Abstract
The gut microbiome is deeply involved in numerous aspects of human health; however, it can be readily perturbed by environmental toxicants, such as arsenic. Meanwhile, the interaction among host, gut microbiome, and xenobiotics is a very complex dynamic process. Previously, we have demonstrated that gut microbiome phenotypes driven by host genetics and bacterial infection affect the responses to arsenic exposure. The role of host sex in shaping the gut microbiome raises the question whether sex plays a role in exposure-induced microbiome responses. To examine this, we used 16S rRNA sequencing and metagenomics sequencing to analyze the changes of the gut microbiome and its associated functional metagenome in both female and male C57/BL6 mice. Our results clearly demonstrated that arsenic exposure perturbed the trajectory and function of the gut microbiome in a sex-specific manner.
Graphical abstract
Arsenic exposure is a global public health issue, with more than 100 million people being exposed primarily from drinking water.1 Arsenic exposure has been linked to a series of human diseases, including cancers, diabetes, and cardiovascular disorders.2–4 Different molecular mechanisms and modes of action are associated with arsenic toxicity. For example, arsenic activates inflammatory signaling and alters DNA methylation and gene expression.5–7 Recently, the role of the gut microbiome in arsenic metabolism and toxicity has been demonstrated in different model systems.8–10 The gut microbiome is a very complex dynamic system which contains trillions of bacteria and 100-fold more unique genes than the human genome.11 The gut microbiome is deeply involved in host metabolism and can be readily perturbed by external factors, raising the possibility that arsenic exposure could alter the gut microbiome and its functions.12,13 Our previous research clearly demonstrated that the gut microbiome interplays with arsenic exposure: arsenic can perturb the gut microbiome community structures and its metabolic profile;13 the gut microbiome phenotypes/variations alter the biotransformation of inorganic arsenic.9,14 Likewise, a recent study by Dheer et al. demonstrated that arsenic at environmentally relevant doses altered the gut microbiome and microbiome/host functions.15
The establishment of the gut microflora is a temporal and sensitive process occurring after birth.16 The colonization of gut microbiome during early life is highly sex-dependent.17,18 Meanwhile, sex-specific effects of arsenic metabolism and toxicity have been reported, although with little of the molecular mechanism being identified.19 For example, previous studies have revealed that arsenic methylation is more efficient in females than in males.19 Likewise, males are more susceptible than females to develop skin lesions when exposed to arsenic.20 Taken together, sex is an important factor to be considered when exploring the complex interaction among host, gut microbiome, and arsenic toxicity. However, it remains unclear whether sex influences the gut microbiome response to arsenic exposure. In this study, we employed 16S rRNA gene sequencing and metagenomics to address this intriguing question. Our results reveal that arsenic exposure altered the trajectories (i.e., time-dependent changes of gut microbiome compositions) and functional metagenome of the gut microbiome in a sex-specific manner.
The experimental design and workflow are described in the Supporting Information and Figure S1. Briefly, we divided 20 female mice and 20 male mice into four groups: Group A, 10 female mice as controls; Group B, 10 female mice treated with 10 ppm arsenic as previously described;13 Group C, 10 male mice as controls; and Group D, 10 male mice treated with 10 ppm arsenic. Then, we collected the fecal pellets from individual animals at two different time-points (day 0 baseline and 4 weeks after arsenic treatment) and applied 16S rRNA sequencing technology to determine the fecal microbiome compositions (Figure 1). Finally, the changes of functional metagenome and pathways induced by arsenic exposure were determined by metagenomics sequencing (Figure 2).
Figure 1.
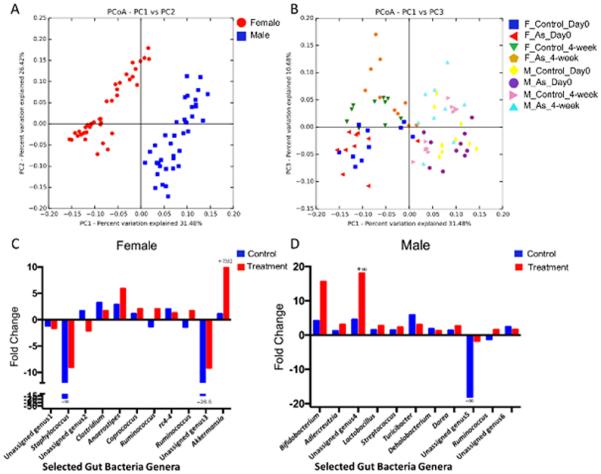
PCoA analysis of the gut microbiome (beta diversity) via 16S rRNA sequencing (A and B) and fold change of some representative genera of female and male mice (C and D). (A) The gut microbiome community structures are dramatically different between male and female mice. (B) The trajectories of the gut microbiome in controls and treated mice over a 4-week period (F, female; M, male). (C) The fold changes of selected significantly changed gut bacterial genera in female mice (p < 0.05). (D) The fold changes of selected significantly changed gut bacterial genera in male mice. Fold changes were calculated using the group means for each bacterial genus (p < 0.05).
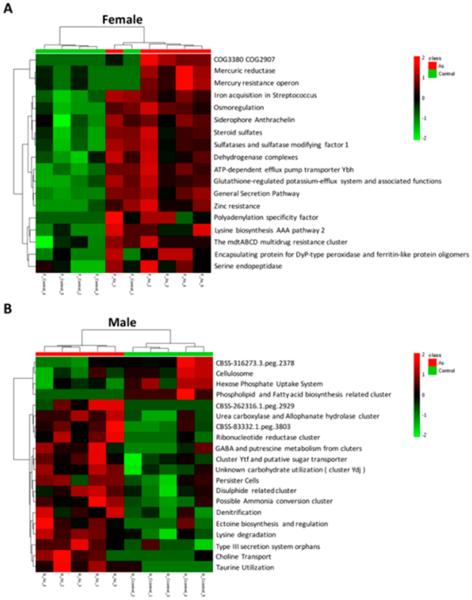
Figure 2.
Sex-selective effects of arsenic exposure on modulating the functional metagenome, as illustrated by the heat map constructed with the results from the functional analysis of the subsystem (A, females; B, males). All functional categories listed here are significantly different between the control and arsenic-treated groups (p < 0.05).
16S rRNA sequencing results reveal that there is a remarkable difference of gut microbiome between male and female mice, as shown in Figure 1A and Figure S2. After a 4-week arsenic treatment, the gut microbiome changed in both male and female mice. According to the principal coordinate analysis (PCoA) of beta diversity (Figure 1B), the difference of gut microbiome community structures in arsenic-treated female mice is more significant than that in male mice when compared to corresponding controls. These results reflect that gut microbiome phenotypes driven by host sex have different sensitivity to arsenic exposure. Meanwhile, time plays a role in shaping the gut microbiome profiles regardless of treatment, as shown in Figure 1B and Figure S2B, raising the possibility that arsenic exposure may perturb the normal trajectory of the gut microbiome. To further examine this, we compared the gut microbiome profiles over time for both controls and treated animals. Figure 1C and D summarizes the fold changes of a few representative genera that were statistically different between day 0 and 4 weeks. Evidently, the change patterns of the gut microbiome were different for controls and treated animals, indicating that arsenic exposure affected the trajectory of the gut microbiome. Moreover, a strong sex-specific response was observed. For example, Dorea decreased in female mice after a 4-week exposure, while it significantly increased in treated males. Likewise, Akkermansia had an ~7000-fold increase in the female arsenic-treated group; however, this genus did not show any significant change in males after arsenic exposure (Figure 1C and D). More detailed information on gut bacteria alterations from arsenic treatment is listed in Tables S1 and S2. Taken together, arsenic exposure altered the trajectory of the gut microbiome in a sex-selective manner.
We next used metagenomics to examine whether arsenic exposure also perturbed the functional metagenome in a sex-specific way, as the functional impact is more relevant to the gut microbiome and host. Figure 2 shows that a number of metabolic pathways were significantly altered by arsenic exposure. Similar to the changes of the microbiome profiles, the functional pathways were also differentially perturbed by arsenic in male and female mice. For example, arsenic treatment up-regulated many functional pathways in females, with several of them being related to metal resistance (such as mercury resistance operon, zinc resistance, and the mdtABCD multidrug resistance cluster) and the cell transport system (such as glutathione-regulated potassium-efflux system, ATP-dependent efflux pump transporter Ybh, general secretion pathway, and iron acquisition in Streptococcus). In arsenic-treated males, the pathways of nitrogen, carbon, and sulfate metabolism were significantly affected. For example, the hexose phosphate uptake system and phospholipid and fatty acid biosynthesis were remarkably down-regulated in arsenic-treated males, whereas functional pathways like ammonia conversion, denitrification, and cluster Ytf and the putative sugar transporter were significantly increased in treated males. Interestingly, the previous study by Dheer et al. found elevated tissue nitrite in hosts, highlighting the potential role of host–microbiome interaction in mediating nitrogen metabolism.15 In summary, arsenic has different effects on the functional metagenome in male and female mice.
The interaction among host, gut microbiome, and xenobiotics is a very complex dynamic process. Previously, we have demonstrated that gut microbiome phenotypes driven by host genetics and bacterial infection largely influence the biotransformation of arsenic in the host.9,14 Here, we show that that host sex also serves as an important factor to influence the gut microbiome compositions and could further affect the gut microbiome response to arsenic exposure. It was previously documented that the female is more resistant to arsenic exposure than males.19–21 It is interesting to notice that gut bacteria in arsenic-treated female mice but not male animals have significantly increased heavy metal resistance and cell-efflux capability, which may partially contribute to the different sex susceptibilities to arsenic exposure. Future studies are needed to further elucidate this intriguing question.
In summary, our results show that arsenic exposure significantly altered the trajectory of the gut microbiome over a 4-week dosing period, with multiple components of gut bacteria being significantly changed in a sex-selective fashion. Consistent to this, metagenomics data also revealed that the functional metagenome was differentially perturbed in male and female mice. Taken together, this study provides strong evidence for the first time that host sex plays a role in the gut microbiome responses to environmental toxicants, such as arsenic. Several limitations are associated with this study, including the high arsenic dose, short exposure time, and the use of fecal microbiome to infer changes of gut microbiome. In particular, the impact of arsenic on the host functions was not assessed in this study, which may largely drive the microbiome changes. Further study is warranted to shed light on these aspects.
Supplementary Material
Acknowledgments
Funding
The authors thank the NIH/NIEHS for financial support (R01ES024950).
ABBREVIATIONS
- PCoA
principal coordinate analysis
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.6b00066.
Detailed methods; experimental workflow; gut bacterial profiles and clustering patterns revealed by 16S rRNA gene sequencing; and gut bacterial abundance changes over a 4-week period in both male and female mice (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol. Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wang C-H, Hsiao CK, Chen C-L, Hsu L-I, Chiou H-Y, Chen S-Y, Hsueh Y-M, Wu M-M, Chen C-J. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol. Appl. Pharmacol. 2007;222:315–326. doi: 10.1016/j.taap.2006.12.022. [DOI] [PubMed] [Google Scholar]
- (3).Rahman M, Tondel M, Ahmad SA, Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am. J. Epidemiol. 1998;148:198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- (4).Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S. Activation of inflammation/NF-κB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- (7).Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- (8).Rubin SSD, Alava P, Zekker I, Du Laing G, Van de Wiele T. Arsenic thiolation and the role of sulfate-reducing bacteria from the human intestinal tract. Environ. Health Perspect. 2014;122:817–822. doi: 10.1289/ehp.1307759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lu K, Mahbub R, Cable PH, Ru H, Parry NM, Bodnar WM, Wishnok JS, Styblo M, Swenberg JA, Fox JG. Gut microbiome phenotypes driven by host genetics affect arsenic metabolism. Chem. Res. Toxicol. 2014;27:172–174. doi: 10.1021/tx400454z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Alava P, Tack F, Laing GD, Wiele TV. HPLC-ICP-MS method development to monitor arsenic speciation changes by human gut microbiota. Biomed. Chromatogr. 2012;26:524–533. doi: 10.1002/bmc.1700. [DOI] [PubMed] [Google Scholar]
- (11).Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- (13).Lu K, Ryan PA, Schlieper KA, Graffam ME, Levine S, Wishnok JS, Swenberg JA, Tannenbaum SR, Fox JG. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014;122:284–291. doi: 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lu K, Cable PH, Abo RP, Ru H, Graffam ME, Schlieper KA, Parry NM, Levine S, Bodnar WM, Wishnok JS. Gut microbiome perturbations induced by bacterial infection affect arsenic biotransformation. Chem. Res. Toxicol. 2013;26:1893–1903. doi: 10.1021/tx4002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dheer R, Patterson J, Dudash M, Stachler EN, Bibby KJ, Stolz DB, Shiva S, Wang Z, Hazen SL, Barchowsky A. Arsenic induces structural and compositional colonic microbiome change and promotes host nitrogen and amino acid metabolism. Toxicol. Appl. Pharmacol. 2015;289:397–408. doi: 10.1016/j.taap.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, Maas K, Graf J. Gut Microbiome Developmental Patterns in Early Life of Preterm Infants: Impacts of Feeding and Gender. PLoS One. 2016;11:e0152751. doi: 10.1371/journal.pone.0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- (19).Lindberg A-L, Ekström E-C, Nermell B, Rahman M, Lönnerdal B, Persson L-Å, Vahter M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res. 2008;106:110–120. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- (20).Rahman M, Vahter M, Sohel N, Yunus M, Wahed MA, Streatfield PK, Ekström E-C, Persson LÅ. Arsenic exposure and age-and sex-specific risk for skin lesions: a population-based case-referent study in Bangladesh. Environ. Health Perspect. 2006;114:1847–1852. doi: 10.1289/ehp.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Watanabe C, Inaoka T, Kadono T, Nagano M, Nakamura S, Ushijima K, Murayama N, Miyazaki K, Ohtsuka R. Males in rural Bangladeshi communities are more susceptible to chronic arsenic poisoning than females: analyses based on urinary arsenic. Environ. Health Perspect. 2001;109:1265–1270. doi: 10.1289/ehp.011091265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.