Abstract
OBJECTIVE
Type 2 diabetes mellitus (DM) has been associated with lung dysfunction, but this association has not been explored in Hispanics/Latinos. The relation between diabetic nephropathy and lung function and symptoms has not been explored.
RESEARCH DESIGN AND METHODS
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a large, multicenter, observational study, recruited 16,415 participants aged 18–74 years (14,455 with complete data on variables of interest), between 2008 and 2011 from four U.S. communities through a two-stage area household probability design. Baseline measurements were used for analyses. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and dyspnea score were compared between individuals with and without DM, overall, and stratified by albuminuria. The analyses were performed separately for those with and without preexisting lung disease (chronic bronchitis, emphysema, asthma). Linear regression with sampling weights was used for all analyses.
RESULTS
Among Hispanics/Latinos without lung disease, those with DM had lower mean FEV1 and FVC values and a higher mean dyspnea score than those without DM (mean [95% CI] FEV1 3.00 [2.96–3.04] vs. 3.10 [3.09–3.11] L, P < 0.01; FVC 3.62 [3.59–3.66] vs. 3.81 [3.79–3.83] L, P < 0.001; dyspnea score 0.60 [0.49–0.71] vs. 0.41 [0.34–0.49], P < 0.001). Hispanics/Latinos with DM and macroalbuminuria showed 10% lower FVC (P < 0.001), 6% lower FEV1 (P < 0.001), and 2.5-fold higher dyspnea score (P = 0.04) than those without DM and with normoalbuminuria. Similar findings but with higher impairment in FVC were found in Hispanics/Latinos with lung disease.
CONCLUSIONS
Hispanics/Latinos with DM have functional and symptomatic pulmonary impairment that mirror kidney microangiopathy. The progression of pulmonary impairment in adults with DM needs to be investigated further.
Introduction
Prior epidemiological (1–3) and clinical studies (4–6) have shown that non-Hispanic whites and blacks with type 2 and type 1 diabetes mellitus (DM) have lower pulmonary function (i.e., forced expiratory volume in 1 s [FEV1], forced vital capacity [FVC], and diffusing capacity of lung for carbon monoxide [DLCO]) than those without DM. Some of these studies found that lung dysfunction correlates with DM control, duration, and microvascular complications (1,4,5).
We summarized the results of these studies in a qualitative review (7). With respect to the magnitude of lung dysfunction, a meta-analysis found that FEV1, FVC, and DLCO are 5–10% lower in adults with DM than in those without DM (8). None of the prior cohort studies evaluated the association between DM and lung dysfunction in Hispanics/Latinos (1–3). Hispanics/Latinos have a higher prevalence of DM (9), poorer DM control (10), and higher prevalence and faster progression of microvascular complications (11) than other populations. In this context, we believe it important to evaluate whether Hispanics/Latinos with DM present lung dysfunction and to assess the degree of this pulmonary impairment.
Lung dysfunction has been associated with DM, but whether this association has clinical consequences, such as dyspnea, is unknown. Adults with DM are more likely to have cardiac diseases, such as coronary artery disease (CAD) and congestive heart failure, leading to dyspnea. Therefore, one can expect that adults with DM are more likely to have dyspnea than adults without DM. Whether the dyspnea is solely due to heart disease or whether lung dysfunction associated with DM can also contribute to dyspnea has not been studied. Lung diseases, such as asthma, chronic bronchitis, and emphysema, and DM have a high prevalence and are likely to coexist in the population (12–14), yet the impact of DM on lung function in adults with preexisting lung disease has not been studied. This can be important in the context of increasing overall morbidity and mortality from lung diseases (15).
The mechanisms that explain the association between DM and lung dysfunction remain unknown. One potential pathway provided by experimental (16,17) and autopsy (18) studies demonstrated glycosylation of the lung tissue collagen and proteins, leading to interstitial fibrosis and alveolar capillary microangiopathy. If lung microangiopathy is responsible for the impairment in lung function observed in DM, it is reasonable to expect that this impairment correlates with other DM microvascular complications, such as diabetic nephropathy.
In this study, we evaluated whether 1) Hispanics/Latinos with DM present functional and symptomatic pulmonary impairment, 2) the pulmonary impairment associated with DM is present in adults without and with preexisting lung disease, and 3) the pulmonary impairment associated with DM correlates with albuminuria.
Research Design and Methods
Participants
The design and conduct of the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) have been described previously (19). The HCHS/SOL is a prospective cohort study of 16,415 men and women who self-identified as Hispanic/Latino (of Cuban, Dominican, Puerto Rican, Mexican, Central American, or South American origin) and were aged 18–74 years at baseline. Participants were recruited through a multistage probability sample design from defined communities in the Bronx, New York; Chicago, Illinois; Miami, Florida; and San Diego, California (20). The first stage was a stratified random sample of census block groups, and the second was a separate stratified sample of household addresses within the first stage. Household members were then screened and sampled from the selected household addresses. Sampling weights were established based on the probability of selection, adjustment for nonresponse, trimming to handle extreme values of the weights, and calibration to the known population distribution and normalized to the entire HCHS/SOL target population.
The analyses of the current study are based on the baseline examination. Of the 16,415 participants, 1,960 were excluded due to missing data on lung function measures, FEV1, and FVC (n = 806); history of asthma or chronic obstructive pulmonary disease (n = 77); albumin-to-creatinine ratio (ACR) (n = 773); dyspnea questionnaire (n = 3); or other covariates (n = 301), leaving a sample of 14,445 for analyses, including 11,496 participants without DM and 2,959 with DM at baseline.
Assessments and Outcome Measures
The definitions and methods used for baseline measurements have been previously described (19). Details on the laboratory collection, processing, and analysis can be found in the laboratory manual (20).
Participants were asked to fast for at least 8 h before the examination. They were classified as having DM if any of the following American Diabetes Association criteria were met: 1) fasting plasma glucose (FPG) ≥126 mg/dL, 2) post–oral glucose tolerance test plasma glucose level ≥200 mg/dL, and 3) glycosylated hemoglobin (HbA1c) ≥6.5%. In addition to the laboratory test criteria, DM was defined based on current use of antidiabetic medications or self-report. The definitions and methods used for other baseline measurements (age, sex, Latino background, height, waist circumference [WC], cumulative smoking) have been previously described (19). Lung disease (chronic bronchitis, emphysema, asthma) was defined based on self-report. In addition, standard digitized spirometric measurements of timed pulmonary function (FEV1, FVC) were performed based on Epidemiology Standardization Project (21) and American Thoracic Society (22) recommendations by using a SensorMedics model 1022 dry-rolling seal volume spirometer (CareFusion, Yorba Linda, CA). The methodology was standardized across the four field centers, and quality control and reproducibility were coordinated by a centralized pulmonary function reading center.
Heart failure (HF) was defined based on self-report. CAD was defined based on electrocardiograms of possible old myocardial infarction and/or self-report of heart attack or procedure (angioplasty, stent, coronary artery bypass). Fasting albumin and creatinine were measured in urine samples collected at the beginning of the examination. Normoalbuminuria was defined as ACR <30 mg/g. Microalbuminuria was defined as ACR in the range of 30–300 mg/g, and macroalbuminuria was defined as ACR >300 mg/g. C-reactive protein (CRP) along with other blood tests were measured in a fasting blood sample collected at the study visit.
Dyspnea score was calculated similarly to other validated instruments (23) based on the following questions: 1) Are you troubled by shortness of breath when hurrying on level ground or walking up a slight hill? 2) Do you have to walk slower than people of your age on level ground because of shortness of breath? 3) Do you ever have to stop for breath when walking at your own pace on level ground? 4) Do you ever have to stop for breath after walking about 100 yards (or after a few minutes) on level ground? 5) Are you too short of breath to leave the house or short of breath on dressing or undressing? If the participants responded negatively to the first question, then the dyspnea score was 0. If they responded affirmatively to the first question, then the dyspnea score was the maximum of the ordered questions (question 1 = 1 point, question 2 = 2 points, question 3 = 3 points, question 4 = 4 points, question 5 = 5 points) because the participants’ answers represented the extent to which they perceived breathlessness to affect their mobility.
Statistical Analysis
Cross-sectional analyses were performed to compare FEV1, FVC, and dyspnea score in participants with and without DM. Analyses were performed separately for participants without and with lung disease. A separate model tested whether the association between DM and lung measures was independent of systemic inflammation (CRP). For individuals with DM, the correlations between FEV1, FVC, and dyspnea score and DM control (HbA1c) and severity (ACR) were evaluated separately for participants without and with lung disease. To further explore the association between lung function and albuminuria, participants with DM were stratified by ACR (normoalbuminuria, microalbuminuria, and macroalbuminuria), and the mean FEV1, FVC, and dyspnea score for each group were compared with the group without DM and with normoalbuminuria (reference).
All analyses were weighted to adjust for sampling probability and nonresponse. Descriptive characteristics were computed for all participants stratified by lung disease and DM status. The means of lung measures were calculated by lung disease and DM status. Survey-specific procedures were used to calculate 95% CIs to account for the two-stage sampling design, stratification, and clustering. Baseline characteristics were computed and compared using t tests for continuous variables and χ2 tests for categorical variables.
Linear regression analyses were used to calculate adjusted means of lung measures and to examine associations of these measures with HbA1c and ACR. Models were adjusted for age, sex, Hispanic/Latino background, height, WC, cumulative smoking, HF (for FEV1 and FVC), and CAD (for dyspnea score). Age, height, WC, CRP, and cumulative smoking were continuous variables, and the remaining variables were categorical. All statistical tests were two-sided at a significance level of 0.05. All analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
Results
Compared with participants without DM, those with DM were older (54 vs. 38 years of age), more likely to be women (54 vs. 51%) and Puerto Rican (20 vs. 15%) and less likely to be South American (3 vs. 5%), had larger WC (105 vs. 96 cm), had more cumulative smoking (8.8 vs. 4.4 pack-years), had a higher level of systemic inflammation (mean CRP 5.6 vs. 3.5 mg/L), and had a higher prevalence of CAD (12.5 vs. 3.2%) and HF (4.6 vs. 0.9%). Baseline characteristics of the population by DM and lung disease status are presented in Table 1.
Table 1.
Baseline participant characteristics (N = 14,455)
| Without lung disease (n = 11,810) |
With lung disease (n = 2,645) |
|||
|---|---|---|---|---|
| Characteristic | Non-DM (n = 9,510) | DM (n = 2,300) | Non-DM (n = 1,986) | DM (n = 659) |
| Age (years) | 38.5 (38.0–39.0) | 53.7 (52.8–54.6) | 39.3 (38.4–40.2) | 53.8 (52.3–55.2) |
| Female (%) | 50.1 (48.9–51.4) | 52.3 (51.7–53.0) | 58.2 (55.8–60.6) | 63.4 (61.9–64.8) |
| Ethnicity (%) | ||||
| Mexican | 41.6 (38.6–44.6) | 40.5 (39.5–41.4) | 19.8 (17.1–22.5) | 22.3 (21.1–23.5) |
| Cuban | 18.6 (16.0–21.2) | 20.5 (19.9–21.2) | 24.5 (20.7–28.3) | 24.7 (23.4–26.0) |
| Puerto Rican | 11.9 (10.8–13.0) | 14.4 (13.9–14.9) | 31.5 (28.4–34.7) | 37.3 (35.9–38.7) |
| Dominican | 10.0 (8.7–11.2) | 10.3 (9.8–10.7) | 10.0 (8.1–11.9) | 7.9 (6.8–9.1) |
| Central American | 8.1 (7.0–9.2) | 7.5 (6.9–8.1) | 5.0 (4.1–6.0) | 4.0 (3.6–4.3) |
| South American | 5.7 (5.0–6.4) | 3.7 (3.4–4.1) | 3.6 (2.8–4.4) | 1.7 (1.3–2.1) |
| Other or mixed | 4.1 (3.5–4.7) | 3.0 (2.7–3.4) | 5.5 (4.4–6.7) | 2.1 (1.8–2.4) |
| Height (cm) | 164.0 (163.7–164.3) | 162.1 (161.5–162.7) | 164.0 (163.3–164.6) | 161.0 (160.2–161.8) |
| WC (cm) | 95.3 (94.8–95.7) | 104.2 (103.4–105.0) | 98.8 (97.5–100.0) | 108.5 (106.8–110.1) |
| Smoking (pack-years) | 4.1 (3.8–4.5) | 7.4 (6.6–8.3) | 6.4 (5.6–7.2) | 14.5 (11.7–17.3) |
| HF (%) | 0.8 (0.7–1.0) | 3.9 (3.7–4.1) | 1.8 (1.2–2.4) | 7.6 (6.9–8.4) |
| CAD (%) | 3.01 (2.46–3.56) | 12.42 (10.16–14.69) | 4.39 (3.22–5.56) | 12.73 (9.59–15.88) |
| CRP (mg/L) | 3.3 (3.1–3.5) | 5.3 (4.9–5.7) | 4.6 (4.1–5.2) | 7.3 (6.3–8.3) |
| FEV1 (L) | 3.2 (3.2–3.2) | 2.6 (2.6–2.7) | 3.0 (2.9–3.0) | 2.2 (2.2–2.3) |
| FVC (L) | 3.9 (3.9–4.0) | 3.3 (3.2–3.4) | 3.7 (3.7–3.8) | 2.9 (2.8–3.0) |
| Dyspnea score | 0.56 (0.52–0.60) | 1.06 (0.96–1.16) | 1.36 (1.23–1.50) | 2.64 (2.42–2.85) |
Data are mean (95% CI). Values are weighted for study design and nonresponse.
After adjustment for covariates, participants with DM had lower mean FEV1 and FVC values and higher dyspnea scores than those without DM (FEV1 3.00 [95% CI 2.96–3.04] vs. 3.10 [3.09–3.11] L, P < 0.01, for participants without lung disease and 2.86 [2.79–2.93] vs. 2.95 [2.92–2.99] L, P < 0.05, for participants with lung disease; FVC 3.62 [3.59–3.66] vs. 3.81 [3.79–3.83] L, P < 0.001, for participants without lung disease and 3.56 [3.48–3.63] vs. 3.74 [3.70–3.77] L, P < 0.001, for participants with lung disease; dyspnea score 0.60 [0.49–0.71] vs. 0.41 [0.34–0.49], P < 0.001, for participants without lung disease and 1.25 [0.94–1.55] vs. 0.77 [0.54–1.00], P < 0.001, for participants with lung disease). Separate models that adjusted for CRP did not change the results (data not shown). Separate analyses were performed that compared outcomes variables (FEV1, FVC, and dyspnea score) between participants without and with DM stratified by age-groups (<45, 45–60, and >60 years old) and smoking status (never and ever). Results showed that independent of the age and smoking strata, participants with DM had a higher impairment in lung function and more dyspnea than those without DM (Supplementary Data).
In participants with DM, impairment in all lung measures (FEV1, FVC, and dyspnea score) correlated with ACR (Table 2). FEV1 decreased by 7 ± 2 and 13 ± 4 mL per 100 mg/g increase in ACR (P < 0.01 for both) in those without and with lung disease, respectively. FVC decreased by 9 ± 2 and 13 ± 5 mL per 100 mg/g increase in ACR (P < 0.01 and P < 0.05, respectively) in participants without and with lung disease, respectively. Dyspnea score increased by 0.02 ± 0.006 and 0.03 ± 0.01 per 100 mg/g increase in ACR (P < 0.05 for both) in those without and with lung disease, respectively.
Table 2.
Association among lung measures, HbA1c, and ACR
| Variable | Participants without lung disease | Participants with lung disease |
|---|---|---|
| FEV1 (L) | ||
| HbA1c (%) | −5.81 (6.76) | −15.41 (13.4) |
| ACR (mg/g) | −7 (2)+ | −13 (4)+ |
| FVC (L) | ||
| HbA1c (%) | −15.93 (7.04)* | −12.72 (13.96) |
| ACR (mg/g) | −9 (2)+ | −13 (5)* |
| Dyspnea score | ||
| HbA1c (%) | −0.03 (0.02) | 0.06 (0.05) |
| ACR (mg/g) | 0.0002 (0.00006)* | 0.0003 (0.0001)** |
Data are mean (SE). The estimate is the increase in the mean FEV1 or FVC per 1% increase in HbA1c and 100 mg/g increase in ACR, adjusting for age, sex, height, WC, Latino background, cumulative smoking, and history of HF (for FEV1 and FVC) and CAD (for dyspnea score).
*P < 0.05;
+P < 0.01;
**P < 0.001.
FVC inversely correlated with HbA1c for participants with DM and without lung disease (Table 2). FVC decreased by 16 ± 7 L per 1% increase in HbA1c (P < 0.05).
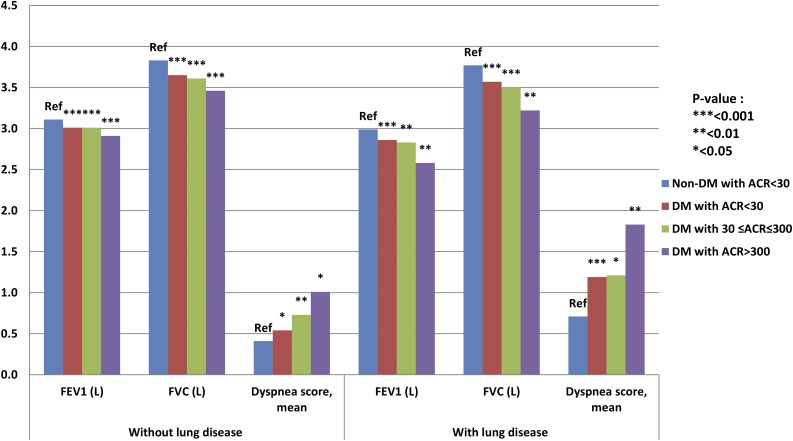
Participants with DM were further stratified into three groups based on ACR (normo-, micro-, and macroalbuminuria) and mean FEV1, FVC, and dyspnea score in each group and compared with the group without DM and with normoalbuminuria. For adults with DM and macroalbuminuria versus without DM and with normoalbuminuria, FVC was 15% and 10% lower for participants with and without lung disease, respectively, and FEV1 was 6% lower and dyspnea score was 2.5-fold higher for both lung disease groups. The impairment in lung measures was twofold higher in individuals with DM and macroalbuminuria versus DM and microalbuminuria or DM and normoalbuminuria (Table 3 and Fig. 1).
Table 3.
FEV1 and FVC in participants with DM stratified by ACR compared with those without DM
| Without lung disease (n = 11,152) |
With lung disease (n = 2,510) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-DM with ACR <30 mg/g (n = 8,852) | DM with ACR <30 mg/g (n = 1,752) | DM with 30 ≤ ACR ≤ 300 mg/g (n = 442) | DM with ACR >300 mg/g (n = 106) | Non-DM with ACR <30 mg/g (n = 1,851) | DM with ACR <30 mg/g (n = 509) | DM with 30 ≤ ACR ≤ 300 mg/g (n = 126) | DM with ACR >300 mg/g (n = 24) | |
| FEV1 (L) | 3.11 (3.10–3.13) | 3.01 (2.97–3.06) | 3.01 (2.95–3.07) | 2.91 (2.83–2.99) | 2.99 (2.96–3.02) | 2.86 (2.79–2.93) | 2.83 (2.72–2.93) | 2.58 (2.31–2.84) |
| P value | Reference | <0.001 | <0.001 | <0.001 | Reference | <0.001 | 0.005 | 0.003 |
| FVC (L) | 3.83 (3.81–3.84) | 3.65 (3.60–3.70) | 3.61 (3.54–3.68) | 3.46 (3.33–3.59) | 3.77 (3.74–3.80) | 3.57 (3.49–3.65) | 3.50 (3.37–3.63) | 3.22 (2.89–3.56) |
| P value | Reference | <0.001 | <0.001 | <0.001 | Reference | <0.001 | <0.001 | 0.002 |
| Dyspnea score | 0.41 (0.34–0.49) | 0.54 (0.42–0.67) | 0.73 (0.53–0.94) | 1.01 (0.45–1.57) | 0.71 (0.47–0.94) | 1.19 (0.86–1.51) | 1.21 (0.71–1.71) | 1.83 (1.00–2.67) |
| P value | Reference | 0.02 | 0.002 | 0.04 | Reference | 0.0002 | 0.04 | 0.008 |
Data are mean (95% CI) unless otherwise indicated. Adjusted for age, sex, height, WC, Latino background, cumulative smoking, and history of HF (for FEV1 and FVC) and CAD (for dyspnea score).
Figure 1.
FEV1, FVC, and dyspnea score in participants with diabetes by ACR compared with those without diabetes. Ref, reference.
Conclusions
In this large cohort of Hispanics/Latinos, we found that individuals with DM have lower FEV1 and FVC (with more impaired FVC than FEV1) and more dyspnea than those without DM. The impairment in all lung measures correlated with ACR in participants with DM. Compared with participants without DM and normoalbuminuria, those with DM and macroalbuminuria had much lower FEV1 and FVC (with more impairment in FVC) and more dyspnea. The negative impact of DM on lung measures overall and stratified by albuminuria was present in participants without and with lung disease (in particular, FVC was more impaired in those with lung disease than in those without lung disease).
The results generally are consistent with those of prior cross-sectional studies that included Caucasian and African American subjects and that demonstrated lower FEV1 and FVC (with higher impairment in FVC) in adults with DM than in those without DM. Of note, although in the Atherosclerosis Risk in Communities study (1) FVC was 3.5% lower in participants with DM versus without DM (mean 3.74 [95% CI 3.71–3.76] vs. 3.87 [3.86–3.88] L, P < 0.001), in the current study, FVC was 5% lower in those with DM versus without DM (3.62 [3.59–3.66] vs. 3.81 [3.79–3.83] L, P < 0.001). In both studies, the mean FVC was similar in participants without DM, and adjustment was performed using similar confounders (age, sex, height, smoking, central obesity); the higher magnitude of impairment in lung function in Hispanics/Latinos likely is related to overall poorer glycemic control and a higher rate and faster progression of microvascular complications compared with Caucasians and African Americans. However, other factors such as a genetic predisposition could also play a role and requires future investigation.
We evaluated the relationship between lung dysfunction and diabetic nephropathy in individuals with DM. Lung dysfunction correlated with ACR, and the impairment in lung function was twofold higher in participants with macroalbuminuria than in those with micro- or normoalbuminuria. Dyspnea score mirrored lung dysfunction. These findings suggest that lung dysfunction and symptoms may increase as disease worsens. Future studies should evaluate the decline in lung function over time in adults with DM and particularly in those with poor glycemic control and faster progression of microvascular complications. Detection of and prevention strategies to limit lung function decline once microalbuminuria is present should also be investigated in future studies.
This study did not investigate the mechanisms explaining lung dysfunction in DM, yet the correlation between impaired lung function and ACR, which was not affected by adjustment for CRP, suggests that biological mechanisms other than systemic inflammation play a role in this association (24–26). The results suggest that intrinsic lung biological changes related to diabetic microangiopathy causes lung dysfunction and symptoms.
The findings demonstrate a negative effect of DM on lung measures not only in individuals without lung disease but also in those with preexisting lung disease. For these individuals, we found that FVC is particularly more impaired than in those without lung disease. These findings have potential clinical consequences. For instance, it is well known that hyperglycemia predisposes to pneumonia and chronic obstructive pulmonary disease exacerbations (27). A potential explanation could be hyperglycemia-induced impairment in the chemotactic and bactericide properties of neutrophils (28). However, the current results suggest that lung function impairment, particularly in adults with advanced DM (and extensive microvascular complications), also contributes to increasing respiratory morbidity in adults with DM. In fact, this hypothesis is supported by one of our prior clinical studies, which demonstrated that patients with diabetes and impaired DLCO had a higher odds of being hospitalized with pneumonia independent of hyperglycemia and comorbidities (29). Future studies should address whether tighter targets for DM control in patients with lung disease limits DM-induced lung dysfunction and decreases respiratory morbidity and mortality.
We found that individuals with DM have more dyspnea than those without DM. Although adults with DM have a higher prevalence of CAD and HF (30) and are expected to have more dyspnea than those without DM (31), we demonstrate that an intrinsic lung disease also plays a role independent of other cardiac etiologies. Strategies to limit not only cardiac but also pulmonary origins of dyspnea should be sought to improve the respiratory and overall quality of life in adults with DM.
The strengths of this study include a community-based population, extensive data on potential confounders, and standardized spirometric techniques. In contrast to prior studies, we adjusted for cumulative smoking rather than smoking status (32), WC rather than BMI (33), and HF. We acknowledge that basing HF on self-report rather than on an objective measurement, such as echocardiography, is a limitation. However, in a prior clinical study comparing DLCO in patients with and without DM, we adjusted for HF based on ICD-9 codes in one model and a combination of ICD-9 codes and echocardiography criteria in a second model (29). Adjusting not only for clinical HF but also for asymptomatic systolic and diastolic dysfunction did not change the results (29).
The main limitation of this study is related to its cross-sectional design. We cannot establish the direction of association between DM and lung dysfunction. Prior longitudinal studies yielded controversial results (7), but the majority showed that lung function continued to decline with DM progression. More-frequent measurements and longer periods of follow-up are needed to better characterize the progression of lung dysfunction in adults with DM. In addition, a good characterization of the population, which is prone to the development of pulmonary microangiopathy and fibrosis, as well as a good understanding of the progression of lung function decline in DM are also needed before considering local therapeutic agents with potential proinflammatory properties, such as inhaled insulin.
In summary, this study brings novel findings to the literature. It shows that Hispanics/Latinos with DM have lung dysfunction that is likely more severe than that of Caucasians and African Americans; reports more lung-related dyspnea in individuals with DM; demonstrates a negative effect of DM on lung function in individuals with lung diseases such as asthma, chronic bronchitis, and emphysema; and shows that lung dysfunction and dyspnea in DM correlate with other DM microvascular complications, suggesting that alveolar-capillary microangiopathy–related mechanisms play a role in this association.
Supplementary Material
Article Information
Funding. The HCHS/SOL was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute to the University of North Carolina (N01-HC-65233), University of Miami (N01-HC-65234), Albert Einstein College of Medicine (N01-HC-65235), Northwestern University (N01-HC-65236), and San Diego State University (N01-HC-65237). The following National Institutes of Health institutes/offices collaborated and cofunded the study: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and Office of Dietary Supplements.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. O.L.K. contributed to the data research and writing of the manuscript. L.A.-S., H.R.C., A.M.K., R.C.K., G.L.K., E.M., L.S., G.T., and M.D. contributed to the discussion and review and editing of the manuscript. J.C. and D.W. contributed to the data analysis, discussion, and review and editing of the manuscript. O.L.K. is the guarantor of this work and, as such, had full access to all the data in the study takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-1170/-/DC1.
References
- 1.Yeh HC, Punjabi NM, Wang NY, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2008;31:741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter RE, Beiser A, Givelber RJ, O’Connor GT, Gottlieb DJ. Association between glycemic state and lung function: the Framingham Heart Study. Am J Respir Crit Care Med 2003;167:911–916 [DOI] [PubMed] [Google Scholar]
- 3.Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and type 2 diabetes: findings from the British Women’s Heart and Health Study. Diabetologia 2004;47:195–203 [DOI] [PubMed] [Google Scholar]
- 4.Chance WW, Rhee C, Yilmaz C, et al. Diminished alveolar microvascular reserves in type 2 diabetes reflect systemic microangiopathy. Diabetes Care 2008;31:1596–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein OL, Kalhan R, Williams MV, et al. Lung spirometry parameters and diffusion capacity are decreased in patients with type 2 diabetes. Diabet Med 2012;29:212–219 [DOI] [PubMed] [Google Scholar]
- 6.Niranjan V, McBrayer DG, Ramirez LC, Raskin P, Hsia CC. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am J Med 1997;103:504–513 [DOI] [PubMed] [Google Scholar]
- 7.Klein OL, Krishnan JA, Glick S, Smith LJ. Systematic review of the association between lung function and type 2 diabetes mellitus. Diabet Med 2010;27:977–987 [DOI] [PubMed] [Google Scholar]
- 8.van den Borst B, Gosker HR, Zeegers MP, Schols AM. Pulmonary function in diabetes: a metaanalysis. Chest 2010;138:393–406 [DOI] [PubMed] [Google Scholar]
- 9.Danaei G, Friedman AB, Oza S, Murray CJ, Ezzati M. Diabetes prevalence and diagnosis in US states: analysis of health surveys. Popul Health Metr 2009;7:16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MK, McKeever Bullard K, Imperatore G, Barker L, Gregg EW; Centers for Disease Control and Prevention (CDC) . Characteristics associated with poor glycemic control among adults with self-reported diagnosed diabetes–National Health and Nutrition Examination Survey, United States, 2007-2010. MMWR Morb Mortal Wkly Rep 2012;15(Suppl.):32–37 [PubMed] [Google Scholar]
- 11.Wang Y, Katzmarzyk PT, Horswell R, et al. Racial disparities in diabetic complications in an underinsured population. J Clin Endocrinol Metab 2012;97:4446–4453 [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich SF, Quesenberry CP Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010;33:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004;27:2478–2484 [DOI] [PubMed] [Google Scholar]
- 14.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis 2014;11:E62 [DOI] [PMC free article] [PubMed]
- 15.Maters GA, de Voogd JN, Sanderman R, Wempe JB. Predictors of all-cause mortality in patients with stable COPD: medical comorbid conditions or high depressive symptoms. COPD 2014;11:468–474 [DOI] [PubMed]
- 16.Yang J, Tan Y, Zhao F, et al. Angiotensin II plays a critical role in diabetic pulmonary fibrosis most likely via activation of NADPH oxidase-mediated nitrosative damage. Am J Physiol Endocrinol Metab 2011;301:E132–E144 [DOI] [PubMed] [Google Scholar]
- 17.Popov D, Simionescu M. Alterations of lung structure in experimental diabetes, and diabetes associated with hyperlipidaemia in hamsters. Eur Respir J 1997;10:1850–1858 [DOI] [PubMed] [Google Scholar]
- 18.Weynand B, Jonckheere A, Frans A, Rahier J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 1999;66:14–19 [DOI] [PubMed] [Google Scholar]
- 19.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris BG Jr, Speizer FE, Bishop Y, Prang G, Weener J. Spirometry for an epidemiologic study: deriving optimum summary statistics for each subject. Bull Eur Physiopathol Respir 1978;14:145–166 [PubMed] [Google Scholar]
- 22.ATS statement—Snowbird workshop on standardization of spirometry. Am Rev Respir Dis 1979;119:831–838 [DOI] [PubMed] [Google Scholar]
- 23.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93:580–586 [DOI] [PubMed] [Google Scholar]
- 24.Festa A, D’Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM; The Insulin Resistance Atherosclerosis Study (IRIS) . Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42–47 [DOI] [PubMed] [Google Scholar]
- 25.Barzilay JI, Abraham L, Heckbert SR, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes 2001;50:2384–2389 [DOI] [PubMed] [Google Scholar]
- 26.Hancox RJ, Poulton R, Greene JM, et al. Systemic inflammation and lung function in young adults. Thorax 2007;62:1064–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labarere J, Stone RA, Obrosky DS, et al. Comparison of outcomes for low-risk outpatients and inpatients with pneumonia: a propensity-adjusted analysis. Chest 2007;131:480–488 [DOI] [PubMed] [Google Scholar]
- 28.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med 1997;14:29–34 [DOI] [PubMed] [Google Scholar]
- 29.Klein OL, Smith LJ, Tipping M, Peng J, Williams MV. Reduced diffusion lung capacity in patients with type 2 diabetes mellitus predicts hospitalization for pneumonia. Diabetes Res Clin Pract 2011;92:e12–e15 [DOI] [PubMed] [Google Scholar]
- 30.Piccini JP, Klein L, Gheorghiade M, Bonow RO. New insights into diastolic heart failure: role of diabetes mellitus. Am J Med 2004;116(Suppl. 5A):64S–75S [DOI] [PubMed] [Google Scholar]
- 31.Guazzi M, Brambilla R, De Vita S, Guazzi MD. Diabetes worsens pulmonary diffusion in heart failure, and insulin counteracts this effect. Am J Respir Crit Care Med 2002;166:978–982 [DOI] [PubMed] [Google Scholar]
- 32.Strand BH, Mishra G, Kuh D, Guralnik JM, Patel KV. Smoking history and physical performance in midlife: results from the British 1946 birth cohort. J Gerontol A Biol Sci Med Sci 2011;66:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wehrmeister FC, Menezes AM, Muniz LC, Martínez-Mesa J, Domingues MR, Horta BL. Waist circumference and pulmonary function: a systematic review and meta-analysis. Syst Rev 2012;1: 55 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.