Abstract
Melanoma is molecularly and structurally heterogeneous, with some tumor cells existing under hypoxic conditions. Our cell growth assays showed that under controlled hypoxic conditions, BRAF(V600E) melanoma cells rapidly became resistant to vemurafenib. By employing both a three-dimensional (3D) spheroid model and a two-dimensional (2D) hypoxic culture system to model hypoxia in vivo, we identified upregulation of HGF/MET signaling as a major mechanism associated with vemurafenib resistance as compared to 2D standard tissue culture in ambient air. We further confirmed that the upregulation of HGF/MET signaling was evident in drug-resistant melanoma patient tissues and mouse xenografts. Pharmacologic inhibition of the c-Met/Akt pathway restored the sensitivity of melanoma spheroids or 2D hypoxic cultures to vemurafenib.
Keywords: Vemurafenib resistance, hypoxia, HGF/MET, 3D spheroid, melanoma
Introduction
Current research efforts have resulted in a better understanding of the genetic complexity of melanoma. Notably, somatic mutations of kinases BRAF and NRAS are present in about 60% and 20% of melanomas (1). A single substitution at V600E (valine to glutamate) accounts for 80% of BRAF mutations in malignant melanoma. Mutated BRAF(V600E) protein has elevated kinase activity and can promote tumor growth and resistance to apoptosis, which led to the development of the potent BRAF(V600E) inhibitor, vemurafenib (PLX4032) (2). Multiple clinical trials of vemurafenib in metastatic melanoma patients with BRAF(V600E) mutations demonstrated a pronounced 80% antitumor response rate (3, 4). Surprisingly, responsive patients’ tumors rapidly and frequently developed resistance to vemurafenib (3, 4).
Aberrant upregulation of PDGFR-β, KIT, MET, EGFR, and MAP3K8 (the gene encoding COT/Tpl2), and dimerization of abnormally spliced BRAF(V600E), have been observed in some PLX4032-resistant melanoma cells (5–8). These studies strongly suggest that the elevated expression of a cluster of kinases is associated with the acquired resistance to BRAF(V600E)-inhibitors in malignant melanoma. Stromal cell secretion of hepatocyte growth factor (HGF) also activates the HGF receptor, c-Met (cellular-mesenchymal to epithelial transition factor), and may contribute to PLX4032 resistance in melanoma (9). On the basis of these data, clinical studies have been proposed to treat melanoma patients with the combination of PLX4032 with MEK or other kinase inhibitors (8, 10). Clinical studies showed that combining mutated BRAF inhibitors (dabrafenib or vemurafenib) and the MEK inhibitors (trametinib or cobimetinib), as compared with BRAF inhibitor alone, significantly enhanced about 8% of overall survival rate in previously untreated patients with metastatic melanoma with BRAF mutations (8, 10). However, these approaches are yet to report significant clinical outcome on preventing or delaying the onset of resistance observed with BRAF inhibitors alone. It is well known that tumor heterogeneity contributes to drug resistance and tumor relapse. The structure of melanoma tumor is highly heterogeneous in vivo compared to standard two-dimensional (2D) cell cultures in CO2 buffered ambient air. As in most solid tumors, some melanoma cells exist under hypoxia in vivo, whereas others survive in regions with increased oxygenation consistent with the normal tissue microenvironment. Numerous studies have shown that tumor hypoxia is an independent prognostic factor for disease progression (11). Hypoxia, as defined as 1% oxygen or lower, induces a wide range of biological changes in tumors, such as increasing the expression of drug-resistance genes, selection of apoptosis-resistant clones, and metastasis (11). However, it is still not clear whether hypoxia specifically contributes to PLX4032 resistance in melanoma. In this study, we hypothesize that melanoma cells survive under hypoxic conditions due to increased expression of uniquegrowth and survival pathways including those that alter their sensitivity to PLX4032.
To date, very few studies have addressed the effect of heterogeneous tumor three-dimensional (3D) architecture on drug resistance in melanoma, particularly using an in vitro model to reproduce the heterogeneity of hypoxic regions. To test this, we employed a 3D model to grow melanoma spheroids with hypoxic centers under standard culture conditions. Based on this model, we identified the hypoxia-driven upregulation of HGF/MET signaling as one pathway responsible for attenuating PLX4032 activity in melanoma cells. We further demonstrated the trend of aberrant upregulation of HGF/MET signaling in drug-resistant melanoma patient tissues and mouse xenografts. Our studies provide valuable insights into the mechanism of vemurafenib resistance and developing more effective treatment strategies to overcome drug resistance in malignant melanoma.
Materials and Methods
Antibodies and reagents
PLX4032 (vemurafenib) was purchased from Selleckchem (Houston, TX) and was dissolved in dimethyl sulfoxide (DMSO) as 100 mM stock. The c-MET specific inhibitor MSC2156119J (Tepotinib, EMD 1214063) was provided by EMD Serono (Rockland, MA) as part of a research collaboration. Structure of MSC2156119J was shown in the supplementary Figure S1. The 4–15% gradient acrylamide gels for Western blot analyses were purchased from Bio-Rad Laboratories (Hercules, CA). Antibodies for human p53, phosphorylated p53, Akt, phosphorylated Akt (Thr308, C31E5E), and c-Met were purchased from Cell Signaling Technology (Danvers, MA). The antibody for human HIF-1α (#610958) was purchased from BD Biosciences (San Jose, CA). Antibodies for human VEGF and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and phosphorylated Met (pY1003, 44-882G) was purchased from Invitrogen (Life Technologies, Grand Island, NY). Neutralizing anti-HGF antibody (MAB294) was purchased from R&D Systems.
Melanoma Cell lines and 2D cultures under hypoxic and standard ambient air conditions
Human BRAF(V600E) melanoma cells, A375, were purchased from American Type Culture Collection (Manassas, VA) in 2013. Human BRAF(V600E) melanoma cells 451Lu and MEL1617 were generously provided by Dr. Meenhard Herlyn (The Wistar Institute, Philadelphia, PA). All three melanoma cell lines were validated via short tandem repeat DNA fingerprinting using the AmpF/STR Identifiler PCR Amplification Kit according to the manufacturer’s instructions (cat 4322288; Applied Biosystems, Foster City, CA), and the analysis was performed by the Characterized Cell Line Core Facility at The University of Texas MD Anderson Cancer Center in September 2014. For 2D monolayer cell cultures with ambient air, melanoma cells were grown in Dulbecco’s modified Eagle medium supplemented with 5% fetal bovine serum, 100 μg/mL glutamine, 100 units/mL penicillin, and 100 units/mL streptomycin (Invitrogen). All cells were grown at 37°C in an atmosphere of 5% CO2 and normal O2 levels (ambient air, ~ 21% O2). For 2D hypoxic cultures, melanoma cells were seeded in culture dishes and placed in a hypoxia chamber under a stable hypoxic environment of 5% CO2, 94% N2, and 1% O2.
3D spheroid culture and application
The inorganic nanoscale scaffolding NanoCulture Plates (NCPs) were purchased from SCIVAX (Woburn, MA). The base of each NCP is constructed with a transparent cycloolefin resinous sheet with a nanoscale indented pattern. 451Lu, A375, or MEL1617 cells were seeded in 24-well NCPs at 4×103 cells/well to form spheroids. The treatment of NCPs before seeding the cells and the culture conditions for the formation of melanoma spheroids were accomplished according to the manufacturer’s protocols (SCIVAX). The NCPs seeded with melanoma cells were incubated in a conventional cell incubator at 37°C in an atmosphere of 5% CO2 and normal O2 levels. The hypoxia probe LOX-1 was also purchased from SCIVAX and dissolved in DMSO to make 1 mmol/L stock solution. The LOX-1 stock solution was diluted with RPMI medium to prepare 4 μmol/L working solution just before use. The LOX-1 working solution was added to the NCPs at a final concentration of 2 μmol/L. After culturing for one day, red phosphorescence was measured via general fluorescent microscopy (Nikon ECLIPSE TS100, G-2A filter block: Ex 510-560, DM575, BA590). On day 3 after melanoma cells being seeded on NCPs, visible spheroids started to form. The formation of spheroids was confirmed via microscopy, and all the spheroids were treated with various concentrations of PLX4032 and/or MSC2156119J as indicated in result section and figures. After drug treatment for 72 h, the cultures were subjected to MTT assay. Immunostaining of 3D cultured spheroids was conducted following the standard protocol of SCIVAX. The dilution of HIF-1α antibody was 1:100.
Western blot analysis
Cells were lysed in buffer containing 50 mM Tris (pH, 7.9), 150 mM NaCl, 1% NP-40, 1 mM EDTA, 10% glycerol, 1 mM sodium vanadate, and protease inhibitor cocktail (Roche, Indianapolis, IN). Proteins were separated via electrophoresis on 4–15% gradient polyacrylamide gels with sodium dodecyl sulfate, transferred to a Hybond electrochemiluminescence (ECL) nitrocellulose membrane (GE Healthcare Biosciences, Piscataway, NJ), and blocked in 5% bovine serum albumin in PBS solution. The membrane was then incubated with primary and secondary antibodies, and target proteins were detected via ECL detection reagent (GE Healthcare Biosciences).
Human phospho-kinase array
The human phospho-kinase antibody array was purchased from R&D Systems. The protein lysates for melanoma spheroids and 2D cultures under ambient air were prepared as described in previous sections. Lysates for each sample (300 μg protein) were subjected to the human phospho-kinase array according to the manufacturer’s protocol (R&D Systems). The signals of blots were developed on films, which were scanned on Kodak Image Station 4000R to determine the average signal (pixel density) of the pair of duplicate spots representing each phosphorylated kinase protein. The clear area of the array was used as background, and the averaged background signal was subtracted from each spot. The averaged signal of internal positive controls was used to normalize each spot to determine and compare the relative change in phosphorylated kinase proteins between different samples.
Cell proliferation and viability assays
Human melanoma cells were plated in 24-well plates at 1 × 104 cells/well and cultured for 24 h before treatment. For MTT assay, cells were treated with drugs for 72 h, and then MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added to the cells to a final concentration of 1 mg/mL. After 3 h, the precipitate formed in the cells was dissolved in DMSO, and the color intensity was measured using an MRX revelation microplate absorbance reader (Dynex Technologies, Chantilly, VA) at 570 nm. As the additional cell growth assay, melanoma cells treated with drugs for 72 h were trypsinized and harvested. The cells were washed with 1× phosphate-buffered saline (PBS) and then diluted in 0.2% trypan blue. The number of viable cells was determined. Each experiment was carried out three times, and the means were used to determine IC50 values. Melanoma cells without any drug treatment for each of three culturing conditions, 2D culture under ambient air, 2D hypoxic culture, or 3D spheroids served as the standard control (100%) individually to compare the cell viability and survival of drug treated cells under that same condition. Data from drug assays were modelled using a nonlinear regression curve fit with a sigmoid dose–response to generate by using GraphPad Prism 6 for Windows, and the 50% inhibition point at were annotated IC50 for drug treatments. All data were analyzed by independent-Sample t-Test. Values between groups were compared by the Student’s t-test, after ANOVA analyses. All experiments were repeated and P ≤ 0.05 was considered statistically significant.
Reverse-transcription PCR (RT-PCR) and real-time PCR analyses
The RT-PCR and real-time PCR primers used in this study are listed in supplementary Table S1. Total cellular RNA of melanoma cells was extracted by using a NucleoSpin RNA II kit (Macherey-Nagel, Bethlehem, PA). First-strand cDNA synthesis was performed with 500 ng of total RNA using an iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s protocol. A 2-μL cDNA product was used for each 20-μL RT-PCR or real-time PCR. The PCR protocol consisted of initial denaturation at 95°C for 5 min; 30 cycles of 95°C for 40 s, 55.5°C for 30 s, and 72°C for 60 s; primer extension at 72°C for 1 min; and a final extension at 72°C for 10 min. We analyzed 20 μL of PCR product on a 1.5% agarose gel. Quantitative real-time PCR was carried out using SYBR Green Mastermixes on a MasterCycler RealPlex4 (Eppendorf).
Animals and in vivo tumor experiments
Experiments were performed using adult female non-obese diabetic/severe combined immunodeficient mice (ages 6–8 weeks, Animal Production Area of the National Cancer Institute-Frederick Cancer Research and Development Center). The mice were housed in an air-conditioned and pathogen-free environment with constant temperature (25°C), a standardized light/dark schedule, and food and water. Every effort was made to minimize the number of animals used and their suffering. All of the animal procedures were carried out according to the protocol approved by MD Anderson’s Institutional Animal Care and Use Committee. For these xenograft experiments, mice were injected with human A375 cells (5×106 cells in 100 μL PBS). After tumors were established, the animals were randomly assigned to experimental groups. When the tumors reached >225 mm2, they were harvested to prepare formalin-fixed paraffin-embedded tissue slides, and sections were cut for immunohistochemistry (IHC). For drug treatment, experimental mice received PLX4032 on day 7 when their tumors reached around 40 mm2. After initial tumor shrinkage in response to PLX4032 treatment for 2 weeks, tumor recurrence was noted at the same sites.
Patient samples
The paraffin-embedded human melanoma tissue slides were provided by the Melanoma Core Bio and Data Repository (MD Anderson Cancer Center). Patients with metastatic melanoma and BRAF(V600E) mutations provided written informed consent for tissue acquisition according to a protocol approved by MD Anderson’s Institutional Review Board. For patient mRNA expression studies, patients with metastatic melanoma containing BRAF(V600E) mutation (confirmed by genotyping) were enrolled on clinical trials for treatment with a BRAF inhibitor (vemurafenib) or combined BRAF + MEK inhibitor (dabrafenib + trametinib) and were consented for tissue acquisition per IRB-approved protocol. Patient characteristics were shown in supplementary Table S2. Tumor biopsies were performed pre-treatment (day 0) and at time of progression. Formalin-fixed tissue was analyzed to confirm that viable tumor was present via hematoxylin and eosin (H&E) staining. Additional tissue was processed for purification of RNA using the RNeasy Mini Protocol (Qiagen).
Immunohistochemical studies
We used an IHC protocol described previously (12) to detect the levels of HIF-1α, HGF, p-Met, and p-Akt. The IHC dilutions of primary antibodies were as follows: HIF-1a (1:100), HGF (1:50), p-Met (1:100), and p-Akt (Thr308) (1:100). All IHC data were manually evaluated and scored independently by 2 researchers (YQ and SE). Dr. Ekmekcioglu reviewed all IHC data as the blinded observer without prior knowledge of the staining conditions.
The Cancer Cell Line Encyclopedia (CCLE)
The Cancer Cell Line Encyclopedia (CCLE) project was developed by the Broad Institute and Novartis, which contains a detailed genetic and pharmacologic characterization of a large panel of human cancer cell lines (13). Based on the integrated computational analyses provided by CCLE, the potential correlation between distinct pharmacologic vulnerabilities with genomic patterns in certain cell lines can be analyzed. The CCLE data used in this study are available online at the Broad Institute CCLE website (14). In this study, we focused on analyzing data of 35 cutaneous melanoma cell lines provided by CCLE. The list of these cell lines is available in Supplementary Table S3.
Results
Hypoxia and heterogeneity in human melanoma
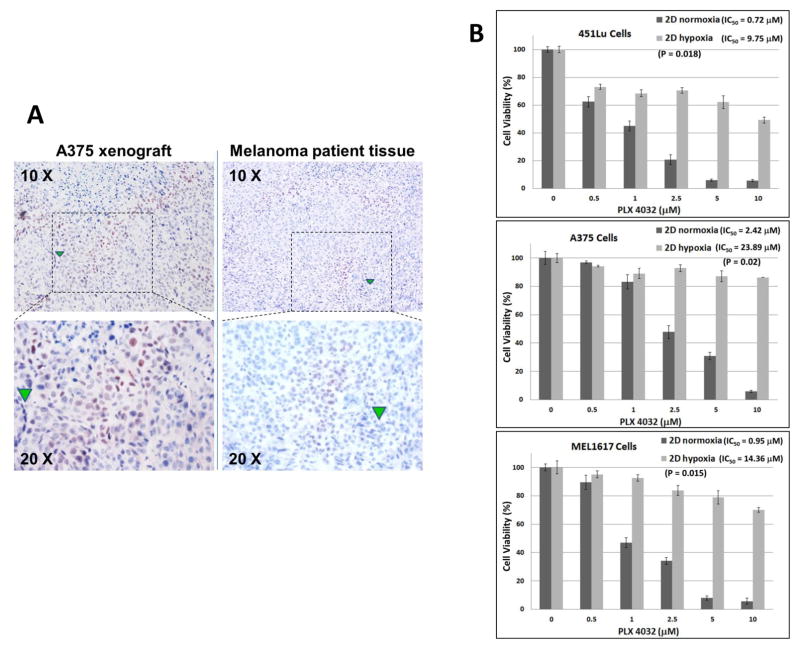
Using IHC, we consistently observed variable expression of hypoxia-inducible factor 1-alpha (HIF-1α), a well-known hypoxia marker, in some areas of A375 mouse xenografts and melanoma patient tumor samples (up to 25% noted in the tissues we assessed) (Figure 1A), indicating these melanoma cells are experiencing hypoxia in vivo. This data confirms that melanoma tissue is molecularly heterogeneous, and non-hypoxic and hypoxic regions co-exist within the same tumor.
Figure 1. Hypoxia in melanoma and growth inhibitory effects of PLX4032 in melanoma cells under hypoxia or ambient air.
(A) Representative IHC staining for HIF-1α in mouse xenograft and melanoma patient tissue. The areas that stained positive for HIF-1α indicated the hypoxic tumor regions. Green triangles indicated the same locations under 10 X and 20 X views.
(B) Dose-dependent inhibition of BRAF(V600E) melanoma cells growth by PLX4032 under normoxia and hypoxia. 451Lu, A375, and MEL1617 cells were treated with PLX4032 at the indicated doses. After 72 h of culture, cell survival was determined via MTT assay. The percent cell survival in each treatment group was calculated relative to cells treated with medium only under the same conditions. As controls, the growth of cells without drug treatment under each condition was normalized as 100% separately. Each experiment was carried out three times, and the means were presented here. Each bar denotes mean ± SD of three experiments. IC50 values were calculated by GraphPad Prism 6. The Student’s t-test was performed to compare IC50 values of the same cell lines under different culture conditions. p ≤ 0.05 was considered statistically significant.
BRAF(V600E) melanoma cells are resistant to PLX4032 under hypoxia
To determine the effect of hypoxia on the efficacy of PLX4032 to inhibit melanoma cell growth, three BRAF(V600E) melanoma cell lines (451Lu, A375, and MEL1617) were treated with variant concentrations of PLX4032 under standard 2D tissue culture of CO2-buffered ambient air or 2D cultures in specialized hypoxia chambers maintained at 1% oxygen. Melanoma cells without drug treatment served as the standard controls (100%), under each conditions, to compare drug effects on cell survival. As shown in Figure 1B, at the concentration of 2.5 μM, PLX4032 inhibited from 60–80% of cell viability in the normoxic cultures of three melanoma cell lines with ambient air. However, in the parallel hypoxic chambers, PLX4032 only inhibited about 10 ~30% of cell viability in all three cell lines. The IC50 for PLX4032 in 2D hypoxic cultures was significantly increased in all three melanoma cell lines compared to relevant 2D cultures with ambient air. The IC50 for PLX4032 in sets of 2D cultures with ambient air ranged from 0.9~2.5 μM, but it increased to 9~24 μM under hypoxic conditions, representing ~10-fold increase (Figure 1B).
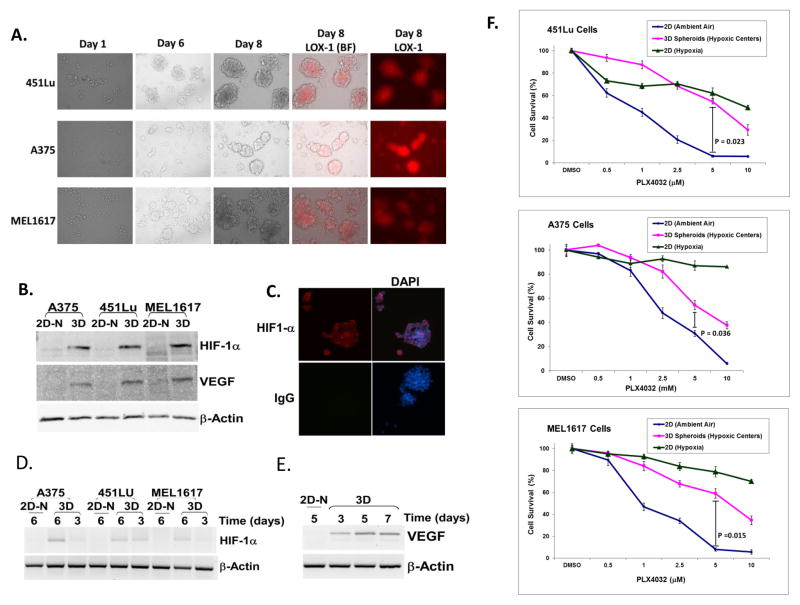
3D human melanoma spheroids are more resistant to PLX4032 treatment than 2D cultures with ambient air, but not 2D cultures under hypoxia
As shown in Figure 1A, melanoma tumor contains a mixture of non-hypoxic and hypoxic regions in vivo. To mimic this heterogeneity in vitro, we applied a 3D model to grow melanoma cells on inorganic nanoscale scaffolding NCPs. Previous studies have shown that multiple cancer cells can naturally form spheroids on NCPs, and the cells at the centers of spheroids are hypoxic (15). All three melanoma cell lines formed spheroids on NCPs in the standard tissue culture under ambient air. By day 6 in the culture plates, large and uniform spheroids with diameters of 100 μm ~ 200 μm consisting of densely packed round melanoma cells were observed (Figure 2A). Tumor cells grown on NCPs were morphologically different from those grown as a 2D monolayer (Supplementary Figure S2).
Figure 2. Formation of melanoma spheroids and their sensitivity to PLX4032 as compared to 2D ambient air cultures.
(A) Formation and morphology of 3D melanoma spheroids on NCPs. 451Lu, A375, or MEL1617 cells were seeded at 4×103 in each well of 24-well NCPs. Periodic analysis showed the formation of human melanoma cell spheroids on NCPs. The majority of melanoma cells in spheroids were identified as hypoxic, as indicated by the specific hypoxic probe LOX-1. BF = Brightfield. LOX-1(BF) = merging images of LOX-1 and BF.
(B) The formation of hypoxic centers in human melanoma spheroids induced the expression of HIF-1α and VEGF. We subjected 50 μg of protein lysates from melanoma 2D cultures with ambient air or 3D spheroids (7 days) to Western blotting for HIF-1α and VEGF. HIF-1α and VEGF were dramatically increased in spheroids compared to 2D cultures with ambient air. 2D-N = 2D cultures with ambient air.
(C) The immunofluorescent staining of HIF-1α in A375 spheroids. The high levels of HIF-1α indicate the formation of hypoxic centers in melanoma spheroids. DAPI staining indicates the location of nuclei.
(D) The expression of HIF-1α mRNA on days 3 and 6 of melanoma spheroids compared to 2D ambient air cultures.
(E) The RT-PCR analysis of VEGF expression in A375 spheroids. The induction of VEGF was observed from A375 cells cultured on NCPs for 3, 5, and 7 days, but the VEGF mRNA was undetectable in 2D ambient air cultures.
(F) The viability of 2D ambient air cultures and spheroids in three melanoma cells treated with various concentration of PLX4032. On day 4, the formation of melanoma spheroids on NCPs was confirmed by microscopy, and then they were treated with different concentrations of PLX4032. After drug treatment for 72 h, the spheroids were subjected to MTT assay. The 2D monolayer cultures of three melanoma cells under hypoxic or ambient air conditions were also subjected to PLX4032 treatment for 72 h. The Student’s t-test was performed to compare cell survival at 5 μM drug concentration. p ≤ 0.05 was considered statistically significant.
Although our melanoma spheroids were cultured under conventional conditions with ambient air, the cells at the center of spheroids were confirmed as hypoxic, indicated by distinct red fluorescent staining of hypoxic probe LOX-1 (Iridium compound) (Figure 2A), which is quenched by oxygen and increases in response to low levels of oxygen (16). In contrast, melanoma cells grown as monolayer cultures under standard ambient air conditions did not show red fluorescent staining of LOX-1 (Supplementary Figure S3). Moreover, the expression of hypoxia markers HIF-1α and VEGF was positive in melanoma spheroids (Figure 2 B–E), but was extremely low or undetectable in control 2D cultures with ambient air (Figure 2 B and D). Interestingly, the expression of low levels HIF-1α and VEGF mRNA was identified in melanoma cells cultured on NCPs as early as 3 days, which was the time when spheroids started to be detected visually (Figure 2D and supplementary Figure S4). This observation suggests that the formation of spheroids also drives the formation of hypoxic centers within them. After day 6 of culture, melanoma cells on NCPs formed larger spheroids, which contained larger hypoxic centers (Figure 2A and supplementary Figure S4). Compared to 3 days cultures, the spheroids that were cultured on NCPs for more than 5 days, expressed high levels of HIF-1α and VEGF (Figure 2 D and E). The expression of hypoxia-relevant genes, such as HIF-1α and VEGF, further confirms the formation of hypoxic centers within melanoma spheroids. Herein, with the NCP system, we established an in vitro 3D model to grow melanoma cells as spheroids containing hypoxic centers under standard ambient air culture conditions, a system available to most laboratories.
We further investigated the sensitivity of melanoma spheroids to PLX4032 compared to parallel 2D monolayer cultures under ambient air or hypoxia conditions. The cell viability assays showed that melanoma spheroids were 2~5 times less sensitive to PLX4032 than respective 2D ambient air cultures (Figure 2F). As shown in Figure 2F, 5 μM PLX4032 inhibited 20~40% of cell viability in all three melanoma cell lines cultured under hypoxia, but it inhibited about 40 ~ 50% of cell viability in the same cell spheroids. This phenomenon may be due to the fact that the melanoma cells on the surface of spheroids are not hypoxic, and are sensitive to PLX4032. Therefore, the melanoma spheroid model could be used to model a heterogeneous 3D structure with melanoma cells in a hypoxic center and surrounded by a layer of non-hypoxic melanoma cells exposed to ambient air culturing environment.
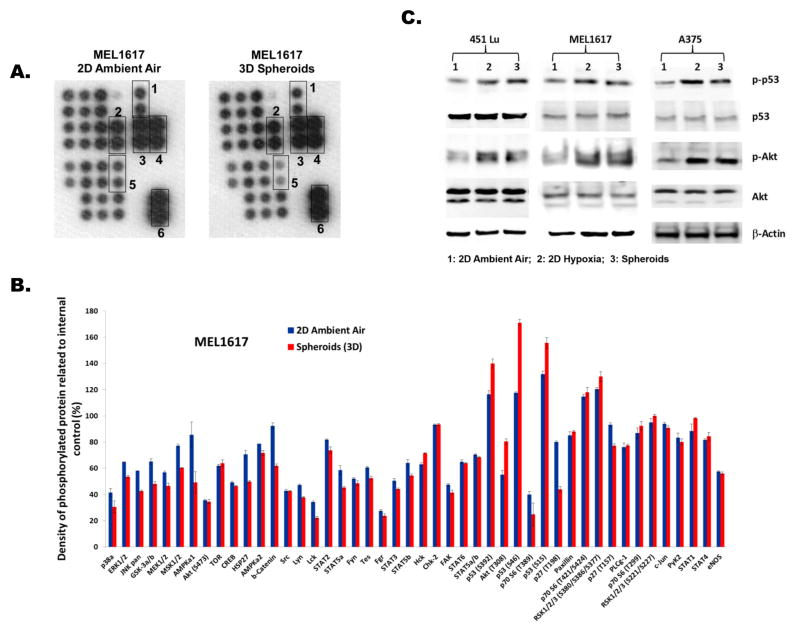
Phosphorylation of Akt and p53 are upregulated in 3D spheroids
To identify the crucial signaling pathways responsible for PLX4032 resistance in melanoma spheroids, we employed a human phospho-kinase array to compare multiple kinase pathways between melanoma spheroids versus 2D standard cultures under ambient air (Figure 3A, supplementary Figure S5 and S6). As demonstrated in MEL1617 cells, levels of phosphorylated AMPKa1, b-Catenin, and p27(T198) were substantially lower in spheroids compared to 2D cultures under ambient air (Figure 3 A and B). Higher levels of phosphorylated p53 (S392, S46, and S15) and AKT (T308) (25~50%) were observed in melanoma spheroids compared to 2D cultures with ambient air (Figure 3A &B). Similar results were also observed in the kinase arrays for the spheroids and 2D cultures with ambient air for A375 and 451Lu cells (supplementary Figure S5 and S6). Since aberrantly upregulated kinase signaling has the potential to support the growth of hypoxic melanoma cells in spheroids, we were interested in evaluating the levels of phosphorylated p53 and Akt (p-53 and p-Akt) in melanoma cells under hypoxic conditions by Western blotting (Figure 3C). Our data clearly demonstrates that p-p53 and p-Akt were upregulated in melanoma spheroids and in 2D hypoxic cultures versus 2D standard cultures under ambient air. Thus, the upregulation of p-Akt and p-p53 in hypoxic BRAF(V600E) melanoma cells may partly represent the hypoxia-driven signaling pathways responsible for resisting the cytotoxic effects of PLX4032, and suggests further studies of these pathways are warranted.
Figure 3. Comparison of kinase signaling between melanoma 3D spheroids and 2D ambient air cultures.
(A) The human phospho-kinase antibody array of MEL1617 spheroids and 2D cultures with ambient air. The duplicate spots in boxes represent: 1. Akt(T308); 2. p53(S15); 3. p53(S46); 4. p53(S392); 5. p27(T198); and 6. reference spots (internal positive controls).
(B) Relative changes in phosphorylated kinase proteins between melanoma 3D spheroids and 2D ambient air cultures. Error bar denotes mean ± SD of replicated samples.
(C) Western blot analyses of p53, p-p53, Akt, and p-Akt levels in 2D ambient air cultures, 2D hypoxic cultures, and 3D spheroids of three melanoma cell lines. β-actin severed as the loading control.
HGF/MET signaling regulates AKT activation and is upregulated in hypoxic melanoma cells
Directly targeting p-Akt as an effective therapeutic strategy in melanoma is challenging due to the broad biological function of Akt and its intermediate position in the kinase cascade. Thus, we hypothesized that selective upstream factors responsible for activating Akt may represent potential targets for preventing hypoxia-driven drug resistance. In previous studies, we found that the HGF/c-Met pathway leads to the activation of Akt signaling in the NRAS-mutated subset of melanoma cell lines (17). Therefore, we conducted studies to determine whether c-Met signaling may be involved in the upregulation of Akt under hypoxia.
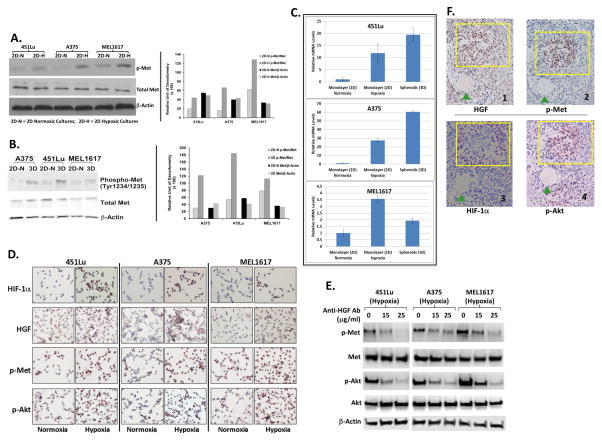
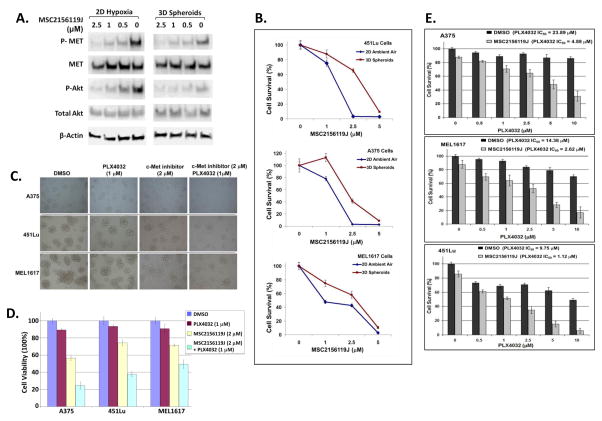
The levels of phosphorylated c-Met (p-Met) were higher in 2D hypoxic cultures (Figure 4A) and in 3D spheroids (Figure 4B) than in respectively 2D standard cultures under ambient air for all three melanoma cell lines tested. These findings suggest that c-Met activation was upregulated in hypoxic melanoma cells. Real-time PCR analyses (Figure 4C) revealed that the levels of HGF mRNA were significantly higher in 2D hypoxic cultures or spheroids than in 2D standard ambient air cultures for all three melanoma cell lines. The upregulation of HGF, p-Akt, and p-Met in melanoma cells under hypoxia conditions compared with respective 2D cultures with ambient air was confirmed via IHC data in Figure 4D, which showed distinct increases of staining insensitivity for these three markers in hypoxic cultures compared to cultures under ambient air. The induction of HIF-1α expression indicated hypoxic conditions were achieved in these cells within 72-hours. We next sought to determine whether HGF/MET signaling contributes to the activation of Akt in hypoxic melanoma cells by using a neutralizing antibody against HGF. Indeed, the addition of 15 μg/ml and 25 μg/ml anti-HGF antibodies led to consistent decreases in p-Met and p-Akt in 2D hypoxic melanoma cell cultures (Figure 4E), confirming that the HGF/MET pathway is an upstream signaling pathway regulating Akt activation under hypoxic conditions.
Figure 4. Upregulation of HGF/MET signaling in hypoxic melanoma cells and spheroids.
(A) Western blot analyses to compare p-Met levels between 2D cultures under hypoxia and ambient air in 451Lu, A375, and MEL1617 cells. Right panel: relative densitometries of p-Met to total Met and total Met to β-actin. 2D-N = 2D cultures with ambient air; 2D-H = 2D hypoxic cultures.
(B) Western blot analyses to compare p-Met levels between spheroids and 2D ambient air cultures in 451Lu, A375, and MEL1617 cells. Right panel: relative densitometries of p-Met to total Met and total Met to β-actin. 3D = 3D spheroids.
(C) Real-time PCR analyses to demonstrate the upregulation of HGF mRNA in 2D hypoxic cultures and spheroids compared to respective 2D ambient air cultures.
(D) IHC analyses to demonstrate the upregulation of protein levels of HIF-1α, HGF, p-Akt, p-Met in 2D hypoxic cultures compared to respective 2D ambient air cultures.
(E) Western blot analyses of p-Met and p-Akt (T308) levels in 2D hypoxic cultures of 451Lu, A375, and MEL1617 cells treated with HGF neutralizing antibodies.
(F) IHC staining of HIF-1α, HGF, p-Akt, and p-Met in the subsequent tissue sections cut from the same tumor. The yellow rectangles indicate similar regions of tumor. The HIF-1α-positive tumor region was also positive for p-Akt, HGF and p-Met.
To determine whether HGF, p-Met, and p-Akt are also upregulated in the hypoxic regions of melanoma tumors in vivo, we conducted IHC staining of HIF-1α, HGF, p-Met, and p-Akt in serial tissue sections cut from the same tumor harvested from an A375 mouse xenograft. The HIF-1α positivity area was identified as hypoxic region in the tissue slide 1 (Figure 4F, yellow rectangle). The yellow rectangle areas in the subsequent tissue sections were also positive for p-Akt, p-MET, and HGF (Figure 4F). Interestingly, the majority of p-Akt negative regions were also negative for p-Met and HGF (Figure 4F). These data suggest that p-Akt, HGF, and p-Met are upregulated in the hypoxic regions of melanoma tumor tissues.
Inhibition of c-Met increases sensitivity to PLX4032 in melanoma spheroids and 2D hypoxic cultures
To determine whether the upregulation of p-Met is responsible for PLX4032 resistance in melanoma spheroids and 2D hypoxic cultures a c-Met specific inhibitor, MSC2156119J (EMD Serono, EMD1214063), was employed to block HGF/MET signaling. MSC2156119J is a small molecule inhibitor that block MET activation by binding to its ATP-binding site. 2 μM of MSC2156119J substantially downregulated the levels of p-Met and p-Akt (Thr308) in all three melanoma cell lines cultured as spheroids, or monolayer (2D) under hypoxic conditions (Figure 5A). As shown in Figure 5B, 2 μM of MSC2156119J inhibited 50~80% of cell growth in standard 2D cultures with ambient air, but inhibited 25~40% of the growth of respective spheroids, consistent with resistance of some hypoxic cells in the spheroids, but not as many as in the controlled 2D chambers, where all are exposed to hypoxia equally. Of note, MSC2156119J also disrupted the formation of spheroids, as indicated by the increase of scattered monolayer cells on NCPs (Figure 5C). The disruption of spheroid structures is also resulted in the breakdown of hypoxic centers and the release of more melanoma cells into the standard air culture environment with higher oxygen levels, increasing sensitivity to PLX4032. Also, as shown in Figure 2F, 1 μM PLX4032 inhibited 5~15% of the growth of melanoma spheroids, suggesting that this drug concentration may be useful to evaluate the ability of c-Met inhibitors to restore the sensitivity of hypoxic melanoma cells to PLX4032. To test this possibility, the combination of MSC2156119J (2 μM) and PLX4032 (1 μM) were used, and found to significantly decrease cell survival by 50 ~80% in melanoma spheroids, which is more potent than either MSC2156119J or PLX4032 alone (Figure 5D). Thus, MSC2156119J substantially potentiated the inhibitory effects of PLX4032 on the growth and formation of melanoma spheroids. We further treated melanoma 2D hypoxic cultures with PLX4032 in the presence or absence of MSC2156119J (0.5 μM). The resultant cell survival was determined via MTT assays. The IC50 for PLX4032 alone was 9~24 μM in the 2D hypoxic cultures, which was reduced to 1~5 μM in the presence of 0.5 μM MSC2156119J (Figure 5E), indicating a 5- to 8-fold increases of PLX4032 sensitivity in hypoxic melanoma cells. These findings confirm that blocking MET signaling potentiates the antitumor effect of PLX4032 in melanoma spheroids and 2D hypoxic cultures. Therefore, our data suggest that the combination of a c-Met inhibitor and vemurafenib is a potential therapeutic strategy for overcoming hypoxia-driven PLX4032 resistance in melanoma patients.
Figure 5. Blocking HGF/MET signaling by MSC2156119J inhibits the growth of melanoma spheroids and disrupts their formation.
(A) Western blot analyses of p-Met/Met and p-AKt/Akt levels in A375 cells treated with Met inhibitor, MSC2156119J, under hypoxia conditions or in 3D spheroids. B-actin served as loading control.
(B) Growth inhibition of melanoma 3D spheroids by MSC2156119J. After the formation of melanoma spheroids on NCPs on day 4, the spheroids were treated with various concentrations of MSC2156119J for 72 h. For 2D ambient air cultures, melanoma cells were treated with the same concentration of MSC2156119J for 72 h under ambient air conditions before being subjected to MTT assay for cell survival analysis.
(C) Light microscopy images of melanoma spheroids treated with MSC2156119J and/or PLX4032 for 72 h.
(D) Percentage of cell viability of cultures from experiment of 5B.
(E) Dose-dependent inhibition of monolayer BRAF(V600E) melanoma cells growth by PLX4032 under hypoxia in the presence or absence of MSC2156119J. Each bar denotes mean ± SD of three experiments.
High levels of HGF/MET signaling are correlated with low sensitivity to BRAF(V600E) inhibitor in melanoma cell lines
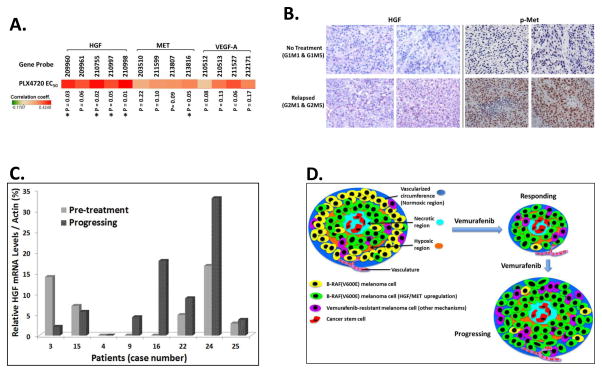
Genetic profiles (gene expression levels and mutations) of 947 human cancer cells lines along with their sensitivity to 24 common anticancer drugs were available through the CCLE (14). Based on the available data in CCLE, we analyzed the sensitivity to PLX4720 in 35 human cutaneous melanoma cell lines across the gene expression patterns of HGF, MET, and VEGF-A. PLX4720 is also a potent and selective inhibitor of B-RAF(V600E). For each of the tested genes, usually there were more than 3 probes applied to measure the mRNA levels, what was considered more reliable for the predictive analysis. Unfortunately, only one probe to measure HIF-1a expression is in the CCLE data, which limits us to perform a statistical analysis to predict drug sensitivity for this gene. Since VEGF-A is a direct target of HIF-1α with 4 probes in CCLE data, we used it as an alternative hypoxia marker in our analysis of CCLE data. As shown in Figure 6A, the Pearson’s correlation coefficients of HGF, MET, and VEGF-A are all positive against PLX4720 EC50 across all reported primer probes. Thus, the higher expression level of HGF, MET, and VEGF-A correlates with higher EC50 of PLX4720 in 35 tested melanoma cell lines. The positive correlation of VEGF-A levels with PLX4720 EC50 indicates that the hypoxia-driven upregulation of VEGF-A expression is correlated with increasing resistance to PLX4720 in melanoma cells. It is noted that the correlation of HGF expression levels and EC50 of PLX4720 are statistically significant among 4 different HGF probes, and one MET probe is also statistically significant for PLX4720 EC50. These CCLE data confirmed that high level of HGF/MET signaling correlates with low sensitivity to BRF(V600E) inhibitor in melanoma cells.
Figure 6. Correlation between HGF/MET signaling levels and sensitivity to vemurafenib in melanoma cell lines and tumor tissues.
(A) Pearson’s correlation coefficients between the expression levels of three genes (HGF, MET, and VEGF-A) and relative efficacy to PLX4720 (EC50) in 35 melanoma cell lines. The red color in the heat map represents positive correlation, which indicates higher gene expression correlates with higher EC50 of drug. For each gene, the mRNA levels were detected by multiple probes. p value of coefficient for each probe (gene expression levels) and PLX4720 EC50 was shown at the bottom of the panel. * p ≤ 0.05.
(B) IHC staining of HGF and p-Met in melanoma tissues from A375 xenografts. G1M1 and G1M5 mice were not treated with any drug. The recurrent tumor biopsy samples were obtained from G2M1 and G2M5 mice after the initial response to PLX4032 treatment while the mice had tumor progression during treatment.
(C) The levels of HGF mRNA in 8 patients treated with BRAF(V600E) inhibitors. Paired cDNA samples from the same patient were prepared from the pre-treatment and progressing tumor sample.
(D) Schematic depiction of hypoxia-driven upregulation of HGF/MET signaling contributing to vemurafenib resistance in BRAF(V600E) melanoma. Within hypoxic regions of tumor, some melanoma cells can sustain aberrant high levels of HGF/MET signaling and are resistant to the cytotoxic effects of vemurafenib. Upon treatment with vemurafenib, most sensitive BRAF(V600E) cells were killed. However, the melanoma cells that can genetically or epigenetically inherit upregulated HGF/MET signaling or other vemurafenib-resistant signaling pathways will survive treatment with vemurafenib and recur as drug-resistant tumors.
HGF/MET signaling is upregulated in PLX4032-resistant tumor tissues from patients and mouse xenografts
As a reasonable extension of our main hypothesis, we expected that BRAF(V600E) cells with aberrant high levels of HGF/MET signaling would be resistant to PLX4032 and that this resistance would contribute to melanoma relapse. This led to the assumption that the upregulation of HGF/MET signaling may be a phenomenon in some PLX4032-resistant melanoma cells in vivo. Therefore, we investigated the levels of HGF and p-Met in PLX4032-resistant melanoma tissues from patients and mouse xenografts. The tissues of relapsed melanoma from two A375 xenografts (G2M1 and G2M5) were compared to tumors of two A375 xenografts (G1M1 and G1M5) without PLX4032 treatment. Our IHC studies showed that the levels of HGF and p-Met in relapsed melanoma tissues were substantially higher than in the tumor samples without treatment (Figure 6B). Moreover, tumor biopsies were also obtained from 8 BRAF(V600E)-positive melanoma patients at pre-treatment and at time of progression on BRAF(V600E) inhibitors (patient characteristic table, Supplemental Table 1). mRNA analysis of these samples showed that the levels of HGF in progressing tumors were higher in five patients than in their pre-treatment tumors (Figure 6C). For one patient, the HGF levels were undetectable in both pre-treatment and the progressing tumor. In two patients, HGF expression was lower in the progressing tumor than in the pre-treatment tumor. It was noted there was no specific selection for biopsies in hypoxic areas and as a result these mRNA extracts were from random tumor-rich biopsies and contained combinations of relapsed tumor and stromal cells, which may not be able to completely reflect the HGF levels within the cancer cells within hypoxic regions. Although only a small number of tumor specimens were tested in our studies, we still observed a distinct upregulation of HGF/MET signaling in 5 out of 8 BRAF(V600E)-inhibitor-resistant melanoma tumors. This data supports our contention that upregulation of HGF/MET signaling may represent a crucial drug-resistance mechanism for BRAF(V600E) inhibitors in many melanomas.
Discussion
It is known that solid tumors are structurally and molecularly heterogeneous. Tumor tissues often contain hypoxic regions, and studies have shown that tumor cells respond differently to chemotherapeutic agents in hypoxic conditions (≤ 1% O2) compared to tumor cells with physiological oxygen supply (5~7% O2) (18, 19). Moreover, tumor hypoxia is significantly associated with lower overall survival and disease-free survival in several cancers (20–22), and hypoxic tumor cells are known to be more resistant to conventional chemotherapies and radiotherapy than non-hypoxic tumor cells within the same tumor (23, 24). Our findings show that BRAF(V600E) melanoma cells are highly resistant to vemurafenib (PLX4032) when cultured under hypoxia compared to ambient air cultures, indicating that melanoma cells can escape vemurafenib inhibition through hypoxia-driven signaling. At the same time, the activity of vemurafenib to inhibit BRAF signaling did not show any significant difference between cultures under ambient air or hypoxia (supplementary Figure S7). Thus, instead of directly affecting BRAF signaling, hypoxia may modulate melanoma cells response to BRAF inhibition through other by-pass mechanisms. A previous study showed that a hypoxia-induced phenotype shift from ROR1-positive to ROR2-positive in melanoma cells leads to a 10-fold decrease in sensitivity to BRAF inhibitors (25). Moreover, a study by Pucciarelli et al. showed that melanoma cells responded to vemurafenib under hypoxia in a cell type specific manner, suggesting that hypoxia increases the heterogeneity of melanoma cell populations and affects the response to vemurafenib (26). These findings indicate that hypoxic melanoma cells play a crucial role in the development of resistance to BRAF(V600E) inhibitors, and may be amenable to biologic manipulation for a more favorable therapeutic outcome. Thus, we tested whether the upregulation of HGF/MET signaling was one of hypoxia-driven mechanisms for vemurafenib resistance in melanoma, which led us to propose a new therapeutic strategy for overcoming vemurafenib resistance via the combination of a c-Met inhibitor and vemurafenib.
In the present study, we employed for the first time a 3D culture system to more closely mimic the heterogeneous mixture of hypoxic and normoxic melanoma cells in vivo. Although 2D cell cultures have been used extensively for drug development and cancer research, the limitations of standard ambient air 2D cultures (~ 21% O2) are widely recognized. 3D in vitro models are now gaining popularity in cellular studies to mimic the features of an in vivo environment (27, 28). In this study, we applied a 3D culture system to grow melanoma cells as spheroids containing hypoxic cores in the standard incubator with ambient air conditions, which allowed us to closely model the heterogeneous characteristics of tumor in vivo. The cancer cell spheroids cultured on NCPs have shown good permeability for small molecule drugs, which is also applicable for conventional assays to analyze cellular proliferation and viability in the presence or absence of anticancer drugs (29, 30). We were able to culture uniform and reproducible melanoma spheroids based on this method, and gain novel insight into the signaling of melanoma cell growth under normally ambient air conditions but still being able to sustain a hypoxic center.
Previous studies from our laboratory and other groups showed that the upregulation of c-Met/Akt signaling was associated with melanoma progression and metastatic spread (17, 31, 32), which prompted us to investigate this pathway under hypoxia conditions and spheroids. Our published data from both melanoma cell lines and patient samples showed that c-Met is preferentially activated in a subclass of melanoma cells without mutated BRAF that are known to be resistant to vemurafenib (17). These findings led us to assume that the upregulation of c-MET/Akt signaling in BRAF(V600E) melanoma cells may drive cell growth under hypoxic conditions and decrease their sensitivity to vemurafenib. In fact, our studies confirm that the hypoxia-driven activation of HGF/MET signaling contributes to the upregulation of p-Akt and resistance to vemurafenib in melanoma spheroids and 2D hypoxic cultures. We further observed upregulation of HGF mRNA expression and the activation of c-Met in progressing tumors from melanoma patients experiencing relapse after vemurafenib treatment, as well as in vemurafenib-resistant melanoma xenografts. Therefore, the aberrant upregulation of HGF/MET signaling reflects a cellular signature of a subgroup of melanoma cells in vivo that contributes to vemurafenib resistance. Herein, we propose a hypoxia-driven mechanism contributing to BRAF(V600E) melanoma progression after vemurafenib treatment (Figure 6D). During treatment with vemurafenib, most normoxic growing BRAF(V600E) cells are rapidly killed; however, some melanoma cells, which can genetically or epigenetically inherit upregulated HGF/MET signaling (such as via hypoxia) or other vemurafenib-resistant signaling pathways, will survive and grow out as drug-resistant tumors.
Under hypoxic conditions, we found that many melanoma cells and melanoma tissues stained positively with p-Met [Y1003] with predominantly nuclear staining (Figure 4D, 4F, and 6B); this observation is consistent with a previous report in non-small cell lung cancer (33). Moreover, nuclear localization of active Met has been found not only playing a critical role in initiating calcium signaling (34), but also correlating with an aggressive invasive/metastatic phenotype of breast carcinoma cells (35). Our studies suggest that nuclear p-Met may have a role enhancing signaling responsible for drug resistance under hypoxic conditions. Several studies have shown that c-Met expression could be induced by hypoxia in various cancer cells due to the fact that c-Met promoter contains multiple HIF-1α binding sites (36, 37). Moreover, previous reports showed that HIF-1α could induce HGF expression and further promote the proliferation and tube formation of endothelial progenitor cells (38). The studies of glioma and endothelial cells revealed that hypoxia could upregulate HGF expression by stabilizing its mRNA (39). It is known that HGF mediated signaling promotes cell proliferation and migration in a variety of cell types through activating MET/AKT signaling pathway. In lung endothelial cells, HGF induces phosphorylation of c-Met, PI3K, and Akt (T308 and S473) in a dose-dependent manner (40). Based on these studies, we propose a similar mechanism for hypoxia upregulating HGF/MET signaling and increasing activation of Akt in melanoma cells. Under hypoxic conditions, c-Met expression is upregulated by HIF-1α, and the levels of HGF are also increased due to its stable mRNA with long half-life, which lead to increasing activation of HGF/MET signaling. Consequently, as one of major downstream targets of HGF/MET signaling, the activation of Akt is increased under hypoxia.
In our phospho-kinase arrays, we observed that the phosphorylation of Akt T308 was higher in 3D spheroids containing hypoxic centers compared to relevant 2D ambient air cultures (Figure 3B and S5). However, there was no significant difference of phosphorylation of Akt S473 between 2D ambient air cultures and spheroids (Figure 3B and S5). It indicates that Akt phosphorylation on T398 but not on S473 correlates with hypoxia driven upregulation of HGF/MET signaling. Akt activation involves the phosphorylation of two residues, threonine 308 (T308) and serine 473 (S473). These two distinct sites can be activated independently (41). Phosphorylation of T308 in the activation loop by PDK1 is essential for Akt activation, and phosphorylation of S473 at the C-terminal tail by either autophosphorylation or by DNA-PK is required for maximal activation of the kinase [41]. Studies in breast and lung cancer cells showed that Akt phosphorylation on T308 but not on S473 correlated with Akt kinase activity (42, 43). Moreover, the study in lung cancer cells confirmed that Akt signaling was reactivated through a feedback-induced Akt species phosphorylated on T308 but lacking S473 (42). The phosphorylation of Akt site is essential for downstream target specification. The most prevalent downstream targets of Akt T308 are TSC2 and GSK3 (39). However, phosphorylation of Akt S473 selectively affects substrates FOXO1 and FOXO3a, with little effect on GSK3 and TSC2 (41). Thus, we suspect that hypoxia mainly activates Akt T308 to upregulate its downstream TSC2 and GSK3 signaling in melanoma cells. Although our data suggests that Akt T308 is the major site responds to hypoxia signaling but not S473, further studies are needed to investigate the complex PI3K-Akt-mTOR signaling in melanoma cells under hypoxia.
Besides Akt, several markers also showed substantial differences between melanoma spheroids versus 2D ambient air cultures in our phospho-kinase arrays (Figure 3A, supplementary Figure S5 and S6). The levels of phosphorylated AMPKa1, b-Catenin, and p27(T198) were lower in spheroids compared to 2D cultures under ambient air. Higher levels of phosphorylated p53 (S392, S46, and S15) were observed in spheroids compared to 2D cultures under ambient air. It remains unclear whether the changes of these markers are due to the hypoxic environment within the centers of spheroids, or simply caused by the unique morphology of spheroids compared to monolayer cultures. Interestingly, a recent study by Zhang et al. showed that the levels of p53 were significantly increased in response to hypoxia, resulting in reducing the stimulating effect of hypoxia on glycolysis in A549 and H460 cells (44). Moreover, the study by Parmenter et al. identified a network of BRAF-regulated transcription factors that control glycolysis in melanoma cells (45). Remarkably, this network of transcription factors included HIF-1α, MYC, and MODDOA. This study showed that BRAF inhibition suppressed glycolysis via the network of transcription factors, which were critical for complete responses to BRAF inhibitor. Parmenter’s group found that hypoxia could significantly increase IC50 of vemurafenib in melanoma cells compared to normoxia (45), which is consistent with our study. Based on these studies, similar regulatory mechanisms may occur in melanoma cells, and hypoxia-driven activation of p53 may play a critical role in antagonizing the stimulating effect of hypoxia on glycolysis, and further affects the response of cancer cells to BRAF inhibition. Further studies will be needed to resolve related mechanisms.
Although we observed a trend of HGF/MET signaling upregulation in multiple vemurafenib-resistant melanoma specimens derived from patients and from mouse xenografts, we cannot conclude a significant association between HGF/MET signaling upregulation and vemurafenib resistance due to the small number of patients included in our study. However, this data now provides the rationale to prospectively and critically investigate the levels of HGF/MET signaling in a large number of melanoma patient samples. Interestingly, a reported study showed that increased plasma HGF was associated with worse outcome in BRAF-mutated metastatic melanoma patients treated with PLX4032 (46). Although that study did not statistically confirm whether higher HGF levels confer PLX4032 resistance in patients, it suggested a clinical implication.
A study by Straussman et al. showed that the tumor microenvironment elicits innate resistance to BRAF(V600E) inhibitors through HGF secretion from stromal cells and activation of MET/Akt signaling in melanoma cells (9). Another study by Wilson et al. also showed that HGF significantly attenuated vemurafenib sensitivity in five BRAF(V600E) melanoma cell lines (46). Moreover, their study showed that inhibiting MET enhanced the effect of PLX4032 on melanoma tumor regression in mice (46). Together with our findings, these data support a mechanism whereby vemurafenib resistance is mediated by aberrant HGF/MET signaling either through autocrine effects of HGF on melanoma tumor cells or through microenvironment-derived HGF. Consistent with previous reports from other groups (9, 46), our study showed that the inhibition of hypoxia-driven c-Met/Akt signaling by the specific inhibitor MSC21562 not only led to a marked 40~60% decrease in spheroid formation and growth, but also significantly increased the efficacy of vemurafenib under hypoxic conditions. Collectively, studies from three groups showed that dual inhibition of mutated BRAF and MET results in overcoming vemurafenib resistance.
HGF/Met signaling is emerging as one of the critical signaling pathways contributing to tumorigenesis, metastasis, and resistance to targeted therapies in cancer cells. Aberrant MET activation is frequently implicated in driving resistance to different kinase inhibitors in multiple tumor types (47, 48). With the FDA approval of Crizotinib, a c-Met inhibitor, to treat patients with non-small cell lung cancer (49), the clinical usage of MET inhibitor in combination therapy to enhance the efficacy of other targeted therapies is becoming more feasible. Moreover, several small-molecule MET inhibitors are in clinical trials for treating melanoma and other solid tumors. For example, one of these trials is investigating combination therapy with cabozantinib-s-malate (a potent VEGF and c-Met inhibitor) and vemurafenib for late-stage melanoma (ClinicalTrials.gov identifier: NCT01835184) (50). The results from these trials will provide valuable insight into the therapeutic strategy of combining MET inhibition with vemurafenib, which is expected to effectively overcome drug resistance in BRAF(V600E) melanoma.
Herein, we confirmed that hypoxia-driven upregulation of HGF/MET plays an important role in in vemurafenib resistance in melanoma. Furthermore, pharmacologic inhibition of the c-Met/Akt pathway restores the sensitivity of melanoma spheroids or 2D hypoxic cultures to vemurafenib.
Supplementary Material
Acknowledgments
Grant Support:
E.A. Grimm: The UT MD Anderson Cancer Center SPORE in Melanoma (NCI, P50 CA093459); Aim at Melanoma Foundation; the Miriam & Jim Mulva Research Funds, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; CCSG grant (NCI, P30 CA016672).
We thank Dr. Meenhard Herlyn for providing BRAF(V600E)-mutated cell lines 451Lu and MEL1617. We also thank Ms. Sandra A. Kinney for her excellent technical assistance for our IHC experiments. The authors are grateful to Markeda Wade for proofreading and editing the manuscript and figures. We thank Dr. Victoria R. Greene for assistance in Immunofluorescence cell staining.
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic BRAF kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–96. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to BRAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 9.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumor micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 11.Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33:1743–1754. doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- 12.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119:861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 13.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broadinstitute.org. Cambridge (MA): Broad-Novartis Cancer Cell Line Encyclopedia (CCLE); p. c2015. [cite 2015 August 3]. Available from: http://www.broadinstitute.org/ccle. [Google Scholar]
- 15.Yoshii Y, Waki A, Yoshida K, Kakezuka A, Kobayashi M, Namiki H, et al. The use of nanoimprinted scaffolds as 3D culture models to facilitate spontaneous tumor cell migration and well-regulated spheroid formation. Biomaterials. 2011;32:6052–6058. doi: 10.1016/j.biomaterials.2011.04.076. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Hosaka M, Yoshihara T, Negishi K, Iida Y, Tobita S, Takeuchi T. Phosphorescent light-emitting iridium complexes serve as a hypoxia-sensing probe for tumor imaging in living animals. Cancer Res. 2010;70:4490–4498. doi: 10.1158/0008-5472.CAN-09-3948. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyay C, Ellerhorst JA, Ekmekcioglu S, Greene VR, Davies MA, Grimm EA. Association of activated c-Met with NRAS-mutated human melanomas. Int J Cancer. 2012;131:E56–65. doi: 10.1002/ijc.26487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer A, Vaupel P. Hypoxia, lactate accumulation, and acidosis: siblings or accomplices driving tumor progression and resistance to therapy? Adv Exp Med Biol. 2013;789:203–209. doi: 10.1007/978-1-4614-7411-1_28. [DOI] [PubMed] [Google Scholar]
- 19.Cuvillier O, Ader I, Bouquerel P, Brizuela L, Gstalder C, Malavaud B. Hypoxia, therapeutic resistance, and sphingosine 1-phosphate. Adv Cancer Res. 2013;117:117–141. doi: 10.1016/B978-0-12-394274-6.00005-4. [DOI] [PubMed] [Google Scholar]
- 20.S, Evans M, Koch CJ. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003;195:1–16. doi: 10.1016/s0304-3835(03)00012-0. [DOI] [PubMed] [Google Scholar]
- 21.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 22.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 23.Nordsmark M, Overgaard J. Overgaard, Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004;43:396–403. doi: 10.1080/02841860410026189. [DOI] [PubMed] [Google Scholar]
- 24.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 25.O’Connell MP, Marchbank K, Webster MR, Valiga AA, Kaur A, Vultur A, et al. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Discov. 2013;3:1378–1393. doi: 10.1158/2159-8290.CD-13-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pucciarelli D, Lengger N, Takáčová M, Csaderova L, Bartosova M, Breiteneder H, et al. Hypoxia increases the heterogeneity of melanoma cell populations and affects the response to vemurafenib. Mol Med Rep. 2016;13:3281–8. doi: 10.3892/mmr.2016.4888. [DOI] [PubMed] [Google Scholar]
- 27.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Haycock JW. 3D cell culture: a review of current approaches and techniques. Methods Mol Biol. 2011;695:1–15. doi: 10.1007/978-1-60761-984-0_1. [DOI] [PubMed] [Google Scholar]
- 29.Arai K, Sakamoto R, Kubota D, Kondo T. Proteomic approach toward molecular backgrounds of drug resistance of osteosarcoma cells in spheroid culture system. Proteomics. 2013;13:2351–2360. doi: 10.1002/pmic.201300053. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi RU, Takeshita F, Honma K, Ono M, Kato K, Ochiya T. Ribophorin II regulates breast tumor initiation and metastasis through the functional suppression of GSK3β. Sci Rep. 2013;3:2474. doi: 10.1038/srep02474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz J, Reis-Filho JS, Silva P, Lopes JM. Expression of c-met tyrosine kinase receptor is biologically and prognostically relevant for primary cutaneous malignant melanomas. Oncology. 2003;65:72–82. doi: 10.1159/000071207. [DOI] [PubMed] [Google Scholar]
- 32.Jubb AM, Ribas A, Sosman JA, McArthur GA, Yan Y, Rost S, et al. Impact of MET expression on outcome in BRAF(V600E/K) advanced melanoma. Histopathology. 2013;63:351–361. doi: 10.1111/his.12169. [DOI] [PubMed] [Google Scholar]
- 33.Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 34.Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, et al. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2008;283:4344–4351. doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matteucci E, Bendinelli P, Desiderio MA. Nuclear localization of active HGF receptor Met in aggressive MDA-MB231 breast carcinoma cells. Carcinogenesis. 2009;30:937–945. doi: 10.1093/carcin/bgp080. [DOI] [PubMed] [Google Scholar]
- 36.Eckerich C, Zapf S, Fillbrandt R, Loges S, Westphal M, Lamszus K. Hypoxia can induce c-Met expression in glioma cells and enhance SF/HGF-induced cell migration. Int J Cancer. 2007;121:276–83. doi: 10.1002/ijc.22679. [DOI] [PubMed] [Google Scholar]
- 37.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 38.Yu F, Lin Y, Zhan T, Chen L, Guo S. HGF expression induced by HIF-1α promote the proliferation and tube formation of endothelial progenitor cells. Cell Biol Int. 2015;39:310–7. doi: 10.1002/cbin.10397. [DOI] [PubMed] [Google Scholar]
- 39.Chu SH, Feng DF, Ma YB, Zhu ZA, Zhang H, Qiu JH. Stabilization of hepatocyte growth factor mRNA by hypoxia-inducible factor 1. Mol Biol Rep. 2009;36:1967–75. doi: 10.1007/s11033-008-9406-1. [DOI] [PubMed] [Google Scholar]
- 40.Usatyuk PV, Fu P, Mohan V, Epshtein Y, Jacobson JR, Gomez-Cambronero J, et al. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J Biol Chem. 2014;89:13476–91. doi: 10.1074/jbc.M113.527556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briest F, Grabowski P. PI3K-AKT-mTOR-signaling and beyond: the complex network in gastroenteropancreatic neuroendocrine neoplasms. Theranostics. 2014;4:336–65. doi: 10.7150/thno.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–59. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent EE, Elder DJ, Thomas EC, Phillips L, Morgan C, Pawade J, et al. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br J Cancer. 2011;104:1755–61. doi: 10.1038/bjc.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Liu J, Wu R, Liang Y, Lin M, Liu J, et al. Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD. Oncotarget. 2014;5:5535–46. doi: 10.18632/oncotarget.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parmenter TJ, Kleinschmidt M, Kinross KM, Bond ST, Li J, Kaadige MR, et al. Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov. 2014;4:423–33. doi: 10.1158/2159-8290.CD-13-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corso S, Giordano S. Cell-autonomous and non-cell-autonomous mechanisms of HGF/MET-driven resistance to targeted therapies: from basic research to a clinical perspective. Cancer Discov. 2013;3:978–992. doi: 10.1158/2159-8290.CD-13-0040. [DOI] [PubMed] [Google Scholar]
- 48.Moschetta M, Basile A, Ferrucci A, Frassanito MA, Rao L, Ria R, et al. Novel targeting of phospho-cMET overcomes drug resistance and induces antitumor activity in multiple myeloma. Clin Cancer Res. 2013;19:4371–4382. doi: 10.1158/1078-0432.CCR-13-0039. [DOI] [PubMed] [Google Scholar]
- 49.Malik SM, Maher VE, Bijwaard KE, Becker RL, Zhang L, Tang SW, et al. U.S. Food and Drug Administration approval: crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014;20:2029–2034. doi: 10.1158/1078-0432.CCR-13-3077. [DOI] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; c 1993–01. [updated: 2012 November 26; cite 2015 August 10]. Available from https://clinicaltrials.gov/ct2/show/NCT01835184?term=NCT01835184&rank=1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.