Abstract
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with a poorly understood preclinical stage of immune dysregulation and symptom accrual. Accumulation of antinuclear autoantibody (ANA) specificities is a hallmark of impending clinical disease. Yet, many ANA-positive individuals remain healthy, suggesting that additional immune dysregulation underlies SLE pathogenesis. Indeed, we have recently demonstrated that interferon (IFN) pathways are dysregulated in preclinical SLE. To determine if other forms of immune dysregulation contribute to preclinical SLE pathogenesis, we measured SLE-associated autoantibodies and soluble mediators in samples from 84 individuals collected prior to SLE classification (average timespan = 5.98 years), compared to unaffected, healthy control samples matched by race, gender, age (± 5 years), and time of sample procurement. We found that multiple soluble mediators, including interleukin (IL)-5, IL-6, and IFN-γ, were significantly elevated in cases compared to controls more than 3.5 years pre-classification, prior to or concurrent with autoantibody positivity. Additional mediators, including innate cytokines, IFN-associated chemokines, and soluble tumor necrosis factor (TNF) superfamily mediators increased longitudinally in cases approaching SLE classification, but not in controls. In particular, levels of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL) were comparable in cases and controls until less than 10 months pre-classification. Over the entire pre-classification period, random forest models incorporating ANA and anti-Ro/SSA positivity with levels of IL-5, IL-6, and the IFN-γ-induced chemokine, MIG, distinguished future SLE patients with 92% (± 1.8%) accuracy, compared to 78% accuracy utilizing ANA positivity alone. These data suggest that immune dysregulation involving multiple pathways contributes to SLE pathogenesis. Importantly, distinct immunological profiles are predictive for individuals who will develop clinical SLE and may be useful for delineating early pathogenesis, discovering therapeutic targets, and designing prevention trials.
Keywords: Systemic Lupus Erythematosus, Disease Progression, Autoantibodies, Cytokines, Biomarkers, Forecasting
1. Introduction
Systemic lupus erythematosus (SLE) is a clinically and serologically heterogeneous systemic autoimmune disease which causes significant morbidity and early mortality, especially in young women and minorities (1). Immune dysregulation in the form of pathogenic autoantibodies and chronic inflammation contributes to a wide range of clinical manifestations, including skin rashes, arthritis, and life-threatening renal and/or central nervous system damage [1]. A number of antinuclear autoantibody (ANA) specificities have been shown to accumulate prior to SLE classification [2-4]; preclinical use of hydroxychloroquine may abrogate autoantibody accumulation and delay clinical disease onset [4]. Early intervention is an attractive approach to SLE treatment. However, our understanding of pathogenic mechanisms in preclinical SLE is inadequate. Closing this knowledge gap would improve our ability to identify individuals with preclinical SLE, define windows of opportunity for early intervention, and facilitate the development of pathway-targeted treatments.
Current biomarkers in preclinical SLE have limited utility for forecasting the transition to classified disease [2, 3, 5]. Although SLE-associated autoantibody specificities such as anti-dsDNA, anti-spliceosome and anti-Ro/SSA, accumulate in SLE patients years before classification [3], their presence is not sufficient to predict SLE. ANAs are also found in sera from patients with other systemic rheumatic diseases [6], and from healthy individuals who do not go on to develop SLE, including some unaffected family members of SLE patients [7], and up to 14% of the general population [8]. Because individuals may remain healthy despite being ANA-positive, ANA positivity alone is likely not the sole pathogenic driver of SLE [2, 9, 10]. In addition to ANA positivity, the dysregulation of various immune pathways driven by soluble mediators may contribute to the development of clinical disease. High expression of type I interferon (IFN)-related genes has been associated with SLE, yet an elevated IFN signature is not present in all patients [5]. Evidence stemming from lupus-like animal models and SLE patients suggests that breaks in tolerance leading to the activation and persistence of autoreactive B cells arise from amplified crosstalk between innate and adaptive immunity [11, 12]. Key mediators of such crosstalk, including Th-type cytokines IFN-γ (Th1), interleukin (IL)-4 and IL-5 (Th2), and IL-17 and IL-21 (Th17) facilitate lymphocyte recruitment to germinal centers [13-15] and pathogenic autoantibody production [16, 17] with the help of T-follicular helper (Tfh) cells [17]. We have recently demonstrated that type II IFN (IFN-γ) becomes elevated prior to and concurrent with the development of lupus-associated autoantibodies [18]. The tumor necrosis factor (TNF) superfamily member B lymphocyte stimulator (BLyS), secreted in response to type I and type II IFNs [19, 20], further supports and propagates autoantibody production as a survival factor for self-reactive B-lymphocytes [21]. In addition to driving the production of pathogenic autoantibodies, these mediators also contribute to inflammation associated with SLE disease flare [22] and organ damage [23]. Although these mediators contribute to SLE disease activity, their role in preclinical autoimmunity and transition to clinical disease are not well understood.
No single factor or mechanism is likely sufficient to explain the complexity and heterogeneity of SLE pathogenesis; thus a multivariate, longitudinal approach is warranted to delineate mechanisms of early disease pathogenesis and discern unique parameters that forecast SLE classification. In the current study, we leveraged longitudinal serum samples from the Department of Defense Serum Repository (DoDSR) to compare levels and determine temporal relationships between autoantibodies and immune mediators from multiple immune pathways in individuals who subsequently developed SLE compared to matched, healthy controls. Our findings shed light on potential mechanisms of early preclinical SLE immunopathogenesis, whereby dysregulation of immune mediators occurs prior to and concurrent with autoantibody accumulation, and is amplified leading up to SLE classification. Further, this study informs the design of reliable and sensitive tools to predict SLE onset. Such tools can be used to identify high risk patients in need of rheumatology referral and enrollment in prospective, preclinical intervention studies, as well as inform the development of novel treatment strategies to avert or delay tissue damage that often accompanies transition to classified disease [24-27].
2. Materials and Methods
2.1 Study population and serum samples
Experiments were performed in accordance with the Helsinki Declaration and approved by the Institutional Review Boards of the Oklahoma Medical Research Foundation and the Walter Reed National Military Medical Center. Samples were obtained from the DoDSR. Demographic and clinical information, including medication history and American College of Rheumatology (ACR) criteria for SLE classification, were extracted from medical records by study personnel. All patients with available serum samples covering periods before and at/after SLE classification (n=84) were selected from a cohort comprised of 130 previously identified individuals [2, 28] and 75 newly identified individuals with classified SLE (≥ 4 ACR criteria for SLE [29, 30]). Cases were compared to healthy controls matched by race, sex, age (± 5 years), and time of sample procurement relative to SLE disease classification, as well as sample availability (n=86; Supplemental Table 1). Individuals selected as matched healthy controls had no signs or symptoms of autoimmune disease in their medical record during the time span assessed. In total, 416 samples were analyzed (246 from cases and 170 from controls). Cases had an average of 2.96 available samples (range, 2-3), and controls had an average of 2 available samples (range, 1-3). For sequential longitudinal analysis, samples from SLE cases and their matched controls were divided into four time periods relative to SLE classification, such that each time period included approximately 60 case samples (range, 61 - 63) (Supplemental Fig. 1).
2.2 Soluble mediator and autoantibody assays
Serum levels of BLyS (R&D Systems, Minneapolis, MN) and a proliferation-inducing ligand (APRIL) (eBioscience/ Affymetrix, San Diego, CA) were assessed using enzyme-linked immunosorbent assay (ELISA) per manufacturer's protocol. Normalized fluorescence intensity values for an additional 30 immune mediators, including cytokines, chemokines, and soluble TNFR superfamily members (Supplemental Table 2), were determined by xMAP multiplex assays (eBioscience/Affymetrix) [31]. After performing quality control as described previously [32], four mediators (IFN-α, TNF-α, IL-10, and IL-15) were excluded from further analysis due ≥ 50% of cytokine measurements falling below the lowest level of detection [33]. The average inter-assay coefficient of variance (CV) of the assays performed in this experiment was 10.5%, comparable to the previously reported CV values (10% - 14%) for multiplexed bead-based cytokine assays [34, 35]. Intra-assay precision was high, with an average CV of <10% for duplicate wells in each 30-plex assay. The BioPlex 2200® system (Bio-Rad Technologies) was used to simultaneously detect levels of multiple autoantibody specificities within a single serum sample: dsDNA, chromatin, Ro/SSA, La/SSB, Sm, SmRNP, and RNP [7, 36]. Semi-quantitative values for anti-dsDNA were reported as IU/mL (positive ≥ 10 IU/mL). All other autoantibody specificities were reported in autoantibody index (AI) units based on the fluorescence intensity (range 0-8) using the manufacturer-specified positive cutoff (positive ≥ 1 AI). Factor XIIIb levels were evaluated as a quality control measure, serving as both a serum confirmation and an indicator of sample integrity.
2.3 Statistical analysis
Samples from SLE cases and their matched controls were divided into quartiles based on time of sample procurement relative to SLE classification (Supplemental Fig. 1). Z-scores reflecting the number of standard deviations (SD) away from the mean of values for case vs. control samples were calculated and displayed as a heatmap using R (version 2.15.0). Non-parametric rank-based analysis was performed using GraphPad Prism 6.0 (La Jolla, CA) for variables with asymmetric distribution. P-values were adjusted for multiple comparison by false discovery rate (FDR) using the fdrtools package (version 1.2.12) in R (version 2.15.0). Categorical factors were compared by odds ratios with 95% confidence intervals and chi-square tests. Mixed linear regression models were fitted on normalized FI values of each soluble mediator over time using the lme4 package in R (version 2.15.0). Using mixed models, intercepts were modeled as random effects to account for the initial soluble mediator level of each study participant. Disease status was applied as a fixed effect (or population effect) on change of soluble mediator over time. Optimal positive/negative cut-off values for each soluble mediator that best distinguished cases from controls were determined by maximizing the sum of sensitivity and specificity among all possible soluble mediator levels (Youden index/J statistic) from receiver operating curves (ROC) [37]. The timing of soluble mediator dysregulation or autoantibody positivity was visualized by Kaplan-Meier survival curve analysis, using autoantibody positivity or soluble mediator elevation as the event of interest. Across the entire pre-classification period, the likelihood of soluble mediator dysregulation compared to autoantibody positivity was determined by hazard ratios calculated using a cox proportional hazard model. Statistical significance was determined by robust log-rank test.
A random forest (RF) classification algorithm [38] was implemented using the randomForest R packages (version 4.6-7) to identify factors differentiating individuals who would transition to classified SLE (Supplemental Fig. 2). Default settings were used (mtry = , importance = TRUE, and proximity = TRUE) except that ntree was set to 2,000. For each forest, a randomly selected training set (2/3 of total samples) was used to generate an ensemble of decision trees. The performance of each RF was evaluated using accuracy (1 – out of bag (OOB) error; Supplemental Fig. 2A). Variables were selected using the stepwise-like algorithm of Genuer and Tuleau-Malot [38] to predict cases in each quartile time bin relative to time of sample procurement relative to SLE classification (using R package “fifer” [39]): (a) ANA positivity alone (categorical variable), (b) soluble mediator levels alone (continuous variables), and (c) ANA positivity (categorical variable), SLE-associated autoantibodies to dsDNA, chromatin, Ro/SSA, La/SSB, Sm, and RNP (categorical variables), and soluble mediator levels (continuous variables; Supplemental Fig. 2B). Final RF models identified the set of predictors that independently contributed to the differentiation of future SLE patients. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated based on the averaged misclassification (2×2 chi-square like) matrix of 50 forests generated using the best model. To generate a predictive model for future SLE onset, a single pre-SLE classification sample from each individual (84 cases and 86 controls) was randomly selected to construct a set of independent pre-SLE samples. To ensure the precision of prediction modeling, ten such datasets were generated with replacement, and the best RF model was selected from each dataset. The final overall pre-SLE model consisted of predictors appearing in at least five of the best RF models. The reliability of the final model was confirmed by calculating the average prediction accuracy using the ten independent pre-SLE datasets (Supplemental Fig. 2C). Multidimensional scaling plots of resulting RF proximity matrices were subsequently created using the randomForest R packages (version 4.6-7). Three dimensional scatter plots of cases and controls identified via Random Forest were created using Spotfire [40] and cases contained within clusters compared for differences in age and number of ACR criteria at SLE classification (unpaired t-test), as well as race, medication history, and the presence of individual ACR classification criteria by Fisher's Exact test or Chi-square test, as appropriate.
3. Results
3.1 Innate and adaptive soluble immune mediators are dysregulated more than 3.5 years before SLE classification
Altered levels of multiple adaptive-type soluble mediators, including inflammatory Th1-, Th2-, and Th17-type cytokines, as well as innate and regulatory mediators, have been observed in established SLE [31, 41, 42]. To elucidate the possible involvement of soluble mediators in various stages of preclinical SLE pathogenesis, longitudinal changes in serum cytokine levels were compared in samples spanning pre- and post-classification time periods in cases and controls matched by demographics and time of sample procurement (Supplemental Table 1). Samples were grouped into four time periods (<−3.5, −3.5 to −0.9, −0.9 to 0.1, and >0.1 years relative to disease classification), such that each time period included approximately 60 case samples (Supplemental Fig. 1).
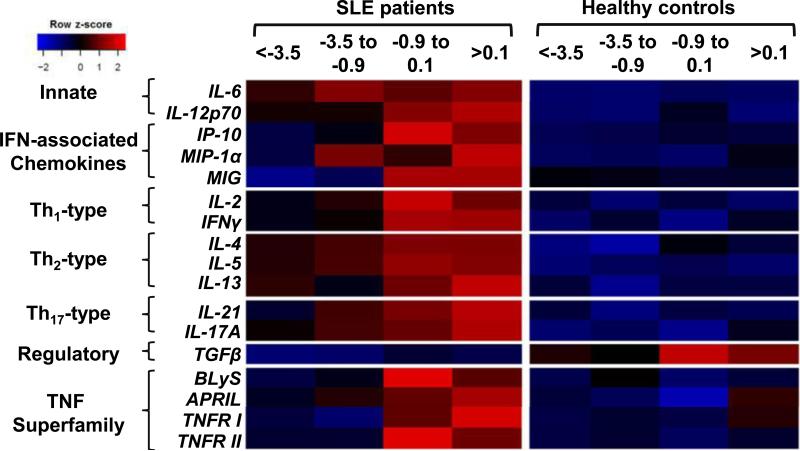
Cases who later developed SLE exhibited increased inflammatory mediators from multiple immune pathways more than 3.5 years pre-classification (Fig. 1 and Table 1). Innate mediators that influence adaptive immune responses were altered in case vs. controls at this earliest time period, including the T-helper (Th) Th2/Th17/Tfh-associated mediator IL-6 (1.69 [1.40-2.15] vs. 1.14 [0.88-1.44], q=6.83×10−6) and Th1-associated mediator IL-12p70 (1.5 [1.33-1.83] vs. 1.24 [1.00-1.60], q=0.013). Additional Th-type mediators elevated in case samples included Th1-type mediator IFN-γ (2.56 [2.2-2.98] vs. 2.20 [1.74-2.75], q=0.035), as well as Th2-type mediators IL-4 (1.60 [1.31-2.00] vs. 1.25 [1.00-1.61], q=0.01) and IL-5 (1.38 [1.24 – 1.62] vs. 0.86 [0.64-1.26], q=3.6×10−6). In addition, the IFN-associated chemokine IFN-γ-inducible protein 10 (IP-10; 2.83 [1.89-4.67] vs. 2.08 [1.45-3.2], q=0.023) was elevated in case samples. Concurrently, case samples had significantly lower levels of the regulatory mediator TGF-β (1.84 [1.47 - 2.54] vs. 2.69 [1.58 - 4.93], q=0.023). These results suggest that early preclinical SLE pathogenesis is marked by an accumulation of dysregulated innate and adaptive mediators, superimposed on a background of deficient regulatory mechanisms.
Figure. 1. Individuals moving toward SLE classification have distinct preclinical soluble mediator profiles compared to healthy controls.
Heat map color type and intensity were determined by median normalized fluorescence intensity values in cases vs. race, gender, age (± 5 years), and time of sample procurement-matched healthy controls at four different quartile periods relative to SLE classification. Blue is lower expression and red is higher expression.
Table 1.
Altered Preclinical Soluble Mediators in Individuals Who Develop SLE
| >3.5 years before classification | 0.9 years before to 0.1 years after classification | ||||||
|---|---|---|---|---|---|---|---|
| Soluble Mediator (Normalized FI) | Case (n=61) Median (IQR) | Control (n=56) Median (IQR) | q-value | Case (n=63) Median (IQR) | Control (n=23) Median (IQR) | q-value | |
| Innate | IL-6 | 1.69 (1.4 - 2.15) | 1.14 (0.88 - 1.44) | 8.26E-06 | 1.86 (1.5 - 3.29) | 1.21 (0.81 - 1.43) | 1.72E-05 |
| IL-12p70 | 1.5 (1.33 - 1.83) | 1.24 (1 - 1.6) | 1.54E-02 | 1.75 (1.5 - 2.17) | 1.39 (1.07 - 1.77) | 1.86E-02 | |
| IFN-Associated Chemokines | IP-10 | 2.83 (1.89 - 4.67) | 2.08 (1.45 - 3.2) | 2.45E-02 | 18.75 (6.09 - 51.5) | 4.08 (2.63 - 5.79) | 8.79E-05 |
| MIP1α | 2.41 (1.51 - 4.81) | 2.13 (1.49 - 3.95) | 9.27E-01 | 3.34 (2.16 - 8.52) | 2.06 (1.38 - 4.51) | 1.91E-01 | |
| MIG | 0.33 (0.16 - 0.6) | 0.54 (0.34 - 0.77) | 1.54E-02 | 0.82 (0.26 - 1.91) | 0.48 (0.38 - 1) | 4.85E-01 | |
| Th1-type | IL-2 | 1.4 (1.14 - 2) | 1.33 (1.17 - 1.81) | 9.27E-01 | 1.8 (1.2 - 2.5) | 1.33 (1.12 - 2.62) | 4.75E-01 |
| IFN-γ | 2.56 (2.2 - 2.98) | 2.2 (1.74 - 2.75) | 3.72E-02 | 3.43 (2.75 - 4.38) | 2.06 (1.81 - 2.87) | 2.01E-03 | |
| Th2-type | IL-4 | 1.6 (1.31 - 2) | 1.25 (1 - 1.61) | 1.25E-02 | 1.8 (1.43 - 2.4) | 1.5 (1.17 - 1.9) | 7.05E-02 |
| IL-5 | 1.38 (1.24 - 1.62) | 0.86 (0.64 - 1.26) | 4.35E-06 | 1.75 (1.31 - 2.08) | 1 (0.61 - 1.14) | 7.43E-07 | |
| IL-13 | 1.32 (1.02 - 1.86) | 1.21 (0.91 - 1.63) | 1.16E-01 | 1.4 (1.11 - 2.33) | 1.2 (0.95 - 1.6) | 3.28E-02 | |
| Th17-type | IL-21 | 0.69 (0.51 - 1.08) | 0.67 (0.43 - 1.01) | 6.72E-01 | 0.99 (0.55 - 2.3) | 0.65 (0.4 - 1.34) | 7.05E-02 |
| IL-17A | 1.87 (1.32 - 2.29) | 1.51 (1.25 - 1.9) | 1.08E-01 | 2.15 (1.75 - 2.95) | 1.42 (1.25 - 2.02) | 8.29E-03 | |
| Regulatory | TGF-β | 1.84 (1.47 - 2.54) | 2.69 (1.58 - 4.93) | 2.45E-02 | 2.21 (1.69 - 3) | 3.67 (2.47 - 6.54) | 2.01E-03 |
| TNF Superfamily | BLyS* | 1049.55 (883.31 - 1319.46) | 1042.96 (905.99 - 1241.23) | 9.27E-01 | 1374.12 (1018.56 - 1788.81) | 1014.64 (792.86 - 1225.4) | 3.02E-03 |
| APRIL* | 5524 (0 - 18877.33) | 4869.57 (0 - 14735.83) | 6.72E-01 | 9010.05 (3232.99 - 22773.56) | 1615.03 (0 - 7540.67) | 1.94E-02 | |
| TNFRI | 1.05 (0.94 - 1.14) | 1.03 (0.91 - 1.17) | 9.27E-01 | 1.22 (1.02 - 1.5) | 1.04 (0.91 - 1.14) | 2.28E-02 | |
| TNFRII | 1.01 (0.92 - 1.21) | 1 (0.9 - 1.11) | 5.94E-01 | 1.24 (1.09 - 1.42) | 0.97 (0.85 - 1.08) | 2.41E-04 | |
Units in pg/ml
3.2 Increasing dysregulation of innate and adaptive immune pathways culminates in elevation of TNF superfamily mediators near SLE classification
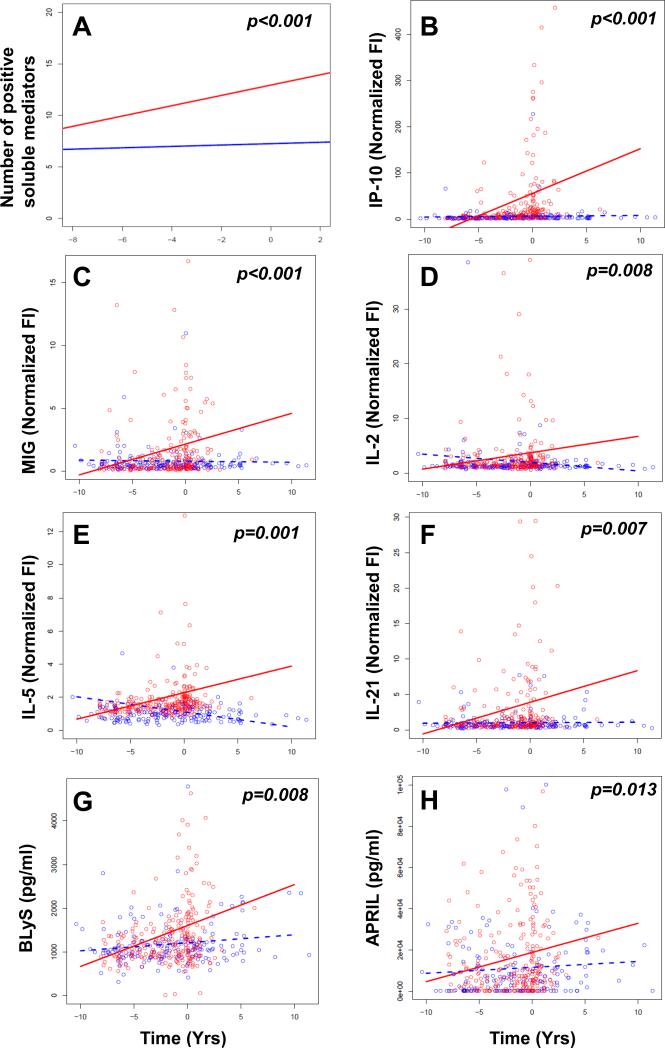
Soluble mediators that were altered in cases > 3.5 years prior to SLE classification remained so throughout the preclinical period, with additional immune dysregulation noted as patients approached disease classification (Fig. 1 and Table 1). We therefore assessed the temporal progression of cytokine dysregulation during SLE development. Consistent with the model that SLE pathogenesis entails a deficient regulatory setting [43, 44], the regulatory cytokine TGF-β was significantly decreased in cases compared to controls at all time periods, with no significant longitudinal changes in either group (Fig. 1 and Table 1). However, cases moving toward SLE classification gained an average of 0.5 dysregulated mediators per year, compared to only 0.06 in controls (p<0.001; Fig. 2A and Table 2). Cases exhibited a mean of 12.7 elevated mediators at the time of SLE classification (increased from 8.8 mediators > 3.5 years prior to classification), compared to 6.3 in controls (increased from 5.7 mediators) during the comparable time period. Similarly, cases moving toward SLE classification gained an average of 0.3 SLE-associated autoantibody specificities per year, compared to no gain in autoantibody positivity in controls (p<0.001; Supplemental Fig. 3 and Table 2). Cases exhibited positivity for an average of 3.0 autoantibody specificities at the time of SLE classification (increased from a mean of 1.0 autoantibody specificities > 3.5 years prior to classification), compared to controls, who were consistently positive for an average of 0.1 autoantibody specificities over the matched evaluation period. Cases showed evidence of expanding IFN activity, including increasing levels of the IFN-associated mediators IP-10 (p<0.001; Fig. 2B) and monocyte induced by IFN-γ (MIG, p<0.001; Fig. 2C). Growing dysregulation of innate and adaptive immune pathways throughout the pre-classification period was evidenced by increasing levels of innate and Th-type mediators, including Th1-type IL-2 (p=0.008; Fig. 2D), Th2-type IL-5 (p=0.001; Fig. 2E), and Th17-type IL-21 (p=0.007; Fig. 2F), compared to low and stable levels of these mediators in healthy controls (Table 2).
Figure 2. Select soluble mediators increase in cases as they approach SLE classification, but not in healthy controls.
(A) Number of positive soluble mediators over time in patients prior to SLE classification (red), vs. race, gender, age (± 5 years) and time of sample procurement-matched healthy controls (blue). P-values for the fixed effect of disease status are shown. Normalized FI of IP-10 (B), MIG (C), IL-2 (D), IL-5 (E), and IL-21 (F), with pg/ml concentration of BLyS (G), and APRIL (H) are compared in cases (red) vs. controls (blue) over time relative to SLE classification by mixed linear regression models. Slope of line for cases (red) vs. matched healthy controls (blue) is presented in Table 2.
Table 2.
Soluble mediator levels increase prior to SLE classification
| Type | Soluble mediator | Slope (Case) | Slope (Control) | p-value |
|---|---|---|---|---|
| Innate | IL-12p70 | 0.26 | −0.26 | 0.011 |
| IL-23 | 0.13 | −0.20 | 0.041 | |
| IFN-associated chemokines | IP-10 | 9.68 | 0.17 | <0.001 |
| MIG | 0.25 | −0.01 | <0.001 | |
| Th1-type | IL-2 | 0.30 | −0.15 | 0.008 |
| IFN-γ | 0.22 | −0.23 | 0.035 | |
| Th2-type | IL-5 | 0.16 | −0.09 | 0 001 |
| Th17-type | IL-21 | 0.45 | 0.01 | 0.007 |
| TNF superfamily | BLyS | 93.93 | 18.38 | 0.008 |
| APRIL | 1415 | 288 | 0.013 | |
| TNFRI | 0.05 | 0.01 | <0.001 | |
| TNFRII | 0.03 | 0.00 | <0.001 | |
| # Positive Mediators | 0.50 | 0.06 | <0.001 | |
| # DNA/RNA-Binding AutoAbs | 0.30 | −0.01 | <0.001 | |
Of note, multiple TNF superfamily members, including TNFRI, TNFRII, BLyS, and APRIL, were dysregulated only as patients approached SLE classification (Fig. 1 and Table 1). Mixed linear regression models confirmed that cases had significant longitudinal increases in the levels of these mediators (Table 2), including BLyS (p=0.008, Fig. 2G) and APRIL (p=0.013, Fig. 2H), compared to minimal changes in controls during the same period. Together, these results support a model in which innate and adaptive immune pathways initiate pathogenic inflammation during early preclinical SLE pathogenesis, followed by expanded immune dysregulation encompassing altered TNF superfamily members as patients approach SLE classification.
3.3 Dysregulated adaptive immune mediators precede autoantibody accumulation in preclinical SLE
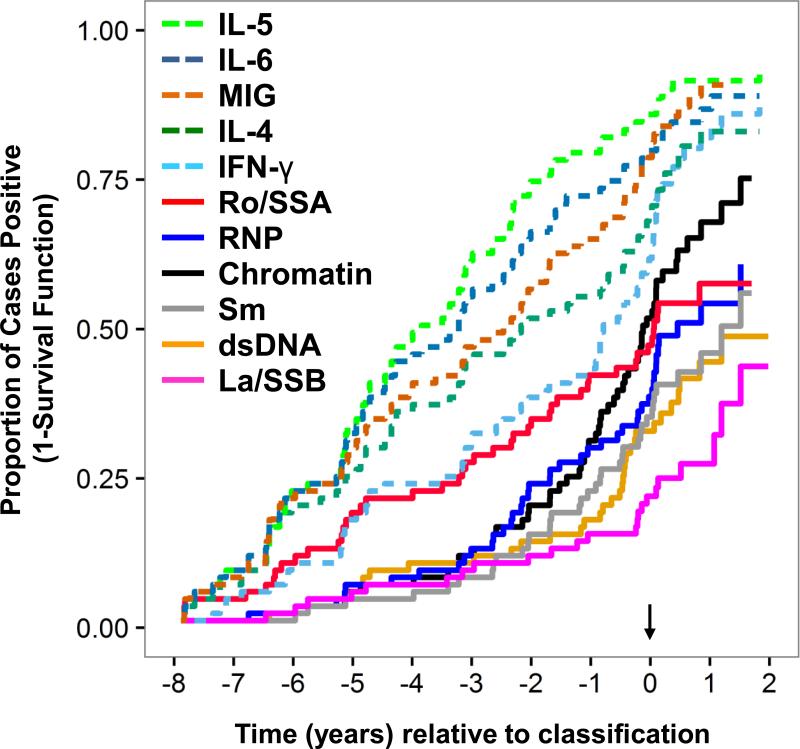
To better understand the temporal relationship between soluble mediator dysregulation and autoantibody production, we next compared the timing of autoantibody specificity detection and soluble mediator dysregulation as patients moved toward SLE classification. The proportion of cases with elevated levels of IL-4 and IL-5 (Th2-type), as well as IL-6 (Th2 and Th17-type), increased rapidly throughout the pre-classification period (Fig. 3). Consistent with our previous findings, IFN-γ (Th1-type) levels also increased rapidly during the pre-classification period, as did the IFN-γ induced chemokine, MIG ([18] and Fig. 3). Each of these mediators was elevated in more than 50% of cases by 2 years pre-classification, and in 85-95% of cases by two years after SLE classification (Fig. 3). In addition, cases continued to accumulate autoantibody specificities as they approached SLE classification [18, 45], with anti-Ro/SSA being among the first lupus-associated autoantibody specificities to be detected, followed by autoantibodies reactive to RNP, chromatin, Sm, dsDNA, and La/SSB as patients approached SLE classification (Fig. 3). Of interest, the detection of most lupus-associated autoantibody specificities occurred significantly later than the onset of IL-4, IL-5, IL-6, IFN-γ, or MIG dysregulation (Fig. 3 and Table 3). These results suggest that early dysregulation of innate and adaptive immune pathways may contribute to autoantibody development during SLE pathogenesis.
Figure 3. Dysregulation of Innate and Th-type mediators occurs prior to or concurrent with lupus-associated autoantibodies during early SLE pathogenesis.
Kaplan-Meier plots demonstrating proportion of cases positive for serum cytokines IL-5 (green dotted line), IL-6 (blue dotted line), MIG (orange dotted line), IL-4 (green dotted line), and IFN-γ (teal dotted line) vs. SLE-associated autoantibody specificities against Ro/SSA (red solid line), RNP (blue solid line), chromatin (black solid line), Sm (grey solid line), dsDNA (orange solid line), and La/SSB (pink solid line) relative to time of SLE classification (arrow) are shown. Hazard ratios are presented in Table 3.
Table 3.
Dysregulation of T-helper-type mediators detected prior to autoantibody positivitya
| IL-4 | IL-5 | IL-6 | IFN-γ | MIG | |
|---|---|---|---|---|---|
| anti-dsDNA | 3.14 (2.13, 4.63) | 5.28 (3.56, 7.82) | 4.22 (2.85, 6.25) | 3.06 (2.11, 4.44) | 4.11 (2.80, 6.06) |
| p=9.65E-08 | p=2.92E-13 | p=1.29E-10 | p=7.67E-09 | p=4.61E-13 | |
| anti-chromatin | 1.69 (1.22, 2.35) | 3.05 (2.17, 4.29) | 2.35 (1.70, 3.24) | 1.54 (1.12, 2.11) | 2.28 (1.68, 3.10) |
| p=0.002 | p=3.30E-09 | p=1.72E-06 | p=0.008 | p=8.82E-07 | |
| anti-Ro/SSA | 1.86 (1.27, 2.71) | 2.96 (2.01, 4.36) | 2.43 (1.67, 3.52) | 1.74 (1.17, 2.60) | 2.38 (1.71, 3.31) |
| p=0.001 | p=2.56E-08 | p=4.32E-06 | p=0.006 | p=6.49E-07 | |
| anti-La/SSB | 4.44 (2.92, 6.76) | 7.13 (4.57, 11.11) | 5.72 (3.60, 9.07) | 4.31 (2.77, 6.69) | 6.01 (3.99, 9.04) |
| p=4.58E-10 | p=2.18E-14 | p=1.94E-11 | p=2.55E-10 | p=2.62E-13 | |
| anti-RNP | 2.19 (1.50, 3.20) | 3.78 (2.65, 5.39) | 2.91 (2.06, 4.12) | 2.03 (1.45, 2.84) | 2.92 (2.01, 4.23) |
| p=9.30E-05 | p=1.67E-11 | p=2.84E-08 | p=4.51E-05 | p=4.69E-08 | |
| anti-Sm | 2.73 (1.91, 3.90) | 4.59 (3.21, 6.55) | 3.62 (2.57, 5.10) | 2.54 (1.76, 3.66) | 3.67 (2.56, 5.24) |
| p=4.58E-07 | p=9.20E-13 | p=3.29E-10 | p=1.06E-06 | p=1.61E-10 |
Likelihood of soluble mediator dysregulation compared to autoantibody positivity is shown as hazard ratio (95% confidence interval), with p-values determined by robust log-rank test. The hazard ratio is the composite ratio of cases with elevated soluble mediator: cases with positive autoantibody at any given time. A hazard ratio >1 indicates that the soluble mediator is more likely to be positive than the SLE-associated autoantibody.
3.4 Autoantibody positivity and dysregulated soluble mediators together reliably distinguish progression to classified SLE
The data presented above suggest that altered soluble mediators are detected years before patients reach SLE classification and may improve the prognostic accuracy of ANA positivity for identifying individuals at high risk of developing SLE. We used random forest (RF) modeling to determine which biomarkers could reliably demarcate patients as they progress from preclinical SLE to classified disease. RF models were generated based on ANA positivity alone, dysregulated soluble mediator levels alone, or the combination of ANA positivity and soluble mediator levels (Table 4). Although the ability to differentiate cases from controls using ANA status alone improved as patients approached SLE classification, the models incorporating soluble mediators consistently exhibited better specificity than ANA-only models (Table 4). In the early preclinical period (>3.5 years pre-classification), cases were best distinguished from controls by elevated Th1- and Th2-type mediators (IFN-γ, IL-5, IL-6) partnered with ANA and anti-Ro/SSA positivity, with 84% (± 0.12%) accuracy, compared to 58% accuracy using ANA positivity alone and 79% (± 0.6%) accuracy utilizing levels of the soluble mediators IL-5 and IL-6 in the RF models.
Table 4.
Soluble mediators improve predictive accuracy of ANA prior to SLE classification
| Years Pre-SLE Classification | Factors | Independent Predictors of Developing SLE | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy |
|---|---|---|---|---|---|---|---|
| >3.5 | ANA only | ANA | 0.86 (0.71, 0.95) |
0.65 (0.53, 0.76) |
0.58 (0.45, 0.70) |
0.89 (0.77, 0.96) |
58% |
| Soluble Mediators |
IL-5 and IL-6 | 0.79 (0.68, 0.89) |
0.79 (0.65, 0.89) |
0.82 (0.71, 0.91) |
0.76 (0.62, 0.86) |
79% ± 0.60% | |
| Combined |
ANA, IL-5, IL-6, anti- Ro/SSA and IFN-γ |
0.83 (0.72, 0.91) |
0.86 (0.73, 0.94) |
0.89 (0.78, 0.95) |
0.8 (0.66, 0.89) |
84% ± 0.12% | |
| 3.5-0.9 | ANA only | ANA | 0.96 (0.86, 1) |
0.73 (0.58, 0.85) |
0.8 (0.68, 0.89) |
0.94 (0.81, 0.99) |
80% |
| Soluble Mediators |
IL-5 | 0.8 (0.69, 0.89) |
0.76 (0.57, 0.90) |
0.89 (0.78, 0.95) |
0.63 (0.45, 0.79) |
79% ± 0.37% | |
| Combined | IL-12, MIG and ANA |
0.93 (0.83, 0.98) |
0.91 (0.76, 0.98) |
0.95 (0.86, 0.99) |
0.87 (0.71, 0.96) |
92% ± 0.52% | |
| <0.9 | ANA only | ANA | 0.95 (0.85, 0.99) |
0.68 (0.45, 0.86) |
0.88 (0.77, 0.95) |
0.83 (0.59, 0.96) |
88% |
| Soluble Mediators |
IL5, IL6 and TGF-β | 0.94 (0.84, 0.98) |
0.79 (0.55, 0.94) |
0.93 (0.84, 0.98) |
0.81 (0.56, 0.95) |
90% ± 0.98% | |
| Combined | ANA and IL-1RA |
0.92 (0.82, 0.97) |
0.78 (0.52, 0.94) |
0.93 (0.84, 0.98) |
0.74 (0.49, 0.91) |
89% | |
| ALL | ANA only | ANA | 0.92 (0.85, 0.97) |
0.61 (0.48, 0.72) |
0.75 (0.66, 0.83) |
0.86 (0.73, 0.94) |
78% ± 2.42% |
| Soluble Mediators |
IP-10, IL-5 and IL-6 | 0.87 (0.79, 0.92) |
0.77 (0.62, 0.89) |
0.91 (0.84, 0.96) |
0.68 (0.54, 0.81) |
84% ± 2.95% | |
| Combined |
IL-6, anti-Ro/SSA, IL-5, ANA, MIG |
0.93 (0.87, 0.97) |
0.89 (0.77, 0.96) |
0.96 (0.90, 0.99) |
0.84 (0.71, 0.93) |
92% ± 1.78% | |
As patients moved closer to SLE classification (0.9-3.5 years pre-classification), cases were best distinguished from controls with 92% (± 0.52%) accuracy by elevated serum levels of the IFN-γ-induced chemokine, MIG, and the Th1-associated mediator IL-12, as well as ANA positivity. When SLE classification was imminent (<0.9 years pre-classification), levels of IL-5, IL-6 and TGF-β independently and optimally predicted SLE classification, and distinguished cases from controls with 90% (± 0.98%) accuracy, highlighting the importance of soluble mediators in the transition to SLE. Finally, in an RF model spanning the entire preclinical period, a combination of ANA positivity, as well as elevated levels of IL-5, IL-6 and MIG, optimally identified individuals who subsequently developed clinical SLE with 92% (± 1.78%) accuracy, positive predictive value (PPV) of 0.96, and negative predictive value (NPV) of 0.84 (Table 4).
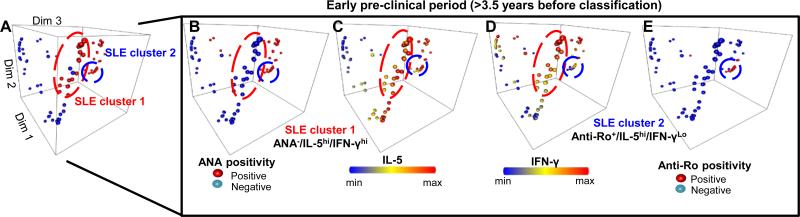
Confirming the above finding that Th-type mediators are dysregulated prior to the appearance of most lupus-associated autoantibody specificities (Fig. 3), a large random forest cluster of cases > 3.5 years prior to SLE classification were ANA negative, but had high levels of IL-5 and IFN-γ (SLE cluster 1, Fig. 4). Compared to cases who were ANA positive > 3.5 years prior to SLE classification, cases who were ANA negative with high levels of IL-5 and IFN-γ demonstrated no difference in sex (p=0.433 by Fisher's exact test), race (p=0.346 by χ2), age at SLE classification (p=0.389 by unpaired t-test), nor medication history as patients approached SLE classification, including hydroxychloroquine (p=0.115), azathioprine (p=0.434), methotrexate (p=0.298), or the use of steroids (p=1.000). However, ANA negative, IL-5 and IFN-γ high cases (SLE cluster 1) were more likely to develop nephritis (p=0.008), while cases who were ANA positive were more likely to develop arthritis (p=0.028) as they transitioned to classified SLE. These results underscore the dual contributions of ANA positivity and progressive, multi-pathway immune dysregulation to preclinical SLE pathogenesis and prognosis.
Figure 4. ANA Negative and ANA/Anti-Ro/SSA Positive, pre-clinical SLE patients show elevated IL-5 and IFN-γ >3.5 years before disease classification.
(A) Scatter plots, showing individual cases (red dots) and matched healthy controls (blue dots) as separate points, were generated using multidimensional reduction analysis of the random forest proximity matrix. ANA positivity (B), IL-5 levels (C), IFN-γ levels (D), and anti-Ro/SSA positivity (E) are shown. SLE cluster 1 (red circle), made up of ANA−/IL-5Hi/IFN-γHi cases, and SLE cluster 2 (blue circle), made up of Anti-Ro+/IL-5Hi/IFN-γLo cases, are highlighted.
4. Discussion
Deciphering immune dysregulation that contributes to early lupus pathogenesis is essential for efforts to thwart the development of tissue and organ damage and ensuing morbidity and early mortality associated with progression to clinical SLE. The goal of the current study was to expand and clarify our understanding of SLE pathogenesis prior to and concurrent with the development of clinical disease by determining the nature and temporal relationship of immune pathway dysregulation and the development and accumulation of SLE-associated autoantibody specificities that lead to clinical disease and SLE classification. To this end, we used a unique resource of well-characterized, longitudinal serum samples collected prior to and at/after SLE classification to determine, for the first time in human patients, the extent and temporal relationship of immune dysregulation relative to the accumulation of autoantibody specificities and SLE classification.
SLE-associated autoantibody specificities can be detected years before SLE classification [2], but these autoantibodies are also present in other autoimmune diseases and in healthy populations [26, 31, 46, 47]. Supporting the paradigm that pathogenic autoantibodies are not the sole drivers of SLE pathogenesis, two independent, randomized clinical trials of B cell depletion therapies demonstrated decreased circulating anti-dsDNA autoantibodies, yet produced only modest clinical improvement over standard of care [48, 49]. We demonstrate in the current study that a model combining IL-5,IL-6, and IFN-γ levels reliably distinguishes individuals in the early preclinical stages of SLE from healthy controls. Indeed, we could identify 79% of future SLE cases by evaluating this combination of factors alone more than 3.5 years prior to classification, compared to only 58% of future SLE cases identified using only ANA status. Furthermore, combining immune factors with ANA status resulted in identifying future SLE patients with 84% accuracy > 3.5 years before they reach SLE classification.
These findings suggest that screening for immune pathway dysregulation in conjunction with ANA positivity may improve our ability to identify individuals at high risk for SLE. Although it is possible for up to 14% of the general population [8] without clinical signs or symptoms of SLE to have other facets of immune dysregulation, we have recently demonstrated that autoantibody-positive healthy individuals do not usually display enhanced dysregulation of those mediators compared to SLE patients, including IL-5, IL-6, and IFN-γ (Table 4), that are dysregulated in patients at the highest risk of developing SLE [50, 51]. In addition, it is possible that immune pathways found to be dysregulated in asymptomatic individuals who develop SLE may also be present in other rheumatologic autoimmune diseases [52], to date, evaluation of serological samples from the DODSR and other community cohorts in asymptomatic patients who develop other diseases such as rheumatoid arthritis have revealed a combination of dysregulated immune pathways and autoantibody specificities distinct from that of preclinical SLE [53-55]. Future prospective, longitudinal studies of individuals with autoantibody positivity ± immune dysregulation, prior to onset of clinical signs and symptoms, will be necessary to determine which autoimmune disease(s) are associated with particular dysregulated immune pathways that are present before/concurrent with particular autoantibody specificities or clinical rheumatic disease.
Aberrant elevation in Th1-, Th2-, and Th17-type cytokines has been reported in multiple SLE cohorts during established disease [22, 47, 56-60]. Our current findings suggest that dysregulation of these cytokines, particularly IL-5 (Th2-type) and IL-6 (Th2/17-type), may be an essential early step in SLE pathogenesis. Indeed, these two mediators were elevated in about 20% of future SLE patients at least 6 years prior to disease transition and in approximately 90% of SLE patients by two years after classification. Further, IL-5 and IL-6 were independent classifiers in most of the multivariate random forest models, revealing that they contribute to all stages of SLE pathogenesis. IL-5 and IL-6 are secreted by both innate and adaptive leukocytes and support T cell survival and antibody production, suggesting that their role in SLE pathogenesis may be to promote autoantibody production. Consistent with this possibility, IL-5 and IL-6 were elevated prior to the development of SLE-associated autoantibodies, and ANA positivity gradually replaced IL-5 as an independent predictor of future SLE classification. Disruption of regulatory mechanisms may also contribute to autoantibody accumulation, as indicated by the observed early decrease in TGF-β and the current literature showing disrupted Th17/Treg homeostasis during established SLE [61-63].
Previous studies have shown that IFN-γ becomes elevated prior to or concurrent with the appearance of autoantibodies [18], and that elevated levels of IFN-γ are associated with the transition from undifferentiated to defined connective tissue disease [64]. Our current study confirms and expands this finding > 3.5 years prior to SLE classification, during the asymptomatic period of pre-clinical disease pathogenesis. In addition to facilitating autoantibody production by perpetuating Th1-type responses and modulating Toll-like receptor regulation, IFN-γ drives the production of IFN-α [65]. In turn, IFN-γ and IFN-α stimulate the production of B cell proliferation and activation factors such as BLyS and APRIL [19, 20, 66, 67], which further reinforce inflammation and B cell activation. Interestingly, ANA positivity did not exclude IFN-γ from multivariate random forest models, suggesting that type II IFN dysregulation and ANA production play distinct roles in SLE pathogenesis. Thus, the early elevation of IFN-γ, followed by significant increases in BLyS and APRIL when within one year of disease classification when SLE is imminent, supports the model that simultaneous dysregulation of T helper, regulatory, IFN-related, and TNF-related pathways may unleash an inflammatory cycle that erodes immune tolerance to a point where clinical disease is inevitable [18]. Such alterations are likely due to abnormalities in receptor-mediated proximal and distal signaling pathways [68], many of which are current targets for novel therapeutic approaches to dampen inflammation and target organ damage in SLE [69]. Additional, future studies will be required to determine if dysregulation of signaling pathways that leads to aberrant cellular activation and secretion of inflammatory mediators is due to genetic [23, 70], epigenetic [71], and/or environmental triggers, such as vitamin D deficiency [51] and/or immune dysregulation caused by latent Epstein-Barr viral infection [72, 73].
Early intervention in SLE may be most effective before the immune system enters a feed-forward, self-sustaining cycle of broken tolerance. Immune homeostasis could potentially be maintained by targeting immune pathways that become dysregulated during early pathogenesis. Although the cellular sources of dysregulated soluble mediators in preclinical SLE remain unknown and are the subject of future study, our data suggest that restoring homeostasis within the IL-5, IL-6, and IFN pathways might be effective interventions prior to SLE classification. Of interest, hydroxychloroquine has been shown to activation of TLR7 pathways [74], pathogenic in SLE [74], as well as decrease production of IL-6 and IFN-γ in several small patient cohorts and in vitro studies [75-78]. A mainstay of treatment in SLE [79], hydroxychloroquine has already been shown to delay SLE onset and slow the accrual of autoantibodies in patients approaching SLE classification [4]. By identifying high-risk patients via the presence of immune dysregulation coupled with one or more lupus-associated autoantibody specificities during the pre-clinical, asymptomatic period, lower doses of hydroxychloroquine may successfully stave off disease and reduce the risk of ocular toxicity [80].
Alternatively, it may be possible, alone or in conjunction with low-dose hydroxychloroquine, to stave off the accumulation of autoantibody specificities and the development of clinical SLE utilizing pathway-specific, biologic, immune modifiers. Given the predictive nature of IL-5, IL-6, and IFN-γ for future disease development > 3.5 years prior to SLE classification, these would be logical pathways to pursue in early intervention trials. Although no studies to date have explored blockade of Th2-type cytokines in SLE patients, a number of studies have been performed in patients with asthma and such therapies have been shown to be well-tolerated and provide some clinical benefit [81]. Blockade of the IL-6 receptor in SLE patients has been shown to decrease both B- and T-lymphocyte activation [82] and there is some evidence of clinical improvement in patient-reported outcomes [83]. Given the role of IL-6 in both Th2 and Th17-type responses, early intervention in patients who exhibit dysregulated levels (89% in the current study) may help delay or prevent both the development of autoantibody specificities and subsequent clinical sequelae, including more serious consequences such as lupus nephritis [83]. For those patients with elevated IFN-γ levels (89% in the current study), treating SLE patients with the anti-IFN-γ monoclonal antibody AMG 811 has been shown to normalized interferon-regulated gene expression and reduce downstream levels of IP-10 [84], which has also become a therapeutic target for rheumatic disease [85]. This may be particularly beneficial as 97% of the future cases in the current study exhibited elevated levels of IFN-γ and/or IP-10 prior to SLE classification. Finally, those patients who may have incomplete lupus, exhibiting signs and symptoms of SLE with concurrent presence of autoantibody specificities and immune dysregulation, may additionally benefit from anti-BLyS therapy, which is elevated proximal to SLE classification and the blockade of which has shown promise clinically, particularly in SLE patients with musculoskeletal and mucocutaneous organ system involvement [86].
5. Conclusions
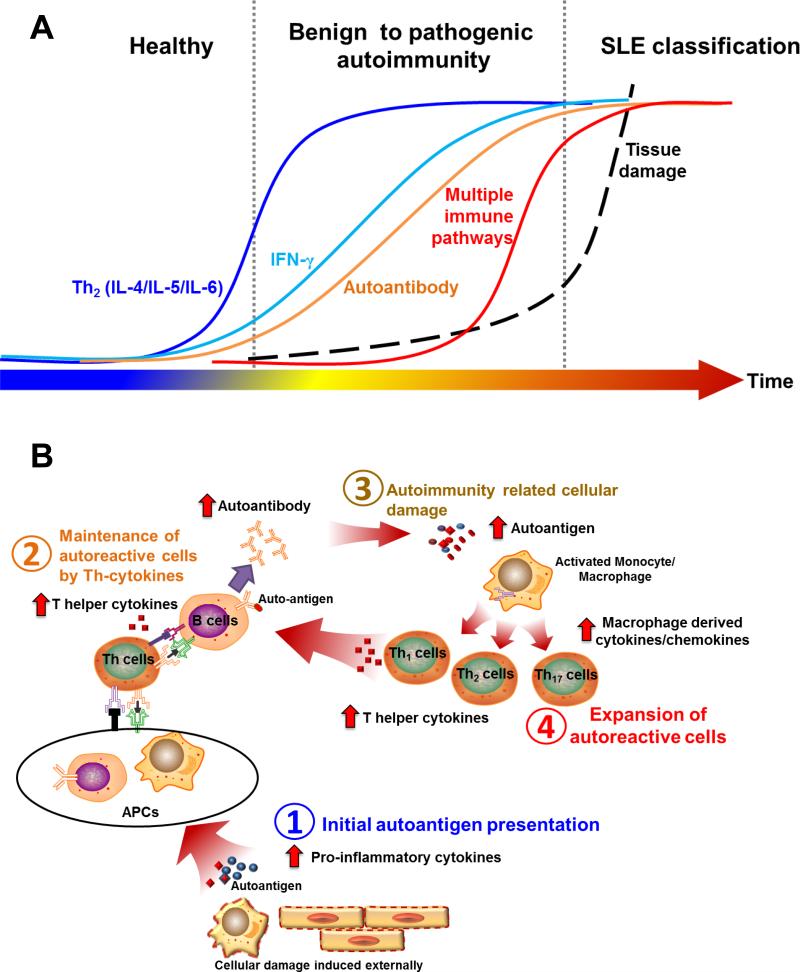
In conclusion, data from our study delineate a complex and cumulative pathogenic process in preclinical SLE, involving a number risk factors and gradual dysregulation of innate and T-helper, adaptive immune pathways (Fig. 5). Abnormalities in multiple Th-type cytokines arise in early preclinical SLE pathogenesis and could be leveraged to identify individuals at highest risk of future SLE clinical onset with >90% accuracy. This study also describes multifactorial models that improve the prediction of SLE classification during early disease development, and thus provides tools to select at-risk individuals for prospective mechanistic studies and clinical prevention trials.
Figure 5. Proposed model of immune dysregulation leading to pathogenic autoimmunity and SLE classification.
(A). Temporal relationship among dysregulated Th-type immune mediators associated with pre-clinical SLE is shown. (B) Hypothesis model of pre-clinical SLE pathogenesis: Genetic predisposition affecting apoptotic clearance, antigen-presentation, and lymphocyte responses may contribute to the appearance and maintenance of autoreactive cells (B1), leading to aberrant elevation of T helper (Th)-type cytokines, providing further co-stimulatory signals for the expansion of auto-reactive cells and potentiating the accrual of lupus-associated autoantibodies (B2). Immune dysregulation results in tissue damage and further exposure to intracellular auto-antigens, which may result in hyperactivation of innate immune cells (B3), leading to further dysregulation of soluble mediators that contribute to enhanced apoptosis and intracellular auto-antigen exposure, perpetuating the cycle of autoimmunity (B4).
Supplementary Material
Acknowledgements
The authors thank the collaborating military rheumatologists for assistance in identifying cases and extracting clinical information; Tim Gross, Nicolas Dominguez, Susan Macwana, Jeannie Te, Jeannette Osban, Wendy Klein, Virginia Roberts, and Jourdan Anderson for technical assistance; Rebecka Bourn, PhD and Angela Andersen, PhD (Life Science Editors) for scientific editing. This work was supported by the National Institute of Allergy, Immunology and Infectious Diseases; National Institute of Arthritis, Musculoskeletal and Skin Diseases; Office of Research on Women's Health; and National Institute of General Medical Sciences; under award numbers U19AI082714, U01AI101934, P30AR053483, P01AR048929, P30GM103510, and U54GM104938. JBH acknowledges support from NIH grants U01HG006828, UL1TR000077, R37AI024717, R21AI103980, P01AR049084, and P01AI083194. This material is also the result of work supported with resources and the use of facilities through the Department of Veterans Affairs. The views and conclusions contained herein are the views of the authors and do not necessarily represent the official views of the Departments of the Army, Navy, or Defense, the Department of Veterans Affairs, the National Institutes of Health, or the United States government. While the military generally excises references to products, companies, manufacturers, organizations, etc. in government produced works, the abstracts produced and other similarly situated research presents a special circumstance when such product inclusions have an integral part of the scientific endeavor.
Footnotes
Author contributions: R.L., M.E.M., M.P.K., J.B.H., and J.A.J designed the study. R.L., M.E.M., and J.A.J. participated in data acquisition. R.L., M.E.M., H.C., K.M.B., J.M.G, S.R.S., D.A.F., J.B.H., and J.A.J. participated in data analysis. All authors assisted with the development of the manuscript and gave final approval for publication. The authors were not paid to write this article by a pharmaceutical company or other agency. M.E.M., R.L., and J.A.J. had full access to the study. J.A.J. had the final responsibility for the decision to submit for publication.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 3.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85–9. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 4.James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain MT, Arbuckle MR, et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16:401–9. doi: 10.1177/0961203307078579. [DOI] [PubMed] [Google Scholar]
- 5.Li QZ, Zhou J, Lian Y, Zhang B, Branch VK, Carr-Johnson F, et al. Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol. 2010;159:281–91. doi: 10.1111/j.1365-2249.2009.04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouwhuis MG, Gast A, Figl A, Eggermont AM, Hemminki K, Schadendorf D, et al. Polymorphisms in the CD28/CTLA4/ICOS genes: role in malignant melanoma susceptibility and prognosis? Cancer immunology, immunotherapy : CII. 2010;59:303–12. doi: 10.1007/s00262-009-0751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruner BF, Guthridge JM, Lu R, Vidal G, Kelly JA, Robertson JM, et al. Comparison of autoantibody specificities between traditional and bead-based assays in a large, diverse collection of patients with systemic lupus erythematosus and family members. Arthritis Rheum. 2012;64:3677–86. doi: 10.1002/art.34651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–27. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vila LM, Mayor AM, Valentin AH, Garcia-Soberal M, Vila S. Clinical outcome and predictors of disease evolution in patients with incomplete lupus erythematosus. Lupus. 2000;9:110–5. doi: 10.1191/096120300678828073. [DOI] [PubMed] [Google Scholar]
- 10.Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst AM, Reimold A, et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27:153–60. doi: 10.1016/j.jaut.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Kil LP, Hendriks RW. Aberrant B cell selection and activation in systemic lupus erythematosus. International reviews of immunology. 2013;32:445–70. doi: 10.3109/08830185.2013.786712. [DOI] [PubMed] [Google Scholar]
- 12.Datta SK. Production of pathogenic antibodies: cognate interactions between autoimmune T and B cells. Lupus. 1998;7:591–6. doi: 10.1191/096120398678920703. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus-an update. Curr Opin Immunol. 2012;24:651–7. doi: 10.1016/j.coi.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, et al. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–92. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Mari N, Hercor M, Denanglaire S, Leo O, Andris F. The capacity of Th2 lymphocytes to deliver B-cell help requires expression of the transcription factor STAT3. Eur J Immunol. 2013;43:1489–98. doi: 10.1002/eji.201242938. [DOI] [PubMed] [Google Scholar]
- 16.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99:5545–50. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HJ, Kim DH, Lim SH, Kim WJ, Youn J, Choi YS, et al. Insights into the role of follicular helper T cells in autoimmunity. Immune network. 2014;14:21–9. doi: 10.4110/in.2014.14.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208140. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–9. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 20.Lopez P, Scheel-Toellner D, Rodriguez-Carrio J, Caminal-Montero L, Gordon C, Suarez A. Interferon-alpha-induced B-lymphocyte stimulator expression and mobilization in healthy and systemic lupus erthymatosus monocytes. Rheumatology (Oxford) 2014;53:2249–58. doi: 10.1093/rheumatology/keu249. [DOI] [PubMed] [Google Scholar]
- 21.Cancro MP, D'Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest. 2009;119:1066–73. doi: 10.1172/JCI38010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munroe ME, Vista ES, Guthridge JM, Thompson LF, Merrill JT, James JA. Pro-inflammatory adaptive cytokines and shed tumor necrosis factor receptors are elevated preceding systemic lupus erythematosus disease flare. Arthritis & rheumatology. 2014;66:1888–99. doi: 10.1002/art.38573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munroe ME, James JA. Genetics of Lupus Nephritis: Clinical Implications. Seminars in nephrology. 2015;35:396–409. doi: 10.1016/j.semnephrol.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoll T, Seifert B, Isenberg DA. SLICC/ACR Damage Index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol. 1996;35:248–54. doi: 10.1093/rheumatology/35.3.248. [DOI] [PubMed] [Google Scholar]
- 25.Rahman P, Gladman DD, Urowitz MB, Hallett D, Tam LS. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus. 2001;10:93–6. doi: 10.1191/096120301670679959. [DOI] [PubMed] [Google Scholar]
- 26.Bruce IN, O'Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74:1706–13. doi: 10.1136/annrheumdis-2013-205171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urowitz MB, Gladman DD, Ibanez D, Fortin PR, Bae SC, Gordon C, et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res. 2012;64:132–7. doi: 10.1002/acr.20648. [DOI] [PubMed] [Google Scholar]
- 28.Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56:2344–51. doi: 10.1002/art.22665. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 30.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 31.Munroe ME, Robertson JM, Fife DA, Zhao YD, Lu R, Guthridge JM, et al. Multiple Autoantibodies Develop Prior to Increases in Interferon Activity Which Precede Systemic Lupus Erythematosus Disease Classification. N Engl J Med. 2014 Submitted. [Google Scholar]
- 32.Rosenberg-Hasson Y, Hansmann L, Liedtke M, Herschmann I, Maecker HT. Effects of serum and plasma matrices on multiplex immunoassays. Immunol Res. 2014;58:224–33. doi: 10.1007/s12026-014-8491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar Y, Liang C, Bo Z, Rajapakse JC, Ooi EE, Tannenbaum SR. Serum proteome and cytokine analysis in a longitudinal cohort of adults with primary dengue infection reveals predictive markers of DHF. PLoS Negl Trop Dis. 2012;6:e1887. doi: 10.1371/journal.pntd.0001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J Immunol Methods. 2009;350:125–32. doi: 10.1016/j.jim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinlen LD, McClain MT, Ritterhouse LL, Bruner BF, Edgerton CC, Keith MP, et al. 60 kD Ro and nRNP A frequently initiate human lupus autoimmunity. PLoS One. 2010;5:e9599. doi: 10.1371/journal.pone.0009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 38.Genuer R, Poggi JM, Tuleau-Malot C. Variable selection using random forests. Pattern Recogn Lett. 2010;31:2225–36. [Google Scholar]
- 39.Fife DA. Package “fifer”. CRAN: CRAN. 2014 [Google Scholar]
- 40.Ladd B, Kenner S. Information visualization and analytical data mining in pharmaceutical R&D. Curr Opin Drug Discov Devel. 2000;3:280–91. [PubMed] [Google Scholar]
- 41.Gomez D, Correa PA, Gomez LM, Cadena J, Molina JF, Anaya JM. Th1/Th2 cytokines in patients with systemic lupus erythematosus: is tumor necrosis factor alpha protective? Semin Arthritis Rheum. 2004;33:404–13. doi: 10.1016/j.semarthrit.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Viallard JF, Taupin JL, Miossec V, Pellegrin JL, Moreau BL. Analysis of interleukin-6, interleukin-10 and leukemia inhibitory factor (LIF) production by peripheral blood cells from patients with systemic lupus erythematosus identifies LIF as a potential marker of disease activity. Eur Cytokine Netw. 1999;10:17–24. [PubMed] [Google Scholar]
- 43.Ohtsuka K, Gray JD, Stimmler MM, Horwitz DA. The relationship between defects in lymphocyte production of transforming growth factor-beta1 in systemic lupus erythematosus and disease activity or severity. Lupus. 1999;8:90–4. doi: 10.1191/096120399678847489. [DOI] [PubMed] [Google Scholar]
- 44.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–8. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Arbuckle MR, James JA, Dennis GJ, Rubertone MV, McClain MT, Kim XR, et al. Rapid clinical progression to diagnosis among African-American men with systemic lupus erythematosus. Lupus. 2003;12:99–106. doi: 10.1191/0961203303lu334oa. [DOI] [PubMed] [Google Scholar]
- 46.Acosta-Rodriguez EV, Craxton A, Hendricks DW, Merino MC, Montes CL, Clark EA, et al. BAFF and LPS cooperate to induce B cells to become susceptible to CD95/Fas-mediated cell death. Eur J Immunol. 2007;37:990–1000. doi: 10.1002/eji.200636698. [DOI] [PubMed] [Google Scholar]
- 47.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–93. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 48.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64:1215–26. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 50.Slight-Webb S, Lu R, Ritterhouse LL, Munroe ME, Maecker HT, Fathman CG, et al. Autoantibody-Positive Healthy Individuals Display Unique Immune Profiles That May Regulate Autoimmunity. Arthritis & rheumatology. 2016 doi: 10.1002/art.39706. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritterhouse LL, Crowe SR, Niewold TB, Kamen DL, Macwana SR, Roberts VC, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis. 2011 doi: 10.1136/ard.2010.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–31. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 53.Hughes-Austin JM, Deane KD, Derber LA, Kolfenbach JR, Zerbe GO, Sokolove J, et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA). Ann Rheum Dis. 2013;72:901–7. doi: 10.1136/annrheumdis-2012-201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deane KD, Striebich CC, Goldstein BL, Derber LA, Parish MC, Feser ML, et al. Identification of undiagnosed inflammatory arthritis in a community health fair screen. Arthritis Rheum. 2009;61:1642–9. doi: 10.1002/art.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deane KD, O'Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62:3161–72. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yap DY, Lai KN. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. J Biomed Biotechnol. 2010;2010:365083. doi: 10.1155/2010/365083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–7. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cash H, Relle M, Menke J, Brochhausen C, Jones SA, Topley N, et al. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol. 2010;37:60–70. doi: 10.3899/jrheum.090194. [DOI] [PubMed] [Google Scholar]
- 59.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–23. [PubMed] [Google Scholar]
- 60.Eilertsen GO, Nikolaisen C, Becker-Merok A, Nossent JC. Interleukin-6 promotes arthritis and joint deformation in patients with systemic lupus erythematosus. Lupus. 2011;20:607–13. doi: 10.1177/0961203310392432. [DOI] [PubMed] [Google Scholar]
- 61.Szodoray P, Nakken B, Barath S, Csipo I, Nagy G, El-Hage F, et al. Altered Th17 cells and Th17/regulatory T-cell ratios indicate the subsequent conversion from undifferentiated connective tissue disease to definitive systemic autoimmune disorders. Hum Immunol. 2013;74:1510–8. doi: 10.1016/j.humimm.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Mesquita D, de Melo Cruvinel W, Araujo J, Pucci F, Salmazi K, Kallas E, et al. Systemic lupus erythematosus exhibits a dynamic and continuum spectrum of effector/regulatory T cells. Scand J Rheumatol. 2011;40:41–50. doi: 10.3109/03009742.2010.489229. [DOI] [PubMed] [Google Scholar]
- 63.Chen XQ, Yu YC, Deng HH, Sun JZ, Dai Z, Wu YW, et al. Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. J Clin Immunol. 2010;30:221–5. doi: 10.1007/s10875-009-9365-x. [DOI] [PubMed] [Google Scholar]
- 64.Szodoray P, Nakken B, Barath S, Gaal J, Aleksza M, Zeher M, et al. Progressive divergent shifts in natural and induced T-regulatory cells signify the transition from undifferentiated to definitive connective tissue disease. Int Immunol. 2008;20:971–9. doi: 10.1093/intimm/dxn056. [DOI] [PubMed] [Google Scholar]
- 65.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 66.Boghdadi G, Elewa EA. Increased serum APRIL differentially correlates with distinct cytokine profiles and disease activity in systemic lupus erythematosus patients. Rheumatol Int. 2014;34:1217–23. doi: 10.1007/s00296-014-3020-4. [DOI] [PubMed] [Google Scholar]
- 67.Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol. 2014;192:906–18. doi: 10.4049/jimmunol.1302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest. 2015;125:2220–7. doi: 10.1172/JCI78087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Comte D, Karampetsou MP, Tsokos GC. T cells as a therapeutic target in SLE. Lupus. 2015;24:351–63. doi: 10.1177/0961203314556139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niewold TB. Advances in lupus genetics. Curr Opin Rheumatol. 2015;27:440–7. doi: 10.1097/BOR.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hedrich CM, Crispin JC, Tsokos GC. Epigenetic regulation of cytokine expression in systemic lupus erythematosus with special focus on T cells. Autoimmunity. 2014;47:234–41. doi: 10.3109/08916934.2013.801462. [DOI] [PubMed] [Google Scholar]
- 72.Poole BD, Scofield RH, Harley JB, James JA. Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39:63–70. doi: 10.1080/08916930500484849. [DOI] [PubMed] [Google Scholar]
- 73.Poole BD, Templeton AK, Guthridge JM, Brown EJ, Harley JB, James JA. Aberrant Epstein-Barr viral infection in systemic lupus erythematosus. Autoimmun Rev. 2009;8:337–42. doi: 10.1016/j.autrev.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clancy RM, Markham AJ, Buyon JP. Endosomal Toll-like receptors in clinically overt and silent autoimmunity. Immunol Rev. 2016;269:76–84. doi: 10.1111/imr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 76.Willis R, Seif AM, McGwin G, Jr., Martinez-Martinez LA, Gonzalez EB, Dang N, et al. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus. 2012;21:830–5. doi: 10.1177/0961203312437270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sacre K, Criswell LA, McCune JM. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther. 2012;14:R155. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costedoat-Chalumeau N, Dunogue B, Morel N, Le Guern V, Guettrot-Imbert G. Hydroxychloroquine: a multifaceted treatment in lupus. Presse medicale. 2014;43:e167–80. doi: 10.1016/j.lpm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Tsang ASMW Bultink IE. Systemic lupus erythematosus: review of synthetic drugs. Expert Opin Pharmacother. 2015;16:2793–806. doi: 10.1517/14656566.2015.1101448. [DOI] [PubMed] [Google Scholar]
- 80.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231–69. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 81.Catley MC, Coote J, Bari M, Tomlinson KL. Monoclonal antibodies for the treatment of asthma. Pharmacol Ther. 2011;132:333–51. doi: 10.1016/j.pharmthera.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Shirota Y, Yarboro C, Fischer R, Pham TH, Lipsky P, Illei GG. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann Rheum Dis. 2013;72:118–28. doi: 10.1136/annrheumdis-2012-201310. [DOI] [PubMed] [Google Scholar]
- 83.Thanarajasingam U, Niewold TB. Sirukumab : a novel therapy for lupus nephritis? Expert opinion on investigational drugs. 2014;23:1449–55. doi: 10.1517/13543784.2014.950837. [DOI] [PubMed] [Google Scholar]
- 84.Chen P, Vu T, Narayanan A, Sohn W, Wang J, Boedigheimer M, et al. Pharmacokinetic and Pharmacodynamic Relationship of AMG 811, An Anti-IFN-gamma IgG Monoclonal Antibody, in Patients with Systemic Lupus Erythematosus. Pharmaceutical research. 2014 doi: 10.1007/s11095-014-1492-2. [DOI] [PubMed] [Google Scholar]
- 85.Yellin M, Paliienko I, Balanescu A, Ter-Vartanian S, Tseluyko V, Xu LA, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:1730–9. doi: 10.1002/art.34330. [DOI] [PubMed] [Google Scholar]
- 86.Zouali M, Uy EA. Belimumab therapy in systemic lupus erythematosus. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2013;27:225–35. doi: 10.1007/s40259-013-0031-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.