Abstract
OBJECTIVE
To determine whether early administration of losartan slows progression of diabetic kidney disease over an extended period.
RESEARCH DESIGN AND METHODS
We conducted a 6-year clinical trial in 169 American Indians with type 2 diabetes and urine albumin/creatinine ratio <300 mg/g; 84 participants were randomly assigned to receive losartan and 85 to placebo. Primary outcome was a decline in glomerular filtration rate (GFR; iothalamate) to ≤60 mL/min or to half the baseline value in persons who entered with GFR <120 mL/min. At enrollment, GFR averaged 165 mL/min (interquartile range 49–313 mL/min). During the trial, nine persons reached the primary outcome with a hazard ratio (HR; losartan vs. placebo) of 0.50 (95% CI 0.12–1.99). Participants were then followed posttrial for up to 12 years, with treatment managed outside the study. The effect of losartan on the primary GFR outcome was then reanalyzed for the entire study period, including the clinical trial and posttrial follow-up.
RESULTS
After completion of the clinical trial, treatment with renin-angiotensin system inhibitors was equivalent in both groups. During a median of 13.5 years following randomization, 29 participants originally assigned to losartan and 35 to placebo reached the primary GFR outcome with an HR of 0.72 (95% CI 0.44–1.18).
CONCLUSIONS
Long-term risk of GFR decline was not significantly different between persons randomized to early treatment with losartan and those randomized to placebo. Accordingly, we found no evidence of an extended benefit of early losartan treatment on slowing GFR decline in persons with type 2 diabetes.
Introduction
An extended benefit of early intensive glycemic control on microvascular complications even after subsequent return to conventional glycemic control is well described. The Epidemiology of Diabetes Interventions and Complications (EDIC) study showed significant sustained reduction in risk of impaired glomerular filtration rate (GFR) (1) and nephropathy during the posttrial period in participants with type 1 diabetes who received intensive glucose control for 6.5 years (2). A similar reduction in incidence and progression of nephropathy with prior tight glycemic control was reported in type 2 diabetes by the UK Prospective Diabetes Study (UKPDS), many years after the conclusion of the clinical trial itself (3). Long-term benefit on nephropathy of early intervention with antihypertensive drugs, however, has not been demonstrated in persons with diabetes, despite the presence of potential mechanisms induced by early treatment with renin-angiotensin system (RAS) inhibitors that might result in a persistent benefit (4). Participants with type 2 diabetes who were randomized to tight blood pressure control with either captopril or atenolol in the UKPDS had a 29% reduction in risk of urinary albumin concentration ≥50 mg/L during the trial (5), but this effect was not sustained long term (6).
In this study, we examine the long-term effect of early treatment with the angiotensin receptor blocker (ARB) losartan on progression of kidney disease in American Indians with type 2 diabetes. Participants in the current study had previously completed a 6-year randomized clinical trial of losartan versus placebo in which few participants reached the primary GFR outcome, and the risk of progression between treatment groups was not statistically significant. In contrast, mesangial fractional volume at the end of the trial was lower in participants with microalbuminuria who were assigned to losartan than in those who were assigned to placebo (7). In this study, we report results from analyses that include the posttrial period. Given the apparent structural preservation associated with early losartan treatment, we hypothesized that early treatment would provide an extended benefit in reducing the risk of GFR decline in diabetic kidney disease, similar to that observed for early intensive glycemic control.
Research Design and Methods
Study Participants and Design
We selected 170 Pima Indians with type 2 diabetes from the Gila River Indian Community (8) to participate in a 6-year, single-center, randomized, double-blind, clinical trial testing the renoprotective efficacy of losartan (Cozaar; Merck) in early diabetic nephropathy. At baseline, 92 participants had normoalbuminuria (albumin/creatinine ratio [ACR] <30 mg/g) and 78 had microalbuminuria (ACR 30 to <300 mg/g). Participants, who were not selected based on GFR at enrollment, were randomized to receive losartan (100 mg/day) or placebo within each albuminuria stratum. Other treatment was provided by the primary care physician. Data on other antihypertensive drugs received during and after the trial were ascertained by self-report. GFR was measured annually, and the primary end point that was specified in the protocol prior to completion of the clinical trial was a decline in GFR to ≤60 mL/min or to half of the baseline value in participants with a baseline GFR <120 mL/min. Progression to macroalbuminuria (ACR ≥300 mg/g) was examined as a secondary outcome. Of the 170 participants randomized in the clinical trial, one had no follow-up measurements and was excluded from analysis (7). Upon trial completion, the study drug was no longer supplied.
The present analysis combines data collected during the clinical trial and data collected at annual research examinations that continued for a maximum of 12 years posttrial. At the end of the 6-year trial, 111 participants agreed to a kidney biopsy to determine the effect of losartan on glomerular structure. Closing date for the clinical trial was determined either by date of last examination during the randomized treatment study or by date of biopsy for those who agreed to a kidney biopsy. Subsequent follow-up was considered posttrial follow-up. We re-evaluated the effect of losartan treatment assignment on the primary GFR outcome and on progression to macroalbuminuria throughout the trial and posttrial period. The effect of treatment on death or the combined end point of end-stage renal disease (ESRD) or death was also examined. ESRD was defined by the initiation of renal replacement therapy or death from diabetic kidney disease if the participant refused dialysis. Vital status and development of ESRD were ascertained in all study participants through 31 December 2015.
This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant provided written informed consent.
Clinical and Anthropometric Measures
GFR was measured after an overnight fast by the urinary clearance of iothalamate (9). Iothalamate concentration was measured in blood and urine samples by high-performance liquid chromatography (Waters, Milford, MA). HbA1c was also measured by high-performance liquid chromatography (Tosoh, Tokyo, Japan). Urine albumin concentration was measured by nephelometric immunoassay and urine creatinine by a modified Jaffé reaction (Siemens, Erlangen, Germany) (10). Urine albumin concentrations below the detection limit of the assay (≤6.8 mg/L) were set to 6.8 mg/L in the analyses.
Blood pressure was measured while the participant was seated. Mean arterial pressure (MAP) was calculated as (2× diastolic blood pressure + systolic blood pressure)/3.
Statistical Analysis
Characteristics of the study population at the beginning of posttrial follow-up were compared between treatment groups using an independent samples t test for normally distributed variables and the Kruskal-Wallis test for nonnormally distributed variables.
During the clinical trial (7), 97.5% of research examinations were conducted according to the prespecified examination schedule. However, during the subsequent follow-up, adherence to annual research examinations declined, and 15 participants progressed to ESRD without documentation of reaching the primary GFR outcome at a research examination. To avoid the bias (informative censoring) that occurs when loss to follow-up is related to the study outcome, we used linear imputation to estimate the date of onset of the study outcomes (GFR and albuminuria). To estimate the date of onset of the primary GFR outcome, a linear GFR slope was computed in each participant based on the last two GFR values, with the last GFR value defined as follows:
In participants who did not reach the primary GFR outcome, the GFR measured at their last examination;
In participants who reached the primary GFR outcome at an examination, the GFR value measured at that examination; and
In participants who progressed to ESRD without a GFR measurement indicating that they had reached the GFR outcome, a GFR of zero was assigned as of the date of onset of renal replacement therapy.
The estimated date of onset of the primary GFR outcome was then imputed for all participants from the GFR slope. This approach permitted us to estimate whether a participant who missed scheduled visits and did not reach the primary GFR outcome by their last examination would have done so if they had remained under observation. To avoid extrapolations over too long an interval, the imputation was truncated at 2 years after the last measured GFR, so that follow-up continued for each participant for 2 years after the last measured GFR or until the primary GFR outcome, death, or 31 December 2015, whichever came first. The 2-year follow-up interval was selected because it represented the median time interval between the last GFR measurement and the onset of ESRD in the study cohort. The same approach was used to compute follow-up time and event status for the albuminuria outcome, assuming that development of ESRD also reflected progression to macroalbuminuria. For outcomes determined independently of the annual research examinations (ESRD and death), follow-up time accumulated from enrollment into the trial until the date of the event or 31 December 2015, whichever came first.
MAP and HbA1c throughout the study period were compared between treatment groups using mixed models to account for serial correlations over time. Times to outcomes were compared by treatment group using Kaplan-Meier survival curves and the log-rank test. Hazard ratios (HRs) were computed using Cox proportional hazards regression. The proportionality assumption was met by each covariate. An interaction term between treatment assignment and baseline albuminuria group was included to test whether the relationship between treatment and outcomes differed by baseline albuminuria status. Where an interaction was present, results were reported separately by baseline albuminuria status. Where no interaction was present, the analysis was stratified by baseline albuminuria status to account for the stratified sampling design, and the overall results were generally reported for both albuminuria groups combined. All analyses were based on intention-to-treat principles.
To account for the acute effects of initiating treatment with RAS inhibitors, GFR measured at each research examination, conducted either during or after the clinical trial at which the participant was treated with a RAS inhibitor, was adjusted upward by 3.75% as described previously (9).
Results
Of the 169 participants in the clinical trial, 149 remained under observation in the posttrial period (12 died and 8 were lost to follow-up during the clinical trial). Subjects who did not participate in the posttrial follow-up did not differ from those who did in terms of age, sex, diabetes duration, BMI, blood pressure, HbA1c, GFR, and ACR at baseline. Characteristics at the last clinical trial visit for the 149 participants who remained posttrial were similar between treatment groups (Table 1). During the clinical trial, 67% of participants in the placebo group were treated with RAS inhibitors at some point (5% with ARB, 47% with ACE inhibitors, and 15% with both), whereas 12% were treated with non-RAS inhibitor antihypertensive drugs (1% were treated solely with non-RAS inhibitor antihypertensive drugs). However, exposure to antihypertensive drugs in the placebo group during the clinical trial was limited to 20% of the total person-time. During posttrial follow-up, 85% of the participants randomized to losartan and 86% to placebo received RAS inhibitors; 6% of those randomized to losartan and 6% to placebo received ARBs alone, 54% of those randomized to losartan and 52% to placebo received ACE inhibitors alone, and 25% of those randomized to losartan and 28% to placebo received both. All participants who received a non-RAS inhibitor antihypertensive drug during the posttrial period also received a RAS inhibitor at some point posttrial. Exposure to RAS inhibitors in the posttrial follow-up was equivalent to 67% of the total person-time in the placebo group and 63% of the total person-time in the losartan group.
Table 1.
Characteristics of the study population at the beginning of posttrial follow-up using data from the last examination of the clinical trial
| Characteristic | Normoalbuminuria at trial enrollment |
Microalbuminuria at trial enrollment |
Total cohort |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 39) | Losartan (N = 41) | P value | Placebo (N = 34) | Losartan (N = 35) | P value | Placebo (N = 73) | Losartan (N = 76) | P value | |
| Age (years) | 47.6 ± 12.0 | 45.2 ± 10.6 | 0.34 | 48.6 ± 10.7 | 47.1 ± 8.7 | 0.53 | 48.0 ± 11.4 | 46.1 ± 9.8 | 0.21 |
| Male, n (%) | 11 (28.2) | 9 (22.0) | 0.52 | 10 (29.4) | 8 (22.9) | 0.54 | 21 (28.8) | 17 (22.4) | 0.37 |
| Diabetes duration (years) | 14.4 (12.6–22.0) | 14.0 (12.0–15.6) | 0.23 | 17.6 (12.9–24.1) | 14.5 (12.5–20.5) | 0.09 | 15.0 (12.6–23.0) | 14.2 (12.0–18.1) | 0.04 |
| BMI (kg/m2) | 34.3 (30.3–41.1) | 37.0 (31.1–41.8) | 0.57 | 35.1 (29.9–39.2) | 35.2 (30.2–38.9) | 0.92 | 34.3 (30.3–39.6) | 36.0 (30.6–40.9) | 0.72 |
| Blood pressure (mmHg) | |||||||||
| Systolic | 124 (117–135) | 124 (113–131) | 0.92 | 127 (110–134) | 125 (107–136) | 0.74 | 125 (114–135) | 124 (110–133) | 0.72 |
| Diastolic | 78 (70–83) | 76 (70–84) | 0.99 | 78 (71–85) | 77 (67–84) | 0.51 | 78 (71–85) | 77 (69–84) | 0.64 |
| HbA1c | 0.17 | 0.16 | 0.85 | ||||||
| % | 9.0 (7.0–10.7) | 10.0 (7.9–11.1) | 9.2 (8.3–10.4) | 8.1 (7.0–10.5) | 9.2 (7.5–10.7) | 9.3 (7.5–11.0) | |||
| mmol/mol | 75 (53–93) | 86 (63–98) | 77 (67–90) | 65 (53–91) | 77 (58–93) | 78 (58–97) | |||
| GFR (mL/min) | 139 (111–199) | 135 (107–191) | 0.86 | 114 (80–183) | 129 (93–159) | 0.56 | 129 (99–192) | 131 (101–170) | 0.78 |
| Urinary ACR (mg/g) | 23 (10–46) | 20 (11–58) | 0.42 | 108 (39–468) | 53 (11–318) | 0.12 | 39.4 (15.6–145.3) | 30.2 (11.0–191.8) | 0.57 |
Data are mean ± SD, median (25th–75th percentiles), or n (%).
Primary GFR Outcome
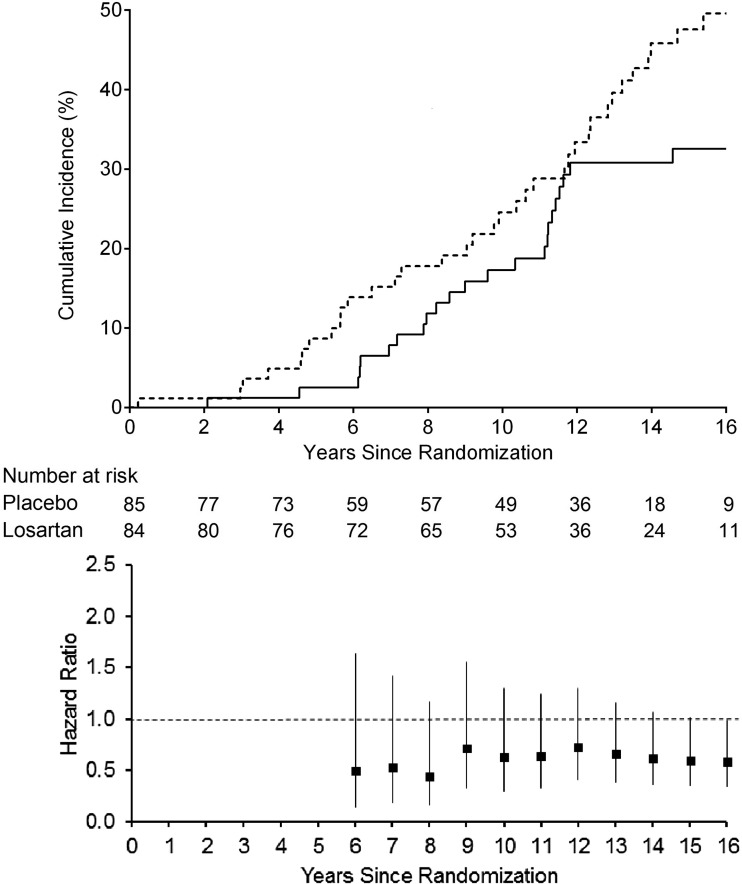
During the trial and posttrial follow-up, 29 participants randomized to losartan and 35 to placebo reached the primary GFR outcome. The median follow-up to the primary GFR outcome was 12.8 years (interquartile range 8.2–16 years). As no significant interaction was found between treatment assignment and albuminuria group (P = 0.20), the overall treatment effect was estimated. The HR for the primary GFR outcome in those receiving losartan versus placebo was 0.72 (95% CI 0.44–1.18). Adjustment for age, sex, diabetes duration, MAP, GFR, and ACR did not significantly alter our results (HR 0.88 [95% CI 0.52–1.48]). When analyzed separately, the HR was 1.04 (95% CI 0.48–2.25) for the normoalbuminuria group and 0.56 (0.29–1.07) for the microalbuminuria group. The cumulative incidence for the primary GFR outcome and the serial HRs are presented in Fig. 1. Although the cumulative HR increased initially following completion of the clinical trial, it then began to decline again, but remained not statistically significant during the follow-up period. Adjustment for the acute effects of RAS inhibitor use did not significantly alter the HR for the primary GFR outcome (HR 0.74 [0.46–1.21]).
Figure 1.
Cumulative incidence of the first occurrence of the primary GFR outcome by treatment group (top panel). Dashed line, placebo; solid line, losartan. Log-rank test for the GFR outcome yielded P = 0.28. Cumulative HRs and 95% CIs for the primary GFR outcome at trial closeout and each year of the posttrial follow-up (bottom panel). Cumulative HRs were not shown prior to the end of the trial because of the few number of events and the absence of events prior to year 4 in the losartan group.
Other Outcomes
Eighty-six participants developed macroalbuminuria (Supplementary Fig. 1); 16 were randomized to placebo and 18 to losartan in the normoalbuminuria group (P = 0.14) and 28 to placebo and 24 to losartan in the microalbuminuria group (P = 0.26). The median follow-up time to development of macroalbuminuria was 10.1 years (interquartile range 3.3–15.6 years). No interaction was found between treatment assignment and albuminuria group (P = 0.11). However, because a significant interaction was found during the clinical trial (P = 0.02), and the risk of macroalbuminuria continued to be in the opposite direction by albuminuria group, the HR for macroalbuminuria was examined separately in these groups. In the normoalbuminuria group, the HR for the first appearance of elevated albuminuria (ACR ≥30 mg/g) among those receiving losartan versus placebo was 1.02 (95% CI 0.65–1.62), and for the appearance of macroalbuminuria, the HR was 1.40 (95% CI 0.71–2.78). In the microalbuminuria group, the HR for developing macroalbuminuria was 0.68 (95% CI 0.40–1.18).
Twenty-six participants progressed to ESRD during follow-up (11 were randomized to placebo and 15 to losartan). Death occurred in 58 participants (32 were randomized to placebo and 26 to losartan) and in 11 was preceded by ESRD. Although the number of ESRD events was insufficient for informative analyses, the HR for death in those receiving losartan versus placebo was 0.79 (95% CI 0.47–1.32) and for either ESRD or death was 0.88 (95% CI 0.56–1.40). There was no interaction between treatment assignment and albuminuria group in predicting death (P = 0.22) or the combined end point of ESRD or death (P = 0.08). HRs for the various outcomes in each baseline albuminuria stratum and for the combined strata are shown in Table 2.
Table 2.
HRs (95% CI) for the effect of early treatment with losartan on long-term outcomes in each baseline albuminuria stratum and for the combined strata
| Outcome | Baseline stratum |
||||
|---|---|---|---|---|---|
| N events | Normoalbuminuria | Microalbuminuria | P value for interaction | Combined | |
| Primary GFR outcome* | 52 | 1.04 (0.48–2.25) | 0.56 (0.29–1.07) | 0.20 | 0.72 (0.44–1.18) |
| Elevated albuminuria (ACR ≥30 mg/g) | 75 | 1.02 (0.65–1.62) | — | — | — |
| Macroalbuminuria | 86 | 1.40 (0.71–2.78) | 0.68 (0.40–1.18) | 0.11 | 0.90 (0.59–1.39) |
| Death | 58 | 1.07 (0.53–2.17) | 0.54 (0.25–1.18) | 0.22 | 0.79 (0.47–1.32) |
| End-stage renal disease or death | 73 | 1.32 (0.70–2.51) | 0.57 (0.29–1.11) | 0.08 | 0.88 (0.56–1.40) |
*Decline in GFR to ≤60 mL/min or to half of the baseline value in persons who entered with GFR <120 mL/min.
Annual Means of Continuous Variables
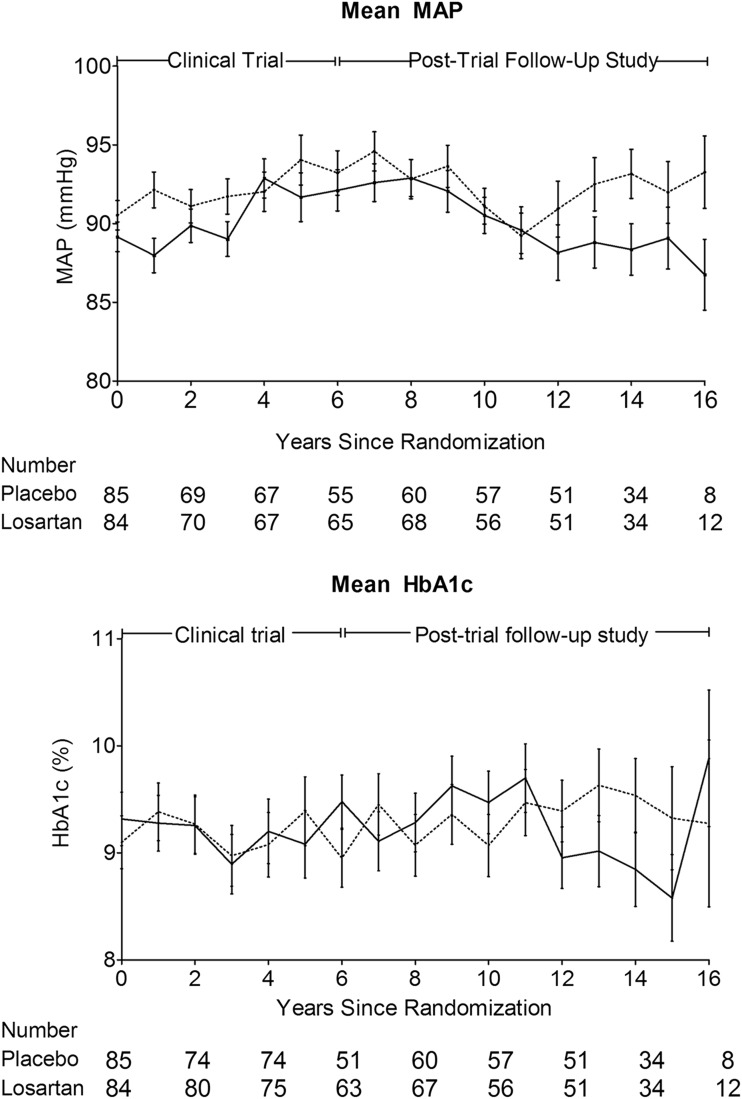
Annual mean MAP and HbA1c are shown by treatment group assignment in Fig. 2. A borderline statistically significant interaction was found between treatment group and baseline albuminuria status when examining annual mean HbA1c (P = 0.05), with losartan treatment being associated with higher HbA1c in those with normoalbuminuria but lower HbA1c in those with microalbuminuria. There was no such interaction for MAP (P = 0.42), but there was a significant difference in MAP by treatment assignment (P = 0.04) that was most apparent in the last 3 years of observation, with lower MAP in those assigned to losartan. The primary GFR finding, however, was unchanged when the Cox model was adjusted for the 2-mmHg difference in MAP between treatment groups (HR 0.74 [95% CI 0.45–1.21]).
Figure 2.
Annual mean ± SE of MAP and HbA1c by treatment group (dashed line, placebo; solid line, losartan). There was a significant difference in MAP by treatment group throughout the study period (P = 0.04), but not for HbA1c.
Conclusions
At the end of our 6-year clinical trial, nine participants had reached the primary GFR outcome for an HR of 0.50 (95% CI 0.12–1.99) in those assigned to losartan versus placebo (7). After up to 12 additional years of follow-up, 64 participants reached the primary GFR outcome—35 originally randomized to placebo and 29 to losartan—and the HR was 0.72 (95% CI 0.44–1.18). Although these HRs are not directly comparable, they both suggest no beneficial effect of early treatment with losartan on progression of diabetic kidney disease in Pima Indians with type 2 diabetes.
RAS inhibition reduces the risk of ESRD in persons with type 1 (11) and type 2 diabetes (12–14) who have chronic kidney disease and in those with other causes of chronic kidney diseases (15), but its effect on protection from ESRD in early diabetic kidney disease is less well established. One trial reported that treatment with losartan slowed the rate of estimated GFR decline in patients with macroalbuminuria (16), whereas another found that a reduction in the development of elevated albuminuria in irbesartan-treated patients with microalbuminuria was independently associated with reduced estimated GFR decline (17), but neither study demonstrated reduction in clinical outcomes such as ESRD.
We had expected that the early structural differences seen on kidney biopsy at the end of the clinical trial might lead to an extended functional benefit of early treatment in our cohort (7). Among the 51 participants with microalbuminuria who had a kidney biopsy at the end of the clinical trial, those who received losartan during the 6-year trial had lower mesangial fractional volume and higher filtration surface area than those who received a placebo. The lack of a statistically significant reduction in early kidney disease progression in the current study suggests that combined beneficial effects of RAS inhibition in early diabetic kidney disease are, at best, modest. The current study further illustrates the challenges of establishing whether RAS inhibition clearly provides renoprotection in early type 2 diabetes, because a statistically significant reduction in clinical outcomes was not observed even after ∼14 years of follow-up, and long-term follow-up of larger antihypertensive drug trials is rarely attempted.
During the clinical trial, we found that the HR for macroalbuminuria in those treated with losartan versus placebo was 8.12 (95% CI 1.02–64.98) among participants with normoalbuminuria and 0.54 (95% CI 0.26–1.10) among those with microalbuminuria at enrollment (7). In the current study, longer follow-up attenuated these HRs, so that neither effect was statistically significant. The current consensus, based on several clinical trials, is that RAS inhibition provides no benefit for primary prevention in normoalbuminuric, normotensive patients with diabetes and may actually lead to harm (18). Consistent with previous findings in antihypertensive drug trials in type 2 diabetes (12–14,19), risk of all-cause mortality in our study did not differ between those randomized to losartan or placebo. Additional follow-up of this cohort is needed to determine the long-term effect of early treatment on the risk of ESRD or death.
RAS inhibitors acutely lower GFR during the first 1–3 months of treatment, but may chronically slow the rate of GFR decline. Because the acute and chronic effects are different, accounting for them is difficult, particularly when change in GFR is the outcome. In this setting, a commonly used approach of fitting the regression model with RAS inhibitor treatment as a binary variable is not valid (20). Accordingly, we used a preferred approach of adjusting the observed GFR in each participant according to changes in RAS inhibitor treatment during follow-up, and we found that this adjustment did not alter our results.
The main strengths of this study include the use of measured GFR and the long follow-up period. Of those alive at the end of the clinical trial, 95% participated in the posttrial follow-up study. Apart from the UKPDS, which had a median posttrial follow-up duration of 8 years, to our knowledge, no previous long-term follow-up of ACE inhibitor or ARB trials beyond 2–4 years of observation has been reported (19,21,22). The current study additionally included long duration of treatment and a minority population with a high frequency of type 2 diabetes and diabetic kidney disease (23,24), which is not represented in most clinical trials. Limitations of this study include its modest sample size, the small number of events, and the inclusion of participants from only a single center, which might limit the generalizability of the findings. Intervals between research examinations sometimes increased as kidney disease progressed, which could lead to differential misclassification of the study-based outcomes (GFR and albuminuria), requiring an imputation method to compute these outcomes. Furthermore, the risk of kidney disease progressing to ESRD in this population may differ from that in other populations because of poor glycemic control and because of the lower risk of competing cardiovascular deaths prior to the onset of renal replacement therapy (25). In addition, a decision was made midway through the clinical trial to suggest that those who managed these patients consider using other RAS inhibitors in their treatment regimens. Standards of care for people with diabetic kidney disease were evolving, and this modification was required by the ethics committee overseeing the study. Although exposure of those in the placebo arm to these additional agents was low, the study ultimately examined efficacy of losartan versus standard care, and this change may have reduced the magnitude of any long-term treatment effect. Ultimately, in this underpowered study, it is difficult to disentangle whether our findings indicate no benefit of early RAS blockade on diabetic kidney disease or whether any benefit that may be present, particularly if small, was masked by the use of RAS inhibitors in the placebo group.
In conclusion, we found that early treatment with losartan in American Indians with type 2 diabetes did not lead to a statistically significant reduction in the risk of renal function loss relative to placebo during extended follow-up that included a median of ∼8 years of observation following 6 years of randomized treatment. Our study highlights the need for larger studies and long-term follow-up to evaluate the renoprotective efficacy of RAS inhibitors in persons with early diabetic kidney disease or with no clinically apparent kidney disease if currently accepted outcomes are used. Reliance on renal function changes or on surrogate markers such as albuminuria may not be sufficient to adequately evaluate renoprotection in early diabetic kidney disease even after many years of follow-up. Alternative end points, such as structural end points from kidney biopsies, may be required to demonstrate renoprotection in early diabetic kidney disease.
Supplementary Material
Article Information
Funding. This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, the American Diabetes Association (Clinical Science Award 1-08-CR-42), and Merck, which provided the study drug and placebo tablets.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.K.T. wrote the draft of the report and performed the statistical analysis. P.-J.S. and K.M.W. wrote the draft of the report. G.D.F. performed the statistical analysis. E.J.W. contributed to the data collection and critical revision of the manuscript for intellectual content. R.L.H., W.C.K., and P.H.B. designed the clinical trial. R.G.N. wrote the draft of the report and designed the clinical trial. All authors contributed to the revision of the paper and approved the final version. R.G.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
Clinical trial reg. no. NCT00340678, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0795/-/DC1.
References
- 1.de Boer IH, Sun W, Cleary PA, et al.; DCCT/EDIC Research Group . Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Bayless M, Cleary P, et al.; DCCT/EDIC Research Group . Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study at 30 years: advances and contributions. Diabetes 2013;62:3976–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 4.Volpe M, Cosentino F, Tocci G, Palano F, Paneni F. Antihypertensive therapy in diabetes: the legacy effect and RAAS blockade. Curr Hypertens Rep 1022;13:318–324 [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–1576 [DOI] [PubMed] [Google Scholar]
- 7.Weil EJ, Fufaa G, Jones LI, et al. . Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 2013;62:3224–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett PH, Burch TA, Miller M. Diabetes mellitus in American (Pima) Indians. Lancet 1971;2:125–128 [DOI] [PubMed] [Google Scholar]
- 9.Fufaa GD, Weil EJ, Lemley KV, et al. . Structural Predictors of Loss of Renal Function in American Indians with Type 2 Diabetes. Clin J Am Soc Nephrol 2016;11:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol 1960;30:207–212 [PubMed] [Google Scholar]
- 11.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 12.Brenner BM, Cooper ME, de Zeeuw D, et al.; RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 13.Lewis EJ, Hunsicker LG, Clarke WR, et al.; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 14.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870–878 [DOI] [PubMed] [Google Scholar]
- 15.Maschio G, Alberti D, Janin G, et al.; The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group . Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 1996;334:939–945 [DOI] [PubMed] [Google Scholar]
- 16.Holtkamp FA, de Zeeuw D, Thomas MC, et al. . An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011;80:282–287 [DOI] [PubMed] [Google Scholar]
- 17.Hellemons ME, Persson F, Bakker SJ, et al. . initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria: a post hoc analysis of the IRMA-2 trial. Diabetes Care 2011;34:2078–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 19.Menne J, Ritz E, Ruilope LM, Chatzikyrkou C, Viberti G, Haller H. The Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) observational follow-up study: benefits of RAS blockade with olmesartan treatment are sustained after study discontinuation. J Am Heart Assoc 2014;3:e000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005;24:2911–2935 [DOI] [PubMed] [Google Scholar]
- 21.Bosch J, Lonn E, Pogue J, Arnold JM, Dagenais GR, Yusuf S; HOPE/HOPE-TOO Study Investigators . Long-term effects of ramipril on cardiovascular events and on diabetes: results of the HOPE study extension. Circulation 2005;112:1339–1346 [DOI] [PubMed] [Google Scholar]
- 22.Cherney DZ, Zinman B, Kennedy CR, et al. . Long-term hemodynamic and molecular effects persist after discontinued renin-angiotensin system blockade in patients with type 1 diabetes mellitus. Kidney Int 2013;84:1246–1253 [DOI] [PubMed] [Google Scholar]
- 23.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 2007;30:1758–1763 [DOI] [PubMed] [Google Scholar]
- 24.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–426 [DOI] [PubMed] [Google Scholar]
- 25.Pavkov ME, Bennett PH, Sievers ML, et al. . Predominant effect of kidney disease on mortality in Pima Indians with or without type 2 diabetes. Kidney Int 2005;68:1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.