Abstract
The insulin-like growth factor (IGF) axis may be implicated in glucose homeostasis, but its longitudinal profile across gestation in relation to the development of gestational diabetes mellitus (GDM) is largely unknown. We prospectively investigated IGF axis biomarkers in early-to-midpregnancy in relation to subsequent GDM risk in a case-control study of 107 case subjects with GDM and 214 control subjects without GDM, with blood sample collection at gestational weeks 10–14, 15–26, 23–31, and 33–39. Conditional logistic regression was used, adjusting for major risk factors including prepregnancy BMI. Plasma IGF-I and IGF binding protein 3 (IGFBP-3) concentrations and molar ratio of IGF-I to IGFBP-3 increased, whereas IGFBP-2 decreased throughout pregnancy. At gestational weeks 10–14, both IGF-I and IGF-I/IGFBP-3 were positively associated with GDM risk; adjusted odds ratio (OR) comparing the highest versus lowest quartile (ORQ4-Q1) was 2.93 (95% CI 1.18, 7.30) for IGF-I and 3.31 (1.10, 9.98) for IGF-I/IGFBP-3. In contrast, higher IGFBP-2 levels were related to a substantially lower risk of GDM (ORQ4-Q1 0.04 [0.01, 0.06]). Similar results were observed at gestational weeks 15–26. In sum, the IGF axis, IGFBP-2 in particular, may be implicated in the pathogenesis of GDM, with significant associations and incremental predictive value detected as early as gestational weeks 10–14, ∼10–18 weeks earlier before GDM is typically screened for.
Introduction
Gestational diabetes mellitus (GDM), one of the most common pregnancy complications, affects up to ∼15% of pregnant women worldwide (1), which parallels the growing global epidemic of type 2 diabetes (2). Despite that the underlying etiology remains to be elucidated, β-cell dysfunction and thus failure to compensate for insulin resistance induced by pregnancy are thought to be relevant (3). Given the structural homology and similarities of downstream signaling pathways with insulin, the insulin-like growth factor I (IGF-I) may be implicated in glucose homeostasis (4,5) and etiology of GDM. Further, emerging, yet sparse, data indicate that IGF axis biomarkers, including IGF-I and IGF binding proteins (IGFBPs), undergo notable changes during pregnancy (6,7). Thus, a comprehensive understanding of the role of IGF axis biomarkers in the etiology of GDM requires longitudinal investigations throughout pregnancy. Moreover, the conventional screening time for GDM is toward late pregnancy (i.e., gestational weeks 24–28), leaving little room for effective interventions or treatment. Further, fetal exposure to increased amniotic fluid glucose before 15 weeks of gestation underscores the occurrence of early metabolic perturbations before GDM diagnosis (8). Thus, identifying a prediagnostic marker for GDM in early pregnancy is warranted, which may be used to inform early diagnostic or prevention strategies.
Longitudinal and prospective studies of IGF axis markers throughout pregnancy and their relation to GDM are lacking. Previous data are typically based on single measurements in retrospective or cross-sectional studies with inconsistent findings (9–11). To date, we are aware of only one study of 47 GDM cases prospectively investigating the associations between IGF-I and IGFBP-1, with single measurements in early pregnancy and subsequent risk of GDM (12). Inference of findings from the study was hindered by its small sample size and measurements obtained at a single point in time. Further, roles of other IGF axis biomarkers in the pathogenesis of GDM remain to be elucidated. In particular, IGFBP-3 is bound to ∼75% of circulating IGF-I and the formed complex serves as the major reserves and buffer of IGF-I. Therefore, the molar ratio of IGF-I to IGFBP-3 is used as an indicator of IGF-I bioavailability (13). In addition, among all the IGFBPs, IGFBP-2 has recently received increasing research interests given its pleiotropic functions (i.e., both pericellular IGF-regulatory and intracellular IGF-independent activities) and lack of postprandial fluctuation (14). Despite its implication in glucose homeostasis (15), data on its association during early-to-midpregnancy with subsequent GDM risk are lacking.
To address the critical data gaps, in the current study, we aimed to prospectively investigate 1) the longitudinal physiological changes of plasma IGF-I, IGFBP-2, and IGFBP-3 concentrations and molar ratio of IGF-I to IGFBP-3 throughout pregnancy and 2) the associations of IGF-I, IGFBP-2, IGFBP-3, and ratio of IGF-I to IGFBP-3 during early to midpregnancy with subsequent risk of GDM.
Research Design and Methods
Study Population and Design
The case-control study was nested among participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Study–Singletons cohort, a multicenter, multiracial prospective cohort study of healthy (without prepregnancy hypertension, diabetes, renal/autoimmune disease, psychiatric disorder, cancer, and HIV or AIDs) women aged 18–40 years with low-risk, singleton pregnancies to 2,334 nonobese (16) and 468 obese women. In total, 2,802 pregnant women were enrolled between 8 weeks 0 days and 13 weeks 6 days of gestation at 12 clinical centers across the U.S. (2009–2013). During pregnancy, maternal blood samples were longitudinally collected during four selected study visits at gestational weeks 8–13 (enrollment visit), 16–22 (visit 1), 24–29 (visit 2), and 34–37 (visit 4). The study was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all the participants.
In the NICHD Fetal Growth Study–Singletons cohort, we identified 107 case subjects with GDM via review of medical records according to the Carpenter and Coustan criteria as recommended by the American College of Obstetrics and Gynecologists (17). The average gestational age at the 100-g, 3-h oral glucose tolerance test (OGTT) for GDM diagnosis among case subjects was 27 weeks (range 11–36). Among 2,695 women without GDM in the whole cohort, 2,477 (92%) had successful biospecimen collection at the first two visits, from which a random sample of 214 control subjects without GDM were selected and matched 2:1 to case subjects according to age (±2 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or Asian/Pacific Islander), and gestational age at blood sample collection (±2 weeks). Of note, the majority of control subjects (n = 195) were screened for GDM using the 50-g glucose challenge test. For those without routine GDM screening (n = 19), 12 went through an OGTT with normal glucose values below the Carpenter and Coustan criteria thresholds, and the remaining were free of hospital discharge diagnosis of GDM.
All biospecimens were processed immediately after the collection and stored at −80°C until being thawed immediately before assay according to a standardized protocol. Samples at visit 1 were obtained after an overnight fast of 8–14 h among both case and control subjects. The fasting duration prior to biospecimen collection at all visits was similar between case and control subjects. By design, participants were randomized within each time window of blood collection to capture weekly biomarker data. As some women came late for the scheduled visit, the actual gestational weeks at blood collection spread beyond the original planned visit time window. Specifically, the actual range of gestational weeks at blood collection prior to the screening or diagnosis of GDM were 10–14 and 15–26 at the enrollment visit and visit 1, respectively.
Exposure Assessment
For the two blood collections prior to the GDM screening test (i.e., at enrollment visit and visit 1), biomarkers were measured among all the case (n = 107) and control subjects (n = 214). At the following blood collections at visits 2 and 4, biomarkers were measured among all the case subjects (n = 107) and one of their control subjects (n = 107). Plasma concentrations of total IGF-I, IGFBP-2, and IGFBP-3 were measured in ng/mL using ELISAs (ALPCO Diagnostics, Salem, NH). Plasma concentrations of glucose, insulin, and C-reactive protein (CRP) were measured using hexokinase, immunosorbent, and immunoturbidimetric assays (Roche Diagnostics, Indianapolis, IN), respectively. Plasma adiponectin levels were measured using a quantitative sandwich enzyme immunoassay (Beckman Coulter, Inc., Fullerton, CA). Plasma lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) were measured using enzymatic assays (Roche Diagnostics, Indianapolis, IN). All the inter- and intra-assay coefficients of variation were <6.7%. All assays were performed without knowledge of GDM status.
Covariates
Data on maternal demographic, lifestyle, and medical characteristics were obtained from questionnaires and extracted from medical records. A priori–selected covariates included conventional risk factors for GDM: family history of diabetes (yes/no) and prepregnancy BMI (<25, 25.0–25.9, 30.0–34.9, or 35.0–44.9 kg/m2). Given that maternal age (years) and gestational age at blood collection (weeks) were matched between case and control subjects within a certain range, these two matching factors were also included as covariates to derive conservative risk estimates. Of note, in this low-risk population, nonobese women who smoked in the 6 months prior to pregnancy were not eligible, and only five obese women smoked in the 6 months prior to pregnancy. Therefore, smoking is not included in multivariable models.
Statistical Methods
Differences in participant characteristics between case and control subjects were assessed by mixed-effect linear regression models for continuous variables and binomial/multinomial logistic regression with generalized estimating equations for binary/multilevel categorical variables, accounting for matched case-control pairs. To compare the concentrations of each IGF axis biomarker throughout pregnancy between case and control subjects, mean concentrations (SE) of each biomarker were plotted against gestational age intervals of 2–3 weeks with P values obtained using mixed-effect linear regression models accounting for matched case-control pairs.
Multivariable conditional logistic regression models adjusting for the above-listed covariates were fitted to assess the associations of each IGF axis biomarker at gestational weeks 10–14 and 15–26 with subsequent risk of GDM. To ensure that biomarker measurements preceded the diagnosis of GDM, we excluded one case subject at weeks 10–14 and five at 15–26 weeks from the final analysis, whose blood samples were collected after the diagnosis of GDM. To assess the independent association of each IGF axis biomarker with GDM risk, we further fitted a multivariable multibiomarker model by mutually adjusting for other IGF axis biomarkers. The levels of IGF-I, IGFBP-2, and IGFBP-3 and ratio of IGF-I to IGFBP-3 were parameterized as quartiles based on the distribution of biomarker concentrations among the control subjects, with the lowest quartile being the reference, and continuously per one SD increase in concentration or ratio. Tests of linear trend were conducted by using the median value for each quartile and fitting it as a continuous variable in the conditional logistic regression models. In addition, we calculated partial Spearman correlation coefficients to prospectively examine association of IGF axis biomarkers at gestational weeks 10–14 with fasting plasma glucose, insulin, CRP, and homeostasis model assessment of insulin resistance (HOMA-IR) (18), at the subsequent study visit 1 (i.e., weeks 15–26) among case subjects with GDM and control subjects without GDM, after adjusting for the above-listed covariates. To estimate crude correlations between IGF axis biomarkers and these metabolic markers implicated in glucose homeostasis, we calculated unadjusted Spearman correlation coefficients among the entire study sample. We also calculated Spearman correlation coefficients of IGF axis biomarkers at weeks 10–14 and 15–26 with the 100-g OGTT fasting, 1-h, 2-h, and 3-h glucose levels among case subjects with GDM, respectively.
We further evaluated the incremental prediction capacity of each IGF axis biomarker at gestational weeks 10–14 and 15–26 in predicting GDM risk, in addition to conventional risk factors, plasma glucose concentrations, and classic biomarkers implicated in glucose homeostasis (i.e., adiponectin, CRP, and lipids [total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides]). We plotted receiver operating characteristic curves and calculated area under the curve statistics using the approach proposed by Pepe et al. (19) for matched case-control studies. The comparison of area under the curve statistics was obtained by DeLong’s test (20). The logarithmic transformed concentrations of plasma IGF axis biomarkers and glucose were parameterized as continuous variables in logistic regression models for receiver operating characteristic curves. To avoid overfitting, leave-one-out cross-validation was performed to derive conservative estimates by successively leaving out each observation from the sample (n) one at a time, and using the model fit based on the remaining observations (n − 1) to compute the predicted probability for the left-out observation. This process was repeated n times until all observations were validated (21).
In addition, to evaluate whether the associations of IGF axis biomarkers with GDM risk varied by major risk factors of GDM (i.e., prepregnancy obesity status, family history of diabetes, and race/ethnicity), we included cross-product (interaction) terms in the multivariable regression model with one SD increase of each IGF axis biomarker or ratio to assess the potential effect modification. To test the robustness of our results against potential residual confounding, we further adjusted for triceps and subscapular skinfold thickness measures at the enrollment visit, as indicators of regional adiposity; gestational weight gain (calculated as the difference between clinically measured or medical chart–abstracted weight at the time of blood collection and self-reported pregravid weight in kg); and moderate-to-vigorous physical activity per week (minutes) during the year preceding the index pregnancy, respectively. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Demographics and Distribution of IGF Axis Biomarkers Before GDM Diagnosis
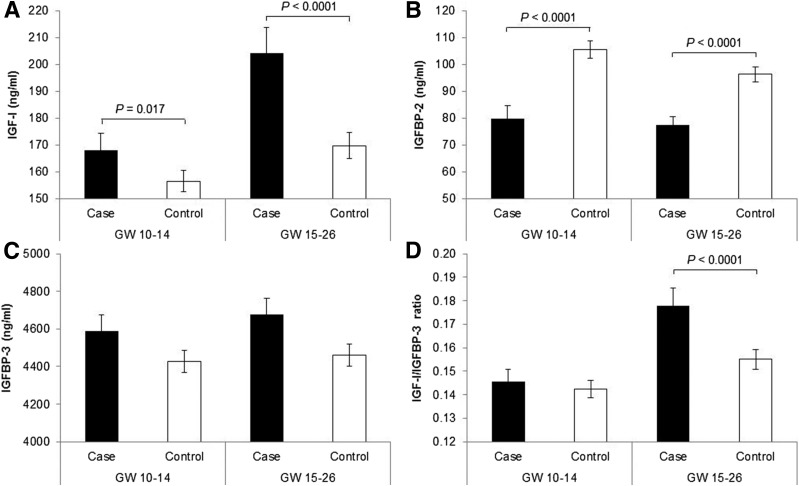
Women with GDM were more likely to have a family history of diabetes and have a higher prepregnancy BMI compared with control subjects without GDM (Table 1). Plasma concentrations of total IGF-I were significantly higher whereas concentrations of IGFBP-2 were significantly lower in case subjects than control subjects at gestational weeks 10–14 and 15–26, respectively (Fig. 1). The molar ratio of IGF-I to IGFBP-3 did not vary by GDM status at weeks 10–14 but was significantly higher among case subjects than control subjects at weeks 15–26. Concentrations of IGFBP-3 did not differ by GDM status at these two times.
Table 1.
Participant characteristics among women with GDM and their matched control subjects, the NICHD Fetal Growth Study–Singletons cohort
| Case subjects with GDM (n = 107) | Control subjects (n = 214) | P* | |
|---|---|---|---|
| Age (years) | 30.5 ± 5.7 | 30.4 ± 5.4 | |
| Race/ethnicity | |||
| Non-Hispanic white | 25 (23.4) | 50 (23.4) | |
| Non-Hispanic black | 15 (14.0) | 30 (14.0) | |
| Hispanic | 41 (38.3) | 82 (38.3) | |
| Asian/Pacific Islander | 26 (24.3) | 52 (24.3) | |
| Education | 0.18 | ||
| Less than high school | 17 (15.9) | 26 (12.1) | |
| High school graduate or equivalent | 15 (14.0) | 23 (10.7) | |
| More than high school | 75 (70.1) | 165 (77.1) | |
| Insurance | 0.43 | ||
| Private or managed care | 68 (63.5) | 143 (66.8) | |
| Medicaid, other | 39 (36.5) | 69 (32.2) | |
| Self-pay | 0 | 2 (0.9) | |
| Marital status | 0.12 | ||
| Never married | 11 (10.3) | 35 (16.4) | |
| Married/living with a partner | 92 (86.0) | 167 (78.0) | |
| Divorced/separated | 4 (3.7) | 12 (5.6) | |
| Nulliparity | 48 (44.9) | 96 (44.9) | 1 |
| Family history of diabetes | 40 (37.4) | 48 (22.4) | 0.003 |
| Prepregnancy BMI (kg/m2) | <0.001 | ||
| <25.0 | 37 (34.6) | 123 (57.5) | |
| 25.0–29.9 | 35 (32.7) | 56 (26.2) | |
| 30.0–34.9 | 20 (18.7) | 17 (7.9) | |
| 35.0–44.9 | 15 (14.0) | 16 (7.5) | |
| Unknown/missing | 0 | 2 (0.9) | |
| Smoking 6 months before pregnancy | 4 (3.7) | 1 (0.5) | 0.06 |
| Alcoholic beverage consumption 3 months before pregnancy | 61 (57.0) | 137 (64.0) | 0.22 |
Data are presented as n (%) for categorical variables and mean (SD) for continuous variables.
*P values for differences between case and control subjects were obtained by mixed-effect linear regression models for continuous variables and binomial/multinomial logistic regression with generalized estimating equations for binary/multilevel categorical variables, accounting for matched case-control pairs. P values are not shown for matching variables (age and race/ethnicity).
Figure 1.
Plasma mean concentrations of IGF-I (A), IGFBP-2 (B), and IGFBP-3 (C) and molar ratio of IGF-I to IGFBP-3 (D) among women with GDM and their matched control subjects at gestational weeks 10–14 and 15–26. P values for case-control comparisons were obtained by mixed-effect linear regression models accounting for matched case-control pairs at gestational weeks 10–14 and 15–26, respectively. GW, gestational weeks.
Longitudinal Profile of IGF Axis Biomarkers Across Gestation
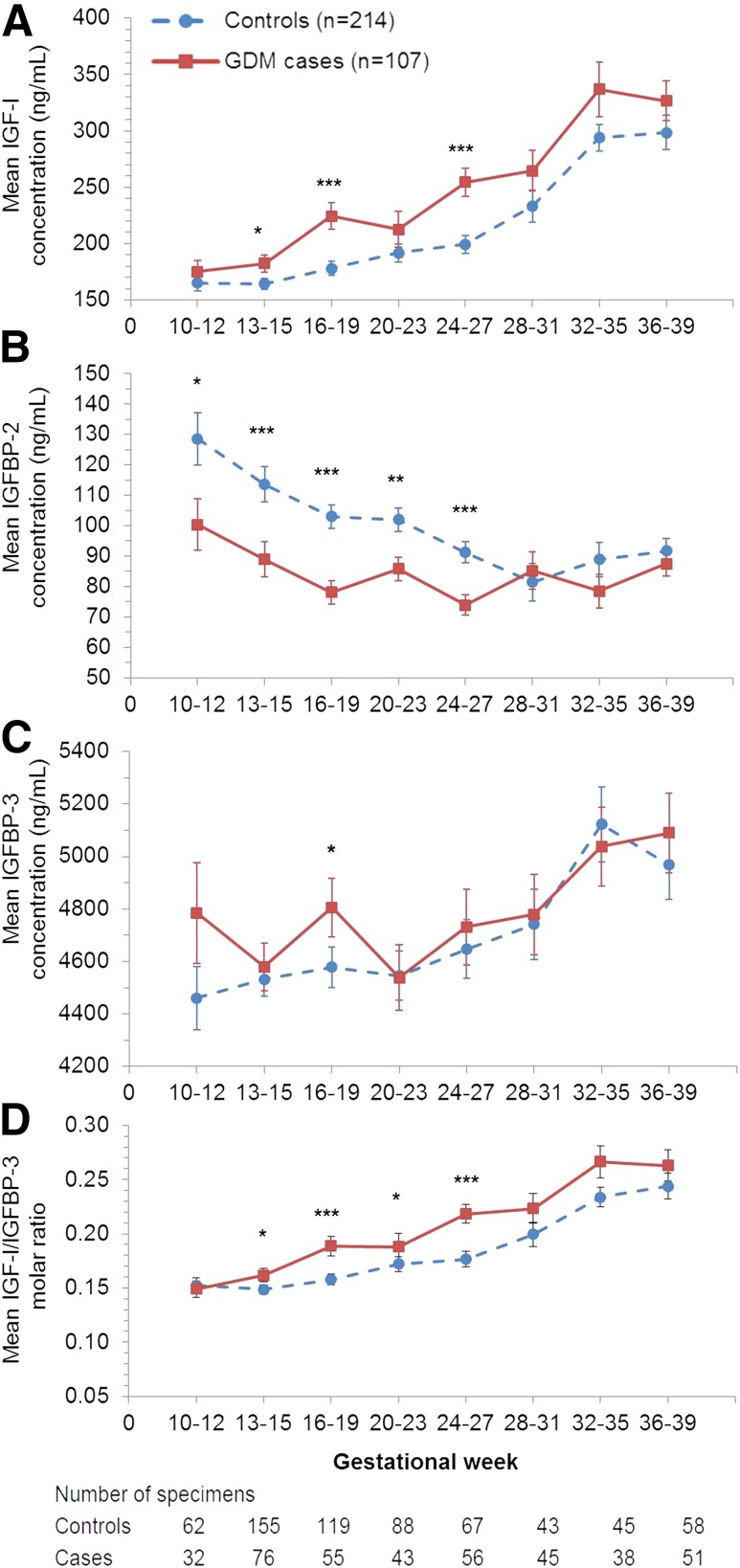
Overall, concentrations of IGF-I and IGFBP-3 as well as molar ratio of IGF-I to IGFBP-3 increased whereas concentrations of IGFBP-2 decreased as pregnancy progressed among both case and control subjects (Fig. 2). With respect to between-group comparisons, mean concentrations of IGF-I were consistently higher in case subjects than control subjects at gestational weeks 13–15, 16–19, and 24–27. A similar trend was observed for the molar ratio of IGF-I to IGFBP-3. Conversely, mean concentrations of IGFBP-2 tended to be significantly lower in case subjects with GDM than control subjects from weeks 10–12 up to 24–27. After weeks 24–27, which was close to the conventional time of GDM screening (i.e., weeks 24–28), the significant difference in IGFBP-2 concentrations between case and control subjects did not persist. Mean concentrations of IGFBP-3 did not differ significantly between the two groups throughout pregnancy except at weeks 16–19.
Figure 2.
Mean concentration (±SEM) of IGF-I (A), IGFBP-2 (B), and IGFBP-3 (C) and molar ratio of IGF-I to IGFBP-3 (D) according to gestational age intervals among women with GDM (squares, solid line) and their matched control subjects (circles, dashed line). *P < 0.05; **P < 0.01; ***P < 0.001 for case-control comparisons obtained by mixed-effect linear regression models accounting for matched case-control pairs at each gestational age interval.
IGF Axis Biomarkers in Relation to Subsequent Risk of GDM
We further examined the associations between IGF axis biomarkers before the GDM screening and subsequent risk of GDM. Overall, plasma concentrations of both IGF-I and IGFBP-2 as well as molar ratio of IGF-I to IGFBP-3 were significantly associated with GDM risk. At gestational weeks 10–14, comparing the highest versus lowest quartile, IGF-I was positively associated with a 2.93-fold increased risk of GDM, after adjusting for major risk factors (Table 2). Similar positive associations were observed for the molar ratio of IGF-I to IGFBP-3, although the P for trend was marginally significant in the multivariable model. In contrast, IGFBP-2 was inversely associated with GDM risk, with the highest quartile associated with a significant 96% decreased risk compared with the lowest quartile (P for trend < 0.001). Overall, associations of IGF axis biomarkers with GDM risk were similar at gestational weeks 15–26, whereas greater effect sizes were observed for IGF-I and ratio of IGF-I to IGFBP-3 compared with weeks 10–14. Further, findings from the continuous models per one SD increase in IGF axis biomarker concentrations or ratio were similar to the quartile models. Additionally, the significant associations persisted for IGFBP-2 at both times and for molar ratio of IGF-I to IGFBP-3 at weeks 15–26 after further mutually adjusting for other IGF axis biomarkers studied (data not shown). Concentrations of IGFBP-3 were not associated with subsequent risk of GDM at either of these two periods. There was no significant effect modification by prepregnancy BMI, family history of diabetes, or race/ethnicity for any of the associations examined. Moreover, results remained robust after additionally adjusting for triceps and subscapular skinfold thickness, gestational weight gain up to blood collection, and physical activity during the previous year of the index pregnancy, respectively.
Table 2.
Adjusted odds ratio (95% CI) for the associations of GDM risk with quartiles of IGF-I, IGFBP-2, and IGFBP-3 concentrations and molar ratio of IGF-I to IGFBP-3 at gestational weeks 10–14 and 15–26, the NICHD Fetal Growth Study–Singletons cohort
| Case subjects (n) | Control subjects (n) | Crude model | Multivariable model* | |
|---|---|---|---|---|
| Gestational weeks 10–14† | ||||
| IGF-I (ng/mL) | ||||
| Q1: 60.0–125.8‡ | 15 | 54 | 1.00 | 1.00 |
| Q2: 126.0–156.5 | 29 | 53 | 2.10 (0.96, 4.59) | 1.94 (0.77, 4.84) |
| Q3: 156.6–194.5 | 24 | 54 | 1.74 (0.80, 3.78) | 2.20 (0.92, 5.28) |
| Q4: 194.6–378.3 | 36 | 53 | 2.87 (1.28, 6.42) | 2.93 (1.18, 7.30) |
| P for trend | 0.018 | 0.023 | ||
| Per SD increment | 1.37 (1.04, 1.81) | 1.37 (1.01, 1.86) | ||
| IGFBP-2 (ng/mL) | ||||
| Q1: 37.3–82.5 | 58 | 54 | 1.00 | 1.00 |
| Q2: 82.6–105.2 | 20 | 53 | 0.24 (0.11, 0.51) | 0.22 (0.09, 0.55) |
| Q3: 105.8–145.8 | 19 | 54 | 0.21 (0.09, 0.46) | 0.14 (0.05, 0.38) |
| Q4: 146.0–311.7 | 7 | 53 | 0.05 (0.02, 0.16) | 0.04 (0.01, 0.16) |
| P for trend | <0.001 | <0.001 | ||
| Per SD increment | 0.40 (0.26, 0.60) | 0.42 (0.26, 0.67) | ||
| IGFBP-3 (ng/mL) | ||||
| Q1: 2,729.0–3,876.4 | 19 | 54 | 1.00 | 1.00 |
| Q2: 3,876.5–4,422.7 | 23 | 53 | 1.29 (0.62, 2.68) | 1.06 (0.46, 2.42) |
| Q3: 4,434.2–4,970.1 | 34 | 54 | 1.93 (0.94, 3.94) | 1.91 (0.84, 4.37) |
| Q4: 4,974.8–7,426.5 | 28 | 53 | 1.76 (0.72, 4.26) | 1.66 (0.61, 4.52) |
| P for trend | 0.122 | 0.158 | ||
| Per SD increment | 1.31 (0.93, 1.84) | 1.35 (0.94, 1.95) | ||
| Molar ratio of IGF-I to IGFBP-3 | ||||
| Q1: 0.035–0.112 | 13 | 54 | 1.00 | 1.00 |
| Q2: 0.113–0.141 | 32 | 53 | 2.85 (1.27, 6.42) | 3.26 (1.26, 8.45) |
| Q3: 0.143–0.175 | 28 | 54 | 2.72 (1.12, 6.58) | 2.69 (0.97, 7.46) |
| Q4: 0.178–0.355 | 31 | 53 | 3.44 (1.33, 8.88) | 3.31 (1.10, 9.98) |
| P for trend | 0.02 | 0.067 | ||
| Per SD increment | 1.27 (0.94, 1.72) | 1.24 (0.88, 1.75) | ||
| Gestational weeks 15–26† | ||||
| IGF-I (ng/mL) | ||||
| Q1: 8.0–134.4 | 11 | 54 | 1.00 | 1.00 |
| Q2: 135.7–169.6 | 19 | 53 | 1.88 (0.80, 4.46) | 2.19 (0.85, 5.63) |
| Q3: 169.8–216.0 | 27 | 54 | 3.06 (1.27, 7.38) | 4.35 (1.55, 12.2) |
| Q4: 218.6–428.7 | 37 | 53 | 5.39 (2.13, 13.6) | 5.53 (1.94, 15.8) |
| P for trend | <0.001 | 0.001 | ||
| Per SD increment | 1.99 (1.41, 2.82) | 2.19 (1.48, 3.23) | ||
| IGFBP-2 (ng/mL) | ||||
| Q1: 37.9–71.6 | 39 | 54 | 1.00 | 1.00 |
| Q2: 73.0–96.2 | 26 | 53 | 0.61 (0.31, 1.23) | 0.55 (0.23, 1.32) |
| Q3: 96.4–127.5 | 25 | 54 | 0.54 (0.26, 1.11) | 0.64 (0.27, 1.56) |
| Q4: 128.1–288.7 | 4 | 53 | 0.07 (0.02, 0.25) | 0.03 (0.001, 0.23) |
| P for trend | <0.001 | <0.001 | ||
| Per SD increment | 0.40 (0.26, 0.60) | 0.34 (0.20, 0.59) | ||
| IGFBP-3 (ng/mL) | ||||
| Q1: 2,575.7–3,977.1 | 18 | 54 | 1.00 | 1.00 |
| Q2: 3,981.2–4,451.1 | 19 | 53 | 1.15 (0.54, 2.47) | 1.11 (0.47, 2.61) |
| Q3: 4,468.8–5,006.0 | 29 | 54 | 1.89 (0.88, 4.05) | 2.05 (0.84, 4.99) |
| Q4: 5,031.5–7,854.6 | 28 | 53 | 2.24 (0.94, 5.33) | 2.27 (0.86, 6.00) |
| P for trend | 0.050 | 0.067 | ||
| Per SD increment | 1.26 (0.89, 1.79) | 1.34 (0.90, 1.99) | ||
| Molar ratio of IGF-I to IGFBP-3 | ||||
| Q1: 0.007–0.120 | 11 | 54 | 1.00 | 1.00 |
| Q2: 0.120–0.154 | 22 | 53 | 2.63 (1.10, 6.24) | 3.27 (1.22, 8.74) |
| Q3: 0.155–0.193 | 24 | 54 | 3.48 (1.32, 9.15) | 3.61 (1.25, 10.4) |
| Q4: 0.194–0.427 | 37 | 53 | 8.31 (2.90, 23.8) | 10.5 (3.26, 34.1) |
| P for trend | <0.001 | <0.001 | ||
| Per SD increment | 2.23 (1.49, 3.33) | 2.33 (1.51, 3.60) |
Q, quartile.
*Adjusted for maternal age (years), gestational age at blood collection (weeks), family history of diabetes (yes/no), and prepregnancy BMI (<24.9, 25.0–25.9, 30.0–34.9, or 35.0–44.9 kg/m2).
†Timing of blood sample collection all preceded the diagnosis of GDM.
‡Range of biomarker concentrations within each quartile among control subjects without GDM.
Correlations Between IGF Axis Biomarkers and Clinical Biomarkers
IGF axis biomarkers in early pregnancy during gestational weeks 10–14 were also prospectively and significantly correlated with fasting clinical biomarkers and indices implicated in glucose homeostasis before GDM screening among case subjects with GDM and control subjects without GDM (Table 3). Specifically, IGF-I and molar ratio of IGF-I to IGFBP-3 at gestational weeks 10–14 were significantly and positively correlated with HOMA-IR and fasting insulin at weeks 15–26. In contrast, IGFBP-2 at weeks 10–14 was significantly and inversely correlated with HOMA-IR and fasting insulin at weeks 15–26, even after adjusting for other factors related to HOMA-IR, including age, gestational age at blood collection, family history of diabetes, and prepregnancy BMI. Before controlling for these factors, Spearman correlation coefficients between IGFBP-2 and HOMA-IR and insulin were as high as −0.47 and −0.48, respectively (P < 0.0001). In addition, IGFBP-2 was significantly and inversely correlated with 100-g OGTT fasting glucose levels, whereas IGF-I, IGFBP-3, and ratio of IGF-I to IGFBP-3 were not significantly associated with any OGTT glucose levels (see Supplementary Table 1).
Table 3.
Partial Spearman correlation coefficients of IGF-I, IGFBP-2, and IGFBP-3 concentrations and molar ratio of IGF-I to IGFBP-3 at gestational weeks 10–14 with subsequent fasting plasma biomarkers at gestational weeks 15–26 among case subjects with GDM and control subjects without GDM*
| HOMA-IR | Glucose | Insulin | CRP | |
|---|---|---|---|---|
| Case subjects | ||||
| IGF-I | 0.24† | 0.12 | 0.25† | −0.22† |
| IGFBP-2 | −0.34‡ | −0.25† | −0.33§ | −0.05 |
| IGFBP-3 | −0.12 | −0.02 | −0.13 | −0.14 |
| Molar ratio of IGF-I to IGFBP-3 | 0.36‡ | 0.13 | 0.37‡ | −0.21† |
| Control subjects | ||||
| IGF-I | 0.17† | 0.002 | 0.18† | −0.07 |
| IGFBP-2 | −0.27‡ | −0.02 | −0.28‡ | −0.11 |
| IGFBP-3 | 0.04 | 0.04 | 0.04 | 0.04 |
| Molar ratio of IGF-I to IGFBP-3 | 0.15† | −0.05 | 0.17† | −0.13 |
*P values were adjusted for age, gestational age at blood collection, family history of diabetes, and prepregnancy BMI.
†P < 0.05.
‡P < 0.001.
§P < 0.01.
Incremental Prediction Capacity of IGF Axis Biomarkers
As for incremental prediction capacity of the IGF axis biomarker, at gestational weeks 10–14, classic biomarkers, including adiponectin, CRP, and lipids, did not significantly improve the prediction capacity beyond conventional risk factors and glucose, whereas IGFBP-2 illustrated significant incremental predictive value in addition to conventional risk factors and, more notably, plasma glucose and classic biomarkers (see Supplementary Fig. 1). At gestational weeks 15–26, IGFBP-2 and classic biomarkers illustrated marginally significant incremental predictive value beyond conventional risk factors and glucose (P = 0.06). IGF-I, IGFBP-3, or molar ratio of IGF-I to IGFBP-3 at gestational weeks 10–14 or 15–26 did not significantly improve the prediction of GDM risk over the conventional risk factors, plasma glucose, and aforementioned classic biomarkers (data not shown).
Discussion
In this longitudinal and prospective study, we profiled longitudinal changes of IGF axis biomarkers throughout pregnancy. In addition, IGF-I concentrations and molar ratio of IGF-I to IGFBP-3 in early and midpregnancy were significantly and positively related to subsequent risk of GDM, whereas IGFBP-2 was inversely and strongly related to the risk, independent of other major risk factors of GDM. Moreover, IGF-I, molar ratio of IGF-I to IGFBP-3, and IGFBP-2 were significantly and prospectively correlated with HOMA-IR and fasting plasma insulin, suggesting their potential pathophysiological roles in glucose homeostasis underway prior to diagnosis of GDM. Notably, we observed that IGFBP-2 significantly improved the prediction capacity of GDM risk in addition to conventional risk factors, plasma glucose, and classic biomarkers (adiponectin, CRP, and lipids) as early as gestational weeks 10–14.
We for the first time longitudinally and prospectively examined the IGF axis markers throughout pregnancy in relation to subsequent risk of GDM. Previous studies were based on retrospective or cross-sectional data with single point blood specimen collection, either coinciding with or after the diagnosis of GDM (9–11,22). Given the notable changes in IGF axis biomarkers across gestation as observed herein, and the potential confounding due to therapeutic effect of GDM intervention after the diagnosis, it is essential to investigate the time-specific associations prior to GDM screening or diagnosis. For instance, in a cross-sectional study of 116 multiracial women with and without GDM (22), concentrations of IGF-I did not differ between case and control subjects at gestational weeks 36–38, which is consistent with our findings at weeks 36–39. On the other hand, our findings of a significant and positive association between total IGF-I concentrations in early pregnancy and GDM risk are in line with findings from emerging, yet limited, available studies (9,23,24). For instance, in a cross-sectional study, IGF-I concentrations at routine screening for GDM at gestational weeks 24–28 were significantly higher among 46 Polish women with GDM than 21 control subjects without GDM (24). In a prospective case-cohort study of 47 GDM events, comparing the highest to lowest tertile, IGF-I at gestational week 13 (interquartile range 8–16 weeks) was associated with a 1.47-fold higher, although nonsignificant, risk of GDM (12). Inference of findings from the scarce, previous studies was limited by either the retrospective/cross-sectional deign or a small sample size.
We were unaware of longitudinal and prospective studies on IGFBP-2 and subsequent risk of GDM. Our findings regarding the inverse association between IGFBP-2 concentrations in early to midpregnancy and subsequent risk of GDM are in agreement with previous observations on type 2 diabetes among nonpregnant individuals. Higher concentrations of IGFBP-2 have been linked to a lower risk of type 2 diabetes (odds ratio comparing the highest vs. lowest quintile 0.17 [95% CI 0.08, 0.35]) (15). In addition, IGFBP-2 overexpression is associated with reduced susceptibility to obesity and diabetes via inhibition of adipogenesis and stimulation of insulin sensitivity in mice (25,26). Taken together, the pleiotropic actions of IGFBP-2 highlight it as a critical molecule implicated in metabolic regulation and homeostasis. The incremental prediction capacity of IGFBP-2 for GDM was not reported previously. In the current study, we observed that as early as 10–14 weeks of gestation, plasma concentrations of IGFBP-2 significantly improved the prediction of GDM in addition to conventional GDM risk factors and plasma glucose, adiponectin, CRP, and lipids levels. Collectively, these data, in addition to the lack of significant postprandial fluctuation of IGFBP-2 (27), may make IGFBP-2 a convenient early marker for subsequent risk of GDM. Future investigation among other study populations is needed to confirm our findings. In addition, assessment of the joint incremental predictive value of IGFBP-2 with other promising pregravid or first-trimester biomarkers implicated in GDM etiology (28–30) is warranted.
The exact biological mechanisms whereby these IGF axis biomarkers are involved in glucose metabolism remain to be elucidated. At the cellular level, IGFBP-2 may exert an inhibitory effect on IGFs by competing with IGF receptors for peptide binding and thus regulating the IGF action (31). In addition, IGFBP-2 may act in an IGF-independent manner by binding to α5β1-integrin receptors and activating a downstream signaling cascade via the phosphatidylinositol-3-kinase and protein kinase β pathway, which is implicated in glucose uptake and insulin sensitivity (32). Indeed, the significant inverse association between IGFBP-2 and GDM persisted after further adjustment for IGF-I and IGFBP-3, as illustrated in this study.
Our study has several notable strengths. First, the longitudinal data collection provided a unique opportunity to prospectively investigate the roles of several components of the IGF axis (i.e., IGF-I, IGFBP-2, and IGFBP-3) in relation to subsequent risk of GDM, within a multiracial pregnancy cohort. In particular, the case-control differences in IGF-I, IGF-I/IGFBP-3, and IGFBP-2 concentrations did not persist after the average gestational age at GDM diagnosis (i.e., approximately week 27), which highlights the importance of temporal precedence in investigating the etiological roles of these biomarkers. Furthermore, longitudinal data on other biomarkers involved in glucose homeostasis are available in the current study, which allows us to gain deeper insight into the pathological roles of these IGF axis biomarkers in development of GDM. In addition, IGF-I and IGFBP-2 are not responsive to short-term fasting or postprandial fluctuations (27,33) and thus would have the potential to serve as convenient markers of subsequent risk of GDM among pregnant women.
Some potential limitations of our study merit discussion. Measurements of other components of the IGF axis (e.g., IGF-II and IGFBP-1), whose metabolic roles in glucose homeostasis have been previously indicated (34,35), are not available for this analysis. However, as opposed to IGFBP-1, plasma concentrations of IGFBP-2 do not significantly fluctuate postprandially but do increase after a prolonged period of fasting, suggesting that IGFBP-2 might mirror long-term status, whereas IGFBP-1 reflects short-term alterations in response to their major regulator, insulin (27). Similarly, we measured total, not free, IGF-I because free IGF-I concentrations are affected by fasting status (36). Nonetheless, we calculated the molar ratio of IGF-I to IGFBP-3 to reflect the bioavailability of IGF-I (13), whose association with GDM risk was similar to total IGF-I, although with greater effect sizes, especially at gestational weeks 15–26. Although we could not completely rule out the possibility of case-control misclassification, among 19 control subjects not routinely screened for GDM using the 50-g glucose challenge test, 12 had normal OGTT glucose levels and all the remaining were free of hospital discharge diagnosis of GDM. Nonetheless, the potential misclassification could have underestimated the incidence of GDM and thus the true effect sizes.
In conclusion, we observed a significantly increased risk of GDM in association with higher concentrations of IGF-I and molar ratio of IGF-I to IGFBP-3, and lower concentrations of IGFBP-2, as early as gestational weeks 10–14, ∼10–18 weeks earlier before GDM is typically screened for. IGFBP-2, particularly during early pregnancy, might serve as a convenient early marker of GDM risk, with significant improvement in risk prediction in addition to established risk factors and plasma glucose concentrations. Collectively, our findings suggest the pathophysiological role of the IGF axis in the development of GDM and highlight its potential to help identify at-risk women as early as the first trimester, an important etiologically relevant time window, for subsequent GDM.
Article Information
Acknowledgments. The authors thank all the research teams at all participating clinical centers, including Christina Care Health Systems; University of California, Irvine; Long Beach Memorial Medical Center; Northwestern University; Medical University of South Carolina; Columbia University; New York Hospital Queens; Saint Peter’s University Hospital; University of Alabama at Birmingham; Women & Infants Hospital of Rhode Island; Fountain Valley Regional Hospital and Medical Center; and Tufts University. The authors also acknowledge the C-TASC Corporation that provided data coordination and the Department of Laboratory Medicine and Pathology, University of Minnesota, that provided laboratory resources essential to test blood samples for biomarkers in this study.
Funding. This research was supported by the NICHD intramural funding and included American Recovery and Reinvestment Act funding via contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z.
Duality of Interest. No potential conflicts of interest relevant to this work were reported.
Author Contributions. Y.Z. analyzed data and wrote the first draft of the manuscript. P.M. interpreted data and revised the manuscript. P.S.A. contributed to data analysis and interpretation and reviewed the manuscript. W.B. coordinated the study, interpreted data, and reviewed the manuscript. S.N.H. interpreted data and reviewed the manuscript. M.Y.T. led laboratory testing and reviewed the manuscript. C.Z. obtained funding, designed and oversaw the study, and revised the manuscript. All authors interpreted the results, revised the manuscript for important intellectual content, and approved the final version of the manuscript. Y.Z. and C.Z. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0514/-/DC1.
See accompanying article, p. 3246.
References
- 1.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract 2014;103:176–185 [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol 2011;8:228–236 [DOI] [PubMed] [Google Scholar]
- 3.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunger DB, Acerini CL. Does recombinant human insulin-like growth factor-1 have a role in the treatment of diabetes? Diabet Med 1997;14:723–731 [DOI] [PubMed]
- 5.Holt RI, Simpson HL, Sonksen PH. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med 2003;20:3–15 [DOI] [PubMed]
- 6.Wilson DM, Bennett A, Adamson GD, et al. Somatomedins in pregnancy: a cross-sectional study of insulin-like growth factors I and II and somatomedin peptide content in normal human pregnancies. J Clin Endocrinol Metab 1982;55:858–861 [DOI] [PubMed] [Google Scholar]
- 7.Giudice LC, Farrell EM, Pham H, Lamson G, Rosenfeld RG. Insulin-like growth factor binding proteins in maternal serum throughout gestation and in the puerperium: effects of a pregnancy-associated serum protease activity. J Clin Endocrinol Metab 1990;71:806–816 [DOI] [PubMed] [Google Scholar]
- 8.Tisi DK, Burns DH, Luskey GW, Koski KG. Fetal exposure to altered amniotic fluid glucose, insulin, and insulin-like growth factor-binding protein 1 occurs before screening for gestational diabetes mellitus. Diabetes Care 2011;34:139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes SCC, Johnson MR, Heinrich G, Holly JMP. Could abnormalities in insulin-like growth factors and their binding proteins during pregnancy result in gestational diabetes? J Endocrinol 1995;147:517–524 [DOI] [PubMed] [Google Scholar]
- 10.Luthman M, Stock S, Werner S, Bremme K. Growth hormone-binding protein in plasma is inversely correlated to placental lactogen and augmented with increasing body mass index in healthy pregnant women and women with gestational diabetes mellitus. Gynecol Obstet Invest 1994;38:145–150 [DOI] [PubMed] [Google Scholar]
- 11.Ramirez VI, Miller E, Meireles CL, Gelfond J, Krummel DA, Powell TL. Adiponectin and IGFBP-1 in the development of gestational diabetes in obese mothers. BMJ Open Diabetes Res Care 2014;2:e000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu C, Vadachkoria S, Meryman L, Frederick IO, Williams MA. Maternal plasma concentrations of IGF-1, IGFBP-1, and C-peptide in early pregnancy and subsequent risk of gestational diabetes mellitus. Am J Obstet Gynecol 2005;193:1691–1697 [DOI] [PubMed] [Google Scholar]
- 13.Juul A, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin Endocrinol (Oxf) 1994;41:85–93 [DOI] [PubMed] [Google Scholar]
- 14.Russo VC, Azar WJ, Yau SW, Sabin MA, Werther GA. IGFBP-2: The dark horse in metabolism and cancer. Cytokine Growth Factor Rev 2015;26:329–346 [DOI] [PubMed] [Google Scholar]
- 15.Rajpathak SN, He M, Sun Q, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes 2012;61:2248–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol 2015;213:449.e1–449.e41 [DOI] [PMC free article] [PubMed]
- 17.American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics . ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes [retracted in: Obstet Gynecol 2013;122:405]. Obstet Gynecol 2001;98:525–538 [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 19.Pepe MS, Fan J, Seymour CW. Estimating the receiver operating characteristic curve in studies that match controls to cases on covariates. Acad Radiol 2013;20:863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845 [PubMed] [Google Scholar]
- 21.Stone M. Cross-validatory choice and assessment of statistical predictions. J R Stat Soc B 1974;36:111–147 [Google Scholar]
- 22.Simmons D, Breier BH. Fetal overnutrition in polynesian pregnancies and in gestational diabetes may lead to dysregulation of the adipoinsular axis in offspring. Diabetes Care 2002;25:1539–1544 [DOI] [PubMed] [Google Scholar]
- 23.Lindsay RS, Westgate JA, Beattie J, et al. Inverse changes in fetal insulin-like growth factor (IGF)-1 and IGF binding protein-1 in association with higher birth weight in maternal diabetes. Clin Endocrinol (Oxf) 2007;66:322–328 [DOI] [PubMed] [Google Scholar]
- 24.Matuszek B, Lenart-Lipińska M, Burska A, Paszkowski T, Smoleń A, Nowakowski A. Increased serum insulin-like growth factor-1 levels in women with gestational diabetes. Adv Med Sci 2011;56:200–206 [DOI] [PubMed] [Google Scholar]
- 25.Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab 2010;11:11–22 [DOI] [PubMed] [Google Scholar]
- 26.Wheatcroft SB, Kearney MT, Shah AM, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes 2007;56:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clemmons DR, Snyder DK, Busby WH Jr. Variables controlling the secretion of insulin-like growth factor binding protein-2 in normal human subjects. J Clin Endocrinol Metab 1991;73:727–733 [DOI] [PubMed] [Google Scholar]
- 28.Hedderson MM, Xu F, Darbinian JA, et al. Prepregnancy SHBG concentrations and risk for subsequently developing gestational diabetes mellitus. Diabetes Care 2014;37:1296–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savvidou M, Nelson SM, Makgoba M, Messow CM, Sattar N, Nicolaides K. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes 2010;59:3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedderson MM, Darbinian J, Havel PJ, et al. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care 2013;36:3930–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 2002;23:824–854 [DOI] [PubMed] [Google Scholar]
- 32.Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol Metab 2009;20:153–162 [DOI] [PubMed] [Google Scholar]
- 33.Bang P, Brismar K, Rosenfeld RG, Hall K. Fasting affects serum insulin-like growth factors (IGFs) and IGF-binding proteins differently in patients with noninsulin-dependent diabetes mellitus versus healthy nonobese and obese subjects. J Clin Endocrinol Metab 1994;78:960–967 [DOI] [PubMed] [Google Scholar]
- 34.Higgins M, Mc Auliffe F. A review of maternal and fetal growth factors in diabetic pregnancy. Curr Diabetes Rev 2010;6:116–125 [DOI] [PubMed] [Google Scholar]
- 35.Ruan W, Lai M. Insulin-like growth factor binding protein: a possible marker for the metabolic syndrome? Acta Diabetol 2010;47:5–14 [DOI] [PubMed] [Google Scholar]
- 36.Frystyk J, Skjaerbaek C, Dinesen B, Orskov H. Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS Lett 1994;348:185–191 [DOI] [PubMed] [Google Scholar]