Abstract
Objective
Inflammation in response to oxidized lipoproteins is believed to play a key role in acute coronary syndromes (ACS), but the pattern of immune activation has not been fully characterized. We sought to perform detailed phenotypic and functional analysis of CD8 T lymphocytes from patients presenting with ACS to determine activation patterns and potential immunologic correlates of ACS.
Approach and Results
We used polychromatic flow cytometry to analyze the cytokine production profiles of naïve, effector, and memory CD8 T cells in patients with ACS compared to control subjects with stable coronary artery disease. ACS was associated with an altered distribution of circulating CD8+ T cell maturation subsets with reduced proportions of naïve cells and expansion of effector memory cells. ACS was also accompanied by impaired interleukin (IL)-2 production by phenotypically naïve CD8 T cells. These results were validated in a second replication cohort. Naïve CD8 cells from ACS patients also had increased expression of programmed cell death (PD)-1, which correlated with IL-2 hypoproduction. In vitro, stimulation of CD8 T cells with oxidized low density lipoprotein (ox-LDL) was sufficient to cause PD-1 upregulation and diminished IL-2 production by naïve CD8 T cells.
Conclusions
In this exploratory analysis, naïve CD8+ T cells from ACS patients show phenotypic and functional characteristics of immune exhaustion: impaired IL-2 production and PD-1 upregulation. Exposure to ox-LDL recapitulates these features in vitro. These data provide the first evidence that ox-LDL could play a role in immune exhaustion and that this immunophenotype may be a biomarker for ACS.
Keywords: Acute Coronary syndrome, oxidized low density lipoprotein, inflammation Subject Codes: Acute coronary syndromes [3], lipid and lipoprotein metabolism [90]
Introduction
Immature T cells undergo positive and negative selection in the thymus and then emerge as naïve T cells. After exposure to their cognate antigen in the context of appropriate co-stimulation, naïve cells become activated and differentiate into effector, and ultimately memory T cells. Effector subsets are hyper-responsive and short-lived. Memory cells are long-lived and can self-propagate to provide an anamnestic response to antigen. Under conditions of antigen persistence, such as human immunodeficiency virus (HIV) infection and cancer, a state of T cell hyporesponsiveness, or T cell exhaustion, can occur1–4. This is accompanied by the expression of the marker programmed cell death (PD)-1 and impaired IL-2 production by antigen specific CD8 T cells.
Acute coronary syndromes (ACS) are generally caused by a localized disruption of atherosclerotic plaque, either by rupture or erosion. Since multiple coronary artery segments can be involved simultaneously5, both local and systemic processes may underlie the complex pathophysiology of ACS. Oxidized lipoproteins within the plaque are potent activators of innate immune responses and appear to play an important role in the conversion of stable coronary artery disease (CAD) to ACS6–8. The role of lymphocyte function in ACS is less clearly defined. Previous studies in humans suggest that ACS may be accompanied by perturbed cytokine production9–13, but the underlying mechanisms accounting for these changes have not been established. We have previously reported that ACS is accompanied by an expansion of inflammatory monocytes akin to findings in HIV disease, however, this was not accompanied by evidence of CD8 T cell activation as measured by the expression of HLA-DR14.
We sought to analyze, in fine detail, lymphocyte phenotypes in patients presenting with ACS. Since T lymphocyte function depends critically on whether the cell has previously been exposed to its cognate antigen, we sought to determine whether ACS might be accompanied by relative differences in maturation populations (naïve, effector, terminal effector, effector memory, and central memory), and whether the cytokine production among corresponding subpopulations is perturbed in ACS.
Materials and Methods
Materials and methods are available in the online-only data supplement.
Results
Imbalance of CD8+Maturation Subsets in ACS
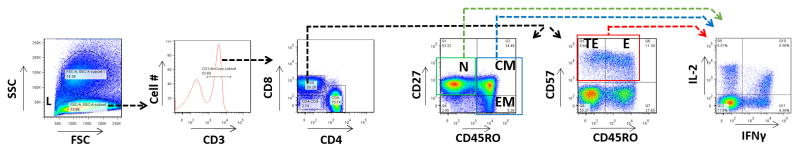
To evaluate the possibility that ACS is associated with changes in the relative frequencies of CD8+ T cell subpopulations, peripheral blood mononuclear cells (PBMC) from patients with ACS and patients with stable CAD were analyzed for phenotype and function by polychromatic flow cytometry. CD8+ naïve, effector, terminal effector, effector memory, and central memory subpopulations were defined using standard phenotypic markers including CD45RO, CD27, and CD57, using the sequential gating strategy shown (Figure 1). To minimize the possibility of type I error, significant differences observed in the pilot cohort were tested in a replication cohort. The baseline demographic and clinical characteristics of these patients are displayed in Table 1.
Figure 1. Flow Cytometry Gating Strategy.
Peripheral blood mononuclear cells from ACS patients and controls were analyzed by polychromatic flow cytometry. Lymphocytes (L) were gated according to forward scatter (FSC) and side scatter (SSC). T cells were selected based upon CD3 expression. CD8+/CD4− T cells were then assigned maturation status according to their expression of CD27, CD45RO, and CD57. Naïve cells are CD27+/CD45RO− (N). Central memory (CM) cells are CD27+/CD45RO+. Effector memory (EM) cells are CD27−/CD45RO+. Terminal effector (TE) cells are CD57+/CD45RO−. Effector (E) T lymphocytes are CD57+/CD45RO+. The expression of IL-2 and IFNγ was then analyzed among naïve, total effector (TE and E), and total memory (CM and EM, shown as example in figure) CD8+ T cells.
Table 1.
Baseline Demographic and Clinical Characteristics
| Pilot Cohort | Replication Cohort | Total Population | |||||
|---|---|---|---|---|---|---|---|
| Stable CAD | ACS | Stable CAD | ACS | Stable CAD | ACS | ||
| n=10 | n=14 | n=56 | n= 20 | n=66 | n= 34 | ||
| Demographic Profile | |||||||
| Median Age (25th, 75th %) | 66 (53, 75) | 64 (51, 68) | 59 (52, 64) | 66 (58, 75) | 59.5 (53.0, 65.2) | 64.5 (56.8, 73.3) | |
| Male | 8 (80 %) | 7 (50 %) | 39 (69%) | 10 (50%) | 47 (71.2 %) | 17 (50 %) | |
| Caucasian | 8 (80 %) | 8 (57 %) | 49 (88%) | 15 (75%) | 57 (86.4 %) | 23 (67.6 %) | |
| Cardiac Risk Factors | |||||||
| DM | 2 (20 %) | 6 (43 %) | 8 (14%) | 5 (25%) | 10 (15.2 %) | 11 (32.4 %) | |
| HTN | 7(70 %) | 9 (64 %) | 32 (57%) | 16 (80%) | 39 (59.1 %) | 25 (73.1 %) | |
| Dyslipidemia | 8 (80 %) | 11 (79 %) | 31 (55%) | 11 (55%) | 39 (59.1 %) | 22 (64.7 %) | |
| Median LDL (25th, 75th %) | 85 (70, 114) | 100.5 (71, 117) | 109.0 (78, 131) | 99.0 (71, 119) | 105.0 (77.0, 129.5) | 100.0 (71.0, 118.0) | |
| Tobacco smoking- active | 2 (20 %) | 3 (21 %) | 5 (4%) | 9 (45%) | 7 (10.6 %) | 12 (35.3 %) | |
| Tobacco smoking- previous | 5 (50 %) | 5 (35 %) | 17 (30%) | 5 (25%) | 22 (33.3 %) | 10 (29.4 %) | |
| FHx- Premature CAD | 2 (20%) | 2 (14 %) | 12 (21%) | 4 (20%) | 14 (21.2%) | 6 (17.6%) | |
| Cardiovascular Disease | |||||||
| Prior CABG | 4 (40 %) | 2 (14 %) | 2 (4%) | 2 (10 %) | 6 (9.1 %) | 4 (11.8 %) | |
| Prior PCI | 4 (40 %) | 8 (57 %) | 2 (4 %) | 2 (10 %) | 6 (9.1 %) | 10 (29.4 %) | |
| Prior LV dysfunction | 2 (20 %) | 3 (21 %) | 3 (5 %) | 1 (5 %) | 5 (7.6 %) | 4 (11.8 %) | |
| Symptomatic PAD | 2 (20%) | 1 (7%) | 0 | 3 (15 %) | 2 (3.0 %) | 4 (11.8 %) | |
| Prior CVA/TIA | 1 (10 %) | 2 (14 %) | 3 (5 %) | 2 (10 %) | 4 (6.1 %) | 4 (11.8 %) | |
| ESRD | 0 | 1 (7 %) | 1 (2 %) | 0 | 1 (1.5 %) | 1 (1.5 %) | |
| Presentation Details | |||||||
| Positive Cardiac Biomarkers | 1/10 (10 %) | 11/14 (79 %) | 0 (0%) | 20 (100%) | 1 (1.5 %) | 31 (91.2 %) | |
| Revascularization | 1/10 (10 %) | 9/14 (64 %) | 0 (0%) | 20 (100%) | 1 (1.5 %) | 29 (85.3 %) | |
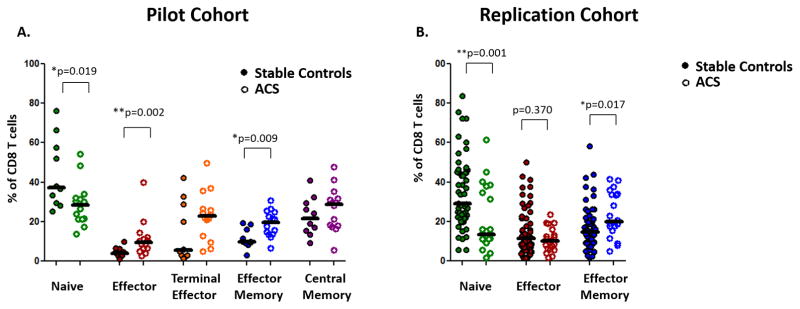
Within the CD8 niche, differences among maturation subsets were apparent in the pilot and replication studies (Figure 2A). The proportion of CD8+ T cells that were phenotypically naïve (CD27+CD45RO−) was lower in ACS patients (ACS: 28.4 vs. control: 37.2%, *p=0.019). In the replication cohort (Figure 2B), the proportion of cells with a naïve surface phenotype was once again reduced in patients with ACS, compared with stable CAD patients (ACS: 13.3% vs. control: 29.1%,** p=0.001). There were no outliers (as defined by having an absolute standardized value greater than 3.29). Taking the pilot and replication cohorts together, the mean difference in the proportion of naïve CD8 T cells was similarly significant after adjustment for age, gender, diabetes, smoking status, and cohort (Table 2).
Figure 2. ACS is associated with altered proportions of maturation subsets among CD8+ T lymphocytes in pilot and replication cohorts.
Peripheral blood mononuclear cells from ACS patients and controls were analyzed by polychromatic flow cytometry, assigning CD8+ cells into maturation subgroups. (A) The relative proportions of CD8+ maturation populations in patients with ACS and stable CAD in the pilot cohort are shown. (B) Significant differences were tested in a replication cohort. (Mann Whitney U; * p<0.05, ** p<0.01)
Table 2. Unadjusted and Adjusted Differences in CD8+ T cell Parameters in Stable CAD versus ACS.
The mean percentage of naïve, effector memory, and IL-2+/IFNγ− naive CD8 T cells in stable and ACS patients are shown. Mean differences are calculated with and without adjustment using multivariate linear regression fitting a generalized linear model with an identity link, adjusting for age, sex, diabetes mellitus, cohort (pilot or replication), and smoking status. After adjustment for these potential confounders, the proportion of naïve CD8+ cells, the proportion of effector memory cells, the proportion of IL-2+/IFNγ− cells among naïve CD8 cells, and the percentage of naïve IL2+/IFNγ− cells among total CD8 T cells remained significantly altered in patients with ACS compared to control patients.
| Stable | ACS | |||||
|---|---|---|---|---|---|---|
| Immune Parameter | Mean (S.D), n | Mean (S.D), n | Unadjusted Difference | Unadjusted p Value | Adjusted c Difference | Adjusted c p Value |
| % Naïve a | 36.2 (18.0), n= 66 | 24.4 (14.9), n=34 | −11.8 | ** 0.002 | − 15.9 | *** < 0.001 |
| % Effector Memory a | 16.1 (10.6), n= 66 | 21.6 (9.9), n=34 | 5.5 | ** 0.003 | 7.6 | ** 0.001 |
| % IL-2+/ IFNγ− Naïve b | 2.7 (2.4), n= 28 | 1.4 (2.6), n=29 | −1.3 | ** 0.002 | − 1.9 | *** < 0.001 |
| % IL-2+/ IFNγ− Naïve a | 0.9 (0.7), n= 28 | 0.4 (0.9), n=29 | −0.5 | *** < 0.001 | −0.8 | *** < 0.001 |
As a percentage of total CD8 T Cells
As a percentage of naive CD8 T Cells
Adjusted for age, sex, diabetes mellitus, cohort, and smoking.
p<0.01,
p<0.001
ACS patients in the pilot cohort had significantly more CD8+ effector memory (CD27−CD45RO+) cells (ACS: 19.5% vs. control: 9.9%, *p=0.009) compared with control subjects. This was also observed in the replication cohort wherein effector memory cells were again found to be increased in ACS (ACS: 19.9% vs. control: 14.7%, *p=0.017). There were no outliers. Taking the pilot and replication cohorts together, the mean difference in the proportion of effector memory CD8 T cells was similarly significant after adjustment for age, gender, diabetes, smoking status, and cohort (Table 2).
ACS patients had significantly more CD8+ effector (CD57+CD45RO+) cells (ACS 9.4% vs. control: 4.0%, *p=0.002) in the pilot study but this was not observed in the replication cohort (ACS: 10.2% vs. control: 11.4%, p=0.370).
The percentage of CD8+ central memory (CD27+CD45RO+) (ACS: 28.8% vs. control: 21.7%, p=0.312) and terminal effector (CD57+CD45RO−) cells (ACS: 22.8%, vs. control: 5.5%, p=0.096) was not significantly different in ACS patients compared with control patients.
These data suggest that within the CD8 niche, ACS is associated with an imbalance of maturation distributions with diminished proportions of naïve and a relative expansion of effector memory CD8+ T lymphocytes.
Altered cytokine production by CD8+ T cells in ACS
We next evaluated the profile of cytokine production by CD8+ T cell populations, comparing corresponding maturation subsets in ACS and stable CAD patients. The production of IL-2 and IFNγ after stimulation via the T cell receptor (TCR) in the presence of co-stimulation was determined by intracellular staining and quantified separately for naïve cells, total effector cells (the aggregate of effector and terminal effector subsets), and total memory cells (the aggregate of effector memory and central memory cells).
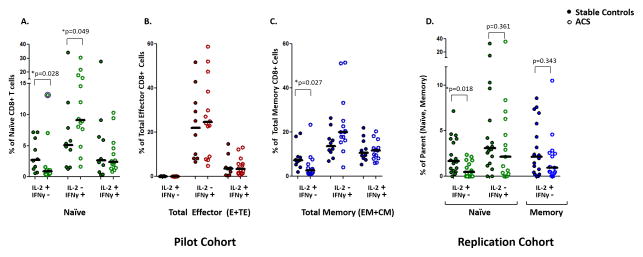
In the pilot study, CD8+ naïve cells from patients with ACS had lower proportions of IL-2+/IFNγ− cells, compared with naïve cells from control patients (ACS 0.9% vs. control 2.7%, *p=0.026) (Figure 3A). Similarly in the replication cohort, the IL2+/IFNγ− response of naïve CD8 T cells in ACS was significantly reduced (ACS: 0.5% vs. control: 1.7%, *p=0.018) (Figure 3D). There was one high outlier among the ACS patients in the pilot cohort as shown whose inclusion/exclusion does not have a notable effect on the calculated significance (**p=0.008 when outlier excluded). Taking the pilot and replication cohorts together, the mean difference in the proportion of IL-2+/IFNγ− CD8 T cells among the naïve population was similarly significant after adjustment for age, gender, diabetes, smoking status, and cohort (Table 2).
Figure 3. ACS is associated with loss of IL-2 production by naïve CD8+ T cells in pilot and replication cohorts.
Peripheral blood mononuclear cells from ACS patients and controls are analyzed by polychromatic flow cytometry, assigning CD8+ cells into maturation subgroups. The percentage of IL2+/IFNγ−, IL2−/IFNγ+, and IL2+/IFNγ+ cells among naïve (A), total effector (B), and total memory (C) CD8+ T cells was compared in a pilot cohort of ACS patients and stable controls. Significant differences were tested in patients enrolled in a replication cohort (D). (Mann Whitney U; * p<0.05) The single outlier (z-score > 3.29) is marked with a purple halo, included in the analysis, and discussed in the text.
Naïve cells from ACS patients tended to have a higher proportion of IL-2−/IFNγ+ cells compared with controls (ACS 9.1% vs. control 5.1%, p=0.048) in the pilot study (Figure 3A) but not in the replication study (ACS: 2.2% vs. control: 3.1%, p=0.361). ACS was not associated with differences in the proportion of multifunctional IL-2+/IFNγ+ cells (ACS 2.4% vs. control 2.6%, p=1.000) within this naïve subpopulation. Thus, CD8+ naïve cells from ACS patients displayed an altered cytokine production profile characterized by reduced frequency of IL-2+/IFNγ− cells compared with CD8+ naïve cells from control patients.
As anticipated, the cytokine profile of total effector CD8+ T cells differed from that of naïve cells in that these subsets expressed no significant IL-2, but exhibited robust IFNγ responses (Figure 3B). There was no difference in the proportions of IL-2+/IFNγ− cells (ACS: 0.03% vs. control: 0.09%, p=0.056), IL-2−/IFNγ+ cells (ACS: 24.6% vs. control: 22.0%, p=0.796), or IL-2+/IFNγ+ cells (ACS: 3.3% vs. control: 3.6%, p=0.666). Thus, CD8+ effector cells did not demonstrate an altered cytokine profile compared to corresponding effector cells in control patients.
Memory cells were capable of efficient production of both IL-2 and IFNγ (Figure 3C). Compared with controls, memory cells from ACS patients had lower proportions of IL-2+/IFNγ− cells (ACS: 2.7% vs. control: 7.4, *p=0.019) in the pilot cohort, but not the replication (Figure 3D) study (ACS: 1.0% vs. control: 2.2%, p=0.343). The proportions of IL-2−/IFNγ+ cells in ACS were not significantly different (ACS: 20.0% vs. control: 13.7, p=0.064). The proportions of multifunctional IL-2+/IFNγ+ cells (ACS: 11.8% vs. control: 10.7, p=0.931) were similar in ACS patients compared with controls.
Naïve CD8+ T Cell Homeostasis in ACS
A consistent feature of both the pilot and replication studies was that naïve CD8 T cells were reduced, both in number and in function vis- à-vis IL-2 production. Taking these two features in combination, the proportion of naïve IL-2+/IFNγ− cells, as a percentage of total CD8 lymphocytes is markedly reduced in ACS (ACS: 0.18% vs. control: 1.04%, **p=0.001, ***p<0.001 excluding outlier), replication cohort (ACS: 0.11% vs. control: 0.71%, **p=0.003) and total population (ACS: 0.16% vs. control: 0.75%, ***p<0.001) (Supplemental Figure 1 A–C).
Oxidized LDL leads to PD-1 Expression and loss of IL-2 Production in vitro
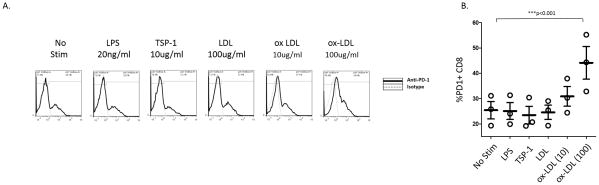
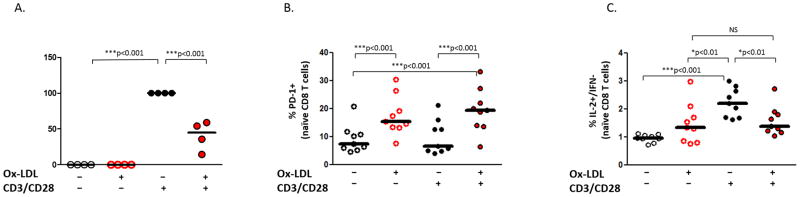
The loss of IL-2 production and apoptosis can be associated with the expression of PD-1 in the setting of immune exhaustion15–19. Since the pathophysiology of ACS is believed to be mediated in part by responses to pro-inflammatory lipids such as oxidized low density lipoproteins (ox-LDL)6,20, we sought to determine whether exposure to ox-LDL might regulate CD8 T cell expression of PD-1 and IL-2. PBMC from healthy volunteers were incubated for 18 hours in medium alone, lipopolysaccharide (LPS, 20ng/ml), thrombospondin (TSP)- 1 (10ug/ml), LDL (100ug/ml), or ox-LDL (10ug/ml, 100ug/ml), and the expression of PD-1 on CD8+ T cells was analyzed by flow cytometry (Figure 4). There is no effect of LPS, TSP-1, or unmodified LDL on PD-1 expression, but exposure to ox-LDL leads to increased expression of PD-1 by CD8 T cells (mean % PD-1 +/− SEM: NS= 25.4 +/− 3.4% vs ox-LDL 100ug/ml= 44.1 +/− 6.5). The effect of oxidized LDL was also apparent on phenotypically naïve (CD27+CD45RO−) CD8 T cells (Supplemental Figure 2). Similar results were obtained when purified CD8 T cells were stimulated with oxidized LDL suggesting the upregulation may be due to a direct effect of oxidized LDL on CD8 T cells (Supplemental Figure 3).
Figure 4. Oxidized LDL exposure leads to expression of PD-1 by CD8 T cells in vitro.
PBMC from healthy volunteers were stimulated for 18 hours in media alone (NS), with lipopolysaccharide (LPS: 20ng/ml), thrombospondin (TSP-1: 10ug/ml), low density lipoprotein (LDL: 100ug/ml), or oxidized LDL (10ug/ml, 100ug/ml) and the expression of PD-1 by CD8 T cells was determined by flow cytometry. Representative flow histograms illustrating PD-1 expression by CD8 T cells (A) and aggregate percentages of PD-1+ CD8 T cells are shown (B). (Repeated measures ANOVA, Bonferroni post hoc tests; ***: p<0.001)
Next, we sought to determine whether exposure to ox-LDL affects TCR stimulated IL-2 production. Purified CD8 T cells from healthy volunteers were incubated for 18 hours in medium with or without ox-LDL (100ug/ml) prior to stimulation with plate bound CD3/CD28 or medium for an additional 18 hours. The supernatants from these conditions were then analyzed for IL-2 protein using cytometric bead assays (Figure 5A). Incubation with ox-LDL led to a significant reduction in IL-2 secretion (40.8 +/− 10.1%). To determine the effects of ox-LDL on naïve CD8 T cells, similar experiments were performed and IL-2 was analyzed by flow cytometry using intracellular staining. PBMC from healthy volunteers were incubated for 18 hours in medium alone or ox-LDL (100ug/ml) prior to stimulation with plate bound CD3/CD28 or medium for an additional 6 hours. Naïve CD8+ T cells were analyzed for PD-1 expression (Figure 5B) and IL-2 expression (Figure 5C). Stimulation of PBMC with ox-LDL, with or without TCR stimulation, led to a significant increase in PD-1 expression by naïve CD8 T cells, an effect which was independent of TCR stimulation (mean % of PD-1+ cells (+/− SEM): NS/NS= 9.2 +/− 1.7%, ox-LDL/NS= 17.5 +/− 2.3%, NS/CD3+CD28= 6.6 +/− 2.0%, ox-LDL/CD3+CD28= 19.3 +/− 2.6%). Stimulation with ox-LDL prior to TCR stimulation lead to a significant reduction in the percentage of IL-2+/IFNγ− naïve CD8 T cells after TCR stimulation (mean % of IL-2+ cells (+/− SEM): NS/NS= 0.96 +/− 0.05, ox-LDL/NS= 1.48 +/− 0.24, NS/ CD3+CD28= 2.23 +/− 0.17%, ox-LDL/CD3+CD28: 1.56 +/− 0.17).
Figure 5. Oxidized LDL exposure leads to the loss of IL-2 responses by CD8 T cells in vitro.
Purified CD8 T cells were incubated for 18 hours with or without oxidized LDL (100ug/ml), and then stimulated with anti-CD28 and plate bound antibodies to CD3 for an additional 18 hours. The supernatant was analyzed for the secretion of IL-2 using cytometric bead assays. IL-2 production was quantified as the percentage of maximal stimulation obtained with CD3/CD28 in the absence of oxidized LDL (A). PBMC were incubated for 18 hours with or without oxidized LDL (100ug/ml), and then cells were stimulated using plate bound antibodies to CD3/CD28 for an additional 6 hours. The expression of PD-1 (B) and IL-2 (C) by naïve CD8+ T cells are plotted. (Repeated measures ANOVA, Bonferroni post hoc tests; * p<0.05, ***: p<0.001)
These results suggest that elevated levels of ox-LDL in ACS, as has been observed both in atheroma21 and systemically22, could impart an immunoregulatory effect on CD8 T cells and account for the reduction in IL-2 production we observe in ACS patients.
ACS is associated with increased PD-1 expression by CD8+ Naïve T cells
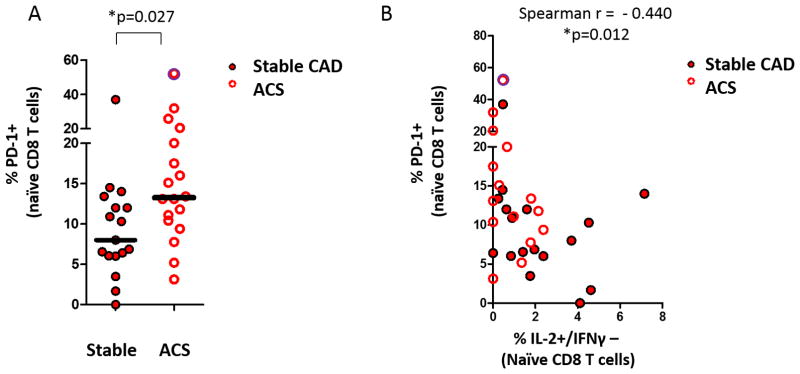
We next sought to determine whether PD-1 expression by naïve CD8 T cells differs in ACS patients compared to controls. Cryopreserved PBMC from patients in the replication cohort were analyzed by flow cytometry for the expression of PD-1 by naïve CD8+ T cells (Figure 6A). ACS was associated with an increase in PD-1+ cells compared to controls (Controls: 8.0% vs ACS: 13.25%, * p=0.023, * p=0.037 with outlier excluded). The PD-1 expression by naïve CD8 T cells correlated inversely with stimulated IL-2 production in naïve CD8+ T cells (Figure 6B) (Spearman r = −0.440, *p=0.012, *p=0.016 with outlier excluded). These results are consistent with the notion that the loss of IL-2 production observed in ACS patients may be indicative of an immune exhaustion phenotype among naïve CD8 T cells.
Figure 6. ACS is associated increased PD-1 expression by naïve CD8+ lymphocytes which correlates with naïve IL-2+/IFNγ− production by intracellular staining.
(A) The expression of PD-1 on naïve CD8+ T cells from patients with ACS is compared to control patients in the replication cohort (Mann Whitney U; * p<0.01). (B) The expression of PD-1 versus IL-2 production among naïve CD8+ T cells is plotted for patients in the replication cohort. (Spearman r, * p<0.05) A single outlier (z-score >3.29) is identified with a purple halo, included in the analysis, and discussed in the text.
Discussion
Herein, we report that ACS is associated with altered proportions of naïve and effector memory subsets within the CD8+ T lymphocyte compartment. ACS patients had a relative reduction in naïve CD8+ T cells and expansion of effector memory cells. Although effector and memory cells were better producers of IFNγ than naïve cells, we observed no consistent difference in IFNγ production by naïve, effector, or memory subsets in ACS, compared with corresponding maturation subsets in stable patients. However, naïve cells from ACS patients were impaired in their production of IL-2, a cytokine of fundamental importance whose autocrine production is required for T cell replication and protective responses to antigen 23,24. These were unexpected findings, leading us to confirm these observations prospectively in a separate cohort. Furthermore, we find that the impairment of IL-2 production by naïve CD8 cells correlates with increased PD-1 expression, a marker of immune exhaustion. In vitro, exposure to oxidized LDL recapitulates these phenotypic and functional features.
The homeostatic maintenance of naïve and memory niches are critical for the capacity to response to new and old antigens, respectively, and thus determine the host response to infection or vaccination25. The production of IL-2 by naïve cells is required for T cell receptor activation, proliferation, and differentiation into effector and ultimately memory cells26. Autocrine IL-2 production is also critical for the recall response and secondary expansion of memory cells.
We hypothesized that ACS might be associated with an altered balance between naïve and effector/memory cells for several reasons. First, ACS has previously been associated with enhanced cytokine production by T lymphocytes27–29. For instance, Liuzzo et al found that T cells display enhanced expression of IFNγ and reduced expression of IL-2 in ACS27. Previous work has also suggested that TCR signaling is hyper-responsive in ACS13. Moreover, ACS has been associated with reduced expression of CCR730, a chemokine receptor that is highly expressed on naïve and central memory T cells but not on effector or effector memory cells. These studies measured changes among total CD4 or CD8 T lymphocytes, but did not account for potential shifts in the balance of naïve and effector/memory T cell subsets. Our hypothesis was that ACS would be accompanied by a relative expansion of effector and memory T cells, which would be consistent with these previous observations.
We did indeed observe a relative decrease in naïve cells, and an increase in effector memory cells. Several processes could account for the altered proportions of CD8+ subsets we observed, including redistribution of cells, expansion of effector memory cells, and decreased proliferation or survival of naïve cells. Additional study is therefore needed to determine the mechanism of this altered homeostasis, whether this is transient or sustained, and its effect on host responses to potential intercurrent illness. Given the temporal association between myocardial infarction and acute upper respiratory tract illnesses31, these data raise the possibility that the immune status of ACS could make patients more susceptible to such illnesses instead of (or in addition to) the reverse.
Chronic inflammation and cancer can be accompanied by a functional impairment of antigen-dependent CD8 T cell responses, a phenomenon termed immune exhaustion. This phenotype refers to a progressive, hierarchical loss of T cell functions including IL-2 production, proliferative potential, inflammatory cytokine production, and ultimately apoptosis of antigen-experienced cells. This phenotype can be attenuated by inhibiting the PD-1 pathway, among other checkpoint receptors, which negatively regulate T cell receptor signaling. This strategy has demonstrated clinical efficacy in the treatment of cancer32 and has the potential to improve the treatment of HIV33,34 and viral hepatitis35.
Whether exhaustion pathways impact the pathogenesis of atherosclerosis has not yet been established. Previous work has demonstrated that genetic deletion of PD-1 or its ligands increases atherosclerosis in LDL-receptor knockout mice36,37. These mice experience increased influx of monocytes, CD4 T cells, and CD8 T cells in association with increased TNFα. However, the role of PD-1 ligation in human atherosclerosis remains uncertain38,39,40. It is against this background that we find phenotypically naive CD8 T cells from ACS patients have a reduced capacity for IL-2 production, and this correlates with increased expression of PD-1. Memory (CD45+) cells from ACS patients also a tended to have reduced IL-2 expression. These data raise the possibility that features of immune exhaustion may pervade the inflammatory milieu in ACS, best demonstrable within the CD27+/CD45RO− niche. It is important to highlight that we used standard surface markers to assign maturation status to control for differences in the proportion of naïve cells when comparing cytokine responses. These markers are imperfect, and thus additional study using expanded panels is warranted to determine with greater granularity the phenotypic and functional differences with particular attention to markers of exhaustion in combination with previous antigen exposure.
Immune exhaustion typically develops under conditions of antigen persistence. Factors such as duration and magnitude of TCR stimulation have been shown to control PD-1 expression2,17. Less well understood are potential extrinsic factors that promote immune exhaustion2,41. We therefore sought to test, in vitro, potential mediators relevant to ACS for effects on PD-1 expression and IL-2 production by CD8 T cells. Our observations that oxidized LDL upregulates PD-1 expression and suppresses IL-2 production raises the possibility that exhaustive pathways can be triggered independent of exposure to cognate antigen. This finding is of potential clinical relevance in cancer and chronic viral infections whose pathogenesis can be complicated by immune exhaustion. For instance the notion that suppression of LDL or the oxidation thereof could limit exhaustion is supported by a recent report that rosuvastatin reduces PD-1 expression in patients with HIV42. This may suggest current and future therapies for LDL lowering might help reverse peripheral tolerance.
Oxidized LDL is capable of activating cells via an expanding array of cell surface receptors including toll-like receptors as well as scavenger receptors such as CD36 and lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1). These pathways have generally been studied in the context of immune activating pathways by oxidized LDL on endothelial cells, platelets, and monocyte/macrophages. Additional study is therefore needed to determine the relevant signaling pathways triggered by oxidized LDL in CD8 T cells. Also, since PD-1 is one of several checkpoint receptors that mediate exhaustion, a more complete understanding of the phenotypic and functional effects of these oxidized lipoproteins is needed. In light of the pro-inflammatory effects of oxidized LDL for innate immune activation, it is tempting to speculate that these negative regulatory effects of oxidized LDL may represent a mechanism to limit the pro-inflammatory effects on monocytes and/or prevent autoimmunity under conditions of oxidative stress.
Lastly, these data and the recent observations of others39,40,42,36,37 raise the possibility that immune exhaustion pathways may have relevance to the progression and activity of atherosclerosis in humans. Additional studies are needed to determine if PD-1 inhibition alters the course of cardiovascular diseases. Proof of either causal or protective relationships between these exhaustion pathways and ACS could signal the potential for novel treatments of atherosclerotic cardiovascular disease.
Limitations and strengths
We sought to assess the feasibility of conducting fine analysis of T lymphocytes in patients with ACS using polychromatic flow cytometry to simultaneously analyze in fine detail both phenotype and function. Several limitations deserve special attention. First, a large number of experimental variables were sampled during the pilot phase of this study, relative to patient sample sizes. To mitigate the likelihood for type 1 error in this setting, we confirmed all significant findings in a separate validation study. Another important limitation is that our ACS and stable CAD patients differed in individual baseline characteristics which may affect immune phenotypes. We adjusted for some of these variables in our generalized linear model, however, additional studies with larger sample sizes are needed to confirm and extend these findings. In addition, the determination of maturation based upon standard surface markers is imperfect and thus additional studies to characterize in finer detail the differences in CD8 T cell subsets in ACS is needed.
Conclusion
We present evidence that ACS is accompanied by altered CD8+ T lymphocyte subpopulations which includes both a relative expansion of antigen-experienced CD8 T cells as well as functional and phenotypic evidence of hyporesponsiveness by naïve CD8 cells. We also describe a heretofore-unrecognized immune effect of oxidized LDL that leads to PD-1 expression and negatively regulates IL-2 production by CD8 cells in vitro. These findings imply that ACS may be a more complex immunologic process than previously recognized with both pro-inflammatory, as well as counter-regulatory immune features. Additional study is needed to determine whether the altered CD8 phenotypes are part cause or consequence of ACS. Additional study is also warranted to determine whether oxidized LDL may contribute to immune exhaustion as part of the pathophysiology of other chronic inflammatory disorders and cancer.
Supplementary Material
Significance.
These findings support the notion that complex immunoregulatory features play a role in the immune pathophysiology of ACS. ACS is accompanied by an imbalance of maturation subsets such that a greater percentage are “antigen-experienced” compared to naïve cells able to recognize new antigens. Furthermore, these naïve CD8 T cells in ACS patients are impaired for IL-2 production and express PD-1 at higher levels, findings that suggest an immune exhaustion phenotype may pervade ACS. These data provide the first evidence to suggest that oxidative stress, mediated through LDL, may be important for the establishment of immune exhaustion. This should provide impetus to establish whether the PD-1 pathway results in a destabilizing versus protective effects within atherosclerotic plaque, which could guide the development of novel treatments for cardiovascular disease.
Acknowledgments
Sources of Funding:
This work was funded by the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- ACS
acute coronary syndromes
- PD-1
programmed cell death
- LDL
low density lipoprotein
- ox-LDL
oxidized LDL
- CAD
coronary artery disease
- TCR
T cell receptor
- IFN
Interferon
- LPS
lipopolysaccharide
- HIV
human immunodeficiency virus
- TSP-1
thrombospondin
Footnotes
Disclosures:
The authors have no conflict of interest related to the contents of this work.
Authorship Contributions:
D.A.Z., J.C.M., S.J., J.P.L., S.S., S.S.P., M.I., R.O., A.O., C.T. performed the experiments and analyzed the primary source data. D.A.Z., J.P.L., M.I., J.B.W., M.A.A., C.O., M.A.C. obtained patient samples and clinical characteristics. D.A.Z., C.C., D.I.S., R.M.C., L.K.N., M.M.L., K.J.W. contributed to experimental design. All authors contributed to data analysis and writing of the manuscript.
References
- 1.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 3.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong M-K, Mintz GS, Lee CW, et al. Comparison of Coronary Plaque Rupture Between Stable Angina and Acute Myocardial Infarction. Circulation. 2004;110:928–933. doi: 10.1161/01.CIR.0000139858.69915.2E. [DOI] [PubMed] [Google Scholar]
- 6.Ehara S, Ueda M, Naruko T, et al. Elevated Levels of Oxidized Low Density Lipoprotein Show a Positive Relationship With the Severity of Acute Coronary Syndromes. Circulation. 2001;103:1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 7.Neri Serneri GG, Coppo M, Bandinelli M, et al. Exaggerated myocardial oxLDL amount and LOX-1 receptor over-expression associated with coronary microvessel inflammation in unstable angina. Atherosclerosis. 2013;226:476–482. doi: 10.1016/j.atherosclerosis.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto N, Nomura S, Kamihata H, Kimura Y, Iwasaka T. Increased level of oxidized LDL-dependent monocyte-derived microparticles in acute coronary syndrome. Thrombosis and Haemostasis. 2004;91:146–154. doi: 10.1160/TH03-04-0247. [DOI] [PubMed] [Google Scholar]
- 9.Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-Cell Repertoire in Patients With Unstable Angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 10.Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER. Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol. 2005;45:1939–1945. doi: 10.1016/j.jacc.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 11.van der Wal AC, Piek JJ, de Boer OJ, et al. Recent activation of the plaque immune response in coronary lesions underlying acute coronary syndromes. Heart. 1998;80:14–18. doi: 10.1136/hrt.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damås JK, Smith C, Øie E, et al. Enhanced Expression of the Homeostatic Chemokines CCL19 and CCL21 in Clinical and Experimental Atherosclerosis: Possible Pathogenic Role in Plaque Destabilization. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:614–620. doi: 10.1161/01.ATV.0000255581.38523.7c. [DOI] [PubMed] [Google Scholar]
- 13.Pryshchep S, Goronzy JJ, Parashar S, Weyand CM. Insufficient Deactivation of the Protein Tyrosine Kinase Lck Amplifies T-Cell Responsiveness in Acute Coronary Syndrome. Circ Res. 2010;106:769–778. doi: 10.1161/CIRCRESAHA.109.206052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei F, Zhong S, Ma Z, et al. Strength of PD-1 signaling differentially affects T-cell effector functions. Proceedings of the National Academy of Sciences. 2013;110:E2480–E2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaitan A, Unutmaz D. Revisiting Immune Exhaustion During HIV Infection. Curr HIV/AIDS Rep. 2011;8:4–11. doi: 10.1007/s11904-010-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment. Journal of Virology. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. International Journal of Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 19.Mühlbauer M, Fleck M, Schütz C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and -γ and mediates T cell apoptosis. Journal of Hepatology. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and Malondialdehyde-Modified LDL in Patients With Acute Coronary Syndromes and Stable Coronary Artery Disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 21.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103(15):1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 22.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 23.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 24.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8+ memory T cells. Nature Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surh CD, Sprent J. Homeostasis of Naive and Memory T Cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Cho J-H, Boyman O, Kim H-O, et al. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. The Journal of Experimental Medicine. 2007;204:1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-Cell Repertoire in Patients With Unstable Angina. 1001999:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 28.Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER. Enhanced T-Helper-1 Lymphocyte Activation Patterns in Acute Coronary Syndromes. 2005;45:1939–1945. doi: 10.1016/j.jacc.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 29.van der Wal AC, Piek JJ, de Boer OJ, et al. Recent activation of the plaque immune response in coronary lesions underlying acute coronary syndromes. 801998:14–18. doi: 10.1136/hrt.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damas JK, Smith C, Oie E, et al. Enhanced Expression of the Homeostatic Chemokines CCL19 and CCL21 in Clinical and Experimental Atherosclerosis: Possible Pathogenic Role in Plaque Destabilization. 272007:614–620. doi: 10.1161/01.ATV.0000255581.38523.7c. [DOI] [PubMed] [Google Scholar]
- 31.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of Myocardial Infarction and Stroke after Acute Infection or Vaccination. New England Journal of Medicine. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 32.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porichis F, Kaufmann D. Role of PD-1 in HIV Pathogenesis and as Target for Therapy. Curr HIV/AIDS Rep. 2012;9:81–90. doi: 10.1007/s11904-011-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 35.Tzeng H-T, Tsai H-F, Liao H-J, et al. PD-1 Blockage Reverses Immune Dysfunction and Hepatitis B Viral Persistence in a Mouse Animal Model. PLoS ONE. 2012;7:e39179. doi: 10.1371/journal.pone.0039179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bu D-x, Tarrio M, Maganto-Garcia E, et al. Impairment of the PD-1 pathway increases atherosclerotic lesion development and inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1100–1107. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. The Journal of Clinical Investigation. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baban B, Liu JY, Qin X, Weintraub NL, Mozaffari MS. Upregulation of Programmed Death-1 and Its Ligand in Cardiac Injury Models: Interaction with GADD153. PLoS ONE. 2015;10:e0124059. doi: 10.1371/journal.pone.0124059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann J, Shmeleva EV, Boag SE, et al. Myocardial Ischemia and Reperfusion Leads to Transient CD8 Immune Deficiency and Accelerated Immunosenescence in CMV-Seropositive Patients. Circulation Research. 2015;116:87–98. doi: 10.1161/CIRCRESAHA.116.304393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu M-K, Wang S-C, Dai Y-X, Wang S-Q, Ou J-M, Quan Z-W. PD-1 and Tim-3 Pathways Regulate CD8+ T Cells Function in Atherosclerosis. PLoS ONE. 2015;10:e0128523. doi: 10.1371/journal.pone.0128523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.