Abstract
Diabetes is associated with a deficit of circulating endothelial progenitor cells (EPCs), which has been attributed to their defective mobilization from the bone marrow. The basis for this mobilization defect is not completely understood, and we sought to determine if hyperglycemic conditions enhanced EPC adhesion. We found that culturing EPCs in high glucose media increased adhesion to bone marrow stromal cells. This enhanced adhesion was associated with decreased expression of protein kinase A regulatory subunit 1β (PRKAR1β), activation of protein kinase A (PKA), and phosphorylation of α4-integrin on serine 988. This potentiated adhesion was reversed by treatment with a PKA inhibitor, overexpression of PRKAR1β, or expression of a phosphorylation-defective α4-integrin variant (α4[S988A]). Using a model of type 1 diabetes, we showed that α4(S988A)-expressing mice have more circulating EPCs than their wild-type counterparts. Moreover, diabetic α4(S988A) mice demonstrate enhanced revascularization after hind limb ischemia. Thus, we have identified a novel signaling mechanism activating PKA in diabetes (downregulation of an inhibitory regulatory subunit) that leads to deficits of circulating EPCs and impaired vascular repair, which could be reversed by α4-integrin mutation.
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide (1). A dysfunctional endothelium contributes to the development of CVD by promoting or exacerbating atherosclerosis, hypertension, and thrombosis. Damage to the vasculature is repaired in part by a population of bone marrow stem cells, the endothelial progenitor cells (EPCs). Several previous studies reported that levels of circulating EPCs are directly associated with vascular health (2), and their abundance and functionality are negatively associated with advanced age (2), smoking (2,3), and a sedentary lifestyle (4). Furthermore, several animal (5–7) and human (8–10) studies have demonstrated an inverse relationship between circulating EPC number and incidence of diabetes.
EPCs are mobilized from the bone marrow by cytokines and growth factors such as stromal cell–derived factor-1 (SDF-1α) (6,11) and vascular endothelial growth factor (VEGF) (12,13). Signaling pathways downstream of these agonists disrupt adhesive interactions mediated by C-X-C chemokine receptor 4 (CXCR4) and c-kit, which are partly responsible for maintaining EPCs in the bone marrow. In addition, signals from the sympathetic nervous system have also been implicated in the mobilization of EPCs (14) as well as hematopoietic stem cells (HSCs) (14,15). Diabetes is associated with defective progenitor cell mobilization. Prior reports suggest this may result from deficits of mobilizing agonists (5,16), altered bone marrow structure and responsiveness (7,17), or induced neuropathy and altered expression of intracellular signaling molecules (14).
Stem cell populations are also retained in the bone marrow through α4β1-integrin vascular cell adhesion molecule-1 (VCAM-1) interactions. Ablation of these interactions or conditional α4-integrin knockdown enhanced circulating levels of both HSCs (18–20) and EPCs (21). Given this prominent role of α4β1 in bone marrow retention, we hypothesized that diabetes might impact the functional properties of this integrin to limit EPC mobilization. To test this possibility, we studied the effects of hyperglycemia on the adhesion of cultured EPCs. We found that growth in high glucose enhanced the adhesion of EPCs to bone marrow stromal cells. This potentiated adhesion was associated with downregulation of the regulatory subunit 1β of protein kinase A (PRKAR1β), consequent activation of protein kinase A (PKA), and phosphorylation of α4-integrin on serine 988. Enhanced adhesion was blocked by a PKA inhibitor and PRKAR1β overexpression. EPCs with an alanine substitution at serine 988 (S988A) in the α4-integrin subunit were also resistant to high glucose–potentiated adhesion. Furthermore, using a model of type 1 diabetes, we observed that mice expressing the α4(S988A) variant had increased levels of circulating EPCs and enhanced revascularization when compared with their wild-type counterparts. Thus, hyperglycemia limits EPC mobilization through PRKAR1β downregulation, activation of PKA, phosphorylation of α4-integrin, and potentiated adhesion in the bone marrow. Ablation of this signaling pathway enhanced circulating EPC levels and vascular repair capacity.
Research Design and Methods
Reagents
Antibodies for α4-integrin immunoprecipitation (PS/2 and HP2/1) and blotting (C-20) were from Millipore, GeneTex, and Santa Cruz Biotechnology, respectively. The phospho-α4-integrin antibody was generated as previously described (22). PKA subunit antibodies were supplied by Becton Dickinson. An anti–VCAM-1 antibody was from Southern Biotech. H89 was obtained from Millipore, and 8-bromoadenosine cAMP (8-Br-cAMP) was from Enzo Life Sciences. The PRKAR1β cDNA was obtained from GeneCopoeia. Streptozotocin (STZ) and anti-actin and control antibodies were from Sigma-Aldrich.
Cells and Mice
Endothelial colony-forming cells (ECFCs) were obtained from the Angiogenesis, Endothelial, and Proangiogenic Cell Core of the Simon Cancer Center at the Indiana University School of Medicine (23). They were maintained in EGM-2 media (Lonza) supplemented with 10% FCS and used between passages 3 and 10. To study the effects of high glucose, media was supplemented with 20 mmol/L d-glucose (Sigma-Aldrich) whereas osmotic control media contained an additional 20 mmol/L l-glucose (VWR). Exogenous activators (8-Br-cAMP; 500 μmol/L) or inhibitors (H89; 10 μmol/L) of PKA were added 1 h prior to cell lysis or biochemical assay. ECFCs were transfected using the TransFectin reagent (Bio-Rad), according to the manufacturer’s recommendations, and used for experiments 48 h later.
Mice expressing the S988A α4-integrin variant α4(S988A) were generated as previously described (24). To induce diabetes, STZ was injected intraperitoneally at a dose of 65 mg/kg for 6 consecutive days. Blood glucose levels were analyzed after 7 days, and only mice having a glucose concentration of >350 mg/dL were used for subsequent analyses. Control mice received injections of citrate.
Murine bone marrow cells were isolated by flushing femurs and tibiae with media. EPCs from these preparations were cultured on fibronectin-coated plates for 1 week in the above media. Circulating EPCs were detected in lysed whole blood by flow cytometry using APC-conjugated anti-Flk and PE-conjugated anti-Sca antibodies (eBioscience).
Biochemical Assays
Measurements of α4-integrin expression and α4β1-integrin affinity were accomplished by flow cytometry using a FITC-conjugated anti-α4 antibody (eBioscience) and a FITC-conjugated affinity ligand (gift from Dr. Larry Sklar, University of New Mexico), respectively. To measure adhesion, harvested cells were incubated with 5 μmol/L calcein AM (Life Technologies) for 30 min at 37°C. The cells were then washed, incubated for an additional 30 min, and washed again. Then 105 cells were added in triplicate to confluent S17 bone marrow stromal cells (gift from Dr. Kenneth Dorschkind, University of California, Los Angeles) in a 24-well plate. At various time points, the wells were aspirated and washed and fluorescence was determined in a plate reader. A percent adhesion was calculated based upon the initial fluorescence of 105 cell aliquots. Background fluorescence was determined using wells coated with BSA. PKA activity in cell homogenates was determined using the PepTag nonradioactive cAMP-dependent protein kinase assay (Promega). Migration toward SDF-1α (100 ng/mL) was determined using modifications of a previously described assay (25).
Hind Limb Ischemia
Revascularization was determined by laser Doppler perfusion imaging (LDPI) in mice subjected to hind limb ischemia (HLI). For HLI, male mice were anesthetized by isoflurane inhalation (3% isoflurane mixed with 100% O2), an incision was made through the skin, and the femoral artery and vein were exposed. Sterile 7.0 sutures were threaded under and around the femoral artery and vein and knotted to permanently ligate both blood vessels. Sham HLI surgery was conducted in the same manner, except that blood vessels were not ligated. Three weeks after surgery, mice were anesthetized as above and LDPI (MoorLDI2; Moor Instruments) was recorded for both ischemic and nonischemic hind limbs. LDPI values scale linearly with the product of red blood cell velocity and the number of blood cells within the tissue and are listed in arbitrary units. Two images were taken for every mouse and imported into ImageJ, and areas of blue, green, and red in the region of interest spanning the entire hind limb were enumerated using the moorFLPI-2 Review Software. The value for the ischemic limb was then normalized to the nonischemic limb to calculate a percent recovery of blood perfusion. All procedures were approved by the University of Louisville Institutional Animal Care and Use Committee.
Bone Marrow Transplantation
For bone marrow transplantation, 7-week recipient male C57BL/6 mice (The Jackson Laboratory) were first conditioned with 950 cGy total body irradiation (γ-cell 40; Nordion, Ontario, Canada). Bone marrow was isolated from tibias and femurs of donor α4(WT) or α4(S988A) male mice (12 weeks) by flushing with PBS. The cells were resuspended in PBS by gentle aspiration through an 18-gauge needle, filtered through sterile nylon mesh with 100-μM pores, centrifuged at 1,000 rpm for 10 min at 4°C, and resuspended in PBS. Twenty-four hours after irradiation, recipients were injected with 0.1 mL PBS containing 1 × 107 cells through the retro-orbital plexus with a 27-gauge needle. Five weeks after transplant, recipients were characterized for hematopoietic recovery by assessing complete blood cell counts using a HemaVet 950 (Drew Scientific Inc., Oxford, CT).
Immunofluorescence
Skeletal muscle from ischemic limbs was collected, formalin fixed, and paraffin embedded. Sections (5 μm) were stained with fluorescently conjugated isolectin (Griffonia, Bandeiraea simplicifolia Lectin I; Vector Laboratories) and nuclear counterstained with DAPI. Images were acquired with a fluorescent microscope. Additional sections were stained with Sirius Red (0.1%; RA Lamb LLC, Apex, NC) and Fast Green FCF (0.1%; Sigma-Aldrich). Up to three sections were viewed on an Olympus IOM microscope and imaged with a SPOT camera using SPOT advanced image-capture software. Analysis of isolectin staining and fibrosis was performed using the National Institutes of Health ImageJ software, version 1.45s. Each digital photomicrograph (up to three different sections per slide) was analyzed as previously reported (26). The extent of fibrosis was measured as the percentage of the total area assessed (available at https://imagej.nih.gov/ij/docs/examples/stained-sections/index.html).
Statistical Analysis
Values are expressed as means ± SE. Statistical significance (P < 0.05) was evaluated using an unpaired Student t test. One-way ANOVA with Bonferroni post hoc test was used for comparing multiple groups.
Results
Growth in High Glucose Potentiates EPC Adhesion
Diabetes has been associated with a deficit of circulating EPCs (5–10). One possible reason for this deficit is enhanced retention of these cells in the bone marrow, as has been suggested for HSCs (17). To test this, we first examined the adhesive properties of cultured EPCs, the ECFCs. To approximate the serum conditions of diabetes, we grew these cells in media containing 25 mmol/L d-glucose for 1 week. These cells demonstrated enhanced adhesion to a monolayer of S17 bone marrow stromal cells compared with those cells grown in the osmotic control media (Fig. 1A). Growth in high glucose media, however, does not induce general cellular dysfunction. Cells cultured in both types of media displayed equivalent SDF-1α–mediated migration (Fig. 1B).
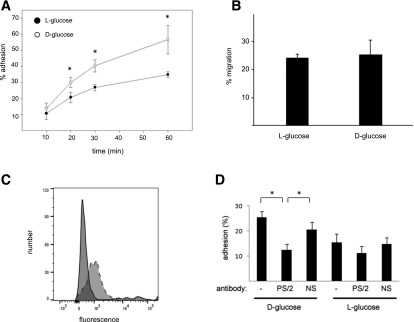
Figure 1.
Growth in high glucose stimulates ECFC adhesion. A: ECFCs were grown in 25 mmol/L d-glucose or the l-glucose osmotic control media for 1 week, and a time course of their adhesion to an S17 cell monolayer was determined. n = 4. *P < 0.05. B: Cells grown for 1 week in the indicated media were harvested, and SDF-1α–stimulated migration was analyzed in a transwell assay. C: ECFCs express the α4-integrin. Solid line, isotype control antibody; dashed line, α4-specific antibody. D: Cells grown for 1 week in the indicated media were harvested and allowed to adhere for 30 min in the absence or presence of an anti-α4β1 blocking antibody (PS/2) or a nonspecific antibody (NS). n = 5. *P < 0.05.
α4β1-Integin–mediated adhesion is crucial for maintaining HSCs (19,27) and EPCs (21) in the bone marrow, and this integrin is expressed in ECFCs (Fig. 1C). To determine whether hyperglycemia-stimulated ECFC adhesion is also mediated by α4β1, we performed the adhesion assay in the presence of a function-blocking antibody (PS/2; 10 μg/mL). Addition of this antibody reduced ECFC adhesion to background levels (Fig. 1D). Inclusion of a nonspecific antibody had no effect on high glucose–stimulated adhesion. Incubation with either antibody did not affect the adhesion of the osmotic control cells. Thus, growth in high glucose stimulates α4β1-dependent adhesion.
Growth in High Glucose Stimulates PKA-Dependent α4-Integrin Phosphorylation
Enhanced α4β1-mediated adhesion might result from an increase in α4β1 expression or ligand binding affinity. However, growth in high glucose media did not appear to change either of these properties (Fig. 2A and B). Alternatively, α4β1-mediated adhesion can be impacted by PKA-dependent phosphorylation of the α4-integrin at serine 988 (22,28). To determine whether growth in high glucose increases adhesion through this mechanism, we first measured PKA activity. Cells grown in 25 mmol/L d-glucose demonstrated a 1.40 ± 0.25–fold increase in activity compared with cells grown in the osmotic control media (Fig. 2C). Consistently, we also found an increase in α4-integrin phosphorylation in the d-glucose–cultured cells (Fig. 2D and E), which was blocked with the PKA inhibitor H89 (Fig. 2D and E). To determine whether there was a causal relationship between PKA activity and increased adhesion, we treated d-glucose–cultured cells with H89 prior to their use in the adhesion assay. We observed that this treatment, but not treatment with vehicle, reduced adhesion (Fig. 2F). To determine whether direct PKA activation stimulated α4 phosphorylation and adhesion, we treated ECFCs with the PKA activator 8-Br-cAMP. Like the high glucose–cultured cells, these cells demonstrated increased α4 subunit phosphorylation (Fig. 3A and B) and adhesion (Fig. 3C) compared with vehicle-treated cells. Moreover, 8-Br-cAMP–stimulated adhesion was blocked with an α4-integrin function–blocking antibody and with H89 (Fig. 3C). Finally, S17 cells express the α4β1 ligand VCAM-1, and an anti–VCAM-1 blocking antibody also reduced ECFC adhesion to background levels (Fig. 3C). Collectively, these observations suggest that high glucose stimulates EPC adhesion by increasing the PKA-mediated phosphorylation of the α4-integrin.
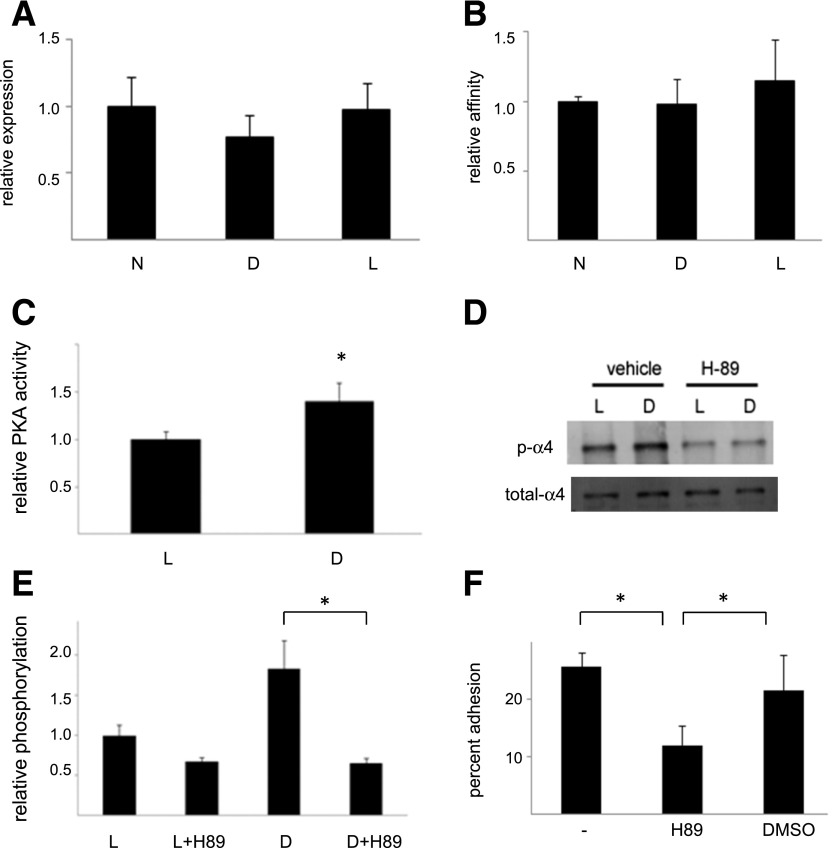
Figure 2.
Growth in high glucose simulates PKA activity, α4-integrin phosphorylation, and PKA-dependent adhesion. A: α4 expression. ECFCs were grown for 1 week in normal media (N), in media containing 25 mmol/L d-glucose (D), or in the osmotic control media (L), and α4-integrin expression was determined by flow cytometry. n = 4. B: α4β1 affinity. The same cells were used to determine α4β1 affinity by flow cytometry. n = 3. C: PKA activity. ECFCs were grown in media containing 25 mmol/L d-glucose and the osmotic control media for 1 week, and PKA activity was then determined. n = 10. *P < 0.05. D: α4 phosphorylation. Cells grown for 1 week in the same media as indicated were treated with DMSO (vehicle) or the PKA inhibitor H89 and levels of phospho-α4 were determined by Western blotting. Illustrated is a representative blot. E: Illustrated are group data for high glucose–stimulated α4 phosphorylation. n = 8. *P < 0.05. F: Cell adhesion. Cells grown in d-glucose media were left untreated or were treated with either H89 or DMSO, and then adhesion to an S17 cell monolayer was determined after 30 min. n = 4. *P < 0.05.
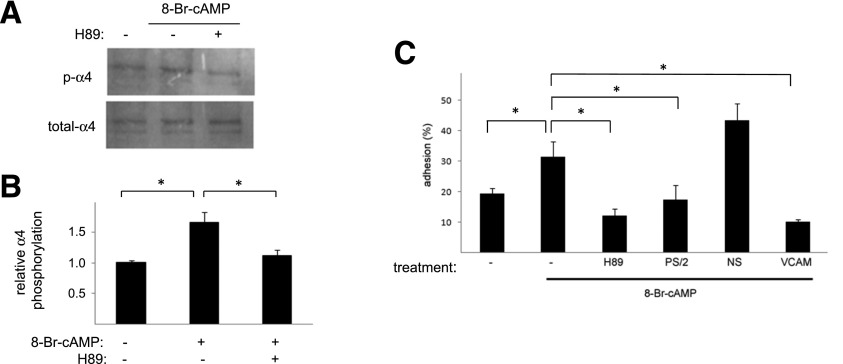
Figure 3.
Direct PKA stimulation enhances α4 phosphorylation and adhesion. A: PKA-stimulated α4 phosphorylation. Untreated ECFCs or those treated with 8-Br-cAMP in the presence or absence of H89 were lysed, and levels of phospho-α4 (p-α4) and total α4 determined by Western blotting. Illustrated are representative blots. B: Illustrated are grouped data of phospho-α4–to–total α4 ratios normalized to untreated samples. n = 4. *P < 0.05. C: Cell adhesion. Cells variably incubated with 8-Br-cAMP as indicated were allowed to adhere to an S17 cell monolayer for 30 min. Adhesion was done in the presence of anti-α4 (PS/2), nonspecific (NS), or anti-VCAM antibodies, as indicated. The PKA dependence of enhanced adhesion was demonstrated by inhibition with H89. n ≥ 4. *P < 0.05.
PRKAR1β Downregulation Enhances PKA Activity and Potentiates Adhesion
PKA activation may result from increased levels of its cellular activator cAMP. However, quantification of cAMP levels by ELISA indicated no change in the concentration of this secondary messenger (Fig. 4A). Alternatively, PKA activity may be influenced by the stoichiometry of its catalytic and regulatory subunits. To test this possibility, we first identified which PKA subunits are expressed in ECFCs. In addition to the catalytic subunit (PKA-C), ECFCs predominantly express the regulatory subunits 1β (PRKAR1β), 2α (PRKAR2α), and 2β (PRKAR2β) (Fig. 4B). Next we determined whether the expression of these subunits was altered by growth in high glucose. Whereas there were no changes in PKA-C, PRKAR2α, or PRKAR2β, we did observe a 33 ± 12% reduction in PRKAR1β expression (Fig. 4C and D).
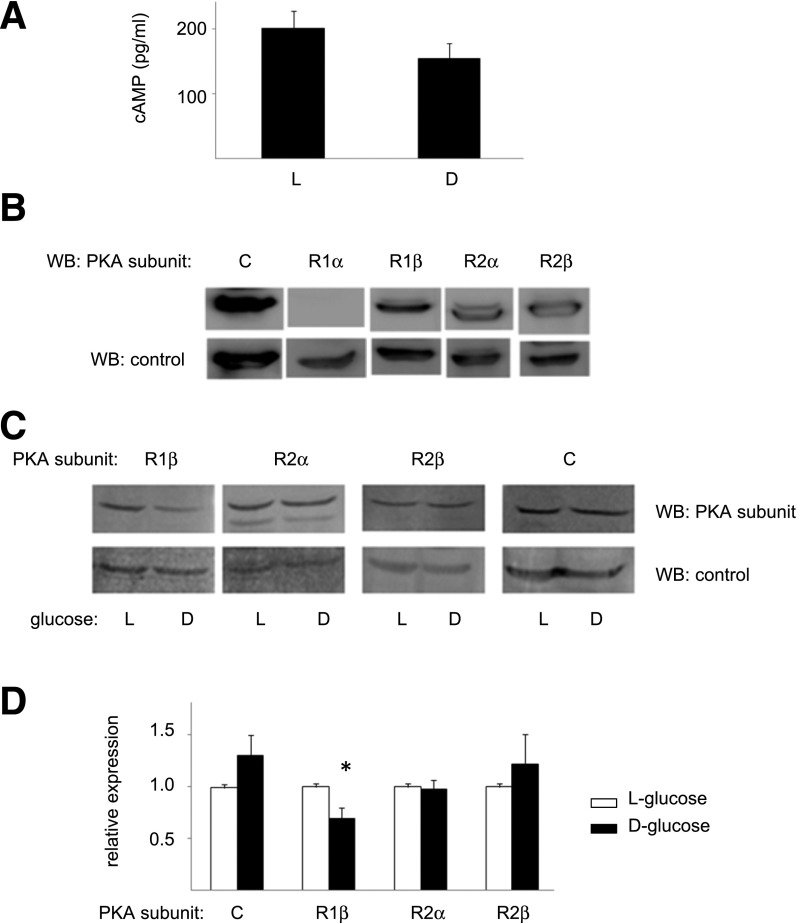
Figure 4.
Hyperglycemia and PKA activation. A: cAMP levels. ECFCs grown for 1 week in 25 mmol/L d-glucose (D) or l-glucose (L) as indicated were analyzed for levels of cAMP by ELISA. n = 3. B: PKA subunits in ECFC. The expression of PKA subunits in ECFC was determined by Western blotting (top panels). In addition to the catalytic subunit C, these cells predominantly express PRKAR1β, PRKAR2α, and PRKAR2β (R1β, R2α, and R2β, respectively). Loading control blots (bottom panels) used antibodies for actin (PRKAR1α [R1α], R1β, R2α, and R2β) or tubulin (PKA-C). C: PKA subunit expression after hyperglycemic growth. ECFCs were cultured for 1 week in 25 mmol/L d-glucose (D) or l-glucose (L) as indicated, and levels of the indicated PKA subunits (top panels) were determined by Western blotting. D: Relative expression levels. Illustrated are the relative expression levels of the PKA subunits after hyperglycemic growth. n = 4. *P < 0.05 vs. l-glucose. C, catalytic subunit C; WB, Western blot.
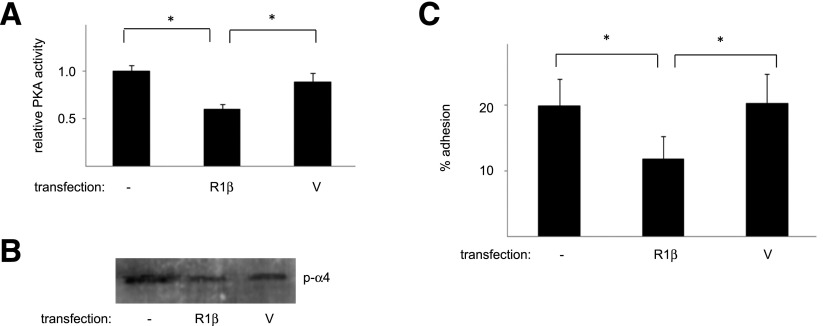
We reasoned that if growth in high glucose downregulates PRKAR1β, resulting in PKA activation, α4 phosphorylation, and enhanced adhesion, then overexpression of PRKAR1β should reverse these effects. To test this, ECFCs cultured in high glucose media were transfected with PRKAR1β or control vectors. PRKAR1β transfectants demonstrated decreases in PKA activity (Fig. 5A), α4 phosphorylation (Fig. 5B), and adhesion (Fig. 5C) compared with vector transfectants or untransfected cells. These observations suggest that attenuated expression of PRKAR1β in the presence of high glucose enhances ECFC adhesion.
Figure 5.
Overexpression of PRKAR1β reverses the effect of hyperglycemic culture. ECFCs grown in 25 mmol/L d-glucose for 5 days were transfected with PRKAR1β (R1β) or control vector (V) and lysed 2 days later. A: PKA activity. The PKA activity of the d-glucose–cultured transfectants was determined as outlined in research design and methods. n = 4. *P < 0.05. B: α4 Phosphorylation (p-α4). Levels of phosphorylated α4-integrin in the transfectants were determined by Western blotting. Illustrated is a representative blot. C: Adhesion. The adhesive properties of the d-glucose–cultured transfectants to an S17 cell monolayer were determined and grouped data illustrated. n = 6. *P < 0.05.
Ablation of α4-Integrin Phosphorylation Reverses Hyperglycemia-Induced Adhesion
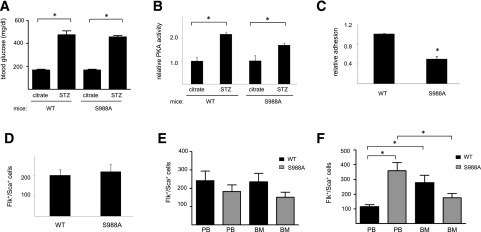
To directly test the idea that α4-integrin phosphorylation enhances EPC adhesion in hyperglycemic conditions, we used cells isolated from mice expressing the α4-integrin S988A variant. This variant has an alanine rather than serine at residue 988 and thus cannot be phosphorylated. Cells from these mice would be predicted to be unresponsive to the effects of hyperglycemia. We tested this possibility by inducing hyperglycemia in α4(WT) and α4(S988A) mice using the STZ model of type 1 diabetes (Fig. 6A). Four weeks after induction of diabetes, bone marrow EPCs were isolated, cultured ex vivo, and used for cellular and biochemical assays. As expected, cells from both STZ-injected mouse strains demonstrated increased PKA activity compared with their control, citrate-injected counterparts (Fig. 6B). However cells isolated from the diabetic α4(S988A) mice demonstrated a 50 ± 5% reduction in adhesion compared with cells isolated from the diabetic α4(WT) mice (Fig. 6C). These findings indicate that increased adhesion of EPCs in hyperglycemic conditions could be attributable to increased α4-integrin phosphorylation.
Figure 6.
Characterization of murine EPCs. A: α4(WT) and α4(S988A) mice were injected with citrate or STZ and blood glucose levels determined after 4 weeks. n ≥8. *P < 0.05. B: Bone marrow–derived EPCs were then isolated from these mice and cultured, and PKA activity was quantified. n = 4. *P < 0.05. C: The adhesion of cells from the diabetic mouse strains to an S17 cell monolayer was determined as above. n = 4. *P < 0.05. D: Levels of Flk+/Sca+ cells were quantified in the peripheral blood of both α4(WT) and α4(S988A) mice prior to injections. The same cells were then quantified in the peripheral blood (PB) and bone marrow (BM) 4 weeks after injections with citrate (E) or STZ (F). n = 8. *P < 0.05.
S988A Mice Demonstrate Enhanced Revascularization Potential
The attenuated adhesion of bone marrow EPCs from diabetic α4(S988A) mice suggests they may be more abundant in circulation. To explore this, we quantified levels of Flk+/Sca+ cells. Prior to any injection (Fig. 6D), or after citrate injection (Fig. 6E), there was no significant difference in the levels of these cells between α4(WT) and α4(S988A) mice. After induction of diabetes, however (Fig. 6F), we observed that STZ-treated α4(S988A) mice had increased levels of circulating (peripheral blood) Flk+/Sca+ cells compared with STZ-treated α4(WT) mice. These levels in the α4(S988A) mice were 3.08-fold greater than levels in the α4(WT) mice. Reciprocal changes in Flk+/Sca+ cells were observed in the bone marrow of STZ-treated mice. These cells were increased in the bone marrow of wild-type mice compared with circulating levels, but they were decreased in the bone marrow of S988A animals compared with circulating levels (Fig. 6F).
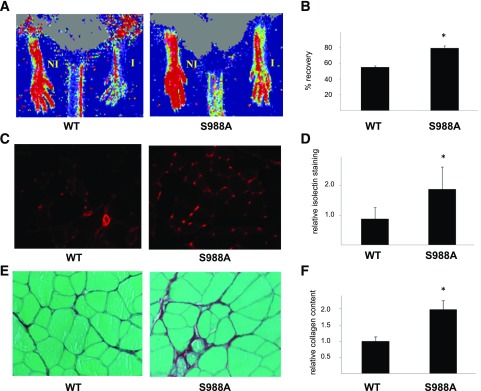
Endogenous or exogenous increases in circulating EPCs promote revascularization (29–32). Thus, increased circulating Flk+/Sca+ cells in diabetic α4(S988A) mice suggests that these mice might be protected from revascularization defects. To test this, we subjected diabetic mice of both strains to HLI and examined the return of vascular perfusion after 3 weeks. We found that blood flow in the ischemic limb of α4(S988A) mice had returned to 79.2 ± 3.1% of that in the uninjured limb. In contrast, the α4(WT) mice demonstrated only a 54.8 ± 2.1% recovery (Fig. 7A and B). Immunohistochemical staining of muscle tissue from the injured limb also demonstrated an increase in vascular capillaries (isolectin-positive structures) in the α4(S988A) mice (1.9 ± 0.7–fold) compared with the α4(WT) mice (Fig. 7C and D). In addition, we observed a greater deposition of collagen fibers in the ischemic muscle of α4(S988A) mice as compared with the α4(WT) mice (Fig. 7E and F).
Figure 7.
S988A-expressing mice have potentiated repair capacity. Diabetic α4(WT) or α4(S988A) mice as indicated were subjected to HLI, and blood perfusion was measured after 3 weeks by LDPI. A: Illustrated are representative scans. NI, nonischemic limb; I, ischemic limb. B: The perfusion in the ischemic limb was normalized to that of the nonischemic limb and a percentage recovery calculated for each mouse. Illustrated are the average recoveries. C and D: Isolated muscles from the ischemic limbs were formalin fixed and sections stained with simplicifolia lectin I (isolectin). Illustrated are representative images (C) and quantitative data (D). The sections were also stained with Sirius Red and illustrated are representative images (E) and quantitative data (F). WT, n = 5; S988A, n = 6. *P < 0.05.
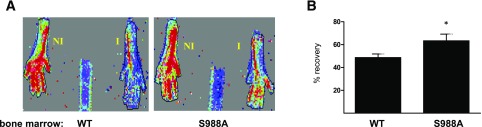
Finally, we assessed whether the enhanced revascularization potential of the α4(S988A) mice was a consequence of the altered adhesive properties of bone marrow EPCs, or whether this was an unrelated, inherent property of the mice themselves. Hence we performed bone marrow transplants with irradiated wild-type recipient mice receiving either α4(WT) or α4(S988A) bone marrow. After repopulation, the mice were made diabetic by STZ injection and subjected to HLI, and vascular perfusion was assessed 3 weeks later. We observed that vascular perfusion in the injured limb of mice receiving the α4(S988A) bone marrow had returned to 63.3 ± 5.8% of that in the uninjured limb (Fig. 8). In contrast mice receiving α4(WT) bone marrow demonstrated only a 48.7 ± 3.1% recovery. These findings suggest that the expression of α4(S988A) in bone marrow alone is sufficient for enhancing tissue revascularization in diabetic mice.
Figure 8.
Mice transplanted with α4(S988A) bone marrow display greater revascularization. Irradiated, wild-type C57 mice transplanted with α4(WT) or α4(S988A) bone marrow as indicated were made diabetic and subjected to HLI. Blood perfusion was measured after 3 weeks by LDPI. A: Illustrated are representative scans. NI, nonischemic limb; I, ischemic limb. B: The perfusion in the ischemic limb was normalized to that of the nonischemic limb and a percentage recovery calculated for each mouse. WT, n = 8; S988A, n = 5. *P < 0.05.
Discussion
In this study, we identified a novel mechanism contributing to a deficit of circulating EPCs in diabetes. Specifically, growth in high glucose led to the downregulation of PRKAR1β, with consequent activation of PKA and α4-integrin phosphorylation. This modification of α4 impacts a pathway regulating cellular adhesion and migration (28). On the basis of these observations, we propose that high levels of phospho-α4, generated under hyperglycemic conditions, promote an overall increase in EPC adhesion, which presents in vivo as a mobilization defect.
This idea is supported by experiments using ECFCs. First, when grown in high glucose, these cells demonstrated enhanced adhesion to bone marrow stromal cells. Second, this potentiated adhesion was dependent upon VCAM-1 and α4β1-integrin, consistent with other studies indicating a major role for this integrin-ligand pair in bone marrow retention (21,33). Third, ECFCs grown in high glucose had enhanced PKA activity and PKA-dependent α4-integrin phosphorylation and adhesion. Finally, the observed increase in PKA activity was associated with the downregulation of PRKAR1β, and overexpression of this subunit in high glucose–cultured cells was sufficient to limit PKA activity and reverse the adhesive phenotype.
These cell culture studies were complemented with in vivo studies using the STZ model of type 1 diabetes. We found that EPCs from STZ-treated α4(S988A) mice had reduced adhesion compared with similar cells from STZ-treated α4(WT) mice. Consistent with this attenuated adhesion, diabetic S988A mice demonstrated greater levels of circulating EPCs than their wild-type STZ counterparts. This supports the notion that persistent and systemic vascular injury resulting from hyperglycemia and signaling molecules thereby released (e.g., VEGF) is sufficient to mobilize a more loosely adherent bone marrow cell population. Finally, consistent with quantitative increases of circulating EPCs, we observed that α4(S988A) mice, and mice transplanted with α4(S988A) bone marrow, displayed enhanced revascularization after injury.
Several mechanisms may contribute to the defective mobilization of EPCs in diabetes. First, as identified here, are increases in progenitor cell adhesiveness. Signaling pathways engaged upon α4-integrin phosphorylation result in Rac1 activation, thereby initiating changes in cytoskeletal organization, promoting stable adhesion (28). This signaling mechanism is crucial in migrating cells where phospho-α4 protein displays a polarized distribution (22). Although we have not examined the spatial orientation of phospho-α4 in diabetic EPCs, we propose that levels are uniformly higher throughout the cell, promoting overall adhesion. It follows then that ablation of α4 phosphorylation, through S988A expression, would decrease adhesive strength, consistent with our observations (Fig. 6C). Further, these loosely adherent cells may be more responsive to mobilizing cues (VEGF and SDF-1α), released as a consequence of persistent, diabetes-induced endothelial damage. Although expression of α4(S988A) limits bone marrow adhesion and promotes mobilization, homing and adhesion to the endothelium may not be impaired. Homing involves primarily β2 family integrins (34) and may be independent of α4β1 (21,34). Thus, the increased availability of EPCs in diabetic S988A mice (Fig. 6F) and their α4β1-independent homing to and incorporation at sites of vascular damage may enable the revascularization we observed in these animals (Figs. 7 and 8). Alternatively, paracrine factors released by recruited EPCs may enhance revascularization potential. Although we did not observe an increase in the expression of the α4 integrin in hyperglycemic conditions, another study has reported this (14). The basis for this discrepancy in α4 expression between studies is not clear. In addition to α4β1, other adhesion molecules may impact progenitor cell retention in diabetes as well. CD11a, CD11c, CD49e, CD62L, and ICAM-1 have all been reported to be increased in bone marrow cells of diabetic mice (14). Interestingly, the same study also reported that CD62L-deficient mice display increased levels of circulating CD34+/Flk+ cells. However, a direct role for CD62L in the bone marrow adhesion of these cells was not demonstrated and there may be other reasons why the CD62L-null animals display enhanced CD34+/Flk+ cell levels.
Second, defective EPC mobilization in diabetes may result from deficits of upstream signaling molecules such as SDF-1α, VEGF, and nitric oxide (NO) (5,6,16), which trigger EPC mobilization. Indeed, means to promote NO production or signaling have been found to enhance mobilization (6,35,36). Among its effects, NO is believed to activate matrix metalloproteinases (37), which cleave membrane-bound kit ligand (35,37). Soluble kit ligand (kitL; stem cell factor) in turn binds its cell surface receptor, c-kit, enabling cellular release from the bone marrow (35,37). In addition, NO has been reported to affect α4-integrin expression (38) and ligand binding affinity (39). Although these properties were not affected in our study, the effects of reduced NO on PKA activity or α4-integrin phosphorylation are unknown. Moreover, it is possible that signals downstream of c-kit may impact PKA activity and α4-integrin phosphorylation. Cooperative signaling between growth factor receptors and α4β1 is not unprecedented. Indeed, it has been demonstrated that interactions between c-kit and α4β1 signaling pathways are essential for HSC mobilization (27) and in regulating neutrophil retention in the bone marrow as well (40).
A final mechanistic basis of diabetes-impaired mobilization may be alterations of bone marrow structure or responsiveness. Neuropathic outcomes (14) or altered bone marrow physiology and β-adrenergic signaling (17) also appear to impede progenitor cell mobilization in diabetes. Altered stromal properties in diabetic mice have been reported in other studies, although a causal relationship to impaired progenitor cell mobilization was not established (7,41). Thus, diabetes may induce effects on either the progenitor cells themselves or the bone marrow to induce selective retention and defective mobilization.
A significant finding in this study is the novel mechanism upregulating PKA activity in diabetes. Prior studies using animal models of type 1 diabetes have demonstrated variable effects on PKA activity. Whereas some have shown increased activity that is associated with neuronal dysfunction and abnormal cardiac contractility (42,43), others have shown decreased activity that impacts vascular reactivity (44). In addition, a previous study using bovine aortic endothelial cells demonstrated that growth in high glucose media led to an increase in cAMP with presumptive increases in PKA activity, although this was not directly assessed (45). Whereas we did not observe changes of cAMP in ECFCs, we did observe changes in PKA subunit expression resulting in measured increases in enzyme activity. PKA is a tetramer composed of two catalytic subunits and two inhibitory, regulatory subunits. Physiological dissociation of the regulatory subunits, such as that mediated by cAMP, activates PKA. Alternatively, decreased expression of the regulatory subunits can alter tetramer stoichiometry, leading to PKA activation. Such a mechanism seems to be operative in pituitary adenomas and the Carney complex pathologies, which demonstrate reduced levels of PRKAR1α (46). Increased PKA signaling in these cases appears to drive tumorigenesis. Alterations in PKA regulatory subunit expression have also been observed in lupus T lymphocytes (47,48) and in Bloom syndrome (49), although no pathological causality has been demonstrated in these cases. Finally, a missense mutation in PRKAR1β has been found to segregate with a familial, late-onset neurodegenerative disorder (50). Alterations of localized PKA activity are hypothesized to underlie these neurological defects. Thus our work adds to this understanding of PKA subunit regulation and disease.
Diabetes is a major CVD risk factor and is associated with impaired wound healing, atherosclerosis, hypertension, and increased risk of myocardial infarction. At their core, these outcomes result from a dysfunctional endothelium. Importantly, our studies identify a novel therapeutic target to promote endothelial repair and maintain its health. Therapeutic strategies to restrain PKA activity and α4-integrin phosphorylation in EPCs would enhance their mobilization. The increased availability of EPCs would promote endothelial repair capacity and could ameliorate the cardiovascular consequences of diabetes.
Article Information
Acknowledgments. The authors thank Luping Guo, Xiaoping Li, and Yuting Zheng (University of Louisville) for their technical support.
Funding. This work is supported by grants from the National Institutes of Health (ES019217, HL31950, and P20 GM103436).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.T.A., M.W., and D.J.C. generated data for the study, contributed to the discussion, and reviewed the manuscript. J.M.C. and M.H.G. provided reagents, contributed to the discussion, and reviewed the manuscript. A.B. contributed to the discussion and reviewed the manuscript. T.E.O. generated data for the study and wrote the manuscript. T.E.O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322 [DOI] [PubMed] [Google Scholar]
- 2.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001;89:E1–E7 [DOI] [PubMed] [Google Scholar]
- 3.Heiss C, Amabile N, Lee AC, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol 2008;51:1760–1771 [DOI] [PubMed] [Google Scholar]
- 4.Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004;109:220–226 [DOI] [PubMed] [Google Scholar]
- 5.Fadini GP, Sartore S, Schiavon M, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia 2006;49:3075–3084 [DOI] [PubMed] [Google Scholar]
- 6.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest 2007;117:1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westerweel PE, Teraa M, Rafii S, et al. Impaired endothelial progenitor cell mobilization and dysfunctional bone marrow stroma in diabetes mellitus. PLoS One 2013;8:e60357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 9.Sibal L, Aldibbiat A, Agarwal SC, et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia 2009;52:1464–1473 [DOI] [PubMed] [Google Scholar]
- 10.Fadini GP, Albiero M, Vigili de Kreutzenberg S, et al. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care 2013;36:943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 2001;97:3354–3360 [DOI] [PubMed] [Google Scholar]
- 12.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 1999;18:3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 2009;4:62–72 [DOI] [PubMed] [Google Scholar]
- 14.Albiero M, Poncina N, Tjwa M, et al. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes 2014;63:1353–1365 [DOI] [PubMed] [Google Scholar]
- 15.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006;124:407–421 [DOI] [PubMed] [Google Scholar]
- 16.Thum T, Fraccarollo D, Schultheiss M, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 2007;56:666–674 [DOI] [PubMed] [Google Scholar]
- 17.Ferraro F, Lymperi S, Méndez-Ferrer S, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med 2011;3:104ra101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez P, Rettig MP, Uy GL, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood 2009;114:1340–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing D, Oelschlaegel U, Ordemann R, et al. CD49d blockade by natalizumab in patients with multiple sclerosis affects steady-state hematopoiesis and mobilizes progenitors with a distinct phenotype and function. Bone Marrow Transplant 2010;45:1489–1496 [DOI] [PubMed] [Google Scholar]
- 20.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci U S A 1993;90:9374–9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin G, Ii M, Silver M, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med 2006;203:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of alpha4 integrin phosphorylation regulates lamellipodial stability and alpha4beta1-dependent cell migration. J Cell Biol 2003;162:731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008;28:1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldfinger LE, Tzima E, Stockton R, et al. Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circ Res 2008;103:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buensuceso CS, Woodside D, Huff JL, Plopper GE, O’Toole TE. The WD protein Rack1 mediates protein kinase C and integrin-dependent cell migration. J Cell Sci 2001;114:1691–1698 [DOI] [PubMed] [Google Scholar]
- 26.Conklin DJ, Kong M; HEI Health Review Committee. Part 4. Assessment of plasma markers and cardiovascular responses in rats after chronic exposure to new-technology diesel exhaust in the ACES bioassay. Res Rep Health Eff Inst 2015:111–139; discussion 141–171 [PubMed] [Google Scholar]
- 27.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood 1998;91:2231–2239 [PubMed] [Google Scholar]
- 28.Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol 2005;7:343–352 [DOI] [PubMed] [Google Scholar]
- 29.Gertz K, Priller J, Kronenberg G, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res 2006;99:1132–1140 [DOI] [PubMed] [Google Scholar]
- 30.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 2000;97:3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 2005;105:1068–1077 [DOI] [PubMed] [Google Scholar]
- 32.Werner N, Priller J, Laufs U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol 2002;22:1567–1572 [DOI] [PubMed] [Google Scholar]
- 33.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A 1995;92:9647–9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavakis E, Aicher A, Heeschen C, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med 2005;201:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002;109:625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang PH, Tsai HY, Wang CH, et al. Moderate intake of red wine improves ischemia-induced neovascularization in diabetic mice--roles of endothelial progenitor cells and nitric oxide. Atherosclerosis 2010;212:426–435 [DOI] [PubMed] [Google Scholar]
- 37.Aicher A, Heeschen C, Dimmeler S. The role of NOS3 in stem cell mobilization. Trends Mol Med 2004;10:421–425 [DOI] [PubMed] [Google Scholar]
- 38.Conran N, Gambero A, Ferreira HH, Antunes E, de Nucci G. Nitric oxide has a role in regulating VLA-4-integrin expression on the human neutrophil cell surface. Biochem Pharmacol 2003;66:43–50 [DOI] [PubMed] [Google Scholar]
- 39.Chigaev A, Smagley Y, Sklar LA. Nitric oxide/cGMP pathway signaling actively down-regulates α4β1-integrin affinity: an unexpected mechanism for inducing cell de-adhesion. BMC Immunol 2011;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petty JM, Lenox CC, Weiss DJ, Poynter ME, Suratt BT. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J Immunol 2009;182:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiba H, Ataka K, Iba K, Nagaishi K, Yamashita T, Fujimiya M. Diabetes impairs the interactions between long-term hematopoietic stem cells and osteopontin-positive cells in the endosteal niche of mouse bone marrow. Am J Physiol Cell Physiol 2013;305:C693–C703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacai H, Sasaki-Hamada S, Sugiyama A, et al. The impairment in spatial learning and hippocampal LTD induced through the PKA pathway in juvenile-onset diabetes rats are rescued by modulating NMDA receptor function. Neurosci Res 2014;81-82:55–63 [DOI] [PubMed] [Google Scholar]
- 43.Vasanji Z, Dhalla NS, Netticadan T. Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 2004;261:245–249 [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto T, Wakabayashi K, Kobayashi T, Kamata K. Diabetes-related changes in cAMP-dependent protein kinase activity and decrease in relaxation response in rat mesenteric artery. Am J Physiol Heart Circ Physiol 2004;287:H1064–H1071 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Apse K, Pang J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem 2000;275:40042–40047 [DOI] [PubMed] [Google Scholar]
- 46.Yin Z, Pringle DR, Jones GN, Kelly KM, Kirschner LS. Differential role of PKA catalytic subunits in mediating phenotypes caused by knockout of the Carney complex gene Prkar1a. Mol Endocrinol 2011;25:1786–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan IU, Laxminarayana D, Kammer GM. Protein kinase A RI beta subunit deficiency in lupus T lymphocytes: bypassing a block in RI beta translation reconstitutes protein kinase A activity and augments IL-2 production. J Immunol 2001;166:7600–7605 [DOI] [PubMed] [Google Scholar]
- 48.Laxminarayana D, Khan IU, Mishra N, Olorenshaw I, Taskén K, Kammer GM. Diminished levels of protein kinase A RI alpha and RI beta transcripts and proteins in systemic lupus erythematosus T lymphocytes. J Immunol 1999;162:5639–5648 [PubMed] [Google Scholar]
- 49.Heyerdahl SL, Boikos S, Horvath A, Giatzakis C, Bossis I, Stratakis CA. Protein kinase A subunit expression is altered in Bloom syndrome fibroblasts and the BLM protein is increased in adrenocortical hyperplasias: inverse findings for BLM and PRKAR1A. Horm Metab Res 2008;40:391–397 [DOI] [PubMed] [Google Scholar]
- 50.Wong TH, Chiu WZ, Breedveld GJ, et al.; Netherlands Brain Bank; International Parkinsonism Genetics Network . PRKAR1B mutation associated with a new neurodegenerative disorder with unique pathology. Brain 2014;137:1361–1373 [DOI] [PubMed] [Google Scholar]