trials in children and adolescents (19,21,22), which were limited by overnight application (19,21) or a shorter intervention period (22). Although benefits of closed-loop in these trials as well as in our previous adult trials (21,26) tended to be greater overnight compared with daytime, results of the current study show consistent improvements in glucose levels overnight and during daytime. Possible explanations include closed-loop militating against missed meal boluses in suboptimally controlled adolescents. In addition, we applied a control algorithm with enhanced adaptivity. Poorly controlled teenagers may be among those most benefiting from closed-loop systems.
Hypoglycemia rates were low, and there was no significant difference in the time spent in hypoglycemia between interventions, in line with findings from our previously published 24/7 closed-loop home trial in adolescents (22). Significant reductions in hypoglycemia risk were observed in populations with greater rates of hypoglycemia or in more challenging environments, such as diabetes camps and during prolonged outpatient closed-loop studies in adults using single-hormone (21,27), or dual hormone (glucagon coadministration) closed-loop approaches across different age groups (16–18).
Prolonged periods of sensor underreading resulting in hypoglycemia overreporting were identified in one participant during the closed-loop intervention, underscoring the challenges associated with quantifying hypoglycemia using glucose sensor data. No similar findings were observed during control intervention. Although the results in Tables 1 and 2 and in the results section are based on the original data, Fig. 2 shows data excluding periods of sensor underreading (see Supplementary Figs. 5–9 for details of excluded data).
Total daily insulin requirements during closed-loop were modestly higher than during control intervention, which was caused by higher basal insulin delivery. Inherent to closed-loop systems, algorithm-directed insulin delivery was more variable than basal insulin delivery during the control period. Previous studies of dual-hormone systems have described more pronounced increases in total insulin delivery during closed-loop intervention of 24% (28) to 33% (16) of total daily insulin dose, where potential insulin overdosing can be mitigated by coadministration of glucagon. Higher insulin requirements during closed-loop in the current study may reflect underinsulinisation in suboptimally controlled adolescents. Of interest, we observed reduced bolus amounts and fewer boluses per day during the closed-loop intervention. We attribute this finding to fewer correction doses, but the observation could also reflect reduced bolus adherence for meals and snacks during closed-loop. The unsupervised design of the study precludes reliable interpretation of the finding.
Closed-loop usage and sensor wear were high. The closed-loop technology was well perceived, in line with previously published data (29). Although the number of devices and system alarms were reported to be drawbacks, participants expressed trust in the technology and reduced burden of diabetes, including less time spent to manage diabetes and fewer worries about glycemic control. Further miniaturization and integration of devices, prolonged sensor life, and simplified meal management are preferable features of future closed-loop systems that may enhance usability. A fully closed-loop system without meal announcement would be particularly applicable in the adolescent population. However, the absorption rate of currently available rapid-acting insulin analogs is not fast enough to effectively control postprandial glucose excursions without anticipatory insulin bolus. Our premise is that present closed-loop systems will benefit from meal announcement but have to be able to cope with missed meal boluses safely and efficaciously, should these occur.
The strengths of our study include the randomized crossover design and the integration of closed-loop into normal life, with participants performing their usual free-living activities while at home or at school and during weekends and holidays. The study was performed without remote monitoring or close supervision to assess real-world applicability of the technology. We did not restrict participants’ dietary intake, physical activity, or geographical movement. The comparator was state-of-the-art sensor-augmented insulin pump therapy. Weaknesses include a small sample size and the need to carry multiple devices during the closed-loop intervention. The study duration was still relatively short.
In conclusion, we found that use of a day-and-night hybrid closed-loop system at home over a period of 21 days during free daily living without close supervision is feasible, safe and effective in suboptimally controlled adolescents with type 1 diabetes. Benefits include increased time when glucose is in the target range and reduced mean glucose. Larger and longer studies are warranted.
Supplementary Material
Article Information
Acknowledgments. The authors are grateful to the study volunteers for their participation, Professor Peter Hindmarsh (University College, London, U.K.) for help in identifying potential recruits, John Lum (Jaeb Center) and Jasdip Mangat for supporting development and validation of the closed-loop system, and Professors John Pickup (Guy’s Hospital, London, U.K.), Irl Hirsch (University of Washington School of Medicine), and Howard Wolpert (Joslin Diabetes Center) for serving on the data safety and monitoring board. The authors acknowledge support by the staff at the Addenbrooke’s Wellcome Trust Clinical Research Facility. Josephine Hayes (University of Cambridge) provided administrative support. Karen Whitehead (University of Cambridge) provided laboratory support. The authors acknowledge support by the staff at Addenbrooke’s Hospital; Sara Hartnell and Sonja Slegtenhorst supported study pump training. The Core Biochemical Assay Laboratory (Keith Burling), University of Cambridge, and the Institute of Life Science (Gareth Dunseath), Swansea University, carried out biochemical analyses.
Funding. This study received support from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01-DK-085621-01). Additional support for the artificial pancreas work was received from JDRF, National Institute for Health Research Cambridge Biomedical Research Centre, and the Wellcome Strategic Award (100574/Z/12/Z). Diasend provided a discounted platform for data upload. Abbott Diabetes Care supplied discounted continuous glucose monitoring devices, sensors, and the communication protocol to facilitate real-time connectivity. Representatives from Abbott Diabetes Care read the manuscript before submission.
No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Duality of Interest. M.T. reports having received speaker honoraria from Novo Nordisk. M.E.W. has received license fees from Becton Dickinson and has served as a consultant to Becton Dickinson. M.E.W. and R.H. report patents and patent applications. R.H. reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panels for Eli Lilly and Novo Nordisk, receiving license fees from B. Braun and Medtronic, and having served as a consultant to B. Braun. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.T. and J.M.A. were responsible for screening and enrollment of participants and arranged informed consent from the participants. M.T., J.M.A., M.E.W., and H.T. provided patient care and/or took samples. M.T., M.E.W., H.T., C.L.A., D.B.D., and R.H. codesigned the studies. M.T., M.E.W., and R.H. carried out or supported data analysis, including the statistical analyses. M.T., H.T., and R.H. contributed to the interpretation of the results. M.E.W. managed randomization. M.T. and R.H. wrote the manuscript. All authors critically reviewed the report. R.H. coordinated the studies and designed and implemented the glucose controller. R.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Results of this trial were presented as an oral communication at the 9th International Conference on Advanced Technologies & Treatments for Diabetes, Milan, Italy, 3–6 February 2016.
Footnotes
Clinical trial reg. no. NCT01873066, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-1094/-/DC1.
References
- 1.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 2.Wood JR, Miller KM, Maahs DM, et al.; T1D Exchange Clinic Network . Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care 2013;36:2035–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 4.Maftei O, Pena AS, Sullivan T, et al.; AdDIT Study Group . Early atherosclerosis relates to urinary albumin excretion and cardiovascular risk factors in adolescents with type 1 diabetes: Adolescent type 1 Diabetes cardio-renal Intervention Trial (AdDIT). Diabetes Care 2014;37:3069–3075 [DOI] [PubMed] [Google Scholar]
- 5.Cho YH, Craig ME, Davis EA, et al.; Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial . Cardiac autonomic dysfunction is associated with high-risk albumin-to-creatinine ratio in young adolescents with type 1 diabetes in AdDIT (adolescent type 1 diabetes cardio-renal interventional trial). Diabetes Care 2015;38:676–681 [DOI] [PubMed] [Google Scholar]
- 6.Burdick J, Chase HP, Slover RH, et al. . Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics 2004;113:e221–e224 [DOI] [PubMed] [Google Scholar]
- 7.Vanderwel BW, Messer LH, Horton LA, et al. . Missed insulin boluses for snacks in youth with type 1 diabetes. Diabetes Care 2010;33:507–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofer SE, Heidtmann B, Raile K, et al.; DPV-Science-Initiative and the German working group for insulin pump treatment in pediatric patients . Discontinuation of insulin pump treatment in children, adolescents, and young adults. A multicenter analysis based on the DPV database in Germany and Austria. Pediatr Diabetes 2010;11:116–121 [DOI] [PubMed] [Google Scholar]
- 9.Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev 2012;1:CD008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergenstal RM, Tamborlane WV, Ahmann A, et al.; STAR 3 Study Group . Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 11.Bergenstal RM, Klonoff DC, Garg SK, et al.; ASPIRE In-Home Study Group . Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 12.Buckingham BA, Raghinaru D, Cameron F, et al.; In Home Closed Loop Study Group . Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care 2015;38:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovorka R, Allen JM, Elleri D, et al. . Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 14.Elleri D, Allen JM, Kumareswaran K, et al. . Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care 2013;36:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nimri R, Danne T, Kordonouri O, et al. . The “Glucositter” overnight automated closed loop system for type 1 diabetes: a randomized crossover trial. Pediatr Diabetes 2013;14:159–167 [DOI] [PubMed] [Google Scholar]
- 16.Russell SJ, El-Khatib FH, Sinha M, et al. . Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haidar A, Legault L, Matteau-Pelletier L, et al. . Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:595–604 [DOI] [PubMed] [Google Scholar]
- 18.Russell SJ, Hillard MA, Balliro C, et al. . Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016;4:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovorka R, Elleri D, Thabit H, et al. . Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2014;37:1204–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimri R, Muller I, Atlas E, et al. . Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes 2014;15:91–99 [DOI] [PubMed] [Google Scholar]
- 21.Thabit H, Tauschmann M, Allen JM, et al. ; APCam Consortium; AP@home Consortium . Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tauschmann M, Allen JM, Wilinska ME, et al. . Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elleri D, Allen JM, Biagioni M, et al. . Evaluation of a portable ambulatory prototype for automated overnight closed-loop insulin delivery in young people with type 1 diabetes. Pediatr Diabetes 2012;13:449–453 [DOI] [PubMed] [Google Scholar]
- 24.Wilinska ME, Budiman ES, Taub MB, et al. . Overnight closed-loop insulin delivery with model predictive control: assessment of hypoglycemia and hyperglycemia risk using simulation studies. J Diabetes Sci Technol 2009;3:1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilinska ME, Chassin LJ, Acerini CL, Allen JM, Dunger DB, Hovorka R. Simulation environment to evaluate closed-loop insulin delivery systems in type 1 diabetes. J Diabetes Sci Technol 2010;4:132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leelarathna L, Dellweg S, Mader JK, et al.; AP@home Consortium . Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care 2014;37:1931–1937 [DOI] [PubMed] [Google Scholar]
- 27.Kropff J, Del Favero S, Place J, et al.; AP@home consortium . 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol 2015;3:939–947 [DOI] [PubMed] [Google Scholar]
- 28.Blauw H, van Bon AC, Koops R, DeVries JH; on behalf of the PCDIAB consortium . Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab 2016;18:671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnard KD, Wysocki T, Allen JM, et al. . Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care 2014;2:e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.