Abstract
Biosynthesis of bacterial natural-product virulence factors is emerging as a promising antibiotic target. Many such natural products are produced by nonribosomal peptide synthetases (NRPS) from amino acid precursors. To develop selective inhibitors of these pathways, we have previously described aminoacyl-AMS (sulfamoyladenosine) macrocycles that inhibit NRPS amino acid adenylation domains but not mechanistically-related aminoacyl-tRNA synthetases. To improve the cell permeability of these inhibitors, we explore herein replacement of the α-amino group with an α-hydroxy group. In both macrocycles and corresponding linear congeners, this leads to decreased biochemical inhibition of the cysteine adenylation domain of the Yersina pestis siderophore synthetase HMWP2, which we attribute to loss of an electrostatic interaction with a conserved active-site aspartate. However, inhibitory activity can be regained by installing a cognate β-thiol moiety in the linear series. This provides a path forward to develop selective, cell-penetrant inhibitors of the biosynthesis of virulence factors to probe their biological functions and potential as therapeutic targets.
Keywords: Antibiotic, Adenylation, Non-ribosomal peptide synthetase, Rational design, Virulence
Graphical abstract
Antibiotic resistance is a major threat to human health and new antibacterials with novel mechanisms of action are urgently needed.1–4 Natural products play key roles in bacterial virulence and communication,5–8 and targeting their biosyntheses has emerged as a promising antibiotic strategy.9,10 Antibacterials that block virulence factor production, as opposed to processes that are essential for bacterial survival, are also attractive in that this may reduce the propensity for the development of antibiotic resistance.11–14 This is based on the hypothesis that drug-resistant bacteria may suffer a relative fitness cost to produce communally beneficial virulence factors for use by their drug-sensitive neighbors. Indeed, proof-of-concept for this hypothesis has been provided in coculture experiments using mutant Pseudomonas aeruginosa strains that mimic drug-sensitive variants and wild-type strains that mimic drug-resistant variants.15,16
Many natural-product virulence factors are biosynthesized by non-ribosomal peptide synthetases (NRPS).17–23 These include a variety of siderophores, which are iron-chelating molecules used by pathogenic bacteria to capture iron, an essential nutrient, from the host organism.10,24–27 NRPS pathways involved in siderophore biosynthesis have been genetically validated as promising antibacterial targets using mutant strains in animal infection models.28–43 Recently, we have also pharmacologically validated siderophore biosynthesis as an effective antibacterial target in a mouse model of Mycobacterium tuberculosis infection.44,45 By definition, all NRPS include at least one amino acid adenylation domain. Thus, to develop inhibitors of NRPS as probes and potential therapeutic leads, we have previously designed an aminoacyl-AMS (5´-O-[N-aminoacylsulfamoyl]-adenosine) macrocycle 5a as a selective inhibitor of amino acid adenylation domains (Figure 1).46 However, although this macrocycle was a potent biochemical inhibitor of the cysteine adenylation domain of HMWP2 (high molecular weight protein 2), a component of the Yersinia pestis yersiniabactin siderophore synthetase, it did not inhibit yersiniabactin production in Y. pestis cell culture (unpublished results). This is in contrast to our earlier demonstration that salicyl-AMS, which inhibits the upstream salicylate adenylation enzyme YbtE, also blocks yersiniabactin production in cell culture.45 We postulate that this lack of cellular activity in the macrocycle is due to its zwitterionic character, which may prevent cell penetration. Indeed, we recently showed that alanyl-AMS (4a) is less cell-penetrant in a variety of bacteria compared to the corresponding α-hydroxy analogue, lactyl-AMS (4b).47 Thus, toward the development of selective, cell-penetrant inhibitors of NRPS amino acid adenylation domains, we report herein the synthesis and evaluation of a series of linear (4b,d) and macrocyclic (5b) α-hydroxyacyl-AMS analogues as inhibitors of HMWP2. While this α-amino-to-α-hydroxy modification results in a significant decrease in biochemical potency, inhibitory activity can be restored by installation of a β-thio group that corresponds to the cognate cysteine side chain recognized by HMWP2. This provides a path forward to develop selective, cell-penetrant NRPS inhibitors to probe the biological functions of bacterial natural products and the therapeutic potential of targeting virulence factor biosynthesis.
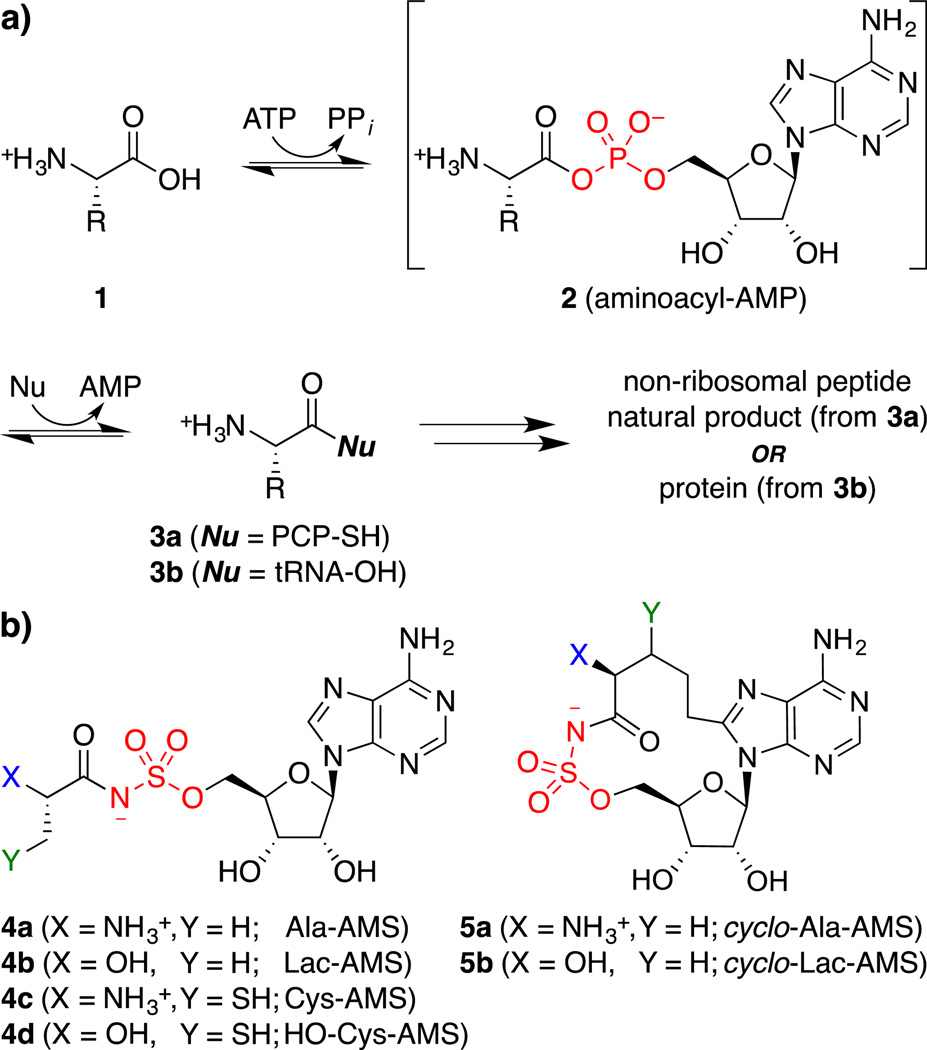
Figure 1. Amino acid adenylation reaction and inhibitors.
(a) Both NRPS amino acid adenylation domains and aminoacyl-tRNA synthetases use a two-step mechanism to activate and couple amino acid substrates via a tightly-bound aminoacyl-AMP intermediate (2). (b) Structures of linear (4) and macrocyclic (5) amino acid adenylation domain inhibitors designed to target the cysteine adenylation domain of Y. pestis HMWP2. AMS = adenosine 5´-O-monosulfamate; PCP = peptidyl carrier protein with phosphopantetheine thiol nucleophile; tRNA = transfer RNA with 2´- or 3´-hydroxyl nucleophile.
Amino acid adenylation domains belong to a mechanistic superfamily of enzymes that catalyze a two-step process involving initial activation of a carboxylic acid substrate (1) to form a tightly-bound acyl adenylate (acyl-AMP) intermediate (2), followed by nucleophilic attack to yield a carboxylate derivative (3, Figure 1a).48 We44–46,49–52 and others53–70 have used this mechanistic information to develop potent inhibitors of a variety of bacterial adenylation enzymes using an acyl-sulfamoyladenosine (acyl-AMS) scaffold,71 which acts as a nonhydrolyzable mimic of the tightly-bound acyl-AMP intermediate (Figure 1b).
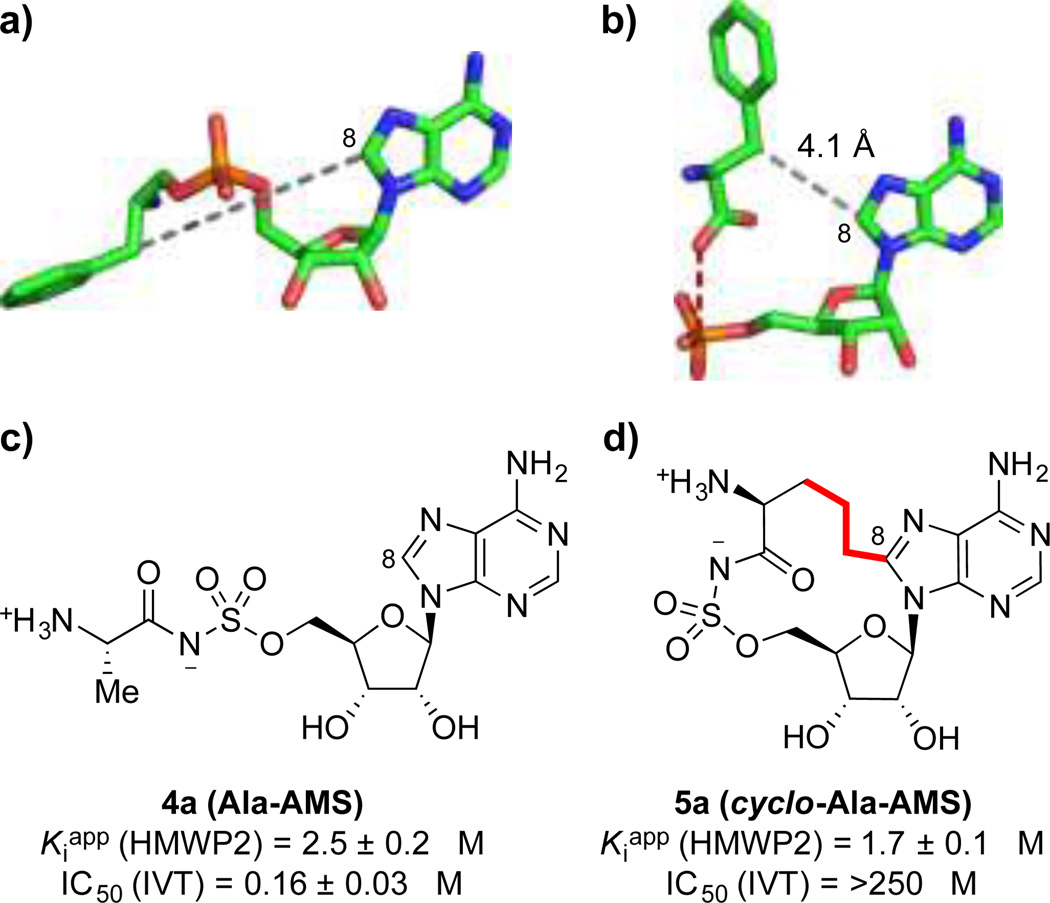
Bacterial NRPS amino acid adenylation domains and aminoacyl-tRNA synthetases, which are ubiquitous in both pathogens and the host, catalyze mechanistically identical reactions (Figure 1a), but have distinct protein folds and active-site interactions. Thus, to develop selective NRPS inhibitors, we have previously exploited conformational differences between ligands observed in cocrystal structures.46 In aminoacyl-tRNA synthetases, the aminoacyl-AMP intermediate 2 adopts an extended, ‘transoid’ conformation (Figure 2a),72,73 whereas in NRPS adenylation domains and related enzymes, this same intermediate is bound in a C-shaped, ‘cisoid’ conformation (Figure 2b).74–77 Thus, while linear aminoacyl-AMS inhibitors54 such as alanyl-AMS (4a, Figure 2c) inhibit both enzyme classes, installation of a macrocyclic constraint linking the C8 position of adenine and the β-carbon of the amino acid in cyclo-alanyl-AMS (5a) (Figure 2d) enforces the cisoid pharmacophoric conformation that is specific to NRPS amino acid adenylation domains and affords highly selective inhibition.46 Notably, the alanyl-AMS-based macrocycle 5a lacks a β-position side chain and was designed as a potential broad-spectrum inhibitor of NRPS amino acid adenylation domains.
Figure 2. Targeting the unique cisoid binding pharmacophore of NRPS amino acid adenylation domains.
(a) Extended conformation of Phe-AMP bound to Phe-tRNA synthetase from Thermus thermophilus (PDB ID: 1B7Y).73 (b) Cisoid conformation of Phe and AMP bound to the Phe adenylation domain of Bacillus brevis gramicidin S synthetase 1 (PDB ID: 1AMU),74 with a 4.1 Å unobstructed space between C8 of the adenine ring and the β-carbon of the amino acid. (c) Structure and non-selective inhibitory activity of linear alanyl-AMS (4a) against both the Cys adenylation domain of Y. pestis HMWP2 and aminoacyl-tRNA synthetases (IVT). (d) Structure and selective inhibitory activity of macrocyclic cyclo-alanyl-AMS (5a) against the Cys adenylation domain of HMWP2. HMWP2 = high molecular-weight protein 2 from Y. pestis yersiniabactin synthetase. IVT = in vitro translation assay with rabbit reticulocyte lysates and luciferase readout.
Although the cyclo-alanyl-AMS macrocycle (5a) was a potent and selective biochemical inhibitor of the cysteine adenylation domain of the Y. pestis yersiniabactin synthetase component HMWP2,46 it did not inhibit yersiniabactin production in Y. pestis cell culture (unpublished results). We posited that this was due to the inability of this zwitterionic molecule to penetrate the bacterial cell membrane. The structural requirements for bacterial cell permeability remain enigmatic,78–81 and our laboratory recently carried out a series of prospective compound accumulation studies using acyl-AMS analogues with diverse structural and physicochemical properties, across various bacterial species.47 This study revealed that alanyl-AMS (4a) did not penetrate any of the bacteria tested (Escherichia coli, Bacillus subtilis, Mycobacterium smegmatis) above the limits of detection, while the corresponding α-hydroxy congener, lactyl-AMS (4b, Figure 1b), penetrated all three strains at significantly higher levels (28–53 µM intracellular concentration vs. 100 µM extracellular concentration applied). Thus, we envisioned that the corresponding macrocycle cyclo-lactyl-AMS (5b) might also be developed as a cell-penetrant inhibitor of NRPS amino acid adenylation domains. Notably, Abell and Aldrich have previously reported α-hydroxyacyl-AMS inhibitors of pantothenate synthetase (PanC), although no cellular activity of these compounds (up to 250 µM) has been observed.63–65
To investigate this hypothesis, we synthesized a series of acyl-AMS analogues to assess systematically the impacts of the α-amino-to-α-hydroxy modification, the amino acid side chain, and the macrocyclic constraint upon inhibition of the cysteine adenylation domain of HMWP2. Thus, alanyl-AMS (4a, Figure 1b), lactyl-AMS (4b), and cysteyl-AMS (4c) were synthesized as previously described.47,82 The corresponding α-hydroxy-cysteyl-AMS analogue 4d was synthesized from (2S)-methyl glycidate (6) by epoxide opening with tritylthiol83 and alcohol protection to form protected hydroxycysteine 7, followed by conversion to the corresponding N-hydroxysuccinimidyl (NHS) ester 8 (Scheme 1). Coupling to 2´,3´-acetonide-protected 5´-O-sulfamoyladenosine (9),82 prepared as previously described,84 and global deprotection with acid afforded α-hydroxy-cysteyl-AMS (4d).
Scheme 1. Synthesis of linear α-hydroxycysteyl-AMS analogue 4d.
Reagents and conditions: a) TrtSH (1.5 equiv), Et3N (1.0 equiv), MeOH, 65 °C, 24 h, 69%; b) TBSCl (4.3 equiv), imidazole (3.5 equiv), CH2Cl2, 0 → 23 °C, 42 h, 100%; c) LiOH (0.4 M aq, 5.0 equiv), THF, 0 → 23 °C, 24 h, then aq HCl (1.0 N) to pH 4, 0 °C, 95%; d) NHS (1.0 equiv), DCC (1.1 equiv), THF, 23 °C, 20.5 h, 76%; e) 8 (1.5 equiv), 9 (1.0 equiv), DBU (1.5 equiv), CH3CN, 23 °C, 2 h, 81%; f) 5:1 TFA/H2O, 0 → 23 °C, 3 h, then MeOH, 0 °C, 79%. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; DCC = N,N´-dicyclohexylcarbodiimide; NHS = N-hydroxysuccinimide; TBS = t-butyldimethylsilyl, TFA = trifluoroacetic acid; THF = tetrahydrofuran; Trt = trityl.
The macrocycle cyclo-alanyl-AMS (5a) was also synthesized as previously described.46 To access the α-hydroxy analogue, cyclo-lactyl-AMS (5b), we envisioned a late-stage macrocyclization of a functionalized AMS precursor 10, which could be accessed by Sonogashira coupling of α-hydroxy-pentynoate 11 with 8-iodoadenosine 12 (Scheme 2a). Synthesis of the pentynoate fragment 11 started from (2S)-methyl glycidate (6), which underwent regioselective epoxide opening with diethyl[(trimethylsilyl)ethynyl]aluminum,84 followed by alkyne desilylation and TBS protection of the secondary alcohol (Scheme 2b). The alkyne 11 then underwent efficient Sonogashira coupling to 8-iodoadenosine 12, prepared as previously described,46 to provide 8-alkynyladenosine 13. Sulfamoylation of the adenosine 5′-hydroxyl group and hydrogenation of the alkyne provided cyclization precursor 10. Saponification of the ester, macrocyclization of the resulting acid, and global deprotection under acidic conditions afforded cyclo-lactyl-AMS (5b).
Scheme 2. Retrosynthetic analysis (a) and synthesis (b) of macrocyclic cyclo-lactyl-AMS analogue 5b.
Reagents and conditions: a) ethynyltrimethylsilane (1.5 equiv), n-BuLi (1.7 M in hexanes, 1.5 equiv), toluene, 0 °C, 15 min, then Et2AlCl (1.5 equiv), 0 °C, 45 min, then 6, 0 → 23 °C, 90 min, 94%; b) TBAF (1.0 M in THF, 1.0 equiv), THF, 23 °C, 2 h, 92%; c) TBSCl (4.3 equiv), imidazole (3.5 equiv), CH2Cl2, 0 → 23 °C, 42 h, 86%; d) 11 (1.5 equiv), 12 (1.0 equiv), i-PrNEt2 (2.0 equiv), CuI (0.70 equiv), Pd(PPh3)4 (0.35 equiv), DMF, 23 °C, 23 h, 94%; e) sulfamoyl chloride (5.6 equiv), DMA, 0 °C, 1 h, 76%; f) 30% Pd/C (2.5 equiv), H2 (balloon), MeOH, 23 °C, 6 h, 100%; g) LiOH (0.4 M aq, 1.5 equiv), THF, MeOH, 0 → 23 °C, 15 h; h) PyBOP (1.8 equiv), HOBt (2.8 equiv), DBU (1.5 equiv), CH3CN, 23 °C, 4.5 h, 48% over 2 steps; i) 5:1 TFA/H2O, 0 → 23 °C, 3 h, then MeOH, 0 °C, 37%. DMA = N,N-dimethylacetamide; DMF = N,N-dimethylformamide; HOBt = hydroxybenzotriazole; Lac = lactyl; PyBOP = benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate; TBAF = tetrabutylammonium fluoride.
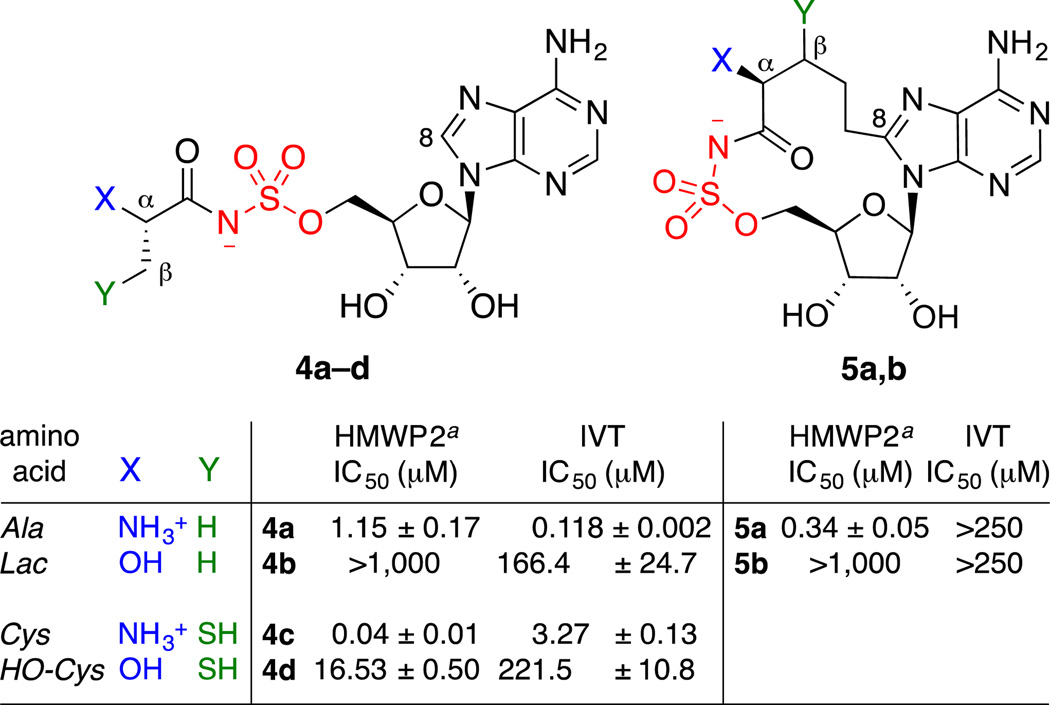
Inhibition of the cysteine adenylation activity of recombinant HMWP2 (residues 1–1491) was evaluated using a coupled, continuous, spectrophotometric 7-methylthioguanosine (MesG) assay recently developed for adenylation domains by Aldrich and coworker (Supplementary Figure 1).86 In contrast to the classical ATP–32PPi isotope exchange assay that we used previously to assess HMWP2 inhibition,46 the MesG assay does not require radioactive reagents and has been applied to several other adenylate-forming enzymes, including those in NRPS pathways (EntE, VibE, BasE, MbtA, GrsA, FadD28).86,87 The MesG assay uses hydroxylamine as an acyl acceptor nucleophile, allowing turnover of the forward adenylation reaction by facilitating release of the acyl-AMP intermediate (2) and pyrophosphate (Figure 1a). The pyrophosphate is then hydrolyzed by a pyrophosphatase to form phosphate, which is, in turn, used by a purine nucleoside phosphorylase to convert MesG into ribose-1-phosphate and 7-methylthioguanine, a UV-active product that can be monitored spectrophotometrically. Using this assay with 30 nM HMWP2, the cognate α-aminoacyl-AMS inhibitor cysteyl-AMS (4c) exhibited an IC50 of 40 ± 10 nM (Figure 3). This recapitulates the tight-binding inhibitor behavior88,89 that we observed previously with the ATP–32PPi isotope exchange assay,46 thus setting the stage for the use of the MesG assay to characterize our other analogues.
Figure 3. Structure–activity relationships at α- and β-positions of acyl-AMS inhibitors.
HMWP2 assay conditions: 30 nM HMWP2(1–1491), 0.04 U inorganic pyrophosphatase, 0.1 U purine nucleoside phosphorylase, 300 mM NH2OH, 0.2 mM MesG, 5 mM cysteine, 2.5 mM ATP, 5 mM MgCl2, 1.5 mM DTT, 50 mM Tris pH 8.0. The in vitro translation (IVT) assay was performed using the Promega TNT T7 Coupled Reticulocyte Lysate System with the luciferase T7 control DNA and luciferin reagent. Values shown for average of three independent experiments, with standard error of the mean. See Supplementary Data for complete details. (ATP–32PPi isotope exchange assay46): 4a = 2.5 ± 0.2 µM, 4c = 0.24 ± 0.02 µM, 5a = 1.7 ± 0.1 µM.
Analysis of the remaining linear and macrocyclic analogues revealed two important trends. First, replacement of the α-amino group with an α-hydroxy group led to decreased HMWP2 inhibition by 2–3 orders of magnitude (4a vs. 4b, 4c vs. 4d, 5a vs. 5b). Second, inclusion of the cognate β-thio group corresponding to the cysteine sidechain recognized by HMWP2 resulted in increased HMWP2 inhibition by ≈2 orders of magnitude (4a vs. 4c, 4b vs. 4d). The decreased inhibitory activity upon replacement of the α-amino group with an α-hydroxy group in both the linear analogues and the corresponding macrocycles can be rationalized by examination of the cocrystal structure of phenylalanine and AMP (presumably hydrolysis products of the cognate phenylalanyl-AMP reaction intermediate) bound to the phenylalanine adenylation domain of Bacillus brevis gramicidin S synthetase 1 (PDB: 1AMU) (Figure 4).74 In the active site, the α-amino group of the amino acid is engaged in an electrostatic interaction with Asp-235. This aspartate is completely conserved in other amino acid adenylation domains, including the cysteine adenylation domain of HMWP2 (Asp-757).46,90 On the other hand, inclusion of the β-thiol side chain that is specifically recognized by the cysteine adenylation domain of HMWP2 increases potency in the linear series, presumably by filling the corresponding specificity pocket in the enzyme.46,90
Figure 4. Electrostatic interaction of α-amino group with conserved active site aspartate.
Structure of phenylalanine (Phe) and AMP, the hydrolysis products of the Phe-AMP reaction intermediate, bound in the active site of the B. brevis gramicidin S synthetase 1 phenylalanine adenylation domain (PDB: 1AMU),74 with key interaction with Asp-235.
To assess the effects of the α-amino-to-α-hydroxy modification on selective inhibition of HMWP2 over aminoacyl-tRNA synthetases, we also evaluated the inhibitory activity of these analogues in a rabbit reticulocyte lysate in vitro translation assay, using translation of luciferase as a readout (Fig. 3).46 Notably, in the linear series, the α-hydroxyacyl-AMS analogues were much weaker inhibitors than the corresponding α-aminoacyl-AMS analogues, by 2–3 orders of magnitude (4a vs. 4b, 4c vs. 4d). This is consistent with engagement of the α-amino group by multiple hydrogen bonds in aminoacyl-tRNA synthetase cocrystal structures.72,91–94 In contrast, both of the macrocycles, cyclo-alanyl-AMS (5a) and cyclo-lactyl-AMS (5b), were inactive at the highest concentration tested (250 µM), indicative of the high specificity of the macrocyclic design.
In conclusion, the advancement of selective adenylation enzyme inhibitors as novel antibacterial agents relies on their ability to permeate bacterial cell membranes to access these intracellular targets. To this end, it is essential to identify rational modifications to biochemically potent inhibitors that confer permeability while maintaining activity.79 We have previously shown that lactyl-AMS (4b) penetrates Gram-positive B. subtilis, Gram-negative E. coli, and M. smegmatis.47 Unfortunately, this α-hydroxy modification also leads to a >2–3 order-of-magnitude decrease in inhibitory potency against the cysteine adenylation domain of HMWP2, when compared to the corresponding α-amino derivatives. While some loss in binding affinity is acceptable if it provides cell permeability, further studies will be required to develop inhibitors that retain sufficient binding affinity to exhibit cellular activity. In the context of the cysteine adenylation domain of HMWP2, inclusion of the β-thiol side chain in cysteyl-AMS (4c) and α-hydroxycysteyl-AMS (4d) increases inhibitory potency by ≈30–60-fold compared to the corresponding alanine-based inhibitors alanyl-AMS (4a) and lactyl-AMS (4b). This brings the IC50 of α-hydroxycysteyl-AMS (4d) to within 15-fold of alanyl-AMS (4a).
Thus, in the macrocycle series, it may similarly be possible to recapture much of the binding affinity lost due to the α-amino-to-α-hydroxy modification by installing this β-thiol side chain (e.g., cyclo-hydroxycysteyl-AMS). This will require development of an efficient, diastereoselective synthetic route to access these significantly more challenging molecules, and determination of the appropriate stereochemical configuration at the β-substituent, which is unclear from inspection of the GrsA phenylalanine adenylation domain structure.74 Other amino acid adenylation domains could then similarly be targeted specifically by incorporating the corresponding β-substituent. If these α-hydroxyacyl-AMS macrocycles also exhibit improved bacterial cell penetration, by analogy to the trends observed in the linear series,47 then this would provide potent, selective, and cell-penetrant inhibitors of NRPS amino acid adenylation domains. Such inhibitors would then be powerful probes to study the biological functions of the NRPS-derived natural products in bacterial processes, including virulence. They could also be used to evaluate the therapeutic potential of targeting natural-product virulence factor biosynthesis, and in particular could be used to assess pharmacologically the propensity for bacteria to develop resistance to such antivirulence factor drugs. Efforts to synthesize and evaluate such fully elaborated inhibitors are underway and will be reported in due course.
Supplementary Material
Acknowledgments
We thank G. Sukenick, R. Wang, H. Liu, H. Fang, and S. Rusli (MSKCC) for expert NMR and mass spectral support. Financial support from the National Institutes of Health (R01 GM100477 to D.S.T. and L.E.N.Q., T32 CA062948-Gudas to T.D.D., and CCSG P30 CA008748 to C. B. Thompson) and endowment support from Carol and Larry Zicklin (to L.E.N.Q.) are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Experimental procedures and analytical data for all new compounds and experimental procedures for biochemical assays are provided. Supplementary data associated with this article can be found, in the online version, at doi: XXXX
References and notes
- 1.Brown ED, Wright GD. Nature. 2016;529:336. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
- 3.World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/
- 4.Pew Charitable Trusts. A Scientific Roadmap for Antibiotic Discovery. 2016 http://www.pewtrusts.org/en/research-and-analysis/reports/2016/05/a-scientific-roadmap-for-antibiotic-discovery. [Google Scholar]
- 5.Keller L, Surette MG. Nat. Rev. Microbiol. 2006;4:249. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 6.Yim G, Wang HH, Davies J. Philos. Trans. R. Soc., B. 2007;362:1195. doi: 10.1098/rstb.2007.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shank EA, Kolter R. Curr. Opin. Microbiol. 2009;12:205. doi: 10.1016/j.mib.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutherford ST, Bassler BL. Cold Spring Harbor Perspect. Med. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cisar JS, Tan DS. Chem. Soc. Rev. 2008;37:1320. doi: 10.1039/b702780j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb AL. Biochim. Biophys. Acta, Proteins Proteomics. 2015;1854:1054. doi: 10.1016/j.bbapap.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clatworthy AE, Pierson E, Hung DT. Nat. Chem. Biol. 2007;3:541. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 12.Rasko DA, Sperandio V. Nat. Rev. Drug Discov. 2010;9:117. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 13.Allen RC, Popat R, Diggle SP, Brown SP. Nat. Rev. Microbiol. 2014;12:300. doi: 10.1038/nrmicro3232. [DOI] [PubMed] [Google Scholar]
- 14.LaSarre B, Federle MJ. Microbiol. Mol. Biol. Rev. 2013;77:73. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellbye B, Schuster M. mBio. 2011;2:e00131. doi: 10.1128/mBio.00131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross-Gillespie A, Gardner A, West SA, Griffin AS. Am. Nat. 2007;170:331. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 17.Fischbach MA, Walsh CT. Chem. Rev. 2006;106:3468. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 18.Nougayrede J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Science. 2006;313:848. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 19.Joyner PM, Liu J, Zhang Z, Merritt J, Qi F, Cichewicz RH. Org. Biomol. Chem. 2010;8:5486. doi: 10.1039/c0ob00579g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyatt MA, Wang W, Roux CM, Beasley FC, Heinrichs DE, Dunman PM, Magarvey NA. Science. 2010;329:294. doi: 10.1126/science.1188888. [DOI] [PubMed] [Google Scholar]
- 21.Wilson DJ, Shi C, Teitelbaum AM, Gulick AM, Aldrich CC. Biochemistry. 2013;52:926. doi: 10.1021/bi301330q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonzo DA, Magarvey NA, Schmeing TM. PLoS One. 2015;10:e0128569. doi: 10.1371/journal.pone.0128569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel P, Vizcaino MI, Crawford JM. Appl. Env. Microbiol. 2015;81:1502. doi: 10.1128/AEM.03283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratledge C, Dover LG. Annu. Rev. Microbiol. 2000;54:881. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 25.Schaible UE, Kaufmann SHE. Nat. Rev. Microbiol. 2004;2:946. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 26.Quadri LEN. Infect. Disord.: Drug Targets. 2007;7:230. doi: 10.2174/187152607782110040. [DOI] [PubMed] [Google Scholar]
- 27.Holden VI, Bachman MA. Metallomics. 2015;7:986. doi: 10.1039/c4mt00333k. [DOI] [PubMed] [Google Scholar]
- 28.Heesemann J. FEMS Microbiol. Lett. 1987;48:229. [Google Scholar]
- 29.Bearden SW, Fetherston JD, Perry RD. Infect. Immun. 1997;65:1659. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rindi L, Fattorini L, Bonanni D, Iona E, Freer G, Tan D, Deho G, Orefici G, Garzelli C. Microbiology. 2002;148:3873. doi: 10.1099/00221287-148-12-3873. [DOI] [PubMed] [Google Scholar]
- 31.Reddy PV, Puri RV, Chauhan P, Kar R, Rohilla A, Khera A, Tyagi AK. J. Inf. Dis. 2013;208:1255. doi: 10.1093/infdis/jit250. [DOI] [PubMed] [Google Scholar]
- 32.Madigan CA, Martinot AJ, Wei J-R, Madduri A, Cheng T-Y, Young DC, Layre E, Murry JP, Rubin EJ, Moody DB. PLoS Pathog. 2015;11:e1004792. doi: 10.1371/journal.ppat.1004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yancey RJ, Breeding SAL, Lankford CE. Infect. Immun. 1979;24:174. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemaitre C, Bidet P, Benoist J-F, Schlemmer D, Sobral E, d'Humieres C, Bonacorsi S. J. Bacteriol. 2014;196:1343. doi: 10.1128/JB.01153-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crouch M-LV, Castor M, Karlinsey JE, Kalhorn T, Fang FC. Mol. Microbiol. 2008;67:971. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- 36.Caza M, Lepine F, Milot S, Dozois CM. Infect. Immun. 2008;76:3539. doi: 10.1128/IAI.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Q, Wang X, Xu H, Xu Y, Ling J, Zhang D, Gao S, Liu X. BMC Microbiol. 2012;12:143. doi: 10.1186/1471-2180-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. Infect. Immun. 2012;80:1015. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson DP, Payne SM. Infect. Immun. 1994;62:5120. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litwin CM, Rayback TW, Skinner J. Infect. Immun. 1996;64:2834. doi: 10.1128/iai.64.7.2834-2838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan W, Verma V, Jeong K, Kim Soo Y, Rhee Joon H, Jung C-H, Lee Shee E. Front. Microbiol. 2014;5:1. doi: 10.3389/fmicb.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer J-M, Neely A, Stintzi A, Georges C, Holder IA. Infect. Immun. 1996;64:518. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takase H, Nitanai H, Hoshino K, Otani T. Infect. Immun. 2000;68:1834. doi: 10.1128/iai.68.4.1834-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lun S, Guo H, Adamson J, Cisar JS, Davis TD, Chavadi SS, Warren JD, Quadri LEN, Tan DS, Bishai WR. Antimicrob. Agents Chemother. 2013;57:5138. doi: 10.1128/AAC.00918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreras JA, Ryu J-S, Di Lello F, Tan DS, Quadri LEN. Nat. Chem. Biol. 2005;1:29. doi: 10.1038/nchembio706. [DOI] [PubMed] [Google Scholar]
- 46.Cisar JS, Ferreras JA, Soni RK, Quadri LEN, Tan DS. J. Am. Chem. Soc. 2007;129:7752. doi: 10.1021/ja0721521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis TD, Gerry CJ, Tan DS. ACS Chem. Biol. 2014;9:2535. doi: 10.1021/cb5003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmelz S, Naismith JH. Curr. Opin. Struct. Biol. 2009;19:666. doi: 10.1016/j.sbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferreras JA, Stirrett KL, Lu X, Ryu J-S, Soll CE, Tan DS, Quadri LEN. Chem. Biol. 2008;15:51. doi: 10.1016/j.chembiol.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu X, Zhang H, Tonge PJ, Tan DS. Bioorg. Med. Chem. Lett. 2008;18:5963. doi: 10.1016/j.bmcl.2008.07.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu X, Zhou R, Sharma I, Li X, Kumar G, Swaminathan S, Tonge PJ, Tan DS. ChemBioChem. 2012;13:129. doi: 10.1002/cbic.201100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matarlo JS, Evans CE, Sharma I, Lavaud LJ, Ngo SC, Shek R, Rajashankar KR, French JB, Tan DS, Tonge PJ. Biochemistry. 2015;54:6514. doi: 10.1021/acs.biochem.5b00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Lee SW, Choi EC, Choi SY. Appl. Microbiol. Biotechnol. 2003;61:278. doi: 10.1007/s00253-003-1243-5. [DOI] [PubMed] [Google Scholar]
- 54.Finking R, Neumueller A, Solsbacher J, Konz D, Kretzschmar G, Schweitzer M, Krumm T, Marahiel MA. ChemBioChem. 2003;4:903. doi: 10.1002/cbic.200300666. [DOI] [PubMed] [Google Scholar]
- 55.Somu RV, Boshoff H, Qiao C, Bennett EM, Barry CE, III, Aldrich CC. J. Med. Chem. 2006;49:31. doi: 10.1021/jm051060o. [DOI] [PubMed] [Google Scholar]
- 56.Duckworth BP, Nelson KM, Aldrich CC. Curr. Top. Med. Chem. 2012;12:766. doi: 10.2174/156802612799984571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.May JJ, Finking R, Wiegeshoff F, Weber TT, Bandur N, Koert U, Marahiel MA. FEBS J. 2005;272:2993. doi: 10.1111/j.1742-4658.2005.04700.x. [DOI] [PubMed] [Google Scholar]
- 58.Miethke M, Bisseret P, Beckering CL, Vignard D, Eustache J, Marahiel MA. FEBS J. 2006;273:409. doi: 10.1111/j.1742-4658.2005.05077.x. [DOI] [PubMed] [Google Scholar]
- 59.Brown PH, Cronan JE, Grotli M, Beckett D. J. Mol. Biol. 2004;337:857. doi: 10.1016/j.jmb.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 60.Duckworth BP, Geders TW, Tiwari D, Boshoff HI, Sibbald PA, Barry CE, III, Schnappinger D, Finzel BC, Aldrich CC. Chem. Biol. 2011;18:1432. doi: 10.1016/j.chembiol.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Callahan BP, Lomino JV, Wolfenden R. Bioorg. Med. Chem. Lett. 2006;16:3802. doi: 10.1016/j.bmcl.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 62.Sikora AL, Wilson DJ, Aldrich CC, Blanchard JS. Biochemistry. 2010;49:3648. doi: 10.1021/bi100350c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuck KL, Saldanha SA, Birch LM, Smith AG, Abell C. Org. Biomol. Chem. 2006;4:3598. doi: 10.1039/b609482a. [DOI] [PubMed] [Google Scholar]
- 64.Ciulli A, Scott DE, Ando M, Reyes F, Saldanha SA, Tuck KL, Chirgadze DY, Blundell TL, Abell C. ChemBioChem. 2008;9:2606. doi: 10.1002/cbic.200800437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Z, Yin W, Martinelli LK, Evans J, Chen J, Yu Y, Wilson DJ, Mizrahi V, Qiao C, Aldrich CC. Bioorg. Med. Chem. 2014;22:1726. doi: 10.1016/j.bmc.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfleger BF, Lee JY, Somu RV, Aldrich CC, Hanna PC, Sherman DH. Biochemistry. 2007;46:4147. doi: 10.1021/bi6023995. [DOI] [PubMed] [Google Scholar]
- 67.Tian Y, Suk D-H, Cai F, Crich D, Mesecar AD. Biochemistry. 2008;47:12434. doi: 10.1021/bi801311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arora P, Goyal A, Natarajan VT, Rajakumara E, Verma P, Gupta R, Yousuf M, Trivedi OA, Mohanty D, Tyagi A, Sankaranarayanan R, Gokhale RS. Nat. Chem. Biol. 2009;5:166. doi: 10.1038/nchembio.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drake EJ, Duckworth BP, Neres J, Aldrich CC, Gulick AM. Biochemistry. 2010;49:9292. doi: 10.1021/bi101226n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson DJ, Shi C, Teitelbaum AM, Gulick AM, Aldrich CC. Biochemistry. 2013;52:926. doi: 10.1021/bi301330q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ueda H, Shoku Y, Hayashi N, Mitsunaga J, In Y, Doi M, Inoue M, Ishida T. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 1991;1080:126. doi: 10.1016/0167-4838(91)90138-p. [DOI] [PubMed] [Google Scholar]
- 72.Brick P, Bhat TN, Blow DM. J. Mol. Biol. 1989;208:83. doi: 10.1016/0022-2836(89)90090-9. [DOI] [PubMed] [Google Scholar]
- 73.Reshetnikova L, Moor N, Lavrik O, Vassylyev DG. J. Mol. Biol. 1999;287:555. doi: 10.1006/jmbi.1999.2617. [DOI] [PubMed] [Google Scholar]
- 74.Conti E, Stachelhaus T, Marahiel MA, Brick P. EMBO J. 1997;16:4174. doi: 10.1093/emboj/16.14.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.May JJ, Kessler N, Marahiel MA, Stubbs MT. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12120. doi: 10.1073/pnas.182156699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hisanaga Y, Ago H, Nakagawa N, Hamada K, Ida K, Yamamoto M, Hori T, Arii Y, Sugahara M, Kuramitsu S, Yokoyama S, Miyano M. J. Biol. Chem. 2004;279:31717. doi: 10.1074/jbc.M400100200. [DOI] [PubMed] [Google Scholar]
- 77.Nakatsu T, Ichiyama S, Hiratake J, Saldanha A, Kobashi N, Sakata K, Kato H. Nature. 2006;440:372. doi: 10.1038/nature04542. [DOI] [PubMed] [Google Scholar]
- 78.O'Shea R, Moser HE. J. Med. Chem. 2008;51:2871. doi: 10.1021/jm700967e. [DOI] [PubMed] [Google Scholar]
- 79.Lewis K. Nature. 2012;485:439. doi: 10.1038/485439a. [DOI] [PubMed] [Google Scholar]
- 80.Tommasi R, Brown DG, Walkup GK, Manchester JI, Miller AA. Nat. Rev. Drug Discov. 2015;14:529. doi: 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- 81.Silver LL. Bioorg. Med. Chem. in press. [Google Scholar]
- 82.Castro-Pichel J, Garcia-Lopez MT, De las Heras FG. Tetrahedron. 1987;43:383. [Google Scholar]
- 83.Deechongkit S, You S-L, Kelly JW. Org. Lett. 2004;6:497. doi: 10.1021/ol036102m. [DOI] [PubMed] [Google Scholar]
- 84.Van de Vijver P, Ostrowski T, Sproat B, Goebels J, Rutgeerts O, Van Aerschot A, Waer M, Herdewijn P. Journal of Medicinal Chemistry. 2008;51:3020. doi: 10.1021/jm8000746. [DOI] [PubMed] [Google Scholar]
- 85.Iwaki Y, Kaneko M, Akita H. Tetrahedron Asym. 2009;20:298. [Google Scholar]
- 86.Wilson DJ, Aldrich CC. Anal. Biochem. 2010;404:56. doi: 10.1016/j.ab.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishikawa F, Miyamoto K, Konno S, Kasai S, Kakeya H. ACS Chem. Biol. 2015;10:2816. doi: 10.1021/acschembio.5b00595. [DOI] [PubMed] [Google Scholar]
- 88.Morrison JF. Biochem. Biophys. Acta, Enzymol. 1969;185:269. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- 89.Williams JW, Morrison JF. Methods Enzymol. 1979;63:437. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]
- 90.Stachelhaus T, Mootz HD, Marahiel MA. Chem. Biol. 1999;6:493. doi: 10.1016/S1074-5521(99)80082-9. [DOI] [PubMed] [Google Scholar]
- 91.Desogus G, Todone F, Brick P, Onesti S. Biochemistry. 2000;39:8418. doi: 10.1021/bi0006722. [DOI] [PubMed] [Google Scholar]
- 92.Perona JJ, Hadd A. Biochemistry. 2012;51:8705. doi: 10.1021/bi301180x. [DOI] [PubMed] [Google Scholar]
- 93.Eiler S, Dock-Bregeon A, Moulinier L, Thierry JC, Moras D. EMBO J. 1999;18:6532. doi: 10.1093/emboj/18.22.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rath VL, Silvian LF, Beijer B, Sproat BS, Steitz TA. Structure. 1998;6:439. doi: 10.1016/s0969-2126(98)00046-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.