Abstract
The influence of adiposity over life course on cancer risk remains poorly understood. We assessed trajectories of body shape from age 5 up to 60 using a group-based modeling approach among 73,581 women from the Nurses’ Health Study and 32,632 men from the Health Professionals Follow-up Study. After a median of approximately 10 years of follow-up, we compared incidence of total and obesity-related cancers (cancers of the esophagus [adenocarcinoma only], colorectum, pancreas, breast [after menopause], endometrium, ovaries, prostate [advanced only], kidney, liver and gallbladder) between these trajectories. We identified 5 distinct trajectories of body shape: lean-stable, lean-moderate increase, lean-marked increase, medium-stable, and heavy-stable/increase. Compared with women in the lean-stable trajectory, those in the lean-marked increase and heavy-stable/increase trajectories had a higher cancer risk in the colorectum, esophagus, pancreas, kidney, and endometrium (relative risk [RR] ranged from 1.22 to 2.56). Early life adiposity was inversely while late life adiposity was positively associated with postmenopausal breast cancer risk. In men, increased body fatness at any life period was associated with a higher risk of esophageal adenocarcinoma and colorectal cancer (RR ranged from 1.23 to 3.01), and the heavy-stable/increase trajectory was associated with a higher risk of pancreatic cancer, but lower risk of advanced prostate cancer. The trajectory-cancer associations were generally stronger for non-smokers and women who did not use menopausal hormone therapy. In conclusion, trajectories of body shape throughout life were related to cancer risk with varied patterns by sex and organ, indicating a role for lifetime adiposity in carcinogenesis.
Keywords: obesity, induction period, group-based modeling, life course epidemiology
INTRODUCTION
The prevalence of overweight and obesity has increased rapidly over the past few decades, creating major public health problems. Given that body mass index (BMI) typically increases with age,1 and obesity during childhood is associated with the persistence of obesity into adulthood,2 a life course perspective is crucial to better understand the health consequences of overweight and obesity, including their influences on cancer risk.
According to systematic reviews by the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR),3, 4 adulthood obesity has been related to an increased risk of cancers in the esophagus (adenocarcinoma), colorectum, pancreas, breast (after menopause), endometrium, kidney, and liver with convincing evidence; and cancers in the ovaries, prostate (advanced only), and gallbladder with probable evidence. Some of the potential mechanisms through which obesity affects cancer development include hyperinsulinemia, excess activation of the insulin-like growth factor (IGF) axis, dysregulated production of sex hormones and adipokines, and chronic low-grade inflammation.5 Other more organ-specific mechanisms linking obesity to cancer have also been proposed, such as gastroesophageal reflux for esophageal adenocarcinoma, non-alcoholic fatty liver disease for liver cancer, and increased glomerular filtration rate and renal plasma flow for kidney cancer. Despite these compelling data, however, little is known about the relationship between body fatness across the lifespan and cancer risk.
Given the long induction period of carcinogenesis, it is plausible that the effects of adiposity on cancer risk may differ over the life course. Although some evidence suggests that obesity in childhood or young adulthood is associated with lower risk of breast6–10 and advanced prostate cancer,11–13 and higher risk of colorectal14–16 and endometrial cancer,17–20 the findings remain inconclusive21–25 and there are little data for other, less common cancers. More importantly, these studies assessed body fatness at select time points individually, making it challenging to separate and interpret the effect of early adiposity from later weight gain due to high correlation.
Therefore, in this study, we employed a novel life course approach to characterize distinct trajectories of body shape across the lifespan. By comparing cancer incidence across these trajectories, our study provides the first prospective data about the relationship between lifetime body shape, as a surrogate of adiposity, and risk of overall and obesity-related cancers.
METHODS
Study population
We included data from two, large ongoing U.S. cohort studies: the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). Details about the two studies have been described elsewhere.26, 27 Briefly, the NHS enrolled 121,701 registered female nurses aged 30–55 years in 1976, and the HPFS enrolled 51,529 male health professionals aged 40–75 years in 1986. Follow-up questionnaires were administered at baseline enrollment and every two years thereafter to collect updated lifestyle and medical information. The follow-up rates of the two cohorts had been 95.4% in the NHS and 95.9% in the HPFS. This investigation was approved by the Institutional Review Board at the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
Assessment of body shape
In 1988, participants in both cohorts were asked to recall their body shape in early and middle life by choosing one of 9 pictorial body diagrams (somatotypes) developed by Stunkard et al28 that best depicted their body outline at ages 5, 10, 20, 30, and 40. The validity of this measure as a surrogate for adiposity in early life has been assessed among 181 participants aged 71 to 76 years in the Third Harvard Growth Study.29 We compared participants’ recalled body shape (somatotypes) with their measured BMI at approximately the same ages. The Pearson correlation coefficients were 0.60 for age 5, 0.65 for age 10, and 0.66 for age 20 in women. The corresponding Pearson correlation coefficients in men were 0.36, 0.66, and 0.53, respectively.29
In both cohorts, body height and weight were queried at baseline enrollment and updated weight was collected via biennial follow-up questionnaires. We used these data to calculate BMI at age 50 and 60 and then converted BMI to the same scale as somatotypes in younger ages. To minimize random variation, we assessed the average BMI from age 47 to 53 as the BMI for age 50, and average BMI from age 57 to 63 as the BMI for age 60. We then divided BMI at these two ages into 9 categories, consistent with the grouping of somatotypes (ranging from 1 to 9) at younger ages. The cutoff points for each category were calculated as the mean BMI of this category at age 40 plus a constant to account for weight gain from age 40 to 50 or 60. For example, in women the mean BMIs at age 40 for the 4th and 5th category of somatotypes were 23.3 and 26.1 kg/m2, respectively, and the mean increment of BMI from age 40 to 50 was 1.5 kg/m2. Therefore, the lower cutoff of BMI for the 5th category of somatotypes at age 50 would be 23.3+1.5=24.8 kg/m2 and the upper cutoff would be 26.1+1.5=27.6 kg/m2. Similar categorizations were conducted for the other categories as well as in men. The BMI cutoffs used to derive somatotype categories at age 50 and 60 in the two cohorts were summarized in Supplementary Table 1.
Trajectory modeling
We used a group-based trajectory modeling approach implemented by SAS Proc Traj to identify subgroups within each cohort that shared a similar underlying trajectory of body shape from age 5 up to 60 among 84,792 women from the NHS and 37,706 men from the HPFS who provided somatotype data for at least 4 different ages. This method represents an application of finite mixture modeling and is designed to identify relatively homogeneous clusters of developmental trajectories within the population. It fits longitudinal data as a discrete mixture of two or more latent trajectories via maximum likelihood using SAS Proc Traj.30 In this study, we used a censored normal model as a polynomial function of the time scale (i.e., age). The optimal number of groups and the shapes of trajectories were selected for best fit to the data using a two-stage approach, as assessed by change in the Bayesian Information Criterion (BIC).31 The first stage was to determine the number of groups using a quadratic form for all trajectory groups. Given the data we had, we considered up to 5 groups and compared the BIC to that with 4, 3, 2, and 1 groups, respectively. Once we had identified that the model with 5 groups fit best, we then determined in the second stage the order of the polynomial function specifying the shape of each trajectory. We compared the BIC of the 5-group models with different functional forms and found that the model with all groups with up to cubic order terms demonstrated the best fit to the data. Therefore, estimation of body shape trajectory throughout life was carried out in the final model using a cubic function of age for each of the 5 trajectories. We then named the trajectory groups to describe their visual patterns (i.e., lean-stable, lean-moderate increase, lean-marked increase, medium-stable, and heavy-stable/increase).
From the final model, we calculated the posterior predicted probability for each individual of being a member of each of the 5 trajectories. Participants were assigned into the trajectory group to which their posterior membership probability was largest. We then assessed the adequacy of our final model by calculating the average posterior probability of assignment for each group. Using ≥0.70 as the recommended criteria,31 our model demonstrated good discrimination in classifying individuals into distinctive trajectory groups: the average posterior probability for each trajectory group was 0.92, 0.86, 0.90, 0.95, and 0.92 in women; and 0.85, 0.92, 0.88, 0.84, and 0.90 in men, respectively. We also calculated the odds of correct classification (OCC), which is the ratio of the odds of correct classification on the basis of the maximum probability classification rule to the odds of correct classification based on random assignment. The OCC for each trajectory group was 100.4, 43.1, 21.4, 23.2, and 66.4 in women; and 28.0, 51.3, 12.5, 29.0, and 55.7 in men, respectively. Using OCC>5.0 for all groups as the recommended criteria, our model demonstrated high assignment accuracy.31
Covariate assessment
In both the NHS and HPFS, we inquired about potential risk factors for cancer in the baseline and biennial follow-up questionnaires, including family history of cancer, smoking, colonoscopy or sigmoidoscopy examination, physical activity, multivitamin use, and use of aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs). Physical activity was calculated by summing the products of time spent on a variety of recreational or leisure-time activities with the average metabolic equivalent (MET) for that activity, except that in 1980 in the NHS a simple questionnaire was used to inquire regular physical activity without collecting detailed information on specific activities and durations. In women, we additionally assessed physical and mammographic examination, menopausal status, and use of menopausal hormone therapy (MHT) in the questionnaires. Prostate-specific antigen test was queried biennially in men.
Participants were asked about their most recent dietary intake using validated food frequency questionnaires (FFQ) in 1980, 1984, 1986 and every 4 years thereafter in the NHS, and in 1986 and every 4 years thereafter in the HPFS. Alcohol consumption was assessed from the FFQs. We also calculated a summary dietary score, the Alternate Healthy Eating Index (AHEI), to represent the overall dietary pattern based on individual food intake. AHEI is designed to target food choices and macronutrient sources associated with reduced chronic disease risk. Adherence to AHEI has been associated with a lower risk of major chronic diseases in the two cohorts.32
Outcome ascertainment
In both cohorts, self-reported diagnoses of cancer were obtained on biennial questionnaires, and participants who reported a cancer diagnosis were asked for permission to acquire their medical records and pathologic reports. We identified deaths through the National Death Index and next-of-kin_ENREF_28. A study physician, blinded to exposure information, reviewed medical records to confirm cancer diagnosis and to extract relevant information, such as histology, grade, and sublocation.33 The outcomes of interest in this study include total cancer and specific cancers that have been related to obesity with probable or convincing evidence in the most recent WCRF/AICR reviews, including cancers of the esophagus (adenocarcinoma only), colorectum, pancreas, kidney, breast (after menopause), endometrium, ovaries, prostate (advanced only), liver, and gallbladder. Because of the small number of cases, liver and gallbladder cancers were not examined individually but included in the obesity-related cancer analysis. Because only advanced prostate cancer was related to obesity in the WCRF/AICR review,4 we only included advanced prostate cancer cases (defined as those that had spread outside the prostate [stage T3b/T4, N1, or M1] or lethal tumors) in our total cancer and prostate cancer analysis.
Association analysis
We used SAS 9.3 for all analyses (SAS Institute Inc., Cary, NC, USA). All statistical tests were two sided and P < 0.05 was considered statistically significant.
Among participants with a trajectory assignment, we excluded those who died or had a history of cancer diagnosis before age 60. After exclusion, 73,581 women and 32,632 men followed for cancer incidence from age 60 onwards were included in the analysis. To minimize the influence of reverse causation arising from undiagnosed cancer-induced weight loss, we allowed for a 2-year lag period and thus follow-up time was calculated from age 62 to the age of cancer diagnosis, death, or the end of the study period (June 1, 2010 for the NHS and January 31, 2010 for the HPFS), whichever came first. Cox proportional hazards model with age as the time scale was used to estimate the hazard ratios (as estimates of relative risk [RR]) and 95% confidence intervals (CIs) for cancer incidence in relation to trajectories. We adjusted for several risk factors for cancer in the multivariable models to control for confounding (see the footnotes of Table 1). Current BMI was not adjusted. We assessed the proportional hazards assumption by including the product term between age and each covariate (including trajectory groups) to the multivariable model, and then tested the statistical significance of the product term via a likelihood ratio test. No deviation from proportional hazards assumption was detected at α=0.05 level.
Table 1.
Basic characteristics of study participants at age 60 according to trajectories of body shape in women (Nurses’ Health Study) and men (Health Professionals Follow-up Study)a
| Variable | Lean-stable | Lean-moderate increase | Lean-marked increase | Medium-stable | Heavy-stable/increase |
|---|---|---|---|---|---|
| Women | |||||
| No. of participants (%) | 13,183 (16) | 18,405 (22) | 18,217 (21) | 23,288 (27) | 11,699 (14) |
| BMI at age 18, kg/m2 | 19.6 | 19.7 | 21.8 | 21.5 | 25.0 |
| BMI at age 40, kg/m2 | 19.8 | 22.0 | 26.0 | 22.1 | 29.3 |
| BMI at age 50, kg/m2 | 20.3 | 23.8 | 28.8 | 23.3 | 31.7 |
| BMI at age 60, kg/m2 | 20.5 | 25.3 | 30.7 | 24.3 | 33.0 |
| Height, inch | 64.7 | 64.5 | 64.3 | 64.5 | 64.5 |
| Physical activity, METs-hours/week b | 20.4 | 16.4 | 13.6 | 18.1 | 12.8 |
| Alcohol consumption, g/d b | 7.6 | 6.1 | 4.5 | 7.2 | 4.3 |
| Pack-years of smoking b | 14.5 | 13.1 | 12.5 | 15.0 | 14.7 |
| Alternative Healthy Eating Index b | 45.4 | 43.8 | 43.3 | 45.5 | 43.7 |
| Family history of cancer, %c | 58 | 60 | 59 | 59 | 58 |
| Multivitamin use, % | 56 | 52 | 51 | 54 | 52 |
|
| |||||
| Men | |||||
| No. of participants (%) | 5,946 (16) | 6,881 (18) | 14,225 (38) | 5,725 (15) | 4,929 (13) |
| BMI at age 21, kg/m2 | 20.5 | 20.4 | 22.3 | 22.3 | 25.0 |
| BMI at age 40, kg/m2 | 22.0 | 23.4 | 25.7 | 23.7 | 28.3 |
| BMI at age 50, kg/m2 | 22.3 | 24.1 | 26.9 | 24.2 | 29.5 |
| BMI at age 60, kg/m2 | 22.6 | 24.8 | 27.7 | 24.5 | 30.1 |
| Height, inch | 70.2 | 70.3 | 70.1 | 70.1 | 70.0 |
| Physical activity, METs-hours/week b | 29.4 | 26.3 | 26.0 | 31.5 | 25.2 |
| Alcohol consumption, g/d b | 11.2 | 11.7 | 11.8 | 11.8 | 10.8 |
| Pack-years of smoking b | 12.0 | 14.0 | 14.8 | 14.2 | 17.4 |
| Alternative Healthy Eating Index b | 42.8 | 41.4 | 40.6 | 43.0 | 41.5 |
| Family history of cancer, %c | 37 | 37 | 36 | 37 | 36 |
| Multivitamin use, % | 51 | 48 | 48 | 51 | 47 |
Abbreviations: BMI, body mass index; MET, metabolic equivalent of task.
All variables are standardized by age at baseline (1976 for the Nurses’ Health Study and 1986 for the Health Professionals Follow-up Study). Mean is presented for continuous variables.
Cumulative average measurements from baseline up to age of 60 years.
Defined as history of cancer diagnosis among biological parents or siblings (including cancers in the colon, rectum, breast, pancreas, ovary, uterus (excluding fibroids or cervical cancer) or prostate, or melanoma).
To examine the influence of early life adiposity on cancer risk, we compared cancer incidence between the two extreme trajectories whose body shape measurements were similar at age 60 years but substantially different at early ages (i.e., the heavy-stable/increase and lean-marked increase groups). We further adjusted for the average body shape levels between age 40 and 60 years to minimize any difference in late life body shape between the two groups. Similarly, to assess the effect for late life body shape, we compared cancer incidence between the lean-marked increase to the lean-stable groups with further adjustment for the average body shape levels from age 5 to 20 years.
Because smoking is a risk factor for many cancers and also decreases adiposity, we assessed whether the adiposity-cancer relationship was stronger among non-smokers than smokers in a stratified analysis and assessed the statistical significance of any interaction via a likelihood ratio test, by comparing the model with the product terms between smoking and trajectories to the model without these terms. Given the prior evidence that obesity is differentially associated with cancer risk according to use of MHT, likely because estrogen is an important risk factor for some female-related cancers, we also performed stratified and interaction analyses by MHT use in women.
RESULTS
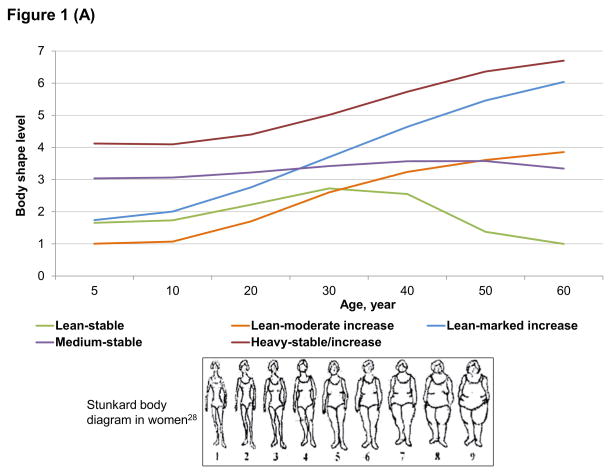
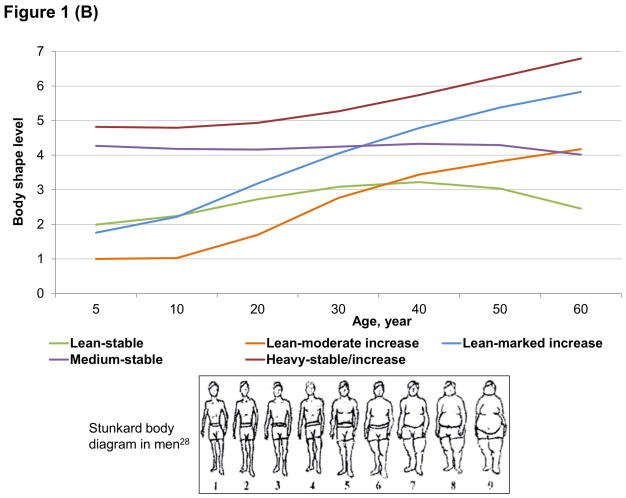
We identified 5 distinct trajectories of body shape from age 5 up to 60 (Figure 1): 16% of women and men maintained a lean body shape across the lifespan (lean-stable group); 22% of women and 18% of men started lean and then experienced a moderate increase in body shape (lean-moderate increase group); 21% of women and 38% of men started lean and then gained a substantial amount of weight (lean-marked increase group); 27% of women and 15% of men maintained a medium body shape throughout life (medium-stable group); and 14% of women and 13% of men started heavy and then maintained or gained weight (heavy-stable/increase group). In general, BMI in each trajectory well tracked from adolescence to late adulthood (Table 1). Participants in the 5 trajectories also demonstrated distinctive patterns in lifestyle factors: those in the lean-stable and medium-stable groups were more likely to exercise, use multivitamins, and follow the Alternate Healthy Eating Index than those in other groups.
Figure 1.
Trajectories of body shape by age in women (A) and men (B)
Table 2 presents the RRs of cancer in the other trajectory groups compared to the lean-stable group. In women, those in the lean-moderate increase, lean-marked increase, and heavy-stable/increase groups had a higher risk of total and obesity-related cancer, with RRs ranging from 1.06 to 1.39. By cancer site, women in the lean-marked increase and heavy-stable/increase groups had a higher risk of cancer in the colorectum, esophagus (adenocarcinoma only), pancreas, kidney, and endometrium (RR ranged from 1.22 to 2.56), although the association was not statistically significant for pancreatic cancer and esophageal adenocarcinoma. For postmenopausal breast cancer, an elevated risk was seen in women in the lean-moderate (RR=1.30, 95% CI, 1.17–1.45) and lean-marked increase (RR=1.41, 95% CI, 1.26–1.58) groups. We did not find any association for ovarian cancer.
Table 2.
Relative risk of cancer according to trajectories of body shape in women (Nurses’ Health Study) and men (Health Professionals Follow-up Study)a
| Lean-stable | Lean-moderate increase | Lean-marked increase | Medium-stable | Heavy-stable/increase | |
|---|---|---|---|---|---|
| Women | |||||
| Total cancer | |||||
| No. of cases (N=11,462) | 1,893 | 2,632 | 2,345 | 3,175 | 1,417 |
| Incidence rateb | 88.8 | 97.3 | 106.7 | 93.5 | 107.7 |
| RR (95% CI) | 1 (reference) | 1.06 (1.00–1.13) | 1.16 (1.09–1.24) | 1.04 (0.98–1.10) | 1.15 (1.07–1.23) |
| Obesity-related cancerc | |||||
| No. of cases (N=6,400) | 1,001 | 1,530 | 1,434 | 1,628 | 807 |
| Incidence rateb | 53.0 | 59.2 | 67.6 | 52.5 | 63.2 |
| RR (95% CI) | 1 (reference) | 1.17 (1.08–1.30) | 1.39 (1.27–1.50) | 1.03 (0.95–1.12) | 1.28 (1.17–1.41) |
| Colorectal cancer | |||||
| No. of cases (N=1,131) | 191 | 250 | 233 | 299 | 158 |
| Incidence rateb | 9.7 | 9.2 | 10.4 | 9.2 | 11.9 |
| RR (95% CI) | 1 (reference) | 0.97 (0.80–1.17) | 1.22 (1.00–1.49) | 1.02 (0.85–1.22) | 1.40 (1.13–1.74) |
| Esophageal adenocarcinoma | |||||
| No. of cases (N=38) | 4 | 6 | 13 | 7 | 8 |
| Incidence rateb | 0.2 | 0.2 | 0.6 | 0.2 | 0.6 |
| RR (95% CI) | 1 (reference) | 1.02 (0.29–3.63) | 2.56 (0.82–8.03) | 1.04 (0.30–3.57) | 2.19 (0.63–7.70) |
| Pancreatic cancer | |||||
| No. of cases (N=324) | 49 | 76 | 69 | 88 | 42 |
| Incidence rateb | 2.6 | 2.9 | 3.2 | 2.8 | 3.3 |
| RR (95% CI) | 1 (reference) | 1.18 (0.82–1.69) | 1.36 (0.93–1.98) | 1.15 (0.81–1.63) | 1.39 (0.91–2.12) |
| Kidney cancer | |||||
| No. of cases (N=212) | 27 | 46 | 57 | 46 | 36 |
| Incidence rateb | 1.4 | 1.8 | 2.7 | 1.5 | 2.8 |
| RR (95% CI) | 1 (reference) | 1.26 (0.78–2.04) | 1.89 (1.19–3.03) | 1.05 (0.65–1.69) | 1.92 (1.15–3.21) |
| Postmenopausal breast cancer | |||||
| No. of cases (N=3,454) | 536 | 898 | 767 | 887 | 366 |
| Incidence rateb | 29.0 | 35.9 | 37.1 | 29.7 | 29.4 |
| RR (95% CI) | 1 (reference) | 1.30 (1.17–1.45) | 1.41 (1.26–1.58) | 1.05 (0.94–1.17) | 1.11 (0.97–1.28) |
| Endometrial cancer | |||||
| No. of cases (N=664) | 95 | 125 | 167 | 144 | 133 |
| Incidence rateb | 5.0 | 4.83 | 7.8 | 4.7 | 10.5 |
| RR (95% CI) | 1 (reference) | 0.99 (0.75–1.29) | 1.57 (1.21–2.03) | 0.94 (0.73–1.22) | 2.08 (1.59–2.73) |
| Ovarian cancer | |||||
| No. of cases (N=476) | 92 | 107 | 95 | 130 | 52 |
| Incidence rateb | 4.8 | 4.1 | 4.4 | 3.3 | 4.1 |
| RR (95% CI) | 1 (reference) | 0.88 (0.66–1.16) | 0.93 (0.70–1.25) | 0.88 (0.67–1.15) | 0.84 (0.59–1.19) |
|
| |||||
| Men | |||||
| Total cancerd | |||||
| No. of cases (N=4,976) | 751 | 1,078 | 1,889 | 706 | 552 |
| Incidence rateb | 108.4 | 118.5 | 115.9 | 110.5 | 123.3 |
| RR (95% CI) | 1 (reference) | 1.06 (0.96–1.16) | 1.07 (0.98–1.16) | 1.04 (0.94–1.15) | 1.22 (1.09–1.36) |
| Obesity-related cancere | |||||
| No. of cases (N=1,832) | 267 | 431 | 689 | 262 | 183 |
| Incidence rateb | 33.8 | 42.4 | 37.4 | 37.0 | 35.0 |
| RR (95% CI) | 1 (reference) | 1.17 (1.00–1.37) | 1.09 (0.95–1.26) | 1.13 (0.95–1.34) | 1.17 (0.97–1.42) |
| Colorectal cancer | |||||
| No. of cases (N=592) | 79 | 141 | 222 | 85 | 65 |
| Incidence rateb | 10.1 | 14.2 | 12.3 | 11.9 | 13.0 |
| RR (95% CI) | 1 (reference) | 1.36 (1.03–1.80) | 1.23 (0.95–1.60) | 1.26 (0.92–1.72) | 1.47 (1.05–2.05) |
| Esophageal adenocarcinoma | |||||
| No. of cases (N=60) | 5 | 13 | 25 | 7 | 10 |
| Incidence rateb | 0.7 | 1.3 | 1.4 | 1.0 | 2.1 |
| RR (95% CI) | 1 (reference) | 1.90 (0.67–5.34) | 2.09 (0.80–5.48) | 1.53 (0.48–4.84) | 3.01 (1.04–9.13) |
| Pancreatic cancer | |||||
| No. of cases (N=231) | 34 | 39 | 94 | 34 | 30 |
| Incidence rateb | 4.5 | 4.0 | 5.4 | 4.9 | 6.2 |
| RR (95% CI) | 1 (reference) | 0.85 (0.54–1.35) | 1.20 (0.81–1.78) | 1.12 (0.70–1.80) | 1.50 (0.92–2.46) |
| Kidney cancer | |||||
| No. of cases (N=202) | 33 | 46 | 72 | 32 | 19 |
| Incidence rateb | 4.4 | 4.7 | 4.1 | 4.6 | 3.9 |
| RR (95% CI) | 1 (reference) | 1.05 (0.67–1.64) | 0.94 (0.63–1.43) | 1.07 (0.66–1.74) | 0.93 (0.53–1.64) |
| Advanced prostate cancerf | |||||
| No. of cases (N=694) | 112 | 182 | 259 | 96 | 45 |
| Incidence rateb | 14.6 | 18.1 | 14.4 | 13.9 | 8.8 |
| RR (95% CI) | 1 (reference) | 1.16 (0.91–1.47) | 0.97 (0.78–1.21) | 1.00 (0.76–1.32) | 0.67 (0.47–0.95) |
Abbreviations: CI, confidence interval; RR, relative risk.
Follow-up started at age 62. RRs were estimated from Cox proportional hazards model adjusted for height (continuous), race (non-white or white), pack-years of smoking (women: 0, 1–<6, 6–≤20, or >20; men: 0, 1–<5, 5–≤25, 26–≤45, or >45), family history of cancer (yes or no), history of lower gastrointestinal endoscopy (yes or no; for analysis of total cancer and colorectal cancer), multivitamin use (yes or no), regular aspirin/NSAID use (yes or no), history of physical exam (yes and for screening, yes and for symptoms, or no), mammography (women only: yes and for screening, yes and for symptoms, or no; for total cancer and breast cancer), prostate-specific antigen test (men only: yes or no), menopausal hormone therapy (women only: past use, current use, or no), physical activity (in quintiles), alcohol consumption (women: 0–<0.5, 0.5–<2, 2–<8, or ≥8 g/d; men: 0–<5, 5–<10, 10–<15, 15–<30, or ≥30 g/d), and AHEI dietary score (in quintiles).
Incidence rate per 100,000 person-years.
Including cancers of the colorectum, esophagus (adenocarcinoma only), pancreas, kidney, breast (postmenopause), endometrium, ovaries, liver, and gallbladder.
Excluding non-advanced prostate cancer.
Including cancers of the colorectum, esophagus (adenocarcinoma only), pancreas, kidney, prostate (advanced cancer only), liver, and gallbladder.
Advanced prostate cancer defined as cases that had spread outside the prostate (stage T3b/T4, N1, or M1) or lethal tumors.
In men, compared with the lean-stable group, the other 4 groups were all at an elevated risk of total and obesity-related cancer as well as colorectal cancer. A higher risk of esophageal adenocarcinoma (RR=3.01, 95% CI, 1.04–9.13) and pancreatic cancer (RR=1.50, 95% CI, 0.92–2.46) was observed among men in the heavy-stable/increase group. In contrast, the heavy-stable/increase group had a lower risk of advanced prostate cancer (RR=0.67, 95% CI, 0.47–0.95). We did not find any association for kidney cancer.
In the sensitivity analysis, we excluded participants whose trajectory assignment probability was below 0.80, and the results remained essentially unchanged (Supplementary Table 2), indicating that our findings are robust to modest trajectory misclassification.
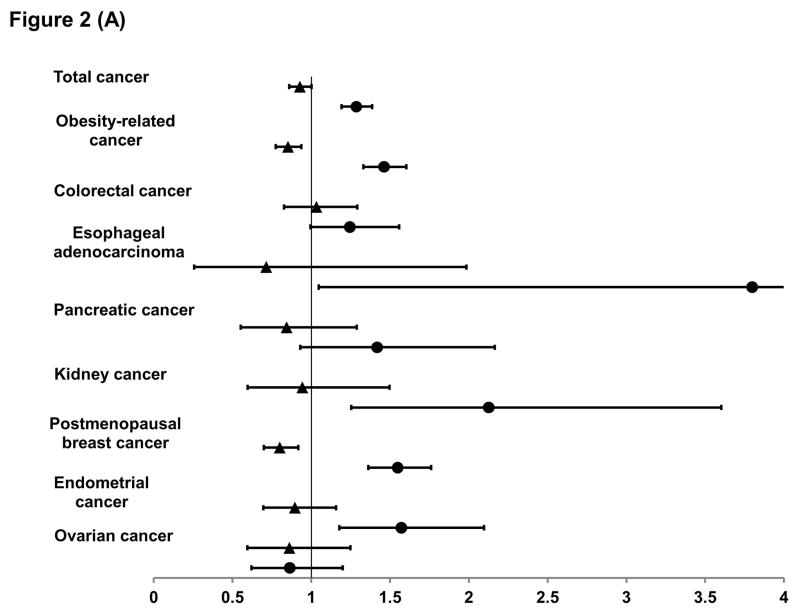
To further investigate the timing effect of body shape on cancer risk, we compared the extreme trajectories whose body shape was similar at one end of life but substantially differed at the other end. As shown in Figure 2A, women with a heavier body shape in early life had a lower risk of total and obesity-related cancer than those with a leaner body shape, and this lowered risk appeared to be largely due to postmenopausal breast cancer, for which the RR was 0.80 (95% CI, 0.70–0.92). In contrast, compared to women with a leaner body shape, those with a heavier body shape in late life had a higher risk of total and obesity-related cancer, especially esophageal adenocarcinoma and cancers of the kidney, endometrium, breast, pancreas and colorectum, with a RR ranging from 1.25 to 3.80.
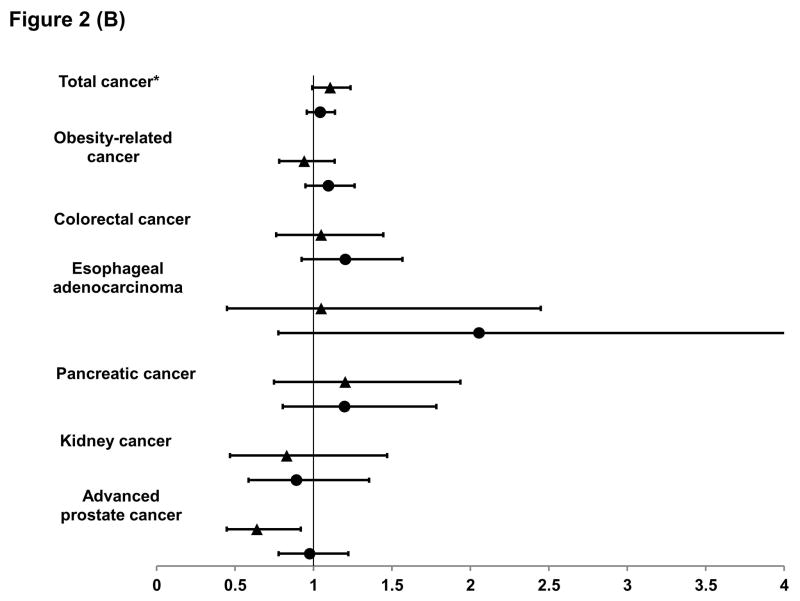
Figure 2. Relative risk and 95% confidence interval of cancer according to early-life (▲) and late-life (●) body shape in women (A) and men (B).
For early life body shape, we compared the risk in the heavy-stable/increase to the lean-marked increase groups in the multivariable model (see footnote of Table 1), with further adjustment for average body shape from age 40 to 60. Similarly, for late life body shape, we compared the risk in the lean-marked increase to the lean-stable groups with further adjustment for average body shape from age 5 to 20.
*For total cancer in men, we excluded non-advanced prostate cancer.
In men (Figure 2B), those with a heavier body shape in early life were at a lower risk of developing advanced prostate cancer than their counterparts with a leaner body shape (RR=0.64, 95% CI, 0.45–0.92); whereas those with a heavier body shape in late life were at a statistically insignificantly higher risk of developing cancer, especially esophageal adenocarcinoma, pancreatic cancer and colorectal cancer.
Because smoking is a risk factor for many cancers and smokers generally have a lower BMI, we then conducted a stratified analysis by smoking history (Table 3). Trajectories were more strongly associated with cancer risk among never smokers than among ever smokers, with a statistically significant interaction for total cancer in women and for pancreatic cancer in men (P for interaction=0.005 and 0.03, respectively). However, unsurprisingly, the absolute incidence rates of cancers were still much higher among ever smokers than among never smokers (Supplementary Table 3).
Table 3.
Relative risk of cancer by smoking according to trajectories of body shape in women (Nurses’ Health Study) and men (Health Professionals Follow-up Study)a
| Lean-stable | Lean-moderate increase | Lean-marked increase | Medium-stable | Heavy-stable/increase | P for interactionb | |
|---|---|---|---|---|---|---|
| Women | ||||||
| Total cancer | ||||||
| Ever smokers (N=7,082) | 1 (reference) | 0.99 (0.92–1.07) | 1.07 (0.99–1.16) | 1.00 (0.93–1.07) | 1.00 (0.92–1.10) | 0.005 |
| Never smokers (N=4,380) | 1 (reference) | 1.22 (1.10–1.34)c | 1.35 (1.22–1.50) | 1.14 (1.03–1.26) | 1.46 (1.30–1.64)e | |
| Obesity-related cancerf | ||||||
| Ever smokers (N=3,591) | 1 (reference) | 1.14 (1.03–1.27) | 1.33 (1.19–1.48) | 0.97 (0.88–1.08) | 1.15 (1.02–1.31) | 0.08 |
| Never smokers (N=2,809) | 1 (reference) | 1.23 (1.08–1.39) | 1.48 (1.30–1.68) | 1.12 (0.99–1.27) | 1.50 (1.30–1.74)d | |
| Colorectal cancer | ||||||
| Ever smokers (N=675) | 1 (reference) | 0.97 (0.76–1.23) | 1.25 (0.97–1.61) | 0.97 (0.77–1.22) | 1.26 (0.95–1.67) | 0.54 |
| Never smokers (N=456) | 1 (reference) | 0.98 (0.72–1.33) | 1.20 (0.87–1.65) | 1.11 (0.82–1.51) | 1.68 (1.19–2.37) | |
| Pancreatic cancer | ||||||
| Ever smokers (N=179) | 1 (reference) | 1.09 (0.67–1.78) | 1.60 (0.98–2.61) | 1.09 (0.69–1.73) | 1.11 (0.61–2.00) | 0.25 |
| Never smokers (N=145) | 1 (reference) | 1.31 (0.76–2.26) | 1.13 (0.63–2.05) | 1.25 (0.72–2.15) | 1.84 (1.00–3.40) | |
| Kidney cancer | ||||||
| Ever smokers (N=120) | 1 (reference) | 1.20 (0.65–2.22) | 1.78 (0.97–3.27) | 1.03 (0.57–1.88) | 1.59 (0.81–3.14) | 0.85 |
| Never smokers (N=92) | 1 (reference) | 1.41 (0.66–3.03) | 2.17 (1.03–4.56) | 1.10 (0.50–2.41) | 2.57 (1.16–5.66) | |
| Postmenopausal breast cancer | ||||||
| Ever smokers (N=1,951) | 1 (reference) | 1.21 (1.05–1.39) | 1.30 (1.12–1.51) | 0.94 (0.81–1.07) | 0.99 (0.83–1.18) | 0.10 |
| Never smokers (N=1,503) | 1 (reference) | 1.47 (1.24–1.75) | 1.60 (1.34–1.92)c | 1.25 (1.05–1.49)d | 1.33 (1.08–1.65)c | |
| Endometrial cancer | ||||||
| Ever smokers (N=324) | 1 (reference) | 1.17 (0.79–1.72) | 1.62 (1.10–2.39) | 1.15 (0.79–1.66) | 2.25 (1.51–3.34) | 0.48 |
| Never smokers (N=340) | 1 (reference) | 0.84 (0.58–1.22) | 1.50 (1.06–2.13) | 0.77 (0.53–1.11) | 1.97 (1.36–2.86) | |
| Ovarian cancer | ||||||
| Ever smoking (N=271) | 1 (reference) | 0.86 (0.59–1.25) | 0.81 (0.54–1.22) | 0.95 (0.68–1.34) | 0.83 (0.52–1.31) | 0.50 |
| Never smoking (N=205) | 1 (reference) | 0.91 (0.59–1.39) | 1.06 (0.69–1.63) | 0.76 (0.49–1.18) | 0.86 (0.50–1.47) | |
|
| ||||||
| Men | ||||||
| Total cancerg | ||||||
| Ever smokers (N=3,235) | 1 (reference) | 0.96 (0.85–1.08) | 1.03 (0.93–1.15) | 0.98 (0.86–1.11) | 1.17 (1.02–1.34) | 0.11 |
| Never smokers (N=1,741) | 1 (reference) | 1.20 (1.03–1.40)c | 1.09 (0.94–1.25) | 1.13 (0.95–1.34) | 1.16 (0.96–1.41) | |
| Obesity-related cancerh | ||||||
| Ever smokers (N=1,125) | 1 (reference) | 1.04 (0.86–1.27) | 1.07 (0.89–1.28) | 0.99 (0.79–1.23) | 1.05 (0.82–1.34) | 0.09 |
| Never smokers (N=707) | 1 (reference) | 1.40 (1.10–1.79)c | 1.12 (0.88–1.41) | 1.38 (1.05–1.82)c | 1.38 (1.01–1.88) | |
| Colorectal cancer | ||||||
| Ever smokers (N=359) | 1 (reference) | 1.25 (0.87–1.79) | 1.21 (0.86–1.69) | 1.08 (0.72–1.64) | 1.55 (1.02–2.35) | 0.55 |
| Never smokers (N=233) | 1 (reference) | 1.51 (0.98–2.35) | 1.26 (0.83–1.91) | 1.57 (0.97–2.53) | 1.33 (0.76–2.34) | |
| Pancreatic cancer | ||||||
| Ever smokers (N=148) | 1 (reference) | 0.62 (0.34–1.12) | 1.18 (0.73–1.89) | 0.97 (0.54–1.74) | 0.90 (0.46–1.75) | 0.03 |
| Never smokers (N=83) | 1 (reference) | 1.27 (0.60–2.70) | 1.04 (0.51–2.10) | 1.34 (0.59–3.05) | 2.68 (1.24–5.80)c | |
| Kidney cancer | ||||||
| Ever smokers (N=130) | 1 (reference) | 1.04 (0.60–1.80) | 0.83 (0.49–1.40) | 0.81 (0.43–1.55) | 1.01 (0.52–1.95) | 0.26 |
| Never smokers (N=72) | 1 (reference) | 0.93 (0.43–2.03) | 1.08 (0.54–2.13) | 1.53 (0.71–3.26) | 0.46 (0.13–1.64) | |
| Advanced prostate canceri | ||||||
| Ever smokers (N=398) | 1 (reference) | 1.00 (0.73–1.37) | 0.93 (0.69–1.24) | 0.93 (0.65–1.32) | 0.49 (0.30–0.81) | 0.18 |
| Never smokers (N=296) | 1 (reference) | 1.40 (0.97–2.03) | 1.03 (0.72–1.47) | 1.13 (0.73–1.74) | 1.04 (0.63–1.73)c | |
Abbreviations: CI, confidence interval; RR, relative risk.
Follow-up started at age 62. RRs were estimated from multivariable Cox proportional hazards model with adjustment for the same set of covariates as in Table 1.
Global likelihood ratio test with 4 degrees of freedom was used to compare the model with the product terms between smoking (binary: ever vs. never smoking) and the trajectory groups (indicator variables for the 4 non-reference groups) to the model without these terms.
P for interaction≤0.05, >0.01 by individual Wald test for each of the non-reference trajectory groups. A product term was created by multiplying the binary variable for each of the trajectory groups (1, non-reference group under test, 0, reference group) and the binary smoking variable (ever vs. never).
P for interaction≤0.01, >0.001 by individual Wald test.
P for interaction≤0.001, >0.0001 by individual Wald test.
Including cancers of the colorectum, esophagus (adenocarcinoma only), pancreas, kidney, breast (postmenopause), endometrium, ovaries, liver, and gallbladder.
Excluding non-advanced prostate cancer.
Including cancers of the colorectum, esophagus (adenocarcinoma only), pancreas, kidney, prostate (advanced cancer only), liver, and gallbladder.
Advanced prostate cancer defined as cases that had spread outside the prostate (stage T3b/T4, N1, or M1) or lethal tumors.
Finally, we examined the trajectory-cancer association in women according to MHT use (Table 4). The increased risk associated with trajectories of the lean-moderate increase, lean-marked increase and heavy-stable/increase was more pronounced among never users of MHT than among ever users for all cancers under study except ovarian cancer, and the interaction P-value was <0.001 for total, obesity-related cancer and endometrial cancer. Interestingly, for ovarian cancer, we found a lower risk in the medium-stable group among never, but not among ever users of MHT (P for interaction=0.008). The absolute incidence rates of cancers among ever and never users of MHT were presented in Supplementary Table 4.
Table 4.
Relative risk of cancer by menopausal hormone therapy (MHT) according to trajectories of body shape in women (Nurses’ Health Study)a
| Lean-stable | Lean-moderate increase | Lean-marked increase | Medium-stable | Heavy-stable/increase | P for interactionb | |
|---|---|---|---|---|---|---|
| Total cancer | ||||||
| Ever used MHT (N=6,834) | 1 (reference) | 1.03 (0.96–1.12) | 1.10 (1.02–1.20) | 1.04 (0.97–1.12) | 1.06 (0.97–1.17) | <0.001 |
| Never used MHT (N=4,594) | 1 (reference) | 1.12 (1.02–1.23) | 1.26 (1.14–1.39)e | 1.04 (0.94–1.14) | 1.27 (1.14–1.42)e | |
| Obesity-related cancerg | ||||||
| Ever used MHT (N=3,776) | 1 (reference) | 1.10 (0.99–1.21) | 1.25 (1.12–1.39) | 1.01 (0.92–1.12) | 1.12 (0.98–1.27) | <0.001 |
| Never used MHT (N=2,606) | 1 (reference) | 1.32 (1.15–1.51)c | 1.64 (1.43–1.87)e | 1.07 (0.93–1.23) | 1.57 (1.35–1.82)e | |
| Colorectal cancer | ||||||
| Ever used MHT (N=572) | 1 (reference) | 0.95 (0.73–1.24) | 1.14 (0.86–1.51) | 1.00 (0.78–1.28) | 1.45 (1.07–1.97) | 0.92 |
| Never used MHT (N=556) | 1 (reference) | 0.97 (0.73–1.29) | 1.30 (0.98–1.72) | 1.03 (0.78–1.35) | 1.36 (0.99–1.85) | |
| Pancreatic cancer | ||||||
| Ever used MHT (N=196) | 1 (reference) | 1.06 (0.68–1.65) | 1.11 (0.69–1.78) | 0.97 (0.63–1.49) | 1.23 (0.72–2.09) | 0.79 |
| Never used MHT (N=127) | 1 (reference) | 1.43 (0.75–2.72) | 1.85 (0.97–3.51) | 1.54 (0.83–2.86) | 1.76 (0.87–3.59) | |
| Kidney cancer | ||||||
| Ever used MHT (N=117) | 1 (reference) | 0.92 (0.50–1.69) | 1.77 (0.99–3.16) | 0.91 (0.51–1.61) | 1.53 (0.78–3.00) | 0.36 |
| Never used MHT (N=94) | 1 (reference) | 2.20 (0.95–5.13) | 2.38 (1.02–5.55) | 1.48 (0.62–3.54) | 2.96 (1.24–7.10) | |
| Postmenopausal breast cancer | ||||||
| Ever used MHT (N=2,137) | 1 (reference) | 1.25 (1.10–1.43) | 1.33 (1.15–1.54) | 1.04 (0.91–1.18) | 0.98 (0.82–1.17) | 0.17 |
| Never used MHT (N=1,307) | 1 (reference) | 1.41 (1.17–1.70) | 1.58 (1.31–1.91) | 1.09 (0.90–1.32) | 1.36 (1.09–1.68)c | |
| Endometrial cancer | ||||||
| Ever used MHT (N=390) | 1 (reference) | 0.77 (0.56–1.06) | 0.93 (0.66–1.29) | 0.87 (0.65–1.17) | 1.41 (1.00–1.99) | <0.001 |
| Never used MHT (N=273) | 1 (reference) | 2.19 (1.23–3.90)e | 4.50 (2.59–7.79)f | 1.42 (0.78–2.59) | 5.25 (2.99–9.23)f | |
| Ovarian cancer | ||||||
| Ever used MHT (N=297) | 1 (reference) | 0.84 (0.58–1.21) | 0.91 (0.62–1.35) | 1.11 (0.80–1.53) | 0.85 (0.53–1.35) | 0.008 |
| Never used MHT (N=178) | 1 (reference) | 0.91 (0.58–1.41) | 0.92 (0.58–1.45) | 0.49 (0.30–0.80)d | 0.79 (0.46–1.34) |
Abbreviations: CI, confidence interval; RR, relative risk.
Follow-up started at age 62. RRs were estimated from multivariable Cox proportional hazards model with adjustment for the same set of covariates as in Table 1.
Global likelihood ratio test with 4 degrees of freedom was used to compare the model with the product terms between MHT use (binary: ever vs. never use) and the trajectory groups (indicator variables for the 4 non-reference groups) to the model without these terms.
P for interaction≤0.05, >0.01 by individual Wald test for each of the non-reference trajectory groups. A product term was created by multiplying the binary variable for each of the trajectory groups (1, non-reference group under test, 0, reference group) and the binary MHT variable (ever vs. never).
P for interaction≤0.01, >0.001 by individual Wald test.
P for interaction≤0.001, >0.0001 by individual Wald test.
P for interaction≤0.0001 by individual Wald test.
Including cancers of the colorectum, esophagus (adenocarcinoma only), pancreas, kidney, breast (postmenopause), endometrium, ovaries, liver, and gallbladder.
DISCUSSION
In the two large cohort studies, we identified 5 distinct subgroups of participants with similar body shape evolution over life course. By comparing cancer risk between these subgroups, we found that, compared to participants who were lean throughout life, those with increased body shape at any life period had an overall higher risk of developing cancer. We found distinct trajectory-cancer association patterns depending on sex and the organ where cancer arose. Our findings extend our understanding of obesity-cancer associations and support a role for adiposity across the lifespan in carcinogenesis.
Building upon the substantial data on adult adiposity and cancer, some studies have related adiposity in early life to subsequent cancer risk. The most compelling evidence is on breast cancer which demonstrates a dual relationship with adiposity: while recent obesity and weight gain in adulthood have been associated with an increased risk of postmenopausal breast cancer,4 high body fatness in childhood and adolescence has been related to lower risk.6–9 This is consistent with our result that women in the lean-moderate increase and lean-marked increase trajectories had the highest risk. While high levels of adipose tissue-derived estrogen after menopause has been suggested as the predominant mechanism for explaining the increased risk of breast cancer associated with adult obesity,4 the mechanism for potential protective effect of early life adiposity remains uncertain. Some evidence suggests that increased estrogen in overweight children may induce early differentiation of mammary glands and eliminate some targets for malignant transformation.34 Further mechanistic investigations are needed to better understand the mechanisms underlying the association between adiposity and breast cancer.
Early life adiposity has been related to an increased for risk of endometrial cancer.17, 18, 23 However, in most studies, this positive association disappeared after concurrently adjusting for current BMI, suggesting greater importance of current fatness.35 This agrees with our result that women with a significant increase in body shape after early adulthood had a higher risk, regardless of their body shape in childhood or adolescence.
For colorectal cancer, a stronger and more consistent positive association with obesity, including that in adolescence and early adulthood,14 has been reported in men than in women, possibly as a result of obesity-related changes in sex hormone levels.36, 37 In this study, we found that in women only the lean-marked increase and heavy-stable/increase trajectories were at higher risk of colorectal cancer, while in men, those who had a heavy body shape in any life period had a higher risk (although some of the relative risk estimates were not statistically significant), indicating that early-life obesity may have a predominant effect on colorectal cancer in women whereas late-life obesity may be more important in men.16 Of note, in contrast to studies that reported a stronger association between late adulthood BMI and colorectal cancer risk in men than in women, other studies using abdominal fatness measures, such as waist circumference and waist-to-hip ratio, have found a similar positive association with colorectal cancer risk between men and women.38 In consideration of the limited ability of BMI in capturing the variation of body fat distribution, these data suggest that differences in body fat distribution between men and women may at least partly explain the observed sex difference in the obesity-colorectal cancer relationship.
Obesity has also been associated with an increased risk of esophageal adenocarcinoma,39 and kidney40 and pancreatic cancer.4 However, the timing effect of adiposity has yet to be determined for these cancers. In this study, we found a substantially increased risk of esophageal adenocarcinoma in relation to late life increase of body shape, but little evidence for early life exposure. For kidney cancer, some studies,41, 42 but not all,40 have found a stronger positive association with obesity in women than in men. Consistently, in the current study the trajectory-kidney cancer association was restricted to women that appeared to be driven by late life body shape. For pancreatic cancer, we found a suggestive positive, albeit statistically non-significant, association with a marked increase in body shape after early adulthood in both men and women, suggesting a more proximate effect of weight gain on promotion of pancreatic carcinogenesis.
For prostate cancer, although obesity at diagnosis has been recognized as a probable cause,4 the findings to date are still conflicting. In addition, obesity earlier in life (age 10–30) has been inversely associated with risk in several studies,11, 43 especially for advanced and lethal prostate cancer, possibly due to delayed puberty and prostate maturation. In this study, men who were lean in early life and experienced moderate increase in body shape appeared to have a higher risk of advanced prostate cancer, while men who were heavy throughout life experienced a lower risk. When the timing of adiposity was examined using body shape as the surrogate, early life adiposity was associated with a lower risk of advanced prostate cancer, while no association was found for late life adiposity. Thus, our results indicate a predominant benefit of early life body fatness over a potential adverse effect of adiposity in later life.
In agreement with the notion that smoking may dampen the obesity-cancer association,39 we found that trajectories were more strongly associated with cancer among never smokers. MHT use in women has also been suggested to modify cancer risk associated with obesity. In the current study, we observed a stronger association of body shape trajectories with the risk of total and obesity-related cancer, especially endometrial cancer, among never users of MHT, compared to ever users. This is consistent with previous evidence that MHT use attenuates the obesity-endometrial cancer association,44 likely due to the central role of unopposed estrogen therapy in endometrial carcinogenesis that overwhelms the effect of adipose tissue-derived estrogen.
Our study has several strengths. First, we applied an innovative statistical method to examine patterns of body shape across the lifespan in two large, well-established cohort studies with long-term follow-up, representing a substantial advantage over previous studies that examined body fatness at select ages. This method has only recently been employed in chronic disease epidemiology. For example, a trajectory assessment of blood pressure across adulthood was found to provide additional information about the future risk for cardiovascular disease.45 Second, we collected detailed data on a range of lifestyle and health-related factors that allowed us to control for confounding and to examine the potential modification by smoking and MHT use.
Some limitations of the study need to be noted. First, we grouped participants into data-derived trajectories that may not accurately reflect each individual’s profile of body shape. However, the good performance of our trajectory-building model and well-tracked change in BMI across trajectories indicate that the trajectories we identified can summarize the distinctive features of lifetime body shape in a parsimonious fashion without a significant loss of information. Second, the recalled body shape and self-reported BMI data were subject to measurement error. However, given the prospective study design, any error would likely have attenuated the observed associations. Third, some of the relative risk estimates were not statistically significant. This may be partly due to several limitations of our study, including the relatively small case numbers in some trajectory groups, the limited difference in body shape between certain trajectories, the presence of other unidentified, less prevalent trajectories (as discussed above), and potential misclassification in trajectory assignment. However, our sensitivity analysis results indicated the robustness of our findings to modest trajectory misclassification. Lastly, although the homogeneity of our study population is a potential limitation, it minimizes the likelihood of uncontrolled confounding. Given that our previously observed associations between overall adiposity and cancer risk have been largely validated in other population, it is unlikely that the relationship between body shape and cancer risk observed here differs substantially from that occurring in the general population. Nevertheless, our findings should be confirmed in other populations.
In conclusion, we identified 5 heterogeneous trajectories of body shape over life course and found distinct patterns of cancer incidence across these trajectories. While early life body shape was inversely associated with the risk of postmenopausal breast cancer and advanced prostate cancer, late life body shape was positively associated with the risk of esophageal adenocarcinoma and cancers of the breast, endometrium, colorectum, pancreas, and kidney (in women only), highlighting the role of weight gain during adulthood in increasing cancer risk. Our results extend the knowledge that obesity is related to cancer risk and suggest an influence of adiposity across the lifespan on carcinogenesis.
Supplementary Material
Brief description of novelty and impact.
Adult obesity has been related to higher risk of several cancers, but the influence of body fatness across the lifespan remains poorly understood. This work represents the first effort to systematically assess the association of lifetime body shape with cancer risk using a novel life course approach. Our findings provide the evidence for a role of lifetime adiposity in carcinogenesis and indicate the importance of body weight management throughout life for cancer prevention.
Acknowledgments
M.S. is a trainee of the Harvard Transdisciplinary Research Center on Energetics and Cancer (TREC). We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Financial support: This work was supported by the National Institutes of Health (UM1 CA186107, P01 CA87969, UM1 CA167552, 1U54CA155626, and K24 DK098311), and Boston Obesity Nutrition Research Center (DK46200). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Abbreviations
- AICR
American Institute for Cancer Research
- BMI
body mass index
- CI
confidence interval
- CRC
colorectal cancer
- HPFS
Health Professionals Follow-up Study
- MHT
menopausal hormone therapy
- NHS
Nurses’ Health Study
- RR
relative risk
- WCRF
World Cancer Research Fund
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Lee JM, Pilli S, Gebremariam A, Keirns CC, Davis MM, Vijan S, Freed GL, Herman WH, Gurney JG. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes (Lond) 2010;34:614–23. doi: 10.1038/ijo.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–8. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; 2007. [Google Scholar]
- 4.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report: Food, Nutrition, Physical Activity, and the Prevention of Cancer. http://www.wcrf.org/int/research-we-fund/continuous-update-project-cup.
- 5.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. JAMA : the journal of the American Medical Association. 1997;278:1407–11. [PubMed] [Google Scholar]
- 7.Bardia A, Vachon CM, Olson JE, Vierkant RA, Wang AH, Hartmann LC, Sellers TA, Cerhan JR. Relative weight at age 12 and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:374–8. doi: 10.1158/1055-9965.EPI-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–94. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki R, Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, Tsugane S. Body weight at age 20 years, subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status--the Japan public health center-based prospective study. Int J Cancer. 2011;129:1214–24. doi: 10.1002/ijc.25744. [DOI] [PubMed] [Google Scholar]
- 10.Fagherazzi G, Guillas G, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S. Body shape throughout life and the risk for breast cancer at adulthood in the French E3N cohort. Eur J Cancer Prev. 2013;22:29–37. doi: 10.1097/CEJ.0b013e328355ec04. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:557–63. [PubMed] [Google Scholar]
- 12.Discacciati A, Orsini N, Andersson SO, Andren O, Johansson JE, Wolk A. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: a population-based prospective study. Br J Cancer. 2011;105:1061–8. doi: 10.1038/bjc.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moller E, Adami HO, Mucci LA, Lundholm C, Bellocco R, Johansson JE, Gronberg H, Balter K. Lifetime body size and prostate cancer risk in a population-based case-control study in Sweden. Cancer Causes Control. 2013;24:2143–55. doi: 10.1007/s10552-013-0291-0. [DOI] [PubMed] [Google Scholar]
- 14.Renehan AG, Flood A, Adams KF, Olden M, Hollenbeck AR, Cross AJ, Leitzmann MF. Body mass index at different adult ages, weight change, and colorectal cancer risk in the National Institutes of Health-AARP Cohort. Am J Epidemiol. 2012;176:1130–40. doi: 10.1093/aje/kws192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi Z, Kark JD, Barchana M, Liphshitz I, Zavdi O, Tzur D, Derazne E, Furman M, Niv Y, Gordon B, Afek A, Shamiss A. Measured body mass index in adolescence and the incidence of colorectal cancer in a cohort of 1. 1 million males. Cancer Epidemiol Biomarkers Prev. 2011;20:2524–31. doi: 10.1158/1055-9965.EPI-11-0531. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Wu K, Giovannucci EL, Ma J, Colditz GA, Fuchs CS, Willett WC, Stampfer MJ, Nimptsch K, Ogino S, Wei EK. Early life body fatness and risk of colorectal cancer in u.s. Women and men-results from two large cohort studies. Cancer Epidemiol Biomarkers Prev. 2015;24:690–7. doi: 10.1158/1055-9965.EPI-14-0909-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst. 2004;96:1635–8. doi: 10.1093/jnci/djh291. [DOI] [PubMed] [Google Scholar]
- 18.Levi F, La Vecchia C, Negri E, Parazzini F, Franceschi S. Body mass at different ages and subsequent endometrial cancer risk. Int J Cancer. 1992;50:567–71. doi: 10.1002/ijc.2910500413. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Risch H, Irwin ML, Mayne ST, Cartmel B, Schwartz P, Rutherford T, Yu H. Long-term overweight and weight gain in early adulthood in association with risk of endometrial cancer. Int J Cancer. 2011;129:1237–43. doi: 10.1002/ijc.26046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens VL, Jacobs EJ, Patel AV, Sun J, Gapstur SM, McCullough ML. Body weight in early adulthood, adult weight gain, and risk of endometrial cancer in women not using postmenopausal hormones. Cancer Causes Control. 2014;25:321–8. doi: 10.1007/s10552-013-0333-7. [DOI] [PubMed] [Google Scholar]
- 21.Han D, Nie J, Bonner MR, McCann SE, Muti P, Trevisan M, Ramirez-Marrero FA, Vito D, Freudenheim JL. Lifetime adult weight gain, central adiposity, and the risk of pre- and postmenopausal breast cancer in the Western New York exposures and breast cancer study. Int J Cancer. 2006;119:2931–7. doi: 10.1002/ijc.22236. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan K, Bassett JK, MacInnis RJ, English DR, Hopper JL, McLean C, Giles GG, Baglietto L. Associations between weight in early adulthood, change in weight, and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22:1409–16. doi: 10.1158/1055-9965.EPI-13-0136. [DOI] [PubMed] [Google Scholar]
- 23.Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden) Cancer Causes Control. 2000;11:185–92. doi: 10.1023/a:1008946825313. [DOI] [PubMed] [Google Scholar]
- 24.Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–84. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 25.Bassett JK, Severi G, Baglietto L, MacInnis RJ, Hoang HN, Hopper JL, English DR, Giles GG. Weight change and prostate cancer incidence and mortality. Int J Cancer. 2012;131:1711–9. doi: 10.1002/ijc.27414. [DOI] [PubMed] [Google Scholar]
- 26.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 28.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis. 1983;60:115–20. [PubMed] [Google Scholar]
- 29.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 30.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Method Res. 2007;35:542–71. [Google Scholar]
- 31.Nagin DS. Group-based modeling of development. Cambridge: Harvard University Press; 2005. [Google Scholar]
- 32.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 33.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. Springer; 2010. [Google Scholar]
- 34.Hilakivi-Clarke L, Forsen T, Eriksson JG, Luoto R, Tuomilehto J, Osmond C, Barker DJ. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85:1680–4. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang TY, Cairns BJ, Allen N, Sweetland S, Reeves GK, Beral V. Postmenopausal endometrial cancer risk and body size in early life and middle age: prospective cohort study. Br J Cancer. 2012;107:169–75. doi: 10.1038/bjc.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, Tworoger SS, Hankinson SE, Fuchs C, Gaziano JM, Buring JE, Giovannucci E. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol. 2013;11:419–24. e1. doi: 10.1016/j.cgh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy N, Strickler HD, Stanczyk FZ, Xue X, Wassertheil-Smoller S, Rohan TE, Ho GY, Anderson GL, Potter JD, Gunter MJ. A Prospective Evaluation of Endogenous Sex Hormone Levels and Colorectal Cancer Risk in Postmenopausal Women. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, Halkjaer J, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Guernec G, Bergmann MM, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 39.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2014;135:1673–86. doi: 10.1002/ijc.28813. [DOI] [PubMed] [Google Scholar]
- 41.Pischon T, Lahmann PH, Boeing H, Tjonneland A, Halkjaer J, Overvad K, Klipstein-Grobusch K, Linseisen J, Becker N, Trichopoulou A, Benetou V, Trichopoulos D, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2006;118:728–38. doi: 10.1002/ijc.21398. [DOI] [PubMed] [Google Scholar]
- 42.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–40. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 43.Giovannucci E, Rimm EB, Liu Y, Leitzmann M, Wu K, Stampfer MJ, Willett WC. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95:1240–4. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 44.Chang SC, Lacey JV, Jr, Brinton LA, Hartge P, Adams K, Mouw T, Carroll L, Hollenbeck A, Schatzkin A, Leitzmann MF. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2007;16:723–30. doi: 10.1158/1055-9965.EPI-06-0675. [DOI] [PubMed] [Google Scholar]
- 45.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, Jacobs DR, Jr, Liu K, Lloyd-Jones D. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA : the journal of the American Medical Association. 2014;311:490–7. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.