Abstract
Light exerts a direct effect on sleep and wakefulness in nocturnal and diurnal animals, with a light pulse during the dark phase suppressing locomotor activity and promoting sleep in the former. In the present study, we investigated this direct effect of light on various sleep parameters by exposing mice to a broad range of illuminances (0.2–200 μW/cm2; equivalent to 1–1000 lux) for 1 h during the dark phase (zeitgeber time 13–14). Fitting the data with a three-parameter log model indicated that ~0.1 μW/cm2 can generate half the sleep response observed at 200 μW/cm2. We observed decreases in total sleep time during the 1 h following the end of the light pulse. Light reduced the latency to sleep from ~30 min in darkness (baseline) to ~10 min at the highest intensity, although this effect was invariant across the light intensities used. We then assessed the role of melanopsin during the rapid transition from wakefulness to sleep at the onset of a light pulse and the maintenance of sleep with a 6-h 20 μW/cm2 light pulse. Even though the melanopsin knockout mice had robust induction of sleep (~35 min) during the first hour of the pulse, it was not maintained. Total sleep decreased by almost 65% by the third hour in comparison with the first hour of the pulse in mice lacking melanopsin, whereas only an 8% decrease was observed in wild-type mice. Collectively, our findings highlight the selective effects of light on murine sleep, and suggest that melanopsin-based photoreception is primarily involved in sustaining light-induced sleep.
Keywords: photoreception, sensory processing, circadian rhythms, SCN, locomotion
Introduction
Sleep regulation in mammals has been modeled as being controlled by two interacting systems, the central circadian clock and a homeostatic process (Borbely, 1982). The circadian clock is a self-sustained, near 24-h oscillation that is entrained primarily by ocular light exposure (Rusak & Zucker, 1979). Ocular light exposure can also have a masking effect on sleep/wakefulness through a mechanism independent of the circadian system. For example, in nocturnal animals, a light pulse during the dark phase both suppresses locomotor activity and promotes sleep (Alfoldi et al., 1991; Redlin & Mrosovsky, 1999). In diurnal animals, including humans, a light pulse during the dark phase has an opposite effect, increasing alertness and decreasing sleep in a dose-dependent manner (Cajochen et al., 2000).
The eye serves as the only input for behavioral responses to light in mammals (Nelson & Zucker, 1981). The rods and cones are essential for the visual responses, whereas a combination of the rods and cones and intrinsically photosensitive retinal ganglion cells (ip-RGCs) expressing melanopsin have been suggested to complement each other to mediate the full effect of light on the non-visual responses such as circadian photoentrainment (Lucas et al., 2001; Panda et al., 2003), pupillary light reflex (Hattar et al., 2003), melatonin suppression (Lucas et al., 1999), masking (Mrosovsky et al., 2001; Mrosovsky & Hattar, 2003), and the induction of sleep in nocturnal animals (Mrosovsky & Hattar, 2003; Altimus et al., 2008; Lupi et al., 2008; Tsai et al., 2009).
Recent studies using wheel running have suggested that the suppression of locomotor activity by light induces a series of events, beginning with an initial effect that is extended by additional light (Morin et al., 2010). However, the extent to which light affects the initiation and maintenance of sleep during a light pulse has not been investigated directly. Moreover, the mechanisms involved in the induction of sleep by a light pulse are not well understood. In the present study, we investigated the full extent of the acute modulation of sleep in mice by light pulses of different intensities and durations during the dark phase. We then explored the underlying mechanisms by directly assessing the contribution of melanopsin to the initiation and maintenance of light-induced sleep during the dark phase.
Materials and Methods
Animals
Adult, 3–4-month-old C57BL/6J male wild-type (WT) and melanopsin knockout (MKO) mice (backcrossed for eight generations to the C57BL/6J background in our laboratory before intercrossing for homozygosity) were used. Mice were housed individually in plastic cages, with ad libitum access to food and water, under a 12-h light/12-h dark cycle in a room that contained four cool white fluorescent tubes (34 W; GE Lighting, Pleasanton, CA, USA) producing an intensity of ~40 μW/cm2 on the cage floors. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Protocol
In all experiments, sleep and locomotion were recorded throughout.
Experiment 1
To assess how light influences sleep during the dark period, WT mice (n = 8) were exposed to a short 1:1 light/dark (LD) cycle (200 μW/cm2) for the 12-h dark phase.
Experiment 2
To assess the ability of individual light pulses to induce sleep early and late in the dark phase, one group of WT mice (n = 7) was pulsed with a 1-h pulse of light (20 μW/cm2) at zeitgeber time (ZT) 13, and a separate group (n = 4) was pulsed with a 1-h pulse of light (20 μW/cm2) at ZT19 (note that ZT12 is the start of the dark phase in a nocturnal animal kept on a 12:12 LD cycle).
Experiment 3
To assess whether there is a dose–response relationship for light intensity and sleep during the dark, WT mice (n = 8) were pulsed with light of 0.2, 2, 20 and 200 μW/cm2 (1–1000 lux) for 1 h, beginning at ZT13, on different days. The order of light intensity delivery was randomised. There was a minimum of 3 days between pulses, to ensure normal entrainment prior to administration of experimental light pulses (Fig. S1). As others have also shown, there is rapid re-entrainment following the light pulses (Mrosovsky et al., 1999; Thompson et al., 2008).
Experiment 4
To assess the effects of light pulses shorter than 1 h, WT mice (n = 8) were subjected to a 15-min or 30-min 20 μW/cm2 light pulse, beginning at ZT13.
Experiment 5
To assess the role of melanopsin in the initiation and maintenance of negative masking and sleep induction by light, one group of mice [WT (n = 16) and MKO (n = 13)] and a separate group [WT (n = 7) and MKO (n = 7)] were assessed for negative masking and sleep, respectively, in response to a 6-h light pulse (20 μW/cm2), from ZT13 to ZT19.
Surgery and Sleep Data Acquisition
Sleep recordings were performed as previously described (Colas et al., 2008). In brief, two cortical electrodes (gold-plated screws, 0.4 mm in diameter) were implanted along with two electromyography electrodes (fluorocarbon-coated gold-plated stainless steel wires, 0.03 mm in diameter; Cooner Wire, Chatsworth, CA, USA) into mice between the ages of 2 and 3 months while they were under ketamine/xylazine-induced anesthesia (0.1 mL/10 g, intraperitoneal). The cortical electrodes were inserted bilaterally in the frontal (1 mm lateral and anterior to bregma) and parietal (1 mm lateral to the midline at the midpoint between bregma and lambda) cortices. The electromyography electrodes were inserted into the neck muscles. The electrode assembly was anchored and fixed to the skull with C&BMETABOND Quick System (Parkell, NY, USA), and further secured with dental cement. At least 14 days were allowed for recovery before the mice were connected to lightweight recording cables connected to a commutator/swivel above the cage, allowing for freedom of movement inside cages approximately 25 cm in width, 40 cm in length, and 25 cm in height. Mice were adapted for at least 3 days before the light stimulation experiments began. A Somnologica system (Embla, Denver, CO, USA) was used to acquire the cortical electroencephalographic (EEG) and electromyographic (EMG) signals. All signals were amplified, analog-to-digital converted with a sampling rate of 200 Hz, and digitally filtered (EEG signals, 0.5–70 Hz; EMG signals, 10–70 Hz). The behavioral states of the mice were scored in 4-s epochs by a single reader (F.M.) using visual inspection of EEG and EMG signals as follows: wakefulness – low-voltage, high-frequency EEG signal; non-rapid eye movement sleep (NREMS) – high-voltage, low-frequency EEG signal; and rapid eye movement sleep (REMS) – prominent theta activity, and low-voltage EMG signal. The power spectra of the corresponding EEG signals were then calculated by using fast Fourier transformation (sampling at 256 Hz) for all 4-s epochs. Power in the 0.8–40-Hz range of the recording was averaged, and the mean values were plotted in 0.8-Hz bins as percentage of total power. The latency to sleep was defined as the time from the light pulse onset to the first NREMS episode longer than 2 min. The latency for REMS was the time from the onset of light to the first episode of REMS longer than 30 s. The average length and number of NREMS episodes were also calculated.
Sleep recordings were done as previously described (Colas et al., 2008). In brief, two cortical electrodes (gold-plated screws, 0.4 mm in diameter) were implanted along with two EMG electrodes (fluorocarbon-coated gold-plated stainless steel wires, 0.03 mm in diameter; Cooner Wire, Chatsworth, CA) into mice between the ages of 2 and 3 months while under ketamine/xylazine-induced anesthesia (0.1 mL/10 g, i.p.). The cortical electrodes were inserted bilaterally in the frontal (1-mm lateral and anterior to the bregma) and parietal (1-mm lateral to the midline at the midpoint between the bregma and lambda) cortices. The EMG electrodes were inserted into the neck muscles. The electrode assembly was anchored and fixed to the skull with C&B-METABOND Quick System (Parkell Inc, NY) and further secured with dental cement. At least 14 days were allowed for recovery before the mice were connected to lightweight recording cables connected to a commutator/swivel above the cage allowing for freedom of movement inside ~25W × 40L × 25H cm cages. Mice were adapted for at least 3 days before the light stimulation experiments began. A Somnologica system (Embla, Denver CO) was used to acquire the cortical electroencephalographic (EEG) and electromyographic (EMG) signals. All signals were amplified, analog-to-digital converted with a sampling rate of 200 Hz, and digitally filtered (EEG, 0.5 – 70 Hz; EMG, 10 – 70 Hz). Behavioral states of the animals were scored in 4-s epochs by a single reader (FM) using visual inspection of EEG and EMG signals as follows: wake = low voltage, high frequency EEG; non-REM sleep [NREMS] = high voltage, low frequency EEG; REMS = prominent theta activity, low voltage EMG. Power spectra of the corresponding EEGs were then calculated by using Fast Fourier Transform (FFT) (sampling 256 Hz) for all 4 s epochs. Power in the 0.8 – 40 Hz range of the recording was averaged and the mean values were plotted in 0.8 Hz bins as percent of total power. The latency to sleep was defined as the time from the light pulse onset to the first NREM sleep episode longer than 2 min. The latency for REMS was the time from the onset of light to the first episode of REMS lasting longer than 30 s. The average length and number of NREM sleep episodes were also calculated.
Locomotor Activity
Activity measurement methods were adapted from previous work (Ruby et al., 2008). A passive infrared motion detector was mounted above the cage. Activity bouts were collected in 1-min intervals, and were then summed to obtain total counts of recorded activity for a given hour.
Lighting Design
For all light experiments, separate white, dimmable LED lamps (LumaPro A19 8W; Grainger, San Jose, CA, USA; the spectrum is shown in Fig. S4) positioned above each individual cage were used. A variable autotransformer (model 116B; Superior Electric Company, Bristol, CT, USA) was used to control the light intensity of the white LED lamps. The intensity of the light used was measured at the front, center and back of each cage with an IL-1400 SL021 #385 photodetector (International Light, Newburyport, MA, USA). Measurements at the center of each cage were used to calculate the average light intensity.
Statistical Analysis
GRAPHPAD PRISM (GraphPad Software, Version 5.04/5.0d) was used for all statistical calculations. To construct dose–response curves, three-parameter logistic models were fitted to the data:
such that A is the lower asymptote, B is the inflection point (the value at which 50% of the maximal effect is observed), and C is the upper asymptote. Once the assumptions of normality and variance homogeneity were satisfied, one-way repeated-measures or two-way ANOVA (factors indicated in Results), followed by Bonferonni’s test for post hoc comparisons, was used accordingly to determine differences. All data are presented as mean ± standard deviation (SD).
Results
Phase dependent effects of light pulses on sleep during the dark
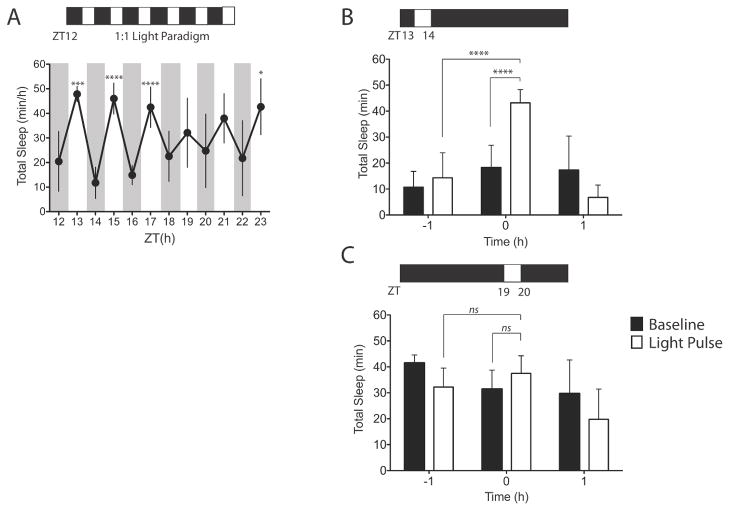
We first confirmed light-mediated sleep induction during the dark phase by maintaining mice (n = 8) under a short 1:1 LD cycle (200 μW/cm2) for the 12-h dark phase (Experiment 1). A one-way repeated-measures ANOVA revealed that the hourly values of total sleep between the hour before and during the light pulses differed significantly (F11,77 = 11.51; P < 0.0001). In particular, a significant increase in sleep during the light pulses was mainly confined to the early portion of the dark period (Fig. 1A). There was no difference in the 12-h total sleep amount between the 1:1 LD protocol (365.9 ± 31.89 min) and baseline during the dark (365.3 ± 37.26 min) (P = 0.96; paired t-test). To establish the phase dependence of induction of sleep by a single light pulse (i.e. without the confound of a prior light history, such as occurs in the 1:1 LD protocol), a separate group of mice (n = 4–7) were exposed to 20 μW/cm2 of light, beginning at ZT13 or ZT19 (Experiment 2). The light pulse beginning at ZT13 increased total sleep time during the pulse (43.2 ± 5.08 min) as compared with baseline at the same circadian phase on the day before (18.3 ± 3.20 min; P < 0.0001, t-test), or the hour before the light pulse (12.9 ± 9.87 min; P < 0.0001, t-test) (Fig. 1B). However, when a light pulse of the same intensity began instead at ZT19, total sleep time (37.5 ± 6.80 min) did not differ from baseline (31.4 ± 7.3 min) on the previous day (P = 0.27, t-test) (Fig. 1C). Similarly, total sleep time during the light pulse did not differ significantly from total sleep time during the previous hour (P = 0.46, t-test). Although there was a decrease in total sleep time following both the light pulse at ZT13 (47.3 ± 37.9%) and that at ZT19 (30.8 ± 27.1%) as compared with baseline, these changes were not statistically significant (P > 0.05, post hoc t-tests). The total sleep time encompassing the light pulse and the hour after (i.e. 2 h) was statistically indistinguishable between baseline (ZT13–15, 35.6 ± 19.9 min; ZT19–21, 61.1 ± 11.7 min) and the ZT13 pulse (49.9 ± 3.88 min; P = 0.10, paired t-test) and the ZT19 pulse (57.3 ± 7.85 min; P = 0.15, paired t-test), respectively. This implies that the light pulse changed the temporal organisation of sleep rather than the amount of sleep expressed.
Figure 1. Phase dependent effects of light pulses on sleep during the dark.
(A) Hourly total sleep time across an LD 1:1; 200 μW/cm2 during the 12-hr dark phase. *p < 0.05, ***p < 0.001, ****p < 0.0001; by Bonferroni’s post-hoc test. Single one-hour exposure to 20 μW/cm2 light at ZT13 (n=7) (B) and single one-hour exposure to 20 μW/cm2 light at ZT19 (n=4) (C). Data were plotted for the hour before the pulse, during the pulse, and for the hour following the pulse. White bars represent the light pulse day and the black bars represent baseline day. ****p < 0.0001, Paired Student’s t test. Data represents mean ± SD.
Dose-response relationship for light intensity and sleep induction
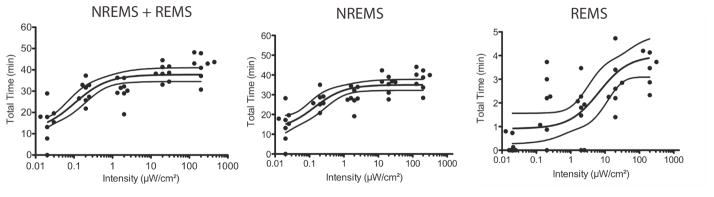
To assess whether there is a dose–response relationship for light intensity and sleep early in the dark phase, mice were exposed to different intensities of light for 1 h, starting at ZT13. Given the utility of logistic regression in modeling a variety of functions, including the effects of light on sleep in humans (Cajochen et al., 2000), we applied this analysis method to our data (see Materials and methods). Residual analysis revealed a normal distribution of the data (Shapiro–Wilk test), and the dependencies between the estimated parameters were below 0.5. All intensity values had 0.01 added to them before fitting. Weighted r2 values for the models, which constitute an estimation of the goodness of fit, indicated an appropriate fit to the chosen regression model (total sleep, r2 = 0.64; NREMS, r2 = 0.63; REMS, r2 = 0.52).
Total sleep, NREMS and REMS each showed changes that were dependent on light intensity. The regression model for total sleep time (Fig. 2A) indicated that 0.13 ± 0.06 μW/cm2 generated half the sleep response seen at 200 μW/cm2, that the mice got, at most, approximately 37.8 ± 1.61 min of sleep during the hour of the highest light exposure, and that, at baseline in darkness at ZT13, they got approximately 13.6 ± 1.64 min of sleep. The high-intensity light, therefore, had the capacity to induce an increase of ~25 min of sleep in this paradigm. A similar relationship was observed for the regression model for NREMS (Fig. 2B), where 0.13 ± 0.08 μW/cm2 generated half the NREMS seen during the 200 μW/cm2 pulse. The mice got 34.9 ± 1.37 min of NREMS during the hour of the highest light exposure, and got approximately 13.6 ± 2.73 min of NREMS when in darkness at ZT13. In contrast, a much higher intensity of light (6.23 ± 4.87 μW/cm2) was required to generate half the REMS response that was produced by the 200 μW/cm2 pulse, with the mice getting 3.98 ± 0.44 min of REMS during the hour of the highest light exposure (Fig. 2C). In comparison, the mice got approximately 0.87 ± 0.31 min of REMS when in the dark at ZT13. The high-intensity light was, therefore, able to induce an increase of ~3 min of REMS. All subsequent analyses of sleep amounts are summations of both NREMS and REMS (i.e. total sleep).
Figure 2. Dose-response relationship for light intensity and sleep induction.
Individual responses to light (filled circles) are plotted and fit with a three-parameter logistic regression model with 95% confidence intervals (solid lines) for total sleep (A; a= 13.6 min, b= 0.12 μW/cm2 and c= 37.8 min), NREMS (B; a= 13.6, b= 0.13 and c= 34.9) and REMS (C; a= 0.87 min, b= 6.23 μW/cm2 and c= 3.98 min). Note: a is the lower asymptote, b is the inflection point (value at which 50% of the maximal effect is observed), and c is the upper asymptote.
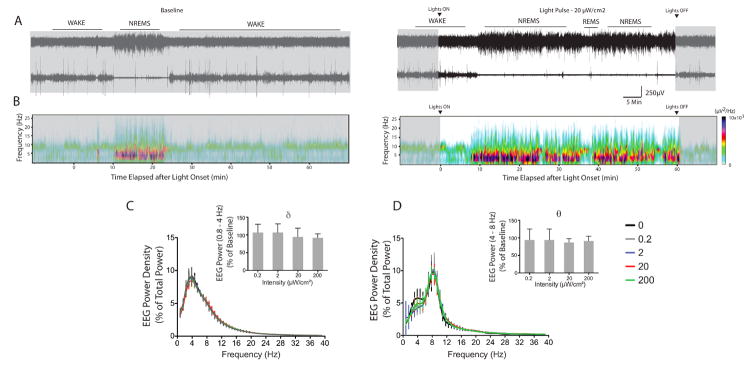
Effects of light on sleep microarchitecture
To assess the changes on sleep parameters in response to light pulses of different intensities, we assessed NREMS and REMS power spectra, latency, episode number, and episode length. We first determined whether an effect of intensity existed, by using one-way or one-way repeated-measures ANOVA accordingly, and then fitting a three-parameter logistic regression model to the data. A representative EEG trace and power spectrum illustrates the temporal profile of changes in sleep during a 20 μW/cm2 light pulse (Fig. 3A and B). Altogether, there were no light-induced changes in the power density in the delta band of NREMS (F3,23 = 0.86; P = 0.47; Fig. 3C) or theta band of REMS (F3,8 = 0.06; P = 0.98; Fig. 3D). The effects of light on the power spectrum of wakefulness at the highest intensity (200 μW/cm2) are shown in Fig. S2.
Figure 3. Effects of light on the power spectra of NREMS (A) and REMS (B) during the 1-h light pulse across intensities.
(A, B) Representative EEG and EMG traces and spectrograms during baseline and light pulse hour are shown (C,D). Power in the 0.8 – 40 Hz range was averaged, and the mean values plotted in 0.8 Hz bins as percent of total mean power expressed. Signature EEG bands (delta for NREMS, theta for REMS) are plotted. Data represents mean ± SD (n=4–8).
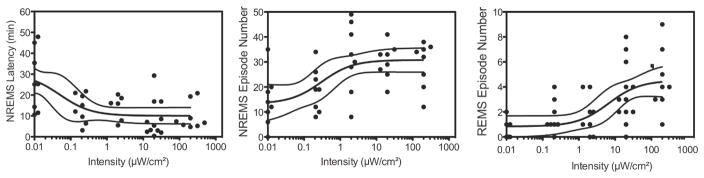
NREMS latency (F4,32 = 4.38; P = 0.0062), NREMS episode number (F4,28 = 6.53; P = 0.0008) and REMS episode number (F4,28 = 11.8; P < 0.0001) all showed changes that were dependent on light intensity. The regression model (weighted r2 = 0.30) for NREMS latency (Fig. 4A, left) indicated that light reduced sleep latency from 33.4 ± 13.2 min in darkness (baseline) to 11.1 ± 2.05 min at the highest intensity, although this effect was invariant across the light intensities used in this paradigm (F3,26 = 0.58; P = 0.63). The regression model for NREMS episode number (weighted r2 = 0.28) indicated that light increased the number of episodes from 13.3 ± 3.95 in darkness to 30.8 ± 2.38 at the highest intensity (Fig. 4, center). The regression model for REMS episode number (weighted r2 = 0.45) indicated that light increased the number of episodes from 0.79 ± 0.48 in darkness to 4.86 ± 0.59 at the highest intensity (Fig. 4, right). Notably, the change in the number of REMS episodes was less sensitive to light than the change in the number of NREMS episodes (half-maximum of 4.04 ± 3.37 μW/cm2 for REMS vs. 0.31 ± 0.33 μW/cm2 for NREMS; Fig 4). No significant changes were observed in REMS latency (F4,25 = 1.04; P = 0.41), which had an average of 26.7 ± 4.79 min across intensities. NREMS episode length, which averaged 1.40 ± 0.30 min across intensities, also did not change with an increase in light intensity (F4,28 = 2.30; P = 0.0834). Similarly, REMS episode lengths, which averaged 0.99 ± 0.21 min, did not change with an increase in light intensity (F4,28 = 1.18; P = 0.34).
Figure 4. Effects of light on sleep parameters.
Shown are three-parameter logistic regression models with 95% confidence intervals (lines), fitted to the data points (filled circles) for NREMS latency (left; a= 33.4 min, b= 0.03 μW/cm2. and c= 11.1 min), NREMS episode number (center; a= 13.3, b= 0.31 μW/cm2 and c= 30.8) and REMS episode number (right; a= 0.78, b= 4.04 μW/cm2 and c= 4.86).
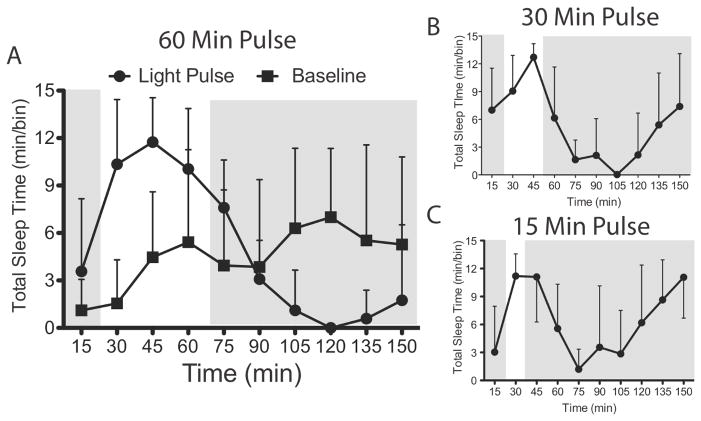
We also performed a time course analysis of total sleep in 15-min bins before, during and after a 1-h 20 μW/cm2 light pulse (Fig. 5A). As compared with baseline, total sleep during the light pulse was increased across the entire pulse (factor ‘light condition’: (F1,20 = 44.21; P < 0.0001), although there was no main effect of time (F3,60 = 0.09; P = 0.97) or significant interaction between time and light condition (F3,60 = 0.56; P = 0.10), indicating that the occurrence of sleep was uniformly distributed over the hour in both conditions. To assess changes in total sleep with light pulses of different durations, mice were pulsed with a 20 μW/cm2 light pulse lasting either 15 or 30 min on different days (Experiment 4). In all cases, the induction of sleep was followed by a characteristic decrease in sleep in the hour following a light pulse before it returned to baseline (Fig. 5B and C).
Figure 5. Time course of sleep before, during and following light pulses of different durations.
Total sleep time in 15-min bins beginning 15 min before a 20 μW/cm2 light pulse lasting 1 hour (A) 30 min (B) and 15 min (C). The grey area shows the time of darkness and the white area indicates times of light. Filled squares are data from baseline days, during which it was dark during all times shown, and filled circles are data from light pulse days. Data represents mean ± SD (n=8).
Homeostatic decrease in sleep duration in response to light induced sleep
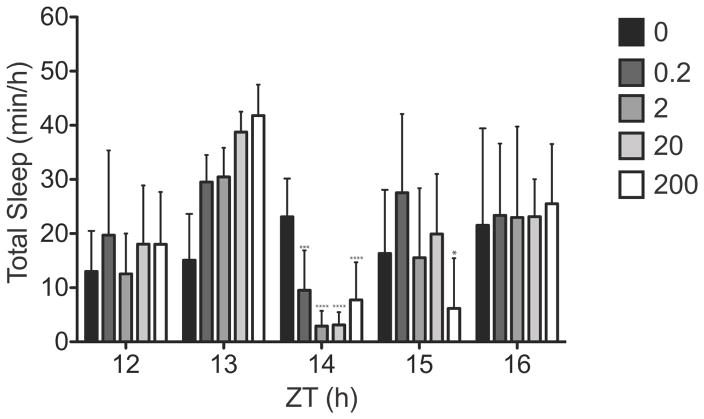
To investigate the long-term effects of a 1-h light pulse on sleep, we analysed the sleep recordings from 10 h of darkness following 1-h light pulses (ZT13–14) of varying intensities (0, 0.2, 2, 20 and 200 μW/cm2). For all intensities, total sleep time in the hour immediately following the light pulse (ZT14–15) was lower (P < 0.001, post hoc t-tests) than in the same hour during baseline in darkness (F4,28 = 14.7; P < 0.0001) (Fig. 6). Only in the 200 μW/cm2 condition was there a continued decrease in sleep amount in the second hour after the pulse (ZT15–16) (P < 0.05, post hoc t-test). For all other times (ZT16–24) and light conditions, sleep times were not different from baseline (data not shown).
Figure 6. Homeostatic response to light induced sleep.
Hourly analysis of total sleep time from ZT12 through ZT16 following light pulse ZT13–14. Mean total sleep time at ZT14 (1-h post-pulse) was decreased compared to baseline at all intensities at ZT14 but returned to baseline levels by ZT 16. Asterisks indicate significant differences from baseline. *p < 0.05, ***p < 0.001; versus baseline at ZT14 and ZT15 by a one-way repeated measures ANOVA followed Bonferroni’s post hoc test. Data represents mean ± SD.
Role of melanopsin in negative masking and sleep induction
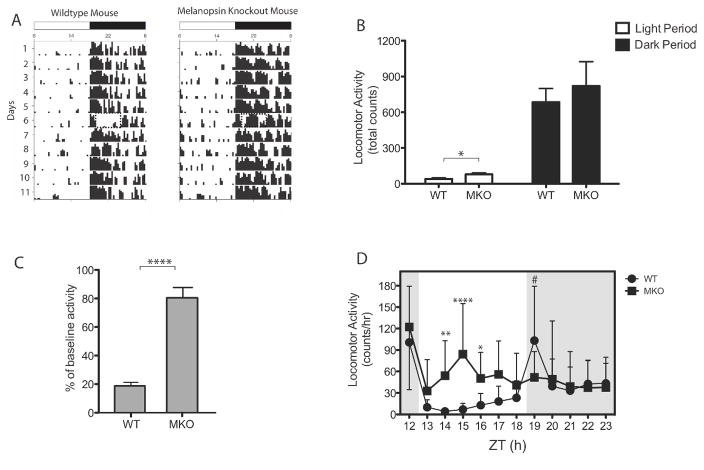
We hypothesised that the maintenance of light-induced sleep is controlled by the non-image-forming retinal pathway involving the ip-RGCs. We tested this hypothesis by characterising the initiation and maintenance of both negative masking and sleep induction by light in MKO mice (Experiment 5). First, we confirmed normal activity levels during the light and dark under the 12 : 12 LD cycle in the WT and MKO mice. In both MKO and WT mice, we observed normal entrainment (Fig. 7A), as reported previously (Ruby et al., 2002; Panda et al., 2003). Analyses of total activity counts (Fig. 7B) during the light period revealed slightly higher amounts of locomotor activity in MKO mice (78.9 ± 12.8 counts) than in WT mice (40.4 ± 9.09 counts) (P < 0.05, t-test). However, total activity count during the dark was statistically indistinguishable between the genotypes (P = 0.54, t-test).
Figure 7. The maintenance of negative masking in mice lacking melanopsin.
(A) Actograms from representative WT (n = 16) and MKO mice (n=13): Days are indicated on the left and time during the day is indicated on the top. Light period was between 0600–1800 hrs and the dark period was between 1800-0600 hrs. The dashed rectangle (Day 6) indicates the period when the light pulse was given (1900-0100 hrs). (B) Total counts/hr during the light and dark periods is shown in both WT and MKO mice. *p < 0.01, by unpaired Student’s t test. (C) Masking of locomotor activity given as percentage of baseline in WT and MKO mice. ****p < 0.0001, by unpaired Student’s t test. (D) Hourly activity count is shown across the dark period in WT and MKO mice. *p < 0.01, **p < 0.01, ****p < 0.0001, by Bonferroni’s post-hoc test. #p < 0.01, by unpaired Student’s t test. Data represents mean ± SD.
To investigate masking, WT (n = 16) and MKO (n = 13) mice were subjected to a 6-h 20 μW/cm2 light pulse, beginning at ZT13. In response to the light pulse, activity was reduced to 18.8 ± 2.50% of baseline levels in WT mice, whereas it was only reduced to 80.9 ± 6.59% of baseline levels in MKO mice (P < 0.001, t-test; Fig. 7C). We next assessed the temporal extent to which the 6-h light pulse changed activity levels in the two genotypes (Fig. 7D). A two-way repeated-measures ANOVA revealed a main effect of genotype (F1,27 = 26.18; P < 0.0001) such that the average locomotor activity was significantly higher for MKO mice than for WT mice. The main effect of time was non-significant (F5,135 = 2.00; P = 0.0829); however, the interaction effect was significant (F5,135 = 3.50; P = 0.0053), indicating a differential distribution of locomotor activity during the light pulse between the genotypes. Differences in hourly activity were observed between ZT14 and ZT17 (P < 0.01; post hoc t-tests), with higher levels of activity in MKO mice. No significant differences in activity were observed between the genotypes during the first hour and the last hour of the light pulse at ZT13 and ZT18, respectively. The total activity count of WT mice (103.3 ± 18.9) was significantly increased in the hour following the pulse as compared with the activity count in MKO mice (51.9 ± 9.99; P = 0.033, t-test), but they were indistinguishable for the remainder of the dark period.
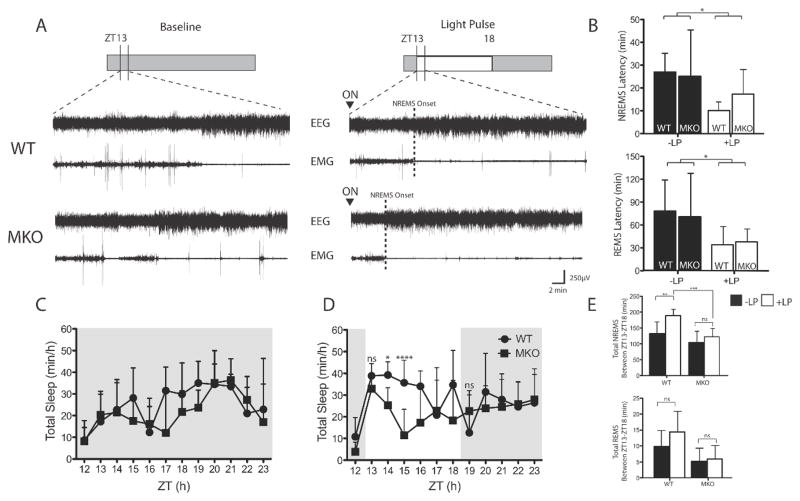
To more closely examine the differences revealed by locomotor activity data, we analysed polysomnographically defined sleep latencies and sleep amounts in WT (n = 7) and MKO (n = 7) mice. We first determined changes in NREMS and REMS sleep latencies after the onset of the light pulse (Fig. 8A and B). NREMS latencies were shortened during the light pulse condition for both genotypes (WT, 26.9 ± 8.29 min at baseline to 10.1 ± 3.77 min during the pulse; MKO, 25.1 ± 20.8 min at baseline to 17.4 ± 11.7 min during the pulse). There was a significant effect of light (F1,22 = 5.97; P = 0.02), but no effect of genotype (F1,22 = 0.30; P = 0.59) or interaction between genotype and light condition (F1,22 = 0.82; P = 0.38). Although there was a decrease in sleep latency in MKO mice from baseline, the difference did not reach statistical significance (P = 0.54; post hoc t-test). A similar reduction was also observed in REMS latency (WT, 78.1 ± 40.8 min at baseline to 34.1 ± 23.8 min during the pulse; MKO, 70.7 ± 57.2 min at baseline to 37.9 ± 16.9 min during the pulse), with an effect of light (F1,23 = 7.79; P = 0.01), but no effect of genotype (F1,23 = 0.01; P = 0.91) or interaction (F1,23 = 0.17; P = 0.68).
Figure 8. Initiation and maintenance of sleep in the absence of melanopsin.
(A). Representative EEG and EMG traces during baseline and at the onset of the light pulse are shown to illustrate the induction of sleep after light onset. (B) NREMS (top) and REMS (bottom) latencies during baseline and in response to the light pulse are quantified. (*light pulse effect: p< 0.02, two-way ANOVA). Total sleep time across the dark period during baseline (C) and the light pulse day (D) in WT and MKO mice. (two-way ANOVA; significant genotype, time and interaction effect between ZT13-ZT15. *p < 0.01, **p < 0.01, ****p < 0.0001, by Bonferroni’s post-hoc test. No interaction between ZT16–18 (see results for further statistics). (E) Summed NREMS (top) and REMS (bottom) is shown for both WT (n=7) and MKO (n=7) mice. **p < 0.01, ***p < 0.001, by Bonferroni’s post-hoc test. Data represents mean ± SD.
We then assessed changes in sleep time across the 6-h pulse. A two-way repeated-measures ANOVA across the entire 6-h pulse revealed a main effect of genotype (F1,12 = 26.74; P < 0.01), indicating that the average total sleep during light exposure was higher for WT mice than for MKO mice. However, there was no main effect of time (F5,60 = 2.29; P = 0.056) or interaction (F5,60 = 1.68; P = 0.15). Given the visual differences in sleep (Fig. 8D), we split the light exposure into two 3-h segments. A two-way repeated-measures ANOVA across the first 3 h of the light pulse revealed an effect of genotype (F1,12 = 41.04; P < 0.001), an effect of time (F2,24 = 6.94; P < 0.01), and an interaction (F2,24 = 3.60; P < 0.05), suggesting a differential distribution of total sleep during the initial 3 h of the pulse (Fig. 8D). Differences in hourly sleep were observed at ZT14 and ZT15 (P < 0.01; post hoc t-tests), with higher levels of total sleep in WT mice. No significant differences in activity were observed between the genotypes during the first hour. Altogether, the data suggest that, although light is able to induce sleep during the first hour, the responses are not dependent on melanopsin.
In examining the latter half of the light pulse, we found an effect of genotype (F1,12 = 5.68; P < 0.05) without an effect of time (F1,12 = 0.29; P = 0.75) or interaction (F1,12 = 1.36; P = 0.27). We also examined total NREMS during the pulse. We found an effect of genotype (F1,24 = 10.59; P < 0.01) and an effect of light pulse (F1,24 = 16.76; P < 0.001), but no interaction (F1,24 = 2.82; P = 0.10). NREMS was increased by almost 1 h in WT mice (131.9 ± 37.07 min at baseline to 188.8 ± 19.77 min during the light pulse; P < 0.01, post hoc t-test), whereas the same pulse increased NREMS by <20 min in MKO mice (103.9 ± 35.54 min at baseline to 122.4 ± 31.06 min during the light pulse; P = 0.55, post hoc t-test) (Fig. 8E, top). In examining REMS, we found an effect of genotype (F1,24 = 11.81; P < 0.01), without an effect of light pulse (F1,24 = 1.97; P = 0.17) or an interaction (F1,24 = 1.06; P = 0.31). The increase in REMS observed in WT mice (9.79 ± 5.02 min at baseline to 14.4 ± 6.44 min during the light pulse) did not reach statistical significance (P = 0.20, post hoc t-test). As was the case for NREMS, no significant changes were observed for REMS in MKO mice (5.21 ± 4.07 min at baseline to 5.93 ± 4.20 min during the light exposure; P > 0.99, post hoc t-test) (Fig. 8E, bottom). No significant difference was found in the amount of sleep in darkness in the first hour after the cessation of the light pulse (P = 0.14, t-test). Note that, although we backcrossed for eight generations to the C57BL/6J background, a confounding effect of flanking genes from the ES donor strain is still possible. One possible solution for future studies could be the use of inducible knockout techniques, which allow temporal control of expression of the gene of interest.
Discussion
Summary
The data here demonstrate that mice are highly responsive to the sleep-inducing properties of light early in the dark phase. The transition to sleep is characterised by rapid induction, followed by a period of sleep maintenance, and ending with a rapid return to wakefulness post-pulse across light intensities and durations. Our data further suggest that, whereas the rods and cones are capable of inducing sleep, the melanopsin-expressing ipRGCs are primarily involved during the maintenance of light-induced sleep early in the dark phase.
Light intensity, rapid sleep induction and sleep homeostasis
Our data suggest that the mouse sleep response early in the dark phase is highly sensitive to light exposure. The degree of the change in sleep is dependent on the intensity of the light stimulus as described by logistic regression models for total sleep, NREMS, and REMS. Our results extend previous results (Borbely, 1976) from a study that used two light intensities during a 1:1 LD cycle to investigate the intensity-dependent differences in sleep in rats. The analysis of sleep episode number/duration suggests that the increase in NREMS and REMS may be achieved by an increase in the number of episodes rather than in the duration of episodes. A visual inspection of the EEG signals revealed regular short arousals across the 1-h light pulses, which supports the idea that the animals are waking up at some frequency during the light exposure and returning to sleep if the light exposure continues.
The dose–response functions described here revealed reduced sensitivity for the light-mediated induction of REMS as compared with NREMS, with the half-maximum sleep-inducing effect of light for REMS being ~60 times that for NREMS. This suggests that REMS is less sensitive to acute light pulses than is NREMS. However, changes in sleep under conditions of additional scotopic (i.e. subsaturating) light amounts will more accurately determine the intensities sufficient to generate half the sleep responses for NREMS and REMS observed at the highest light intensity we used at ZT13. This is important, as the half-maximal intensity for total sleep that our model predicted is lower than that provided by the dimmest light pulse used. Nevertheless, the data suggest that light exerts more potent effects on NREMS than on REMS early in the dark period. Altogether, the findings call into question the underlying mechanisms involved in the changes of NREMS and REMS caused by the presence of light. How changes in light intensity affect the induction of NREMS and REMS across the circadian cycle will be important to understand in the future, as it may reveal additional insights into the control of sleep states by light.
Although a decrease in latency to sleep (~10 min) was observed in response to light of all intensities used in this study, no differences were detected across intensities (Fig. 4A). This is consistent with a previous finding in rats, where it took at least 15–30 min of light to induce a maximum increase in sleep (Borbely et al., 1975; Borbely, 1976). There are both direct and indirect projections of the retinohypothalamic tract to brain regions involved in the regulation of sleep (Hannibal & Fahrenkrug, 2004). However, the precise activation pattern of sleep and/or inhibition of wake centers in the brain involved in the rapid induction of sleep is not well known. This is the first time that the latency to both NREMS and REMS in response to light has been demonstrated across different light intensities. The consistency in the latency to sleep across the range of light intensities used here suggests that the pathways involved are very sensitive to light, as only a small amount of light is needed to cause the transition from wakefulness to sleep in mice. More generally, the low threshold for light to induce changes in sleep as suggested by our data is similar to that observed for phase shifting (Meijer et al., 1992; Benloucif et al., 1997), entrainment (Ebihara & Tsuji, 1980; Altimus et al., 2010), pupillary constriction (Lucas et al., 2001), and negative masking (Mrosovsky et al., 1999; Thompson et al., 2008). With regard to entrainment, light intensities as low as 0.01 lux have been shown to be sufficient for entrainment (Ebihara & Tsuji, 1980). Concerning negative masking, the fast saturation of this response as reported by Mrosovsky et al. (1999) and Thompson et al. (2008) is similar to our sleep data, which show a similar fast saturation of the mouse sleep response early in the dark phase. Taking these findings together, the mouse photic pathways show high sensitivity across a range of non-visual functions. The light-mediated transition from wakefulness to sleep in mice shows a robust behavioral response that should be explored in greater detail in the future.
Our data also demonstrate that, in addition to the circadian phase-dependent effects of light on sleep, robust homeostatic control of sleep duration is also involved in the response to the induction of sleep by light. We found that preceding sleep/wakefulness amounts influenced the ability of light to induce sleep, and that the total sleep time during the light pulse and the hour after was not different from the total sleep time during the equivalent baseline 2 h. This suggests that light adjusts total sleep amounts through homeostatic mechanisms without altering the set-point for sleep need (that is, light changes the temporal distribution of sleep rather than the amount of sleep). The lack of differences in the EEG power density across intensities in the slow-wave and theta range further suggests that the light-induced changes were restricted to the temporal reorganization of sleep rather than changing sleep need. Such homeostatic control has also been demonstrated through the use of short LD cycles ranging from 60 s to 1 h across the 24-h period (Deboer et al., 2007). In that study, total sleep time remained the same across all LD cycle durations. Thus, it is reasonable to conclude that light-mediated changes in sleep are strongly homeostatically regulated, which may explain the immediate post-light pulse decrease in sleep. One cannot rule out the possibility, however, that immediate phase shifts may also be partly involved in the response. Presumably, both circadian and homeostatic processes, as proposed by the two-process model of sleep regulation (Borbely, 1982), are involved in the modulation of light-mediated induction of sleep in the dark.
Melanopsin and the maintenance of masking and sleep
We have further demonstrated that melanopsin is not required for the suppression of locomotor activity or the rapid induction of sleep at the onset of a light pulse, but melanopsin may be required to maintain the response across a long-duration light exposure. Our findings both confirm and extend the results on masking obtained previously with MKO animals (Mrosovsky & Hattar, 2003) and sleep (Altimus et al., 2008; Lupi et al., 2008; Tsai et al., 2009). We found that both NREMS and REMS latencies (~ 15 min and 30 min, respectively) in response to a light pulse are largely unaffected in the absence of melanopsin. As expected, there were no differences in either locomotor activity (Fig. 7D) or total sleep amounts (Fig. 8D) in the first hour, providing further support for the preservation of sleep initiation without melanopsin during the light pulse. A significant reduction (almost 65%) in total sleep was observed by the third hour of the light pulse in MKO mice, suggesting that melanopsin is primarily important for maintaining light-induced sleep over long periods of time. Interestingly, ipRGCs have recently been reported to respond to light continuously for at least 10 h (Wong, 2012). However, whether light signaling is maintained in the absence of melanopsin over a similar time span is not known.
In contrast to the aforementioned studies, we found an increase in locomotor activity and, as expected, a decrease in sleep amount in WT mice during the hour following the light pulse. However, such changes were not observed in MKO mice. A likely explanation for the difference may lie within the homeostatic regulation of sleep duration. Light increased total sleep by only ~18% during the pulse in MKO mice, whereas a ~42% increase was observed in WT mice. Thus, changes in sleep amounts post-pulse would be minimal in MKO mice in comparison with WT mice, in which there are large compensatory changes to ensure the return to baseline sleep amounts. Consequently, total sleep time during the entire 12-h dark period does not change significantly as compared with baseline in either WT or MKO mice. It is likely, then, that such compensatory changes observed almost immediately after the pulse work to maintain 24-h total sleep amounts.
We also confirmed greater suprachiasmatic nucleus (SCN) activation with FOS expression in response to light in WT mice (Fig. S3), whereas a reduced response was observed in MKO mice, similarly to what others have found (Ruby et al., 2002; Lupi et al., 2006; Tsai et al., 2009). This suggests that, in addition to the SCN, other regions are probably involved during the early stages of light-induced sleep, as locomotor activity and sleep amounts were similar in WT and MKO mice during the first hour (Figs 7 and 8). Although it is not well known whether the SCN has a causal relationship with the changes in transition state in response to light, some inferences can be drawn from the existing evidence. First, the transition between vigilance states has been shown to correlate with SCN firing rate, whereby SCN neurons fired at lower rates during NREMS than during REMS (Deboer et al., 2003). Second, electrophysiological recordings within the SCN have also shown a general increase in activity almost immediately at the onset of a light exposure before it reaches a steady state (Meijer et al., 1998). Recent experiments from the same group have similarly demonstrated both transient and sustained responses within the SCN, even in the absence of melanopsin (van Oosterhout et al., 2012). Their data suggest that activity within the SCN can be initiated and maintained in the short term without melanopsin. However, it remains unknown whether the response could be maintained for periods longer than the 5 min used in the study. Finally, the SCN sends both direct and indirect projections to several nuclei involved in the regulation of sleep (Deurveilher & Semba, 2005). These downstream projections represent the SCN-dependent pathways by which light information may influence both short-term and long-term changes in the induction of sleep.
The ipRGCs send their axons to several regions in the brain (Hattar et al., 2006). More extensive studies are required to determine the specific roles of other targets activated by the ipRGCs during both the rapid transition from wakefulness to sleep and the subsequent maintenance of sleep during a light pulse. These targets include the anterior hypothalamic area, ventrolateral preoptic area, lateral hypothalamus, ventral subparaventricular zone, pretectum, intergeniculate leaflet nucleus of the thalamus, and superior colliculus. The data presented here provide a framework for future research into the roles of these neural substrates in the light-mediated induction of sleep in the dark.
Supplementary Material
Figure S1. Mice maintain normal entrainment to the 12:12 LD cycle in between light pulses.
Figure S2. Effects of light on the power spectrum of wake during the 1-h 200 μW/cm2 light pulse.
Figure S3. Effects of a 15-min 200 μW/cm2 light pulse on FOS immunoreactivity in the SCN.
Acknowledgments
A special thanks goes to Grace Hagiwara, Vinh Cao and Norman Ruby for valuable discussions and technical advice. This work was supported by the Down Syndrome Research and Treatment Foundation (DSRTF) and the Research Down Syndrome Organization (RDS).
Abbreviations
- ipRGCs
intrinsically photosensitive retinal ganglion cells
- LD
light/dark
- MKO
melanopsin knockout
- NREMS
non rapid eye movement sleep
- REMS
rapid eye movement sleep
- SCN
suprachiasmatic nucleus
- SD
standard deviation
- WT
wildtype
References
- Alfoldi P, Franken P, Tobler I, Borbely AA. Short light-dark cycles influence sleep stages and EEG power spectra in the rat. Behav Brain Res. 1991;43:125–131. doi: 10.1016/s0166-4328(05)80062-2. [DOI] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Neurobiol Dis. 1982;1:195–204. [PubMed] [Google Scholar]
- Borbely AA. Sleep and motor activity of the rat during ultra-short light-dark cycles. Brain Res. 1976;114:305–317. doi: 10.1016/0006-8993(76)90673-9. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Huston JP, Waser PG. Control of sleep states in the rat by short light-dark cycles. Brain Res. 1975;95:89–101. doi: 10.1016/0006-8993(75)90209-7. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Colas D, Valletta JS, Takimoto-Kimura R, Nishino S, Fujiki N, Mobley WC, Mignot E. Sleep and EEG features in genetic models of down syndrome. Neurobiol Dis. 2008;30:1–7. doi: 10.1016/j.nbd.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Ruijgrok G, Meijer JH. Short light-dark cycles affect sleep in mice. Eur J Neurosci. 2007;26:3518–3523. doi: 10.1111/j.1460-9568.2007.05964.x. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: Implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Tsuji K. Entrainment of the circadian activity rhythm to the light cycle: Effective light intensity for a zeitgeber in the retinal degenerate C3H mouse and the normal C57BL mouse. Physiol Behav. 1980;24:523–527. doi: 10.1016/0031-9384(80)90246-2. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 2004;316:99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Lupi D, Sekaran S, Jones SL, Hankins MW, Foster RG. Light-evoked FOS induction within the suprachiasmatic nuclei (SCN) of melanopsin knockout (Opn4-/-) mice: A developmental study. Chronobiol Int. 2006;23:167–179. doi: 10.1080/07420520500545870. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: Long-term multiunit and single-unit recordings in freely moving rats. J Neurosci. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Rusak B, Ganshirt G. The relation between light-induced discharge in the suprachiasmatic nucleus and phase shifts of hamster circadian rhythms. Brain Res. 1992;598:257–263. doi: 10.1016/0006-8993(92)90191-b. [DOI] [PubMed] [Google Scholar]
- Morin LP, Lituma PJ, Studholme KM. Two components of nocturnal locomotor suppression by light. J Biol Rhythms. 2010;25:197–207. doi: 10.1177/0748730410369890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Lucas RJ, Foster RG. Persistence of masking responses to light in mice lacking rods and cones. J Biol Rhythms. 2001;16:585–588. doi: 10.1177/074873001129002277. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Foster RG, Salmon PA. Thresholds for masking responses to light in three strains of retinally degenerate mice. J Comp Physiol A. 1999;184:423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- Nelson R, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comparative Biochemistry and Physiology. 1981;69A:145–148. [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Redlin U, Mrosovsky N. Masking of locomotor activity in hamsters. J Comp Physiol A. 1999;184:429–437. doi: 10.1007/s003590050342. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105:15593–15598. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Rusak B, Zucker I. Neural regulation of circadian rhythms. The American Physiological Society. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- Thompson S, Foster RG, Stone EM, Sheffield VC, Mrosovsky N. Classical and melanopsin photoreception in irradiance detection: Negative masking of locomotor activity by light. Eur J Neurosci. 2008;27:1973–1979. doi: 10.1111/j.1460-9568.2008.06168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: Circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(−/−) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oosterhout F, Fisher SP, van Diepen HC, Watson TS, Houben T, VanderLeest HT, Thompson S, Peirson SN, Foster RG, Meijer JH. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr Biol. 2012;22:1397–1402. doi: 10.1016/j.cub.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci. 2012;32:11478–11485. doi: 10.1523/JNEUROSCI.1423-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mice maintain normal entrainment to the 12:12 LD cycle in between light pulses.
Figure S2. Effects of light on the power spectrum of wake during the 1-h 200 μW/cm2 light pulse.
Figure S3. Effects of a 15-min 200 μW/cm2 light pulse on FOS immunoreactivity in the SCN.