Abstract
Background
Obsessive-compulsive disorder (OCD) is associated with an abnormally large error-related negativity (ERN), an electrophysiological measure of error monitoring in response to performance errors, but it is unclear if Hoarding Disorder (HD) also shows this abnormality. This study aimed to determine whether the neurophysiological mechanisms underlying error monitoring are similarly compromised in HD and OCD.
Method
We used a visual flanker task to assess the ERN in response to performance errors in 14 individuals with HD, 27 with OCD, 10 with HD and OCD, and 45 healthy controls (HC). Age-corrected performance and ERN amplitudes were examined using analyses of variance and planned pairwise group comparisons.
Results
A main effect of Hoarding on the ERN (p = 0.031) was observed, indicating ERN amplitudes were attenuated in HD relative to non-HD subjects. A group x age interaction effect on the ERN was also evident. In HD positive subjects, ERN amplitude deficits were significantly greater in younger individuals (r=−.479, p = 0.018), whereas there were no significant ERN changes with increasing age in OCD and HC participants.
Conclusions
The reduced ERN in HD relative to OCD and HC provides evidence that HD is neurobiologically distinct from OCD, and suggests that deficient error monitoring may be a core pathophysiological feature of HD. This effect was particularly prominent in younger HD participants, further suggesting that deficient error monitoring manifests most strongly early in the illness course and/or in individuals with a relatively early illness onset.
Keywords: hoarding, obsessive-compulsive disorder (OCD), error-related negativity (ERN), electroencephalography/event-related potentials (EEG/ERP), neurophysiological, error monitoring
Hoarding Disorder (HD), as newly defined in the DSM-5, is characterized by persistent problems with discarding personal possessions, regardless of their value, due to the overwhelming desire to save, and distress or indecision about what to discard. Unless a third party intervenes, these symptoms lead to over-accumulation and clutter, such that use of living or work space is compromised. Many individuals with HD also demonstrate difficulties with executive function tasks, with impairments in procrastination, categorization, concentration and attention, and speed of task completion (i.e., slowed performance) (Grisham, Norberg, Williams, Certoma, & Kadib, 2010; Mathews, Perez, Delucchi, & Mathalon, 2012). Furthermore, accumulated clutter and cognitive deficits are associated with functional impairment, danger, distress, and disability (American Psychiatric Association, 2013). Although HD is clearly an independent disorder (Ayers, Saxena, Golshan, & Wetherell, 2010; Hall, Tolin, Frost, & Steketee, 2013), hoarding symptoms frequently co-occur within an individual across the lifetime and co-segregate with OCD in families (Mathews et al., 2007; Morein-Zamir et al., 2014), complicating the dissection of the etiologies and pathophysiologies of these disorders. However, a new line of evidence suggests the presence of divergent neural substrates corresponding to each of these two disorders (Tolin, Witt, & Stevens, 2014).
We and others have previously hypothesized that HD impairments in cognitive and affective processing following simple errors may arise from abnormalities in the brain’s error detection system, which can be interrogated using electrophysiological methods (e.g.,(Nieuwenhuis, Nielen, Mol, Hajcak, & Veltman, 2005)). The error-related negativity (ERN), a frontocentrally distributed negative deflection in the response-locked event-related potential (ERP) peaks around 80 msec following incorrect responses during reaction time tasks. The ERN is thought to occur when the brain’s online error monitoring system detects a mismatch between actual (incorrect) and intended (correct) responses on error trials (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; W.J. Gehring, Goss, Coles, Meyer, & Donchin, 1993), in response to a reinforcement learning signal (Holroyd & Coles, 2002), or if the amount of response conflict is varied probabilistically (M. M. Botvinick, Braver, Barch, Carter, & Cohen, 2001; Brown & Braver, 2005; Yeung, Botvinick, & Cohen, 2004). Specifically, it has been shown that participants adjust their processing strategy according to a simple probability heuristic (Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988). Using a flanker task, Botvinick et al. (M. Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999) found that incongruent (high-conflict) trials following congruent (low-conflict) trials produced greater error-related ACC activation than did incongruent trials following other incongruent trials. The neural generator of the ERN has been intimately linked to the ACC (Debener et al., 2005; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; van Veen & Carter, 2002), and there are robust findings of error-related ACC abnormalities in OCD patients (Fitzgerald et al., 2005; Maltby, Tolin, Worhunsky, O’Keefe, & Kiehl, 2005; Ursu, Stenger, Shear, Jones, & Carter, 2003). Importantly, the ERN has repeatedly been shown to be hyperactive in individuals with OCD compared to controls (Carrasco et al., 2013; Endrass, Klawohn, Schuster, & Kathmann, 2008; Endrass, Riesel, Kathmann, & Buhlmann, 2014; Endrass et al., 2010; W. J. Gehring, Himle, & Nisenson, 2000; Hajcak, Franklin, Foa, & Simons, 2008; Hanna et al., 2012; Johannes et al., 2001; Klawohn, Riesel, Grutzmann, Kathmann, & Endrass, 2014; Santesso, Segalowitz, & Schmidt, 2006; Weinberg, Dieterich, & Riesel, 2015) reviewed in (Mathews et al., 2012)).
No ERN studies to date have explicitly focused on individuals with a primary diagnosis of HD. However, in a previously published meta-analysis of ERN studies of OCD and obsessive compulsive symptoms, we retrospectively identified individuals who reported hoarding symptoms based on data obtained from the authors of the included studies (Mathews et al., 2012). The results of the meta-analysis comparing the ERN in OCD-affected individuals with co-occurring hoarding symptoms (OCD+H) to those without hoarding symptoms (OCD-H) indicated reduced (though not significantly) ERN in OCD+H relative to OCD-H in response conflict tasks and enhanced (again, not significantly) ERN in OCD+H for probabilistic learning tasks. More recently, work by Reisel et al. (Riesel, Kathmann, & Endrass, 2014) in 72 individuals with OCD suggested that those with hoarding/symmetry symptoms had a reduced ERN relative to those without such symptoms (note that the ERN in these individuals was still enhanced relative to healthy controls, however). Although multiple lines of evidence exist to support the separation of HD and OCD into distinct entities (An et al., 2008; Iervolino et al., 2009; Pertusa et al., 2008; Saxena, 2007; Tolin et al., 2014), as yet little work has been done to investigate the neural substrates of HD independent of OCD.
One critical component of the investigation into the pathophysiological distinctions between HD and OCD involves group age differences. Although hoarding symptoms often begin by age 15, they do not typically become problematic until the 20’s or 30’s, and treatment-seeking for HD is uncommon before age 40 (Frost & Gross, 1993; Grisham, Frost, Steketee, Kim, & Hood, 2006; Kim, Steketee, & Frost, 2001), as symptom severity worsens progressively over time (Ayers et al., 2010). For these reasons, individuals with HD available for study are often in an older age range, usually 40–65 years old (Ayers et al., 2010; Pertusa et al., 2008). In contrast, OCD typically comes to attention much earlier, as symptoms, which also appear during childhood or adolescence, are more often clinically significant or impairing early in life. In addition, neurodevelopmental and aging effects on the neurophysiological activity underlying the brain’s error monitoring system have been reported. ERN amplitudes appear to increase throughout adolescence and into adulthood (Davies, Segalowitz, & Gavin, 2004; Santesso & Segalowitz, 2008), decreasing with normal aging at the other end of the lifespan (Falkenstein, Hoormann, & Hohnsbein, 2001; Nieuwenhuis et al., 2002), although not all studies have found this relationship (Friedman, Nessler, Cycowicz, & Horton, 2009). Therefore, given the changes in ERN with age, and the older age of most HD participants relative to OCD participants, studies comparing ERN in HD and OCD should explicitly investigate putative pathophysiological differences while accounting for possible normal aging effects. In addition, given the increasing severity of hoarding symptoms over time, and the increasing rates of HD with age, possible interactions between HD, age, and ERN amplitude warrant investigation.
Accordingly, the present study examined the ERN in individuals with HD relative to healthy controls (HC), to individuals with OCD but without hoarding symptoms, and to individuals with comorbid HD and OCD diagnoses. We also examined the age trajectory of the ERN in each group. Previous studies of the ERN and OCD have shown that response conflict tasks, particularly the Flanker task, are highly reliable (Meyer, Riesel, & Hajcak Proudfit, 2013; Riesel, Weinberg, Endrass, Meyer, & Hajcak, 2013), and stable (Olvet & Hajcak, 2009); however, studies of the ERN and OCD using probabilistic learning or other tasks are more heterogeneous (Endrass et al., 2010; Grundler, Cavanagh, Figueroa, Frank, & Allen, 2009; Hammer, Kordon, Heldmann, Zurowski, & Munte, 2009; Nieuwenhuis et al., 2005). Given the reliability and stability of response conflict tasks for OCD, and the heterogeneity seen for other tasks, we chose to use a Flanker task for our comparisons.
Based on the results of the meta-analysis, and supported by the Reisel et al (Riesel et al., 2013) findings, we hypothesized that individuals with HD would have a reduced electrophysiological response to errors relative to individuals without HD on a response conflict task. We also hypothesized that individuals with HD would demonstrate an abnormality in the developmental age trajectory of the ERN.
Methods
Participants
Participants included 14 individuals with HD, 28 individuals with OCD, 10 individuals with HD and OCD, and 47 HC spanning the age range of the patient groups. Participants were recruited through mental health clinics, through the Mental Health Association of San Francisco (MHA-SF), and through media advertisements, and were age 18 or older. For all groups, individuals with psychosis, intellectual disability, neurocognitive disorders, active substance use, a history of head trauma with loss of consciousness, or any medical conditions known or suspected to affect cognitive function were excluded. Individuals were compensated $20 per hour for their participation in the study. The study was approved by the Institutional Review Board at UCSF, and all participants provided written informed consent.
Clinical assessments
All participants were assessed for HD using the Structured Interview for Hoarding Disorder (SIHD) (Mataix-Cols, Billotti, Fernandez de la Cruz, & Nordsletten, 2012), the Saving Inventory - Revised (SI-R) (Frost, Steketee, & Grisham, 2004), the UCLA Hoarding Symptom Scale (UHSS) (Saxena, Brody, Maidment, & Baxter, 2007), and the Clutter Image Rating Scale (CIR) (Tolin, Frost, & Steketee, 2007). OCD was assessed using the Yale Brown Obsessive Compulsive Scale (YBOCS) (Goodman et al., 1989), and the Structured Clinical Interview Diagnosis of DSM disorders (SCID) (Spitzer, Williams, Gibbon, & First, 1992). Current symptoms of anxiety and depression were assessed using the Beck Anxiety Inventory (BAI) (A. T. Beck, Steer, R.A., 1993) and the Beck Depression Inventory (BDI) (A. T. Beck, Ward, C.H., Mendelson, M., Mock, J., Erbaugh, J., 1961). Clinical assessments for acute symptoms (YBOCS, BDI, BAI) were conducted within one month of the EEG assessment.
Inclusion/exclusion criteria
All psychiatric diagnoses were assigned blinded to group by a psychiatrist (CAM) with experience in HD and OCD according to DSM-5 criteria. HD participants were eligible for the study if they met DSM-5 criteria for HD but did not have OCD symptoms (YBOCS score of <5). OCD participants were eligible if they met DSM-5 criteria for OCD without significant hoarding symptoms, defined as scores of ≤20 on the SI-R, ≤10 on the UHSS, and ≤ 8 on the CIR. HD+OCD participants were considered eligible if they met DSM-5 criteria for both HD and OCD diagnoses. HC participants were age- and education-matched to both the HD and OCD participants, and were included if they did not have hoarding symptoms (as defined above) or OCD symptoms (as defined above). HC participants with a lifetime history of HD, OCD, or any current DSM-5 psychiatric disorder were excluded; however, HC with a lifetime history of a mood or anxiety disorder other than HD and OCD were included if the disorder was in remission at the time of the assessment. HC participants who had first-degree biological relatives with a diagnosis of HD or OCD or clinically significant hoarding or OCD symptoms by report were also excluded. Participants in all groups were excluded if taking antipsychotic medication. Antidepressants were allowed as they have not been clearly shown to affect the ERN (Stern et al., 2010).
Behavioral Task
A variant of the Eriksen flankers paradigm (Eriksen & Eriksen, 1974) was used to assess error processing in two separate probability conditions. Contextual flanker stimuli consisted of 4 letters (“EE EE” or “FF FF”; “MM MM” or “NN NN”; “UU UU” or “VV VV”) presented for 100 milliseconds (ms), followed immediately by presentation of a central target letter belonging to the same letter set (e.g., “E” or “F” within the “EE EE” or “FF FF” context) for 100 ms. Inter-trial intervals were pseudo-randomly jittered between 1250 and 1400 ms in 50 ms increments. The two probability conditions were defined where probability (P) was manipulated such that one condition had 75% congruent trials (“EEEEE” or “FFFFF”) and 25% incongruent (“EEFEE” or “FFEFF”) while the other condition had 75% incongruent and 25% congruent (referred to as P25 and P75, respectively throughout). Participants were instructed to press the right or left button corresponding to the target letter, using the respective index finger on a response box; response mapping of target letter-to-button location was randomized within subject. Subjects were not informed about the probabilities, but a practice run was completed for each letter set, and probability order was randomized within each of three blocked letter combinations (EF, MN, and UV). There were six runs, three per condition, with 200 trials each. Participants were instructed to respond as quickly as possible without sacrificing accuracy and received feedback at the end of each run based on overall accuracy: >90% correct: “Please try to respond faster”; between 90% and 75%: “Please continue”; <75% correct: “Please try to be more accurate”. For each participant, error rates and median reaction times (RT) were calculated for each trial type (congruent, incongruent) and probability condition (P75 and P25).
Electroencephalographic (EEG) Data Acquisition
EEG data were acquired using a 64-channel, high-impedance BioSemi ActiveTwo recording system. Continuous EEG data were sampled at 1024 Hz, referenced offline to averaged earlobe electrodes, band-pass filtered 0.1 to 15 Hz using zero-phase shift Butterworth filters, and segmented into 1050 ms epochs time-locked to the button press responses (−100 to 950 ms). Electro-oculogram (EOG) data were recorded from electrodes placed above and below the right eye and at the outer canthi of both eyes to capture vertical (VEOG) and horizontal (HEOG) eye movements that were used to correct the EEG for artifacts using a regression-based algorithm (Gratton, Coles, & Donchin, 1983). Following baseline correction (−100 to 0 ms), epochs were rejected if they contained amplitudes greater than ±100 µV in any of the Fz, FCz, Cz, or Pz electrodes. Due to low error rates in congruent trials in the P75 condition (N=63 with <6 errors (Olvet & Hajcak, 2009; Pontifex et al., 2010), ERN analyses assessed incongruent trials only. All participants except three had ≥6 incongruent trial errors in P75 and P25 after artifact rejection. These three participants were excluded from subsequent analyses. The remaining participants included 14 individuals with HD, 27 individuals with OCD, 10 individuals with HD and OCD, and 45 HC as described in Table 1. Response-locked ERP averages for each probability condition (P75, P25) were calculated separately using a 10%-trimmed mean approach (Leonowicz, Karvanen, & Shishkin, 2005). ERN was then measured in each participant’s ERP as the amplitude value at the latency of the most negative peak between 0 and 100 ms in Fz, FCz, and Cz.
Table 1.
Demographic characteristics of the participant sample.
| Hoarding Disorder (HD) |
Obsessive- Compulsive Disorder (OCD) |
Hoarding and Obsessive- Compulsive Disorder (OCD) |
Healthy Controls (HC) |
Hoarding (present vs absent) Effects |
OCD (present vs absent) Effects |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n= 14 | n = 27 | n = 10 | n = 45 | |||||||||
| N | % | N | % | N | % | N | % | X2 or t statistic (df) |
p-value | X2 or t statistic (df) |
p-value | |
| Gender | 7.027 | 0.008 | 0.023 | 0.879 | ||||||||
| Female | 11 | 78.6% | 13 | 48.1% | 9 | 90.0% | 25 | 55.6% | ||||
| Male | 3 | 21.4% | 14 | 51.9% | 1 | 10.0% | 20 | 44.4% | ||||
| Handednessa | ||||||||||||
| Right | 13 | 92.9% | 23 | 85.2% | 8 | 80.0% | 40 | 88.9% | ||||
| Left | 0 | 0.0% | 1 | 3.7% | 2 | 20.0% | 5 | 11.1% | ||||
| Both | 1 | 7.1% | 3 | 11.1% | 0 | 0.0% | 0 | 0.0% | ||||
| Antidepressants | 2 | 14.3% | 15 | 55.6% | 4 | 40.0% | 3 | 6.7% | 0 | 1 | 22.296 | <.0001 |
| M | SD | M | SD | M | SD | M | SD | |||||
| Age, years | 58.1 | 12.6 | 38.5 | 11.5 | 46.5 | 11.3 | 44.2 | 18.1 | 3.089 | 0.002 | −2.061 | 0.042 |
| Education, years | 15.4 | 1.9 | 16.5 | 1.9 | 14.8 | 1.5 | 15.8 | 2.4 | −1.865 | 0.065 | 0.694 | 0.489 |
| SI-R Total | 55.4 | 15.5 | 16.8 | 10.5 | 53.4 | 11.8 | 8.8 | 8.4 | 15.9 | <.0001 | 1.273 | 0.206 |
| YBOCS Total | 2.9 | 7.4 | 27.3 | 9.2 | 26.9 | 8 | 0 | 0 | −2.337 | 0.024 | 11.66 | <.0001 |
| BAI Total | 9.8 | 8.4 | 17 | 13.9 | 14.8 | 13 | 2.6 | 3.3 | 1.619 | 0.109 | 5.681 | <.0001 |
| BDI Total | 9.3 | 7.5 | 10.7 | 10.3 | 26.7 | 18.7 | 2.3 | 3.8 | 4.069 | 0.0001 | 4.8 | <.0001 |
SI-R, Saving Inventory-Revised; YBOCS, Yale-Brown Obsessive-Compulsive Scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory.
The Crovitz-Zener (1962) questionnaire was used to measure handedness.
Correction for Normal Aging Effects
In order to directly compare the patient groups, whose ages significantly differed (Table 1), it was necessary to first remove the effects of normal aging from the ERN. Using a previously published approach (Perez et al., 2012), we derived a single age-corrected value for each participant for ERN peak amplitude at each electrode in incongruent trials, separately for the P75 and P25 probability conditions. First, raw ERN amplitudes were regressed on age in the HC group (age range 18.4 – 78.1 years). Next, the resulting HC age-regression models were used to derive age-specific predicted ERN values that were subtracted from the observed values and divided by the standard error of regression, yielding age-corrected ERN z-scores (ERNz) for each participant at each electrode and for each probability condition. An identical approach was applied to median reaction time and error rate performance measures. The resulting age-corrected z-scores reflect deviations, in standard units, from the values expected for a normal healthy participant of a given age. The method is preferable to using age as a covariate in an ANCOVA because it removes normal aging effects while preserving any pathological aging effects present in the patient data.
Statistical Analysis
Behavioral Flanker task error rates were analyzed using repeated measures analysis of variance (rmANOVA) with two between-subjects factors: HD (Present, Absent) and OCD (Present, Absent), and two within-subjects factors: probability (P75, P25) and trial type (incongruent, congruent). Due to a lack of congruent errors, the error RT rmANOVA model had no trial type factor. Electrophysiological ERN data were analyzed using a 3-way rmANOVA with two between-subjects factors: HD (Present, Absent) and OCD (Present, Absent), and two within-subjects factors: probability (P75, P25) and electrode (Fz, FCz, Cz) for incongruent trials only. Age-corrected ERNz values were used to compare factors in all analyses involving HD and OCD. Because age-corrected ERNz have the expected value of “0” for all HC averages, within-subject effects (probability, electrode, and probability x electrode) were assessed with raw ERN (ERN-raw) scores. We assessed age-related patient group differences in ERNz amplitude using a regression analysis to test patient group (HD, OCD) x ERNz interaction effects. The interaction terms tested for slope differences between patient groups.
Results
Clinical characteristics
Table 1 shows the clinical and demographic characteristics of the sample. The HD group had significantly more females than males relative to the other groups. Because of gender differences between participant groups, we conducted secondary analyses (see Supplement) to test effects in female participants only (since there were too few males in the HD group to permit an analysis of gender effects).
Behavioral data
Error Rate
Table 2 presents the Error Rate descriptive statistics and rmANOVA results. There was a significant probability X trial type interaction (F(1,92) = 237.23, p <.001), driven by a significant increase in the incongruent trial error rate (mean±SE) at the 25% (24.42±1.59%) relative to 75% probability level (12.83±0.97%), but no change in the congruent error rate with change in probability (P25: 3.94±0.48% vs P75: 4.75±0.61%). There was also a significant OCD X HD X probability interaction (F(1,92) = 5.61, p = 0.02). Follow-up models at each probability level were used to parse this effect. While there was no OCD X HD interaction effect in the 25% probability level model (F(1,92) = 0.53, p = 0.4673), there was a marginal OCD X HD interaction effect in the 75% model (F(1,92) = 2.98, p = 0.087). This was driven by a marginally significant reduction in age-corrected error rates in OCD only patients compared to HC (t(70) = −1.93, p = 0.057). HD-only patients did not differ from HD+OCD patients in age-corrected error rates for the 75% probability level (t(22)=0.74, p = 0.465).This was driven by significantly greater age-corrected error rates in HD+OCD patients compared to OCD patients (t(35) = 2.55, p = 0.015). HD patients and HC did not differ in age-corrected error rates for the 75% probability level (t(57)=1.0, p = 0.323).
Table 2.
Error Rate scores comparing Hoarding Disorder (HD), Obsessive-Compulsive Disorder (OCD), Hoarding and Obsessive-Compulsive Disorder, and Healthy Control (HC) groups.
| Table 2a Incongruent Error Rate scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hoarding Disorder (HD) |
Obsessive-Compulsive Disorder (OCD) |
Hoarding and Obsessive-Compulsive Disorder (OCD) |
Healthy Controls (HC) | |||||
| n= 14 | n = 27 | n = 10 | n = 45 | |||||
| M | SE | M | SE | M | SE | M | SE | |
| Congruent | ||||||||
| P25 | 3.8% | 1.07% | 2.6% | 0.77% | 5.2% | 1.27% | 4.1% | 0.60% |
| P75 | 4.0% | 1.35% | 2.8% | 0.97% | 7.8% | 1.60% | 4.4% | 0.75% |
| Incongruent | ||||||||
| P25 | 25.4% | 3.56% | 22.7% | 2.56% | 26.7% | 4.21% | 22.9% | 1.98% |
| P75 | 13.0% | 2.16% | 10.3% | 1.55% | 16.4% | 2.55% | 11.8% | 1.20% |
| Table 2b Group Differences on Age-Corrected Incongruent Error Rate z-scores | |||
|---|---|---|---|
| ANOVA Results | df | F | p value |
| Hoarding (Present, Absent) | 1, 92 | 6.67 | 0.0114 |
| OCD (Present, Absent) | 1, 92 | 0.02 | 0.9001 |
| Hoarding X OCD | 1, 92 | 1.74 | 0.1901 |
| Hoarding X Probability | 1, 92 | 5.91 | 0.017 |
| OCD X Probability | 1, 92 | 1.62 | 0.2063 |
| Hoarding X OCD X Probability | 1, 92 | 5.61 | 0.0199 |
| Hoarding X Trial Type | 1, 92 | 0.07 | 0.7957 |
| OCD X Trial Type | 1, 92 | 0.04 | 0.8371 |
| Hoarding X OCD X Trial Type | 1, 92 | 1.41 | 0.2386 |
| Hoarding X Trial Type X Probability | 1, 92 | 0.12 | 0.7248 |
| OCD X Trial Type X Probability | 1, 92 | 1.6 | 0.2096 |
| Hoarding X OCD X Trial Type X Probability | 1, 92 | 0.02 | 0.8905 |
Reaction Time (RT)
There were no significant between-subjects effects or patient-group X probability interactions on median incongruent error RT. There was a significant effect of probability (F(1,92) = 17.8, p<.001), with longer median error RTs (mean±SE) in the P75 (350.37 ± 7.29) relative to the P25 condition (334.77 ± 5.81). Descriptive statistics and Reaction Time (RT) rmANOVA results are presented in the Supplementary Material.
Group Differences in Error-Related Negativity (ERN)
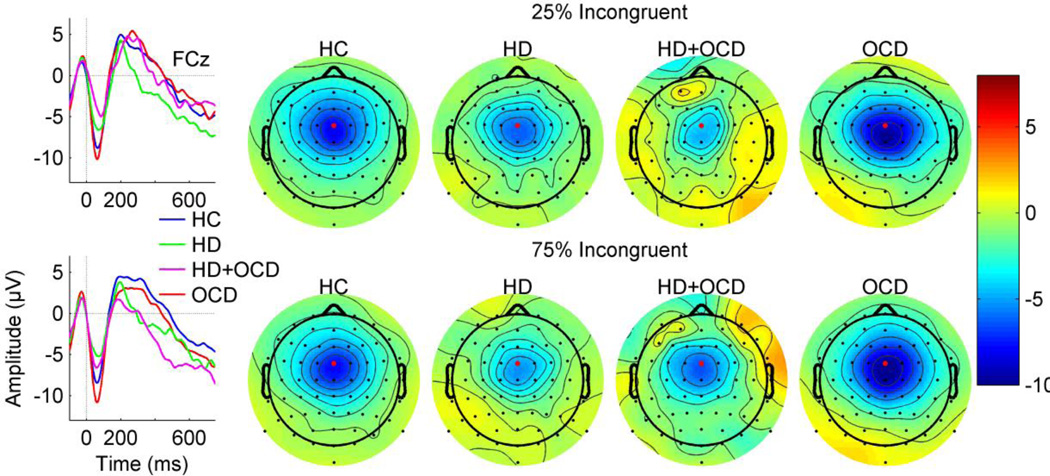
Errors elicited a large frontocentral (Fz, FCz, Cz) ERN peaking at approximately 55.9 ms (SD = 22.3 ms) post-response (Figure 1). Table 3 presents the results of the rmANOVA of ERNz. A significant main effect of hoarding (p = 0.031) was observed, driven by reduced ERNz amplitude in the HD relative to non-HD subjects. Planned group comparisons confirmed that HD-only had significantly reduced ERNz relative to OCD-only participants (t(39) = 2.148, p = 0.038, Cohen’s d= 0.77) and similarly reduced ERNz, though not significantly, relative to HC (t(57) = −2.1497, p = 0.13, Cohen’s d=0 .54) but not relative to HD+OCD (t(22) = 0.1037, p = 0.981, Cohen’s d=0.04) participants. A significant 3-way OCD*Probability*Electrode interaction (p = 0.0162) was driven in part by differences in the OCD*Probability effects at electrode FCz. In particular, subjects without OCD had little, if any, probability effect, whereas those with OCD appeared to have larger probability effects in the opposite direction at FCz in particular (i.e., larger ERNz in the 75% congruent condition). However, the OCD*Probability effect was only marginal (p=0.06) at FCz, and within that individual electrode, there was no OCD effect at either 25% incongruent (p=0.94) or 75% incongruent (p=0.25) probability levels. Secondary analyses limiting the participants to females showed similar results (see Supplement for details). Summary plots in Figure 1 present the ERNz averaged across probability conditions and frontocentral electrodes for the HD, OCD, HD+OCD, and HC groups.
Figure 1.
Left: Grand average ERP waveforms. Grand average ERP waveforms for response-locked error trials for age-matched Healthy Control (HC; blue), Hoarding Disorder (HD; green), comorbid Hoarding Disorder and Obsessive-Compulsive Disorder (HD+OCD; magenta) and Obsessive-Compulsive Disorder (OCD; red) participants. Waveforms are from FCz (indicated by red dot on topographic maps). The x-axis presents time in milliseconds from −100 ms (pre-response) to 750 ms (post-response) relative to the button press at 0 ms (vertical dotted line). The y-axis presents amplitude in microvolts (µV). Right: Scalp topography maps display ERN amplitudes averaged over a time window centered on the grand mean ERP peak latency (50–60 ms after the response event). Color bar indicates amplitude values in microvolts. Note the frontocentral distribution of the ERN, and the attenuated ERN for HD positive individuals relative to HC and OCD groups for the 25% (top panel) and the 75% (bottom panel) probability conditions in incongruent trials.
Table 3.
Results of repeated measures analysis of variance analysis on Age-Corrected Error-Related Negativity (ERN) Amplitude z-scores comparing Hoarding Disorder (HD), Obsessive-Compulsive Disorder (OCD), and Healthy Control (HC) groups.
| Table 3 Group Differences on Age-Corrected ERN z-scores | |||
|---|---|---|---|
| ANOVA Results | df | F | p value |
| Hoarding (Present, Absent) | 1, 92 | 2.88 | 0.031 |
| OCD (Present, Absent) | 1, 92 | 0.2 | 0.658 |
| Hoarding X OCD | 1, 92 | 0.09 | 0.767 |
| Hoarding X Electrode | 2, 92 | 0.75 | 0.477 |
| OCD X Electrode | 2, 92 | 1.42 | 0.248 |
| Hoarding X OCD X Electrode | 2, 92 | 2.67 | 0.075 |
| Hoarding X Probability | 1, 92 | 0.22 | 0.638 |
| OCD X Probability | 1, 92 | 3.13 | 0.08 |
| Hoarding X OCD X Probability | 1, 92 | 1.04 | 0.31 |
| Hoarding X Electrode X Probability | 2, 92 | 0.98 | 0.379 |
| OCD X Electrode X Probability | 2, 92 | 4.31 | 0.016 |
| Hoarding X OCD X Electrode X Probability | 2, 92 | 0.89 | 0.414 |
In the rmANOVA of ERN-raw amplitudes used to assess within-subjects effects of probability, electrode, and their interactions, no significant main effect of probability was found (F(1,92) = 0.23, p = .631). A significant main effect of electrode (F(2,92) = 46.35, p < .001) and simple follow-up contrasts indicated that the ERN was smaller at Fz than at FCz (t(92) = −8.97, p < 0.001) and Cz (t(92) = −4.71, p < 0.001). There was no significant probability*electrode interaction (F(2,92) = 1.84, p = 0.1646).
Effects of Symptom Severity and Age on ERN
There were no significant associations between ERNz and symptom severity within a combined HD, HD+OCD group for anxiety (BAI: r= 0.158, p = 0.506), depressive (BDI: r = −0.059, p = 0.81), hoarding (SI-R: r = −0.28, p = 0.195), or obsessive-compulsive symptoms (YBOCS: r = 0.178, p = 0.439). There was a relationship between age and age-corrected ERNz for the combined HD group such that younger HD had larger ERN abnormalities (r = −0.479, p=0.018). ERNz amplitude showed no age relationship in the OCD (p=0.959) or HC (p=0.66) groups (see Supplement). Similar patterns of correlations were observed using ERN-raw amplitude values.
Discussion
Although impaired error processing has been hypothesized to be associated with hoarding behaviors (Mathews et al., 2012; Riesel et al., 2014; Tolin et al., 2014), this study is the first to directly examine a neurophysiological measure of error detection in individuals with Hoarding Disorder. We found a significant reduction in ERN amplitude in individuals with HD relative to individuals without HD, independent of OCD diagnosis. Moreover, a significant reduction in ERN was detected in individuals with an HD diagnosis only relative to those with only an OCD diagnosis, even after correcting for age. These findings were not related to behavioral performance, since none of the clinical groups showed significantly poorer accuracy on any aspect of the Flanker task than the HC group.
ERN amplitude has been shown to be enhanced in most previous studies of adults with OCD (Endrass & Ullsperger, 2014), with some exceptions (Grundler et al., 2009; Hammer et al., 2009; Nieuwenhuis et al., 2005). Amplification of the error response has been theoretically linked to recurring obsessional thoughts and compulsive actions (Pitman, 1987), though neural correlates of clinical symptoms are inconsistent (Endrass & Ullsperger, 2014). Our previously published meta-analysis suggests that ERN enhancement in OCD may be task-dependent (Mathews et al., 2012), with more consistent enhancement effects shown in response-conflict tasks. Despite using a response-conflict task in the current study, we did not find a significant enhancement of the ERN in the HD+OCD patients relative to HD only patients, nor did we find a significant ERN enhancement in OCD patients relative to HC. The fact that HD+OCD participants had ERN amplitudes that were very similar to the HD participants is particularly striking, as, based on the previous OCD literature, co-occurring OCD would be expected to attenuate the observed differences between these patient groups. While evidence from literature examining reliability of the ERN across various response conflict tasks have demonstrated the Flanker task to be consistently more reliable and stable relative to other response conflict tasks in OCD samples (Riesel et al., 2013), there remains much task-specific variance which may lead to unique influences on error-related brain activity across individuals that are specific to the sample within this study (Grundler et al., 2009; Nieuwenhuis et al., 2005; Riesel et al., 2013).
For example, our study systematically varied the probability of an incongruent trial in the flanker task in order to manipulate the degree to which incongruent trials elicited conflict vs. top-down control (Bartholow et al., 2005; van Veen & Carter, 2002). When incongruent trials are improbable relative to congruent trials, it is thought that the prepotent tendency to be influenced the by the flankers is enhanced with minimal need to recruit top down control processes to respond correctly. This leads to higher conflict and lower cognitive control when an incongruent trial appears. When incongruent trials are probable relative to congruent trials, it is thought the prepotent tendency to be influenced by the flankers is minimized by additional recruitment of top-down control processes, since the conflict induced by incongruent flankers must be overcome on most trials in order to respond correctly. This leads to relatively lower conflict and higher cognitive control on incongruent trials. In our study, the 25% incongruent condition produced a higher error rate and a faster reaction time than the 75% incongruent condition, consistent with its producing higher conflict and recruiting less cognitive control. However, susceptibility to these effects was similar across the three groups. Compared to controls, neither patient group was more prone to heightened conflict when incongruent trials were infrequent relative to when they were frequent. Similarly, neither patient group was deficient in increasing cognitive control when incongruent trials were frequent, relative to when they were infrequent. In other studies manipulating task difficulty, the amplitude of the ERN decreased with increasing difficulty (Kaczkurkin, 2013; Riesel, Richter, Kaufmann, Kathmann, & Endrass, 2015), and these studies also failed to replicate the ERN enhancement in individuals with OCD in tasks with increasing difficulty. However, the apparently normal ability to modulate the ERN amplitude according to task conditions in the patient groups is superimposed on a substantial overall deficit in the ERN amplitude in response to errors seen only in patients with HD, suggesting that the neural substrates of the ERN itself are deficient while the neural inputs that modulate the ERN according to task conditions are intact.
The broad age range represented in our HC sample allowed us to examine the effects of normal aging on error monitoring in healthy adults and to control for this when addressing whether pathological age relationships are evident in either HD or OCD. Early studies have shown smaller ERN amplitudes in older adults relative to younger adults (Falkenstein et al., 2001), although later studies have not identified a relationship between age and ERN amplitude (Friedman et al., 2009). In line with the later studies, we did not observe any significant age-related effects on ERN in our HC participants. However, we did find that the reduced ERN amplitude observed in HD was most prominent in younger participants, and tended to normalize (i.e., become more negative) in older participants. Although younger HD participants were more likely to have OCD, and higher YBOCS scores were associated with younger age in the HD sample, the change in the degree of ERN abnormality with age is not likely to be due to co-occurring OCD symptoms, as the ERN was not attenuated in individuals with OCD, and we did not observe age-related ERN changes in the OCD group. Although requiring further study, the observed group differences in ERN abnormalities with age where only individuals with HD showed age-related ERN trajectories provide further evidence for a pathophysiological distinction between HD and OCD.
Our results converge with the small number of recent neuroimaging studies that have directly compared individuals with HD and OCD and found differential neural abnormalities between these clinical populations (Tolin et al., 2012; Tolin et al., 2014). The current findings also corroborate several studies predating DSM-5 that support a neurobiological distinction between OCD-affected individuals with and without hoarding symptoms (An et al., 2008; Mathews et al., 2012; Saxena et al., 2004). We and others have previously suggested that, for individuals with HD, fear of making an error may lead to an inability to decide what to discard, and ultimately result in hoarding (Mathews et al., 2012). While we did not observe a relationship between the ERN and specific HD or OCD symptoms, Reisel et al (Riesel, Endrass, Auerbach, & Kathmann, 2015) recently demonstrated the stability of the error monitoring system by comparing OCD patients before and after symptom reduction through cognitive-behavioral therapy (CBT). OCD patients consistently displayed abnormal ERN patterns, even after post-intervention symptom reduction. Given that the neural substrates of OCD have been characterized by a hyperactivity of the orbitofrontal-striatal loop (Rotge et al., 2008), and that orbitofrontal cortex and anterior cingulate cortex (ACC) show dissociable roles in decision-making (Luk & Wallis, 2013), it may be that the ACC (strongly supported as the focal neural generator of the ERN (W.J. Gehring et al., 1993; van Veen & Carter, 2002)) may also play a differential role in the pathophysiology of HD and OCD. As suggested by Weinberg et al (2015), the interactions between patient comorbidities may present distinct pathophysiological profiles due to complex differential activation of the ACC. The ERN may reflect variation in the strength of these inputs based on these transdiagnostic classifications (Weinberg et al., 2015).
The diverging ERN patterns seen between individuals with HD and OCD, and the deficient error monitoring system in HD relative to non-HD individuals, indicate that ERN abnormalities may ultimately provide a useful biomarker for establishing diagnostic specificity between OCD and HD, as previously suggested (Riesel, Endrass, Kaufmann, & Kathmann, 2011). Although symptoms often begin in adolescence, HD is typically diagnosed late in life, and symptoms appear to progressively worsen over time (Ayers et al., 2010; Grisham et al., 2006). Interestingly, in the present study, it was evident that the younger HD patients demonstrated the greatest degree of ERN abnormalities. Therefore, it may be that the pathophysiological error monitoring system observed in HD is less related to symptom severity, but instead may be an underlying biomarker of the illness, as Carrasco et al. (2012) suggested in a sample of OCD youth and their unaffected siblings. While there have not yet been reports of evidence of an ERN biomarker predating hoarding symptoms, biomarker identification in young individuals who are at-risk for HD could facilitate early diagnosis and have implications for treatment, as the standard treatments for OCD (SSRIs and behavioral therapy) are generally less effective for HD; treatments that are more specific to HD are currently being developed (Ayers et al., 2013).
Limitations
This study has several potential limitations. Although the healthy comparison group was matched to the patient groups on key characteristics, the HD and OCD groups differed on some factors, including gender, age, and rate of antidepressant use. We have controlled for age differences in our analyses, and feel confident that age is not a confound; in fact, the broad age range in our sample allowed us to identify a relationship between age and ERN within the HD group that was not present in the HC or OCD groups. Although the participants differed in antidepressant use, these medications have not been clearly shown to significantly affect the ERN, either in OCD patients or in healthy individuals (de Bruijn, Sabbe, Hulstijn, Ruigt, & Verkes, 2006; Stern et al., 2010). Given the divergent patterns between OCD and HD, future studies should carefully control for co-occurring OCD and perhaps also for antidepressant use. Additionally, the majority of HD participants were female; thus, we cannot say with confidence whether the same ERN effects are present in males with HD. Finally, the present study included small sample sizes. Further studies that include larger samples may better define the neurophysiological characteristics of comorbid HD+OCD individuals, taking into account our unexpected finding that ERN amplitudes were more similar to HD amplitudes than to OCD amplitudes.
In conclusion, this is the first study to directly examine an electrophysiological measure of error monitoring in individuals with primary Hoarding Disorder. As expected, we found a reduced ERN amplitude in HD patients relative to individuals without HD. Of note, the ERN was significantly attenuated in HD patients relative to OCD patients. We also found a relationship between age and ERN amplitude in the HD group that was not evident in the OCD or HC group. These results add to the emerging evidence that, although they may co-occur, HD and OCD have distinct pathophysiologies. Dissociable HD and OCD error-related brain activity may inform future studies of the pathophysiological mechanisms and aging trajectories across these two illnesses, and perhaps, further the understanding of the divergent courses of illness for these complex disorders.
Supplementary Material
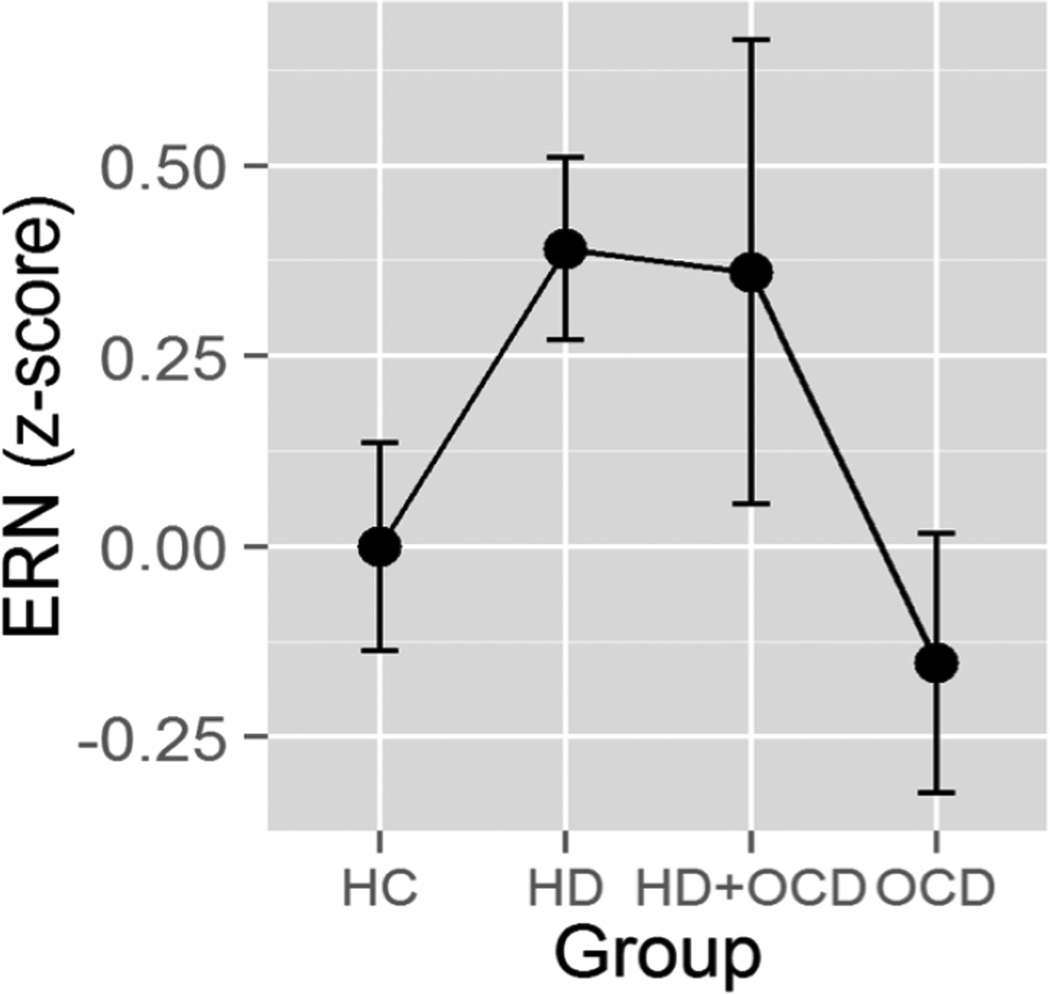
Figure 2.
Mean (± standard errors) ERN amplitude age-corrected z-scores, averaged across probability and electrode in incongruent trials, plotted for each group. Note larger age-corrected ERN deficits (i.e., less negative ERN) in HD positive individuals relative to HC and OCD participants. HC = Healthy Controls; HD = Hoarding Disorder; HD+OCD = comorbid Hoarding Disorder and Obsessive-Compulsive Disorder; OCD = Obsessive-Compulsive Disorder.
Acknowledgments
Financial Disclosures and Acknowledgments. This study was supported by the National Institutes of Mental Health (CAM: R21MH087748) and by the Althea Foundation. Dr. Mathalon has served as a consultant for Amgen and Roche for matters unrelated to this study. Dr. Mathews, Dr. Perez, Mr. Roach, Ms. Fekri, Mr. Vigil, and Dr. Kupferman report no financial relationships with commercial interests.
References
- American Psychiatric Association, A. Disgnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, et al. To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002129. [DOI] [PubMed] [Google Scholar]
- Ayers CR, Saxena S, Espejo E, Twamley EW, Granholm E, Wetherell JL. Novel Treatment for Geriatric Hoarding Disorder: An Open Trial of Cognitive Rehabilitation Paired with Behavior Therapy. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers CR, Saxena S, Golshan S, Wetherell JL. Age at onset and clinical features of late life compulsive hoarding. Int J Geriatr Psychiatry. 2010;25(2):142–149. doi: 10.1002/gps.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G. Strategic control and medial frontal negativity: beyond errors and response conflict. Psychophysiology. 2005;42(1):33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory. San Antonio: Harcourt Assessment, Inc; 1993. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Hong C, Nienhuis JK, Harbin SM, Fitzgerald KD, Gehring WJ, et al. Increased error-related brain activity in youth with obsessive-compulsive disorder and other anxiety disorders. Neurosci Lett. 2013;541:214–218. doi: 10.1016/j.neulet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- de Bruijn ER, Sabbe BG, Hulstijn W, Ruigt GS, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Res. 2006;1105(1):122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25(50):11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46(7):1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Endrass T, Riesel A, Kathmann N, Buhlmann U. Performance monitoring in obsessive-compulsive disorder and social anxiety disorder. J Abnorm Psychol. 2014;123(4):705–714. doi: 10.1037/abn0000012. [DOI] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biol Psychol. 2010;84(2):257–263. doi: 10.1016/j.biopsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Endrass T, Ullsperger M. Specificity of performance monitoring changes in obsessive-compulsive disorder. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention, Perception, & Psychophysics. 1974;16(1):143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Exp Brain Res. 2001;138(2):258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57(3):287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Cycowicz YM, Horton C. Development of and change in cognitive control: a comparison of children, young adults, and older adults. Cogn Affect Behav Neurosci. 2009;9(1):91–102. doi: 10.3758/CABN.9.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RO, Gross RC. The hoarding of possessions. Behav Res Ther. 1993;31(4):367–381. doi: 10.1016/0005-7967(93)90094-b. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behav Res Ther. 2004;42(10):1163–1182. doi: 10.1016/j.brat.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11(1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. J Exp Psychol Hum Percept Perform. 1988;14(3):331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Frost RO, Steketee G, Kim HJ, Hood S. Age of onset of compulsive hoarding. J Anxiety Disord. 2006;20(5):675–686. doi: 10.1016/j.janxdis.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Norberg MM, Williams AD, Certoma SP, Kadib R. Categorization and cognitive deficits in compulsive hoarding. Behav Res Ther. 2010;48(9):866–872. doi: 10.1016/j.brat.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Grundler TO, Cavanagh JF, Figueroa CM, Frank MJ, Allen JJ. Task-related dissociation in ERN amplitude as a function of obsessive-compulsive symptoms. Neuropsychologia. 2009;47(8–9):1978–1987. doi: 10.1016/j.neuropsychologia.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry. 2008;165(1):116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Tolin DF, Frost RO, Steketee G. An exploration of comorbid symptoms and clinical correlates of clinically significant hoarding symptoms. Depress Anxiety. 2013;30(1):67–76. doi: 10.1002/da.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer A, Kordon A, Heldmann M, Zurowski B, Munte TF. Brain potentials of conflict and error-likelihood following errorful and errorless learning in obsessive-compulsive disorder. PLoS One. 2009;4(8):e6553. doi: 10.1371/journal.pone.0006553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna GL, Carrasco M, Harbin SM, Nienhuis JK, LaRosa CE, Chen P, et al. Error-related negativity and tic history in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(9):902–910. doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Iervolino AC, Perroud N, Fullana MA, Guipponi M, Cherkas L, Collier DA, et al. Prevalence and Heritability of Compulsive Hoarding: A Twin Study. Am J Psychiatry. 2009 doi: 10.1176/appi.ajp.2009.08121789. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, et al. Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Res. 2001;108(2):101–110. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN. The effect of manipulating task difficulty on error-related negativity in individuals with obsessive-compulsive symptoms. Biol Psychol. 2013;93(1):122–131. doi: 10.1016/j.biopsycho.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Steketee G, Frost RO. Hoarding by elderly people. Health Soc Work. 2001;26(3):176–184. doi: 10.1093/hsw/26.3.176. [DOI] [PubMed] [Google Scholar]
- Klawohn J, Riesel A, Grutzmann R, Kathmann N, Endrass T. Performance monitoring in obsessive-compulsive disorder: a temporo-spatial principal component analysis. Cogn Affect Behav Neurosci. 2014;14(3):983–995. doi: 10.3758/s13415-014-0248-0. [DOI] [PubMed] [Google Scholar]
- Leonowicz Z, Karvanen J, Shishkin SL. Trimmed estimators for robust averaging of event-related potentials. J Neurosci Methods. 2005;142(1):17–26. doi: 10.1016/j.jneumeth.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Luk CH, Wallis JD. Choice coding in frontal cortex during stimulus-guided or action-guided decision-making. J Neurosci. 2013;33(5):1864–1871. doi: 10.1523/JNEUROSCI.4920-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24(2):495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Billotti D, Fernandez de la Cruz L, Nordsletten AE. The London field trial for hoarding disorder. Psychol Med. 2012:1–11. doi: 10.1017/S0033291712001560. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Nievergelt CM, Azzam A, Garrido H, Chavira DA, Wessel J, et al. Heritability and clinical features of multigenerational families with obsessive-compulsive disorder and hoarding. Am J Med Genet B Neuropsychiatr Genet. 2007;144(2):174–182. doi: 10.1002/ajmg.b.30370. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Perez VB, Delucchi KL, Mathalon DH. Error-related negativity in individuals with obsessive-compulsive symptoms: Toward an understanding of hoarding behaviors. Biol Psychol. 2012 doi: 10.1016/j.biopsycho.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Riesel A, Hajcak Proudfit G. Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology. 2013;50(12):1220–1225. doi: 10.1111/psyp.12132. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Papmeyer M, Pertusa A, Chamberlain SR, Fineberg NA, Sahakian BJ, et al. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res. 2014;215(3):659–667. doi: 10.1016/j.psychres.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Nielen MM, Mol N, Hajcak G, Veltman DJ. Performance monitoring in obsessive-compulsive disorder. Psychiatry Res. 2005;134(2):111–122. doi: 10.1016/j.psychres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MG, Holroyd CB, Kok A, et al. A computational account of altered error processing in older age: dopamine and the error-related negativity. Cogn Affect Behav Neurosci. 2002;2(1):19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46(5):957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, et al. Error monitoring dysfunction across the illness course of schizophrenia. J Abnorm Psychol. 2012;121(2):372–387. doi: 10.1037/a0025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertusa A, Fullana MA, Singh S, Alonso P, Menchon JM, Mataix-Cols D. Compulsive Hoarding: OCD Symptom, Distinct Clinical Syndrome, or Both? Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07111730. [DOI] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry. 1987;28(4):334–343. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu CT, Themanson JR, et al. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47(4):767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Auerbach LA, Kathmann N. Overactive Performance Monitoring as an Endophenotype for Obsessive-Compulsive Disorder: Evidence From a Treatment Study. Am J Psychiatry. 2015;172(7):665–673. doi: 10.1176/appi.ajp.2014.14070886. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am J Psychiatry. 2011;168(3):317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Kathmann N, Endrass T. Overactive performance monitoring in obsessive-compulsive disorder is independent of symptom expression. Eur Arch Psychiatry Clin Neurosci. 2014;264(8):707–717. doi: 10.1007/s00406-014-0499-3. [DOI] [PubMed] [Google Scholar]
- Riesel A, Richter A, Kaufmann C, Kathmann N, Endrass T. Performance monitoring in obsessive-compulsive undergraduates: Effects of task difficulty. Brain Cogn. 2015;98:35–42. doi: 10.1016/j.bandc.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biol Psychol. 2013;93(3):377–385. doi: 10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, et al. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. 2008;33(5):405–412. [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. Developmental differences in error-related ERPs in middle- to late-adolescent males. Dev Psychol. 2008;44(1):205–217. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Dev Neuropsychol. 2006;29(3):431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Saxena S. Is compulsive hoarding a genetically and neurobiologically discrete syndrome? Implications for diagnostic classification. Am J Psychiatry. 2007;164(3):380–384. doi: 10.1176/ajp.2007.164.3.380. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Baxter LR., Jr Paroxetine treatment of compulsive hoarding. J Psychiatr Res. 2007;41(6):481–487. doi: 10.1016/j.jpsychires.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 2004;161(6):1038–1048. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, et al. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Frost RO, Steketee G. An open trial of cognitive-behavioral therapy for compulsive hoarding. Behav Res Ther. 2007;45(7):1461–1470. doi: 10.1016/j.brat.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Stevens MC, Villavicencio AL, Norberg MM, Calhoun VD, Frost RO, et al. Neural mechanisms of decision making in hoarding disorder. Arch Gen Psychiatry. 2012;69(8):832–841. doi: 10.1001/archgenpsychiatry.2011.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Witt ST, Stevens MC. Hoarding disorder and obsessive-compulsive disorder show different patterns of neural activity during response inhibition. Psychiatry Res. 2014;221(2):142–148. doi: 10.1016/j.pscychresns.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14(4):347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Dieterich R, Riesel A. Error-related brain activity in the age of RDoC: A review of the literature. Int J Psychophysiol. 2015 doi: 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.