Abstract
Background
The 1-minute sit-to-stand (STS) test could be valuable to assess the level of exercise tolerance in chronic obstructive pulmonary disease (COPD). There is a need to provide the minimal important difference (MID) of this test in pulmonary rehabilitation (PR).
Methods
COPD patients undergoing the 1-minute STS test before PR were included. The test was performed at baseline and the end of PR, as well as the 6-minute walk test, and the quadriceps maximum voluntary contraction (QMVC). Home and community-based programs were conducted as recommended. Responsiveness to PR was determined by the difference in the 1-minute STS test between baseline and the end of PR. The MID was evaluated using distribution and anchor-based methods.
Results
Forty-eight COPD patients were included. At baseline, the significant predictors of the number of 1-minute STS repetitions were the 6-minute walk distance (6MWD) (r=0.574; P<10−3), age (r=−0.453; P=0.001), being on long-term oxygen treatment (r=−0.454; P=0.017), and the QMVC (r=0.424; P=0.031). The multivariate analysis explained 75.8% of the variance of 1-minute STS repetitions. The improvement of the 1-minute STS repetitions at the end of PR was 3.8±4.2 (P<10−3). It was mainly correlated with the change in QMVC (r=0.572; P=0.004) and 6MWD (r=0.428; P=0.006). Using the distribution-based analysis, an MID of 1.9 (standard error of measurement method) or 3.1 (standard deviation method) was found. With the 6MWD as anchor, the receiver operating characteristic curve identified the MID for the change in 1-minute STS repetitions at 2.5 (sensibility: 80%, specificity: 60%) with area under curve of 0.716.
Conclusion
The 1-minute STS test is simple and sensitive to measure the efficiency of PR. An improvement of at least three repetitions is consistent with physical benefits after PR.
Keywords: sit-to-stand test, COPD, 6-minute walk test, exercise tolerance, pulmonary rehabilitation
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by limitations in the lung airflow with serious public health issues.1 Ventilatory limitation (deficiency) is the first cause of breathlessness and exercise intolerance (incapacity) among these patients impacts their physical activities of daily living, thus leading to social disadvantage.2 Hence, the evaluation of exercise tolerance and the functional capacity of the patients lie within the assessment of disease progression. This evaluation can be done by simple field tests that require minimal technical equipment and could be performed in an environment that is comfortable for the patients. With the availability of various tests such as the 6-minute walk test (6MWT), the incremental shuttle walk test, the endurance shuttle walk test, the stair climbing test, the step test, and the sit-to-stand (STS) test or chair rise test, choosing the right test for these patients could be tricky.3–8 The STS test has been developed for different chronic diseases and elderly people, since the gesture of getting up from a chair is an essential activity of daily living. This ability to stand up from a chair is an important component of maintaining independence among the elderly people, as this movement depends on stability and balance.9 Hence, this test is accepted as an indicator of functional status among the elderly people but scarcity of data prevails for the COPD population.10 Being a validated measure of the functional outcome in COPD patient, the STS test could also be considered a surrogate to the walking test.7,11 Therefore, we aimed at evaluating the 1-minute STS test minimal important difference (MID) among COPD patients undergoing a pulmonary rehabilitation (PR).
Materials and methods
Subjects
Patients were included in this study if they fulfilled the following criteria: a diagnosis of COPD according to the Global initiative for COPD (GOLD) recommendations, having completed a PR and joined the cohort RéhaEffort from the Institut de Recherche en Santé Respiratoire des Pays de la Loire (IRSR) or the Centre Hospitalier des Pays de Morlaix.12 Forty-eight consecutive COPD patients were included (30 males), aged 64.5±9.8 years and mean forced expiratory volume in 1 second (FEV1) 52.3%±16.1%. The patients were recruited in four different centers in two cities: the Lung Function Department, the Pulmonary Rehabilitation Department of the Nantes University Hospital, the Tourmaline Medical Centre in Nantes, the Pulmonology Department of the Angers University Hospital, and the Centre Hospitalier des Pays de Morlaix, Pulmonary Rehabilitation Unit, France. The study was conducted by the RéhaEffort cohort group from the IRSR. Approval was obtained from the University of Angers ethics committee and the Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé (07.207 bis). All patients included in the RéhaEffort cohort provided their written informed consent. For the patients from the Centre Hospitalier des Pays de Morlaix, they signed a written informed consent form in accordance with the Declaration of Helsinki and the current guidelines for Clinical Good Practice after approval by the Institutional Medical Ethics Committees (BE403201317621 – 2009/17 – 07 207 bis).
Study design
All the patients went through a complete outpatient visit in our institutions to assess individual deficiency, incapacity, and social disadvantage in detail as recommended.13 The pulmonary function tests were obtained using a volume/pressure body plethysmograph (MasterScreen® Body, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) according to the European Respiratory Society and American Thoracic Society task force.14,15 The European Coal and Steel Community reference values were used.16 All patients performed a cardiopulmonary exercise test that was obtained using a progressive 10W incremental test on an electromagnetically braked cycle ergometer up to exhaustion as recommended.17 Field exercise and muscle strength tests included a 6MWT and a measure of the quadriceps maximal voluntary contraction (QMVC). The evaluation of quality of life was done using the St George’s Respiratory Questionnaire and the Hospital Anxiety and Depression questionnaire.
One-minute STS test
The 1-minute STS test was performed with a chair of standard height of 46 cm without arm rests. The patient was ensured to be seated upright on the chair positioned against a wall. The patient sat with the knees and hips flexed to 90°, feet placed flat on the floor hip-width apart, and the hands placed on the hips. Every get up from one’s chair was validated to check if complete sit-to-stand-to-sit sequence was achieved. The patient was given the following instructions:
The purpose of the test is to assess your exercise capacity and leg muscle strength. The movement required is to get up from this chair with the legs straight and sit back continuing the repetitions as fast as possible within one minute. I will give you the countdown ‘3, 2, 1 Go’ as an indication to start and also I will tell you when we are at the 15 remaining seconds. If required, you can make a break and resume the test as soon as possible.
The number of repetitions was measured using a dedicated actimeter conceived for this purpose from Bluenight® (SleepInnov Technology, Moirans, France), controlled by the video and expressed in percentage of reference values.18 The Modified Borg Scale was used to assess dyspnea and fatigue. The test was performed at baseline and repeated at the end of PR.
Six-minute walk test
The 6MWT was conducted in a 30 m long corridor following the recommendations.19,20 The test was performed using the Bluenight® oximeter (SleepInnov Technology) to measure the heart rate and oxygen saturation during the procedure. The test was repeated twice, once at the beginning and once at the end of PR, with the best distance recorded.
Quadriceps maximum voluntary contraction
The evaluation of the QMVC was performed by measuring the maximal voluntary isometric contraction using the handheld dynamometer microFET2™ (Hoggan Scientific, LLC, Salt Lake City, UT, USA). To prevent any operator bias, the device was fixed on the leg of the table, with the patient comfortably seated using a high-density corner cushion to maintain their knees at 90°, as recommended.21 At least three measures were registered for each leg (additional measures were taken if the third was better than the previous ones – suggesting a learning effect – up to a lower result, which completed the session). The highest value was expressed in Newton and used in the analysis.22 This measure was reproduced at the end of PR.
Statistical analysis
All results were presented as mean ± standard deviation (SD) for the quantitative variables. The qualitative variables were expressed in percentages. Statistical analyses were performed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). The determination of the dependent variable, the 1-minute STS repetitions at baseline, and other independent variables were made using the univariate linear regression analysis. These included anthropometric parameters, the data of the combined COPD assessment A, B, C, and D categories, the Charlson and body mass index, airflow obstruction, dyspnea and exercise capacity index pulmonary function parameters (from the forced expiratory maneuver, plethysmography, and respiratory muscle strength), cardiopulmonary exercise tests parameters, 6MWT, and QMVC parameters. This was followed by a multivariate analysis, combining the most relevant independent variables from the univariate analysis that showed at least a tendency with the 1-minute STS repetitions, ie, a P-value <0.2. Only independent variables, which increased consistently the explained variance (R2 coefficient of determination) of the 1-minute STS repetitions, were used in the final model.
The variables that significantly changed from baseline to the end of the PR were identified using a paired t-test in case of normality of the distribution or the Wilcoxon test if not.
The analysis of the MID was based on the use of distribution-based methods, using the effect size method (1-minute STS repetitions[end-baseline]/SDbaseline), the standardized response mean method (1-minute STS repetitions[end-baseline]/SD[end-baseline]), the SD method (0.5× SD of 1-minute STS repetitions at baseline) or the standard error of measurement method (SD of 1-minute STS repetitions at baseline × √[1-{test–retest reliability}]). Based on personal data of our team, not yet published, we found the test–retest reliability of the 1-minute STS test to be 0.906. For anchor-based estimation of the MID, we used the change from baseline variables that correlated with the change in 1-minute STS repetitions from baseline to the end with a correlation coefficient of at least 0.3 and a P-value <0.05 as recommended.23 Then, we used the sensitivity- and specificity-based approach with receiver operating characteristic curves to determine the best cutoff for the change in 1-minute STS repetitions with the established MID of 30 m for the 6MWT, according to the recommendations.19,20 A P-value of <0.05 was considered significant.
Results
The characteristics of the subjects included in the study are given in Table 1. Using the GOLD spirometric classification, the repartition was 5% in GOLD 1, 49% in GOLD 2, 28% in GOLD 3, and 18% in GOLD 4. Using the combined COPD assessment A, B, C, and D categories, the repartition was 9% in group A, 30% in group B, 3% in group C, and 58% in group D.12
Table 1.
Baseline characteristics of the patients
| Male/female, n | 30/18 |
| Age, year | 64±9.8 |
| BMI, kg/m2 | 26.4±7 |
| Smoking, pack-year | 44.7±21.8 |
| Dyspnea, mMRC | 2.2±0.7 |
| Charlson index | 3.5±1.7 |
| Lung function: | |
| * FEV1, % predicted | 52.3±16.9 |
| * FVC, % predicted | 77.6±19.6 |
| * FEV1/FVC | 54.4±12.5 |
| * RV/TLC, % predicted | 135.4±27.5 |
| * MIP, % predicted# | 79.8±20.2 |
| Exercise testing: | |
| * 6MWD, m | 387.6±111 |
| * 6MWD, %# | 64±38 |
| * 6MWT desaturation, n (%) | 20 (42) |
| * QMVC, %# | 72.5±21.8 |
| * Peak O2 consumption, ml/min/kg | 13.8±3 |
| * Peak O2 consumption, %# | 61.2±15.3 |
| SGRQ total score | 55.1 ± 15.1 |
| HAD total score | 16±6.8 |
| BODE index | 3.1±1.5 |
Notes: Data are presented as mean ± SD unless otherwise indicated. Post-bronchodilator results are provided in percentage of predicted value.
Predicted values are from Uldry et al35 for MIP, from Troosters et al37 for the 6MWD, from Hogrel et al38 for QMVC and from Hansen et al39 for Peak O2 consumption. 6MWT desaturation, fall of at least 4% of the SpO2, and post-test SpO2 <90% according to Wedzicha et al36.
Abbreviations: BMI, body mass index; BODE, body mass index, airflow obstruction, dyspnoea and exercise capacity index; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; HAD, hospital anxiety depression; MIP, maximal inspiratory pressure; mMRC, modified medical research council scale; 6MWD, 6-minute walk distance; QMVC, quadriceps maximum voluntary contraction; RV/TLC, residual volume/total lung capacity; SGRQ, saint george respiratory questionnaire.
Determinants of 1-minute STS repetitions at baseline
The main parameters linked with 1-minute STS repetitions at baseline in the univariate analysis were as follows: 6-minute walk distance (6MWD) (r=0.574; P<10−3), age (r=−0.453; P=0.001), patients on long-term oxygen treatment (r=−0.454; P=0.017), and QMVC (r=0.424; P=0.031). The height was weakly (but not significantly) and negatively associated with the number of repetitions (r=−0.197; P=0.185). There were no significant results with variables from pulmonary function tests (notably lung hyperinflation parameters) and cardiopulmonary exercise tests. The best prediction model in the multivariate analysis was obtained with a combination of 6MWD, height, and QMVC, without any improvement of the model after adjustment for age and sex. The explained variance of 1-minute STS repetitions from the determination coefficient (R2) was 75.8% (Table 2).
Table 2.
Main determinants of 1-minute STS repetitions at baseline (multivariate analysis)
| Variables | b | Correlation coefficient (r) | P-value |
|---|---|---|---|
| 6MWD, m | 0.038 | 0.834 | <10−3 |
| Height, cm | −0.438 | −0.532 | <10−3 |
| QMVC, Newton | 0.016 | 0.236 | 0.065 |
| b0 | 74.254 |
Notes: The predicted equation is based on a linear regression analysis with the following form: 1-minute STS repetitions = b0 + (b × 6MWD) + (b × height) + (b × QMVC) =74.254 + 0.038× 6MWD −0.438× height + 0.016 × QMVC).
Abbreviations: 6MWD, 6-minute walk distance; QMVC, quadriceps maximal voluntary contraction; STS, sit-to-stand.
Responsiveness to PR
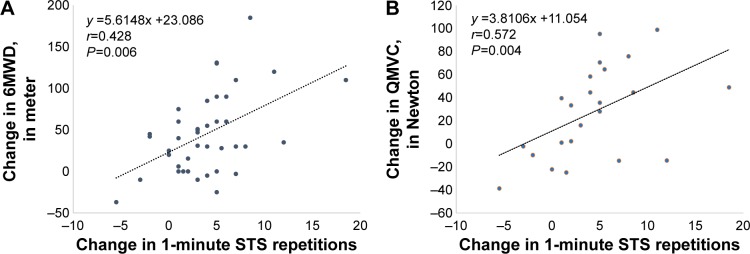
The change in the parameters measured during the 1-minute STS test at baseline and at the end of the PR are listed in Table 3 and compared with the main parameters obtained from the 6MWT and the QMVC in Table 4. The change in 1-minute STS repetitions was correlated with the change in 6MWD (r=0.428; P=0.006) and QMVC (r=0.572; P=0.004) (Figure 1).
Table 3.
Change of the parameters measured during 1-minute STS test at baseline and at the end of PR
| Baseline | End | P-value | |
|---|---|---|---|
| 1-minute STS repetitions | 19.2±6.1 | 23.0±7.7 | <10−3 |
| Dyspnea, Borg scale | 4.9±2.1 | 4.0± 1.7 | 0.025 |
| HR, beats/min | 101.3±15.7 | 101.9±15.3 | 0.705 |
| SpO2, % | 91.6±3.9 | 91.2±4.4 | 0.902 |
Notes: The test was performed at the inclusion (Baseline) and at the end of the PR (End). All the variables were measured at the end of each test (1 minute). Data presented as mean ± standard deviation.
Abbreviations: 6MWD, 6-minute walk distance; HR, heart rate; PR, pulmonary rehabilitation; QMVC, quadriceps maximal voluntary contraction; SpO2, minimal pulse oxygen saturation percentage measured at the end of the test; STS, sit-to-stand.
Table 4.
Comparisons between 1-minute STS repetitions, 6MWD and QMVC at baseline and the end of the PR
| Baseline | Change from baseline (absolute value) | Change from baseline (% baseline value) | P-value | |
|---|---|---|---|---|
| 1-minute STS repetitions | 19.2±6.1 | 3.8±4.2 | 23±28 | <10−3 |
| 6MWD, m | 387.6±111 | 45.3±47.7 | 15±17 | <10−3 |
| QMVC, Newton | 244.6±83.7 | 27.4±39.5 | 14±21 | 0.003 |
Notes: The tests were performed at the inclusion (Baseline) and at the end of the PR. Data presented as mean ± standard deviation.
Abbreviations: 6MWD, 6-minute walk distance; QMVC, quadriceps maximal voluntary contraction; STS, sit-to-stand.
Figure 1.
Association between change in 1-minute STS test and change in (A) 6-minute walk distance (6MWD) and (B) quadriceps maximal voluntary contraction (QMVC) with pulmonary rehabilitation.
Note: (A and B) The black dotted line is the linear regression of the relation between the two variables.
Abbreviations: 6MWD, 6-minute walk distance; QMVC, quadriceps maximal voluntary contraction; STS, sit-to-stand.
Determination of the minimal important difference
Using the distribution-based methods, the MID calculated for the change in the number of repetitions was 3.1 using the SD, 1.9 using the standard error of measurement, with an effect size of 0.6. For the anchor-based method, we use the MID of 30 m from the 6MWT as anchor. The receiver operating characteristic curve identified the point between 2.5 and 3.5 with the best sensitivity (80% and 64%, respectively) and specificity (60% and 67% respectively) with an area under the curve of 0.716 (Figure 2).
Figure 2.
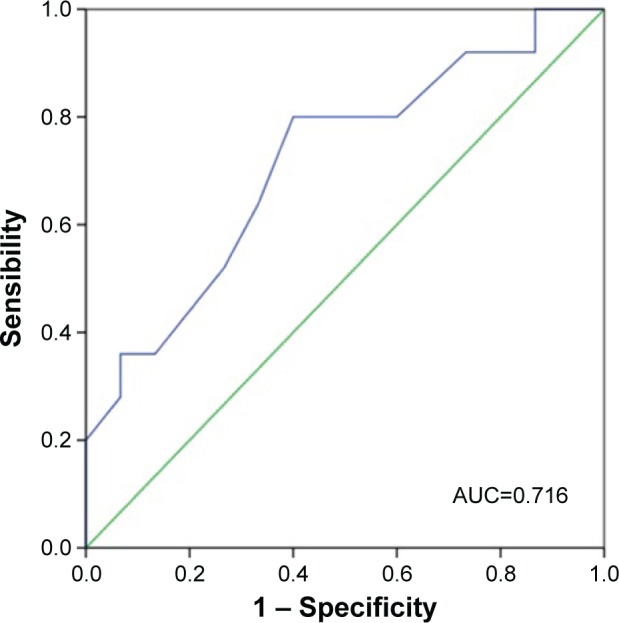
Receiver operating characteristic curve for the identification of the minimal important difference of the 1-minute STS test using a 30 m improvement in 6-minute walk test as anchor.
Abbreviations: STS, sit-to-stand; AUC, area under curve.
Determinants of an improvement of 1-minute STS repetitions between baseline and the end of PR
Only two parameters were significantly associated with the improvement of 1-minute STS repetitions between baseline and end of PR. The univariate analysis identified the change in the distance obtained from the 6MWT (r=0.496; P=0.001) and the change in the QMVC (r=0.503; P=0.015), with an explained variance (R2) of 24.6% and 25.3%, respectively. The combination of both parameters in the multivariate analysis produced a model in which the explained variance increased to 43.9% (r=0.663; P=0.003), with the following equation: Change in 1-minute STS repetitions = 0.041× (change in 6MWD) + 0.049 × (change in QMVC) + 1.052 in which both parameters remained significant (0.018 and 0.047, respectively).
Discussion
The results of this study demonstrate the utility of the 1-minute STS test in the assessment of COPD patients undergoing PR. Its ability to detect a change in exercise tolerance is similar to the 6MWT. The change in the number of repetitions also correlated with the change in the distance from the 6MWT as well as the change in QMVC, which makes this tool a valuable test to assess lower limb strength and the impact on walking capacity. Using different methods to determine the MID of the 1-minute STS test, a change of at least three repetitions provides a good agreement in comparison to the 30 m improvement in 6MWD.
The STS test was first introduced as a measure of lower limb strength and remains an important marker of an independent lifestyle.24 Since then, various studies have been conducted with different modalities of this test. Ozalevli et al was one of the first to compare the 1-minute STS test with the 6MWT, and the results demonstrated a good correlation (r=0.75; P<0.001) among these tests.7 Similar to the 6MWT, the 1-minute STS test was able to determine the functional status correctly.7 Their study presented the 1-minute STS test as an alternative to the 6MWT in COPD patients. Unlike the 6MWT, where a practice test is recommended, the STS test seems less prone to the learning effect.19,20 Recent data show that the number of repetitions a patient is able to do during the 1-minute STS correlated with the level of physical activity, which makes it a good surrogate of physical activity.25 This was also reported by van Gestel et al who showed that the number of steps taken per day related to the number of repetitions in the 1-minute STS test among COPD patients.26 The 1-minute STS test is then a good surrogate of the level of physical activity achieved by a patient in his daily life. In this study, we not only found a correlation with age but also a relation with the patient’s height, which underlines the influence of chair height on lower limb mechanics during rising.27
While the perception of the effort by the patient in terms of dyspnea and fatigue was similar in the 1-minute STS test and 6MWT, the physiological responses measured by the pulse oxygen saturation and heart rate were less important in the 1-minute STS test. This test therefore seems more appropriate for the evaluation of the leg capacity apart from the ventilatory limitations. Consequently, it provides relevant information about the quality of the biomechanical response in terms of postural control and lower limb muscle strength. Because the ventilatory limitations may be more limited than balance in COPD patients, it is not surprising to find a good correlation between the 1-minute STS test and the quadriceps strength as it was reported by others using the 30-seconds or 1-minute STS test.28,29 A more recent modality of the STS test in 3 minutes presented by Aguilaniu et al shows a good interchangeability of the physiological characteristics among the 6MWT and the STS test.30 But this proof of concept study still suffers from weaknesses due to a more complex modality compared with previous STS tests. Moreover, there is no confirmation by other teams or studies of the advantage of such modality, which limits its use in practice.
More interesting is the ability of the 1-minute STS test to detect a change in the functional status of the subjects. In the whole group in average, there was an improvement in the number of repetitions and a reduction in the perception of dyspnea at the end of PR. The differences were not significant for the heart rate and pulse oxygen saturation, which is probably related to the short duration of the test even if it is perceived as a maximal task by the patient. It provides additional information that cannot be compared to more standardized incremental exercise tests, such as the incremental shuttle walk test or cardiopulmonary exercise test. While STS tests explored the balance quality and the efficiency of the biomechanical properties of lower limbs, the incremental exercise tests explored the maximal cardiorespiratory and metabolic capacities of the patient. The lack of these abilities contribute to the worsening of a sedentary lifestyle by patients, which is the hallmark of disability in chronic diseases of the cardiac, respiratory, locomotor, and metabolic systems.1 The study conducted by Jones et al11 showed that the simple five-repetition STS test was responsive to PR, as Panton et al previously demonstrated a significant increase in the number of repetitions in the 1-minute STS test among 17 COPD subjects after an aerobic-based training program.31 As the improvement in the 1-minute STS test presented in our study matched the improvement in 6MWT and QMVC, we were able to estimate an MID between 1.9 and 3.5 with a consistent effect size using different distribution and anchor-based approaches. An operational target of at least 2.5, ie, three more repetitions, represents an acceptable and conservative estimate. We opted for an MID of 30 m for the 6MWD, which corresponds to the last European Respiratory Society and American Thoracic Society task force recommendation in chronic respiratory disease.19,20 However, there could be some variability in the number of repetitions depending on the distance chosen as the MID, ie, a higher number of repetitions for a distance of 33 m and lower using 25 m.
Limitations
This study entailed some limitations partly due to the small number of subjects included. However, the multicenter approach ensures that the significant results obtained provide a robust and realistic picture of the heterogeneity of COPD patients in the daily practice of PR. In another way, some results did not reach the significance probably because of low statistical power that should be confirmed by larger studies. It is particularly important to clarify some issues about the standardization of the test, notably the impact of adjusting the height of the seat to guarantee an optimal position for all patients.32,33 Although the 1-minute STS test remains a good surrogate of lower limb strength, this property could be biased in overweight patients in whom sitting up from a chair represents a higher energy expenditure compared to normal weight patients. But this should be confirmed in further studies, since the weight was not found to be a determinant at least in the five-repetition STS.34 These limitations are partially overcome by the use of reference values for 1-minute STS test that allow intergroup comparisons.18 But one of the most important result is the sensitivity to change the 1-minute STS test to objectively assess the improvement of a given patient after PR.
Conclusion
In conclusion, this study shows that the 1-minute STS test is a simple and sensitive test that is capable of depicting physical improvement after PR. An MID of 3 represents a good target to consider for assessing the benefit gained by patients after an intervention of physical training.
Acknowledgments
The authors would like to acknowledge colleagues from the following teams who were involved in the task of taking care of patients in pulmonary rehabilitation, and assisting with the inclusion and data mining process: C Nogues, MP Humeau, and S Grelier from Explorations Fonctionnelles, Hôpital Laennec, CHU de Nantes in Laennec Hospital, Nantes; L Nourry and C Le Blanc from Pulmonary Rehabilitation Department in Saint-Jacques Hospital, Nantes; members from Tourmaline Medical Centre in Nantes; A Martin from Pulmonary Rehabilitation Unit of the CH des Pays de Morlaix, Morlaix; A Martin from Pulmonary Department of the University of Angers and Pulmonology Department of the University Hospital of Angers; M Le Bras from Centre d’Investigation Clinique Thorax, l’institut du thorax, CHU de Nantes; and C Gosselin, N Meslier, and JL. Racineux from the Institut de Recherche en Santé Respiratoire des Pays de la Loire.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization . The International Classification of Functioning, Disability and Health (ICF) Geneva: WHO Press; 2001. [Google Scholar]
- 2.O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225–236. doi: 10.1080/15412550701480455. [DOI] [PubMed] [Google Scholar]
- 3.Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. 2009;39(8):495–501. doi: 10.1111/j.1445-5994.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 4.Revill SM, Morgan MD, Singh SJ, Williams J, Hardman AE. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax. 1999;54(3):213–222. doi: 10.1136/thx.54.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–1024. doi: 10.1136/thx.47.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villiot-Danger E. A stairclimbing test in COPD patients assessment. Rev Mal Respir. 2009;26(5):530–536. doi: 10.1016/s0761-8425(09)74672-8. [DOI] [PubMed] [Google Scholar]
- 7.Ozalevli S, Ozden A, Itil O, Akkoclu A. Comparison of the sit-to-stand test with 6 min walk test in patients with chronic obstructive pulmonary disease. Respir Med. 2007;101(2):286–293. doi: 10.1016/j.rmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Coquart JB, Lemaitre F, Castres I, Saison S, Bart F, Grosbois JM. Reproducibility and sensitivity of the 6-minute stepper test in patients with COPD. COPD. 2015;12(5):533–538. doi: 10.3109/15412555.2014.974733. [DOI] [PubMed] [Google Scholar]
- 9.Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57(8):M539–M543. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- 10.Gross MM, Stevenson PJ, Charette SL, Pyka G, Marcus R. Effect of muscle strength and movement speed on the biomechanics of rising from a chair in healthy elderly and young women. Gait Posture. 1998;8(3):175–185. doi: 10.1016/s0966-6362(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 11.Jones SE, Kon SS, Canavan JL, et al. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax. 2013;68(11):1015–1020. doi: 10.1136/thoraxjnl-2013-203576. [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 13.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 16.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. report working party standardization of lung function tests, European community for steel and coal. Official statement of the European respiratory society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 17.American Thoracic S, American College of Chest P ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 18.Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health. 2013;58(6):949–953. doi: 10.1007/s00038-013-0504-z. [DOI] [PubMed] [Google Scholar]
- 19.Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 20.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 21.Bachasson D, Villiot-Danger E, Verges S, et al. Maximal isometric voluntary quadriceps strength assessment in COPD. Rev Mal Respir. 2014;31(8):765–770. doi: 10.1016/j.rmr.2013.10.645. [DOI] [PubMed] [Google Scholar]
- 22.Bohannon RW, Kindig J, Sabo G, Duni AE, Cram P. Isometric knee extension force measured using a handheld dynamometer with and without belt-stabilization. Physiother Theory Pract. 2012;28(7):562–568. doi: 10.3109/09593985.2011.640385. [DOI] [PubMed] [Google Scholar]
- 23.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78(1):77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 25.Bisca GW, Morita AA, Hernandes NA, Probst VS, Pitta F. Simple lower limb functional tests in patients with chronic obstructive pulmonary disease: a systematic review. Arch Phys Med Rehabil. 2015;96(12):2221–2230. doi: 10.1016/j.apmr.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 26.van Gestel AJ, Clarenbach CF, Stowhas AC, et al. Predicting daily physical activity in patients with chronic obstructive pulmonary disease. PLoS One. 2012;7(11):e48081. doi: 10.1371/journal.pone.0048081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo YL. The influence of chair seat height on the performance of community-dwelling older adults’ 30-second chair stand test. Aging Clin Exp Res. 2013;25(3):305–309. doi: 10.1007/s40520-013-0041-x. [DOI] [PubMed] [Google Scholar]
- 28.Butcher SJ, Pikaluk BJ, Chura RL, Walkner MJ, Farthing JP, Marciniuk DD. Associations between isokinetic muscle strength, high-level functional performance, and physiological parameters in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:537–542. doi: 10.2147/COPD.S34170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rausch-Osthoff AK, Kohler M, Sievi NA, Clarenbach CF, van Gestel AJ. Association between peripheral muscle strength, exercise performance, and physical activity in daily life in patients with chronic obstructive pulmonary disease. Multidiscip Respir Med. 2014;9(1):37. doi: 10.1186/2049-6958-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguilaniu B, Roth H, Gonzalez-Bermejo J, et al. A simple semipaced 3-minute chair rise test for routine exercise tolerance testing in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:1009–1019. doi: 10.2147/COPD.S59855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panton LB, Golden J, Broeder CE, Browder KD, Cestaro-Seifer DJ, Seifer FD. The effects of resistance training on functional outcomes in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol. 2004;91(4):443–449. doi: 10.1007/s00421-003-1008-y. [DOI] [PubMed] [Google Scholar]
- 32.Janssen WG, Bussmann HB, Stam HJ. Determinants of the sit-to-stand movement: a review. Phys Ther. 2002;82(9):866–879. [PubMed] [Google Scholar]
- 33.Rodosky MW, Andriacchi TP, Andersson GB. The influence of chair height on lower limb mechanics during rising. J Orthop Res. 1989;7(2):266–271. doi: 10.1002/jor.1100070215. [DOI] [PubMed] [Google Scholar]
- 34.Bohannon RW, Bubela DJ, Magasi SR, Wang YC, Gershon RC. Sit-to-stand test: performance and determinants across the age-span. Isokinet Exerc Sci. 2010;18(4):235–240. doi: 10.3233/IES-2010-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uldry C, Fitting JW. Maximal values of sniff nasal inspiratory pressure in healthy subjects. Thorax. 1995;50(4):371–375. doi: 10.1136/thx.50.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wedzicha JA. Domiciliary oxygen therapy services: clinical guidelines and advice for prescribers. Summary of a report of the Royal College of Physicians. J R Coll Physicians Lond. 1999;33(5):445–447. [PMC free article] [PubMed] [Google Scholar]
- 37.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14(2):270–274. doi: 10.1034/j.1399-3003.1999.14b06.x. [DOI] [PubMed] [Google Scholar]
- 38.Hogrel JY, Payan CA, Ollivier G, et al. Development of a French isometric strength normative database for adults using quantitative muscle testing. Arch Phys Med Rehabil. 2007;88(10):1289–1297. doi: 10.1016/j.apmr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 Pt 2):S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]