Abstract
For the past quarter century, scientists at the Center for Family Research at the University of Georgia have conducted research designed to promote understanding of normative developmental trajectories among low-SES African American children, youths, and young adults. In this paper, we describe a recent expansion of this research program using longitudinal, epidemiological studies and randomized prevention trials to tests hypotheses about the origins of disease among rural African American youths. The contributions of economic hardship, downward mobility, neighborhood poverty, and racial discrimination to allostatic load and epigenetic aging are illustrated. The health benefits of supportive family relationships in protecting youths from these challenges are also illustrated. A cautionary set of studies is presented showing that some psychosocially resilient youths demonstrate high allostatic loads and accelerated epigenetic aging, suggesting that, for some, “resilience is just skin deep.” Finally, we end on an optimistic note by demonstrating that family-centered prevention programs can have health benefits by reducing inflammation, helping to preserve telomere length, and inhibiting epigenetic aging.
In 1989, the W. T. Grant Foundation issued an influential report, The Forgotten Half: Non-College Bound Youth in America, an analysis of the life situations of young people who do not attend college. After 27 years, the forgotten half remains largely forgotten in research, given the scarcity of programmatic, longitudinal studies of young people who are not college-bound. The majority of rural African American youths and emerging adults in the southern United States are part of this forgotten half. A minority of African American secondary school graduates in the Southern counties in which we conducted the research program described in this paper complete post-secondary education programs (Boatright, 2005). It was against this backdrop that the first author (GHB) began systematic longitudinal analyses designed to identify the risk and protective processes in the lives of rural African American youths that forecast psychological well-being and the development of academic and self-regulatory competence. This initial work was performed under the auspices of the Program for the Study of Competence of Children and Families at the University of Georgia, which was founded in 1987. Brody expanded this administrative structure to form the Center for Family Research (CFR) in 1994. This expansion reflected scientific growth in this research program, along with an elaboration of its mission to use longitudinal, epidemiological data to inform the development and testing of prevention programs for rural African American preadolescents, adolescents, and young adults (Brody, Kogan, & Grange, 2012; Brody et al., 2004).
The research conducted within the Program for the Study of Competence of Children and Families and at CFR demonstrated that, despite persistent exposure to a plethora of environmental stressors and challenges, many youths demonstrated academic competence, planful self-regulation, and positive psychological adjustment. Naturally occurring influences within family and community networks were identified that buffered youths from the consequences of chronic economic hardship and contextual stressors (Brody, Kogan, et al., 2012). In recent years, CFR expanded its research agenda to understand better the origins of the health disparities that currently plague rural African Americans.
The paper is organized into four sections. We begin by describing the rural Southern “Black Belt” and the ways in which the economic hardships that many who live there experience can sponsor vulnerabilities to the chronic diseases of aging (CDAs) such as coronary heart disease, diabetes, stroke, hypertension, chronic lung diseases, and some cancers. Second, we describe our recent “proof-of-principle” studies that link exposure to socioeconomic status (SES) and race-related stressors to biomarkers that act as indicators of the biological weathering of young, rural African Americans’ bodies. Next, we review evidence that, for many African American youths, resilience is a “skin deep” phenomenon wherein outward indicators of success can mask emerging health problems. Finally, we conclude on an optimistic note by showing that psychosocial interventions designed to enhance parenting and strengthen family relationships can deter contextual risk mechanisms from affecting an array of health-relevant biomarkers such as inflammatory profiles, epigenetic aging, and telomere length (TL).
Socioeconomic Challenges for African Americans Growing Up in the Rural South
Poverty and economic distress are pervasive for many African American families in rural areas, particularly those who reside in a crescent-shaped area of 623 counties in 11 southern states; 34% of the nation’s poor reside here (Wimberly & Morris, 1997). This region has been called the Black Belt, a term first used to describe the color of the rich Southern soil on which enslaved Africans worked. Later, the term came to refer to the large African American population in this area. The rural communities in the Black Belt commonly face chronic poverty, population decline, inadequate education programs, poor health care, substandard housing, and high levels of unemployment. These problems disproportionately affect African American residents (Probst et al., 2002).
Recent estimates indicate that more than half of the African American children in the rural South live in economically distressed households (Mattingly & Bean, 2010). Most of their primary caregivers are employed; their poverty reflects the dominance of low-wage, resource intensive industries in these areas. For families with little discretionary income, rural residence can be more challenging than is life in urban areas due to a restricted range of employment opportunities, a lack of public transportation systems, the absence of recreational facilities for youths, and difficulties in obtaining care for physical and mental health problems (Brody, Neubaum, Boyd, & Dufour, 1997). Many African American families in the rural South thus live under conditions of severe, ongoing economic stress that have the potential to take a toll on health and well-being among children and youths (Miller, Chen, & Parker, 2011; Shonkoff, Boyce, & McEwen, 2009).
Low Socioeconomic Status and Health Across the Lifespan
Because many rural African American children live in low SES circumstances, they are at elevated risk for health problems across their lifespans (Braveman, Cubbin, Egerter, Williams, & Pamuk, 2010; Shonkoff et al., 2009). These disparities likely begin at the earliest stages of life. Children in low-income families experience disproportionately high rates of growth restriction, preterm births, and neonatal mortality (Blumenshine, Egerter, Barclay, Cubbin, & Braveman, 2010). As children from low-SES environments mature, they continue to experience health problems at higher rates than those of their more advantaged peers. Low-SES youths show a heightened prevalence of obesity, insulin resistance, and asthma (Chen, Matthews, & Boyce, 2002; Goodman, Daniels, & Dolan, 2007; Singh, Siahpush, & Kogan, 2010; Wright & Subramanian, 2007). When they reach later life stages, those raised in low-SES families show excessive morbidity and mortality from CDAs (Galobardes, Lynch, & Smith, 2004, 2008; Galobardes, Smith, & Lynch, 2006). These associations are typically independent of SES in adulthood, suggesting that childhood disadvantage can leave a “biological residue” with long-term health consequences.
These data raised challenging questions for the CFR health initiative. How does living in conditions of economic hardship and its associated stressors get “under the skin” to influence the anatomy and physiology of rural African American children and youths in ways that contribute to health disparities? Through what mechanisms do these experiences confer vulnerability to CDAs? To begin to address these questions, we conducted prospective analyses designed to determine whether economic hardship and exposure to racial discrimination presage indicators of cardiometabolic risk and epigenetic aging. These studies are described in the following sections.
Psychosocial Processes Presage Health Vulnerabilities: Five Proof-Of-Principal Studies
Many scientists believe that the well-documented health disparities between African Americans and members of other ethnic groups do not originate in adulthood but result from changes in biological processes at earlier stages of development (Geronimus, Hicken, Keene, & Bound, 2006). Nevertheless, when we began our research program, little if any longitudinal, prospective research had been conducted on the contributions of contextual factors to the development of health vulnerabilities among African American youths. To meet the need for such studies, we added health-relevant biomarkers to our research, embedding them into existing longitudinal cohorts of rural African American youths. We hoped this would allow us to determine whether contextual risks presage the development of health vulnerabilities among rural African American youths and young adults. We also wanted to learn whether supportive family environments could serve as countervailing buffers to deter the expression of contextual challenges in the biomarkers that we added to our study protocols.
Study 1: Can early signs of biological weathering be detected in African American adolescents?
The first study was designed to address two questions. First, do a significant percentage of African American youths show signs of biological weathering? Second, could we detect, during participants’ adolescence, a counterintuitive pattern in the health disparities literature in which African American middle-aged adults evince elevated levels of problems with physical health but unremarkable levels of psychological maladjustment (Kessler et al., 2005; Lantz et al., 2001; Mensah, Mokdad, Ford, Greenlund, & Croft, 2005)? Answering these questions required us to see whether we could identify a substantial number of African American youths who were exposed to high levels of economic hardship and cumulative SES risks, evinced relatively high levels of biological weathering, and experienced few problems with psychological adjustment.
We tested the study questions with a representative sample of 443 African American youths living in the rural South. Parents assessed cumulative SES risk at ages 11 through 13 years; psychological adjustment (drug use, delinquent behavior, depression, and externalizing behavior) was assessed by youths and their mothers across ages 14 to 18 years; and allostatic load (AL) was assessed at age 20 years (Brody, Yu, Chen, et al., 2013). AL is thought to reflect physiological wear and tear that results from the body’s efforts to maintain homeostasis in response to stress (McEwen, 1998). Over time, this strain can manifest in the dysregulation of multiple physiological structures, including the cardiovascular, metabolic, autonomic, endocrine, and inflammatory systems (Seeman, Epel, Gruenewald, Karlamangla, & McEwen, 2010). AL composites based on these systems’ functioning are thought to indicate physical health risk because they have been shown to predict the onset of cardiovascular disease and all-cause mortality later in life (Karlamangla, Singer, & Seeman, 2006; Seeman, McEwen, Rowe, & Singer, 2001; Seeman, Singer, Rowe, Horwitz, & McEwen, 1997). Our protocol for measuring AL was based on procedures that Evans (2003) developed for field studies involving children and adolescents. This protocol included the measurement of overnight cortisol, epinephrine, and norepinephrine from urine voids; resting diastolic and systolic blood pressure; and body mass index (BMI). AL was calculated by summing the indicators on which each participant scored in the top quartile of risk; possible scores ranged from 0 to 6.
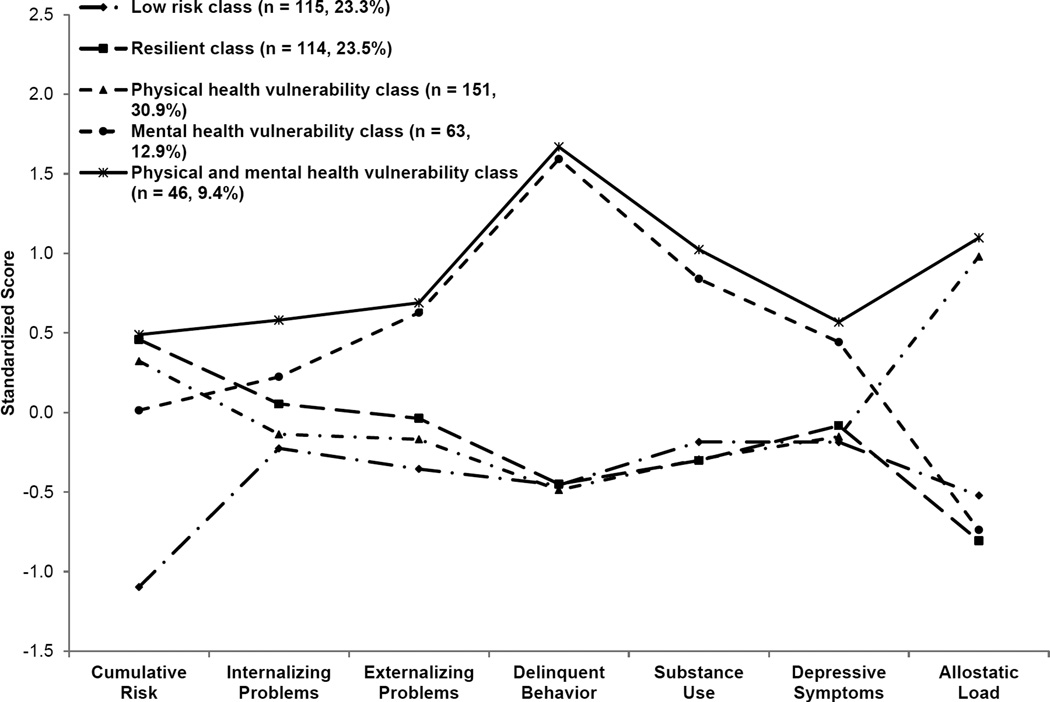
A latent profile analysis identified five profiles that are depicted in Figure 1. The first profile featured high levels of cumulative SES risk, low levels of adjustment problems, and high AL. On the basis of the health disparities literature and theoretical conceptualizations, we classified these youths into a physical health vulnerability profile. This classification included the largest number of youths (n = 151, 30.9%), supporting our hypothesis that a significant percentage of rural African American adolescents would display characteristics described in the adult health disparities literature. Compared with those in the first profile, youths in a second profile experienced equally high exposure to cumulative SES risk and similarly low levels of adjustment problems across adolescence, along with substantially lower (> 4 SD) AL. On the basis of their exposure to high cumulative risk coupled with low levels of adjustment problems and low AL, we classified these youths into a resilient profile (n = 115, 23.5%). The third profile, the physical and mental health vulnerability group, was the smallest (n = 46, 9.4%). These youths displayed high levels of both physical and mental health vulnerability, having been exposed to high cumulative SES risk and demonstrating high levels of adjustment problems and high AL. Youths in this profile were exposed to similar levels of cumulative SES risks as were youths in the physical health vulnerability and resilient profiles, yet they evinced much higher levels of adjustment problems across adolescence than did those in either of the other profiles. Their AL was substantially higher than that of youths in the resilient profile and indistinguishable from the high AL characterizing youths in the physical health vulnerability profile. Youths in the fourth profile were exposed to moderate levels of SES risk coupled with high adjustment problems and low AL. These youths were classified into a mental health vulnerability profile (n = 63, 12.9%). The fifth and final profile was characterized by relatively low levels of exposure to cumulative SES risk, low levels of adjustment problems across adolescence, and low AL. These youths were classified into a low-risk profile (n = 114, 23.3%). Youths in the low-risk profile evinced levels of adjustment problems and AL that were indistinguishable from those of youths in the resilient profile. Youths in the physical health vulnerability profile evinced low levels of adjustment problems indistinguishable from those in the low-risk profile but substantially higher levels of AL (> 2 SD) than did low-risk youths.
Figure 1.
Latent profile membership standardized scores for the study sample. Adapted from “Cumulative Socioeconomic Status Risk, Allostatic Load, and Adjustment: A Prospective Latent Profile Analysis with Contextual and Genetic Protective Factors,” by G. H. Brody, T. Yu, Y. Chen, et al., 2013, Developmental Psychology, 49, p. 921. Copyright 2012 by the American Psychological Association. Adapted with permission.
The results confirmed our conjecture that we could identify empirically two theoretically important focal profiles: a physical health vulnerability profile and a resilient profile. Together, these two profiles accounted for 54.4% of a representative sample of rural African Americans. These findings were consistent with propositions that poor health and health disparities during adulthood are tied to experiences earlier in life, particularly for persons growing up with the stressors associated with low SES (Shonkoff et al., 2009). The findings were also consistent with resilience theory (Luthar, 2006), demonstrating that not all rural African American youths exposed to cumulative SES risks will develop physiological dysregulation.
Study 2: The Great Recession and health risks in African American adolescents: A natural experiment
One reason that statistical associations between a risk factor and an outcome cannot be assumed to reflect environmentally mediated causation is that it is not uncommon for a risk factor to be associated in some way with an individual’s behavior. This rival hypothesis can be eliminated when circumstances beyond the individual’s control create the environmental risk factor. One such circumstance was the Great Recession. In December 2007, the United States officially entered a recession. Starting in the autumn of 2008 and continuing into 2009, the recession intensified, with a dramatic collapse of financial markets and an equally dramatic increase in mass layoffs (Council of Economic Advisors, 2010). The annual unemployment rate increased from 5% in December 2007 to 10% in October 2010. Although the recession was officially over in June 2009, its effects were long lasting and are still being felt in households across the nation.
In the second proof-of-principle study, we took advantage of the Great Recession as an externally occurring macro-economic event that occurred while we were conducting an ongoing longitudinal study of African American youths (Chen, Miller, Yu, & Brody, 2015). Families’ economic circumstances were assessed repeatedly, allowing us to identify prospectively the families who experienced economic decline during the recession and the families who did not. We investigated associations of macro-economic conditions with cellular epigenetic aging (described later), AL, and self-reported health. A sample of 330 African American adolescents in Georgia was followed from pre-recession (2007, M age = 16.6 years) to post-recession (2010, M age = 19.3 years). Three groups were formed to represent family economic trajectories across the period of the Great Recession (stable low economic hardship, downward mobility, and stable high economic hardship). At age 19, measures of cellular epigenetic aging, AL (to which a measure of C-reactive protein, an indicator of systemic inflammation, was added), and youths’ self-reports of health were obtained.
To measure cellular aging, we used a recently developed biomarker derived from the DNA methylation pattern of immune system cells. Modifications in DNA that do not involve changes in the DNA nucleotide sequence are termed epigenetic processes. Methylation is an epigenetic process whereby methyl groups bind to the cytosine residues that comprise DNA; in so doing, the methyl groups alter the cells’ ability to activate particular genes. On the basis of the notion that methylation reflects, in part, the amount of turmoil to which a cell has been exposed, researchers have used methylation to assess cellular aging. This marker has been validated in cells from diverse tissues and reflects the disparity between an individual’s biological and chronological ages (Hannum et al., 2013). The concept of cellular age is closely related to, but not isomorphic with, chronological age. Indeed, research shows that some individuals show more rapid cellular aging than would be expected on the basis of their chronological ages, whereas others show the reverse. Faster epigenetic aging has been documented in tumor-derived cells from more than 20 cancers, as well as in liver biopsies from obese patients (Horvath et al., 2014). Epigenetic aging has also been studied in peripheral blood mononuclear cells (PBMC). Children with more “aged” cells have heightened blood pressure (Simpkin et al., in press), and adults with more aged cells experience higher rates of all-cause mortality (Marioni et al., 2015).
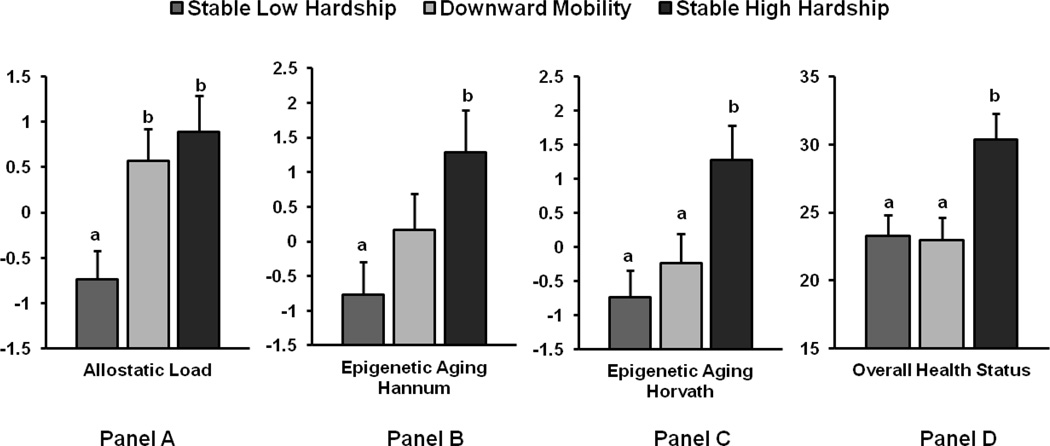
The results for AL, epigenetic aging, and self-reported health are presented in Figure 2. Two different metrics of epigenetic aging, one derived by Horvath et al. (2014) and the other by Hannum et al. (2013), are presented in Figure 2. Both metrics index methylation-based profiles of aging; the number of CPG sites on the genome that are included in their matrices differ (353 sites for Horvath and 71 sites for Hannum). As Figure 2 indicates, the more time adolescents spent in economic hardship (two time points for the high economic hardship group and one time point for the downward mobility group), the higher their epigenetic aging and AL, and the worse their self-reported health. Specific group comparisons revealed that adolescents in the downward mobility group had higher AL then did those in the stable low hardship group. This suggests that the downward mobility group resembled the high economic hardship group on AL but the low economic hardship group on self-reported health and epigenetic aging. Thus, the experience of economic decline may have its most immediate effects on AL. This was the first study of which we were aware to focus on biological mechanisms, including cellular epigenetic aging and AL, among African American adolescents in the context of changing macro-economic conditions. This allowed us to demonstrate that societal-level economic conditions may be related to immune and physiological mechanisms, with implications for later health.
Figure 2.
Differences in Adolescent Outcomes by Economic Trajectory Across the Great Recession. Means of allostatic load (Panel A), epigenetic aging using the Hannum method (Panel B), epigenetic aging using the Horvath method (Panel C), and overall health status (Panel D; higher numbers indicate poorer health) by family economic hardship groups; n = 131 for stable low hardship group, 105 for downward mobility group, and 82 for stable high hardship group. Significant differences are indicated by difference in letters a and b (p < .05). Error bars = +1 standard error. Adapted from “The Great Recession and Health Risks in African American Youth,” by E. Chen, G. E. Miller, T. Yu, and G. H. Brody, 2015, Brain, Behavior, and Immunity, DOI:10.1016/j.bbi.2015.12.015. Copyright 2015 by Elsevier Inc. Adapted with permission.
Study 3: Does living in a neighborhood in which poverty levels increase across time forecast AL?
Our third proof-of-principle study focused on forecasting AL across time from the changing characteristics of neighborhoods in which rural African American youths lived. A hypothesis proposed in the pediatric health disparities literature concerns the possibility that living in a high-poverty neighborhood during childhood, adolescence, or both will engender heightened levels of AL in youths, with downstream health consequences (Shonkoff et al., 2009). In addition, little was known when we did this research about the health of youths, African American or not, who experienced changes within their neighborhoods. Neighborhood socioeconomic conditions shift over time as a consequence of broader macro-economic upheavals. Even without moving out of a neighborhood, many African American children’s residential areas can become progressively lower in SES over time (Braveman & Egerter, 2008). A primary aim of this proof-of-principle study was to determine the effects that changes in neighborhood poverty levels, net of continuities and changes in family financial circumstances, had on rural African American youths’ AL in a cohort followed from ages 11 to 19 years (Brody, Lei, Chen, & Miller, 2014).
This study also addressed another important question: Why can some youths and adults maintain physically healthy profiles despite living in difficult circumstances—that is, what makes them resilient (Luthar, 2006)? Research involving children and adolescents suggests that receipt of emotional support from parents (Chen & Miller, 2012; Chen, Miller, Kobor, & Cole, 2011; Evans, Kim, Ting, Tesher, & Shannis, 2007) and peers (Adams, Santo, & Bukowski, 2012; Brody, Yu, Chen, Kogan, et al., 2013) can offset some of the neuroendocrine, metabolic, inflammatory, and cardiovascular risks that often develop after exposure to adversity (Miller, Chen, et al., 2011; Shonkoff et al., 2009). On the basis of these findings, we explored the possibility that emotional support would have a protective effect by reducing the impact of increases in neighborhood poverty on AL during adolescence.
To address these questions, data on neighborhood concentrations of poverty were obtained from the United States Census Bureau for 420 African American youths living in rural Georgia when they were 11 and 19 years of age. AL was measured at age 19, using our protocol for adolescents (Brody, Yu, Chen, Kogan, et al., 2013; Evans, 2003). When youths were 18, caregivers reported parental emotional support, and youths assessed peer and mentor emotional support. Covariates included family poverty status at ages 11 and 19, their interaction, family financial stress, parental employment status, and youths’ unhealthful behaviors.
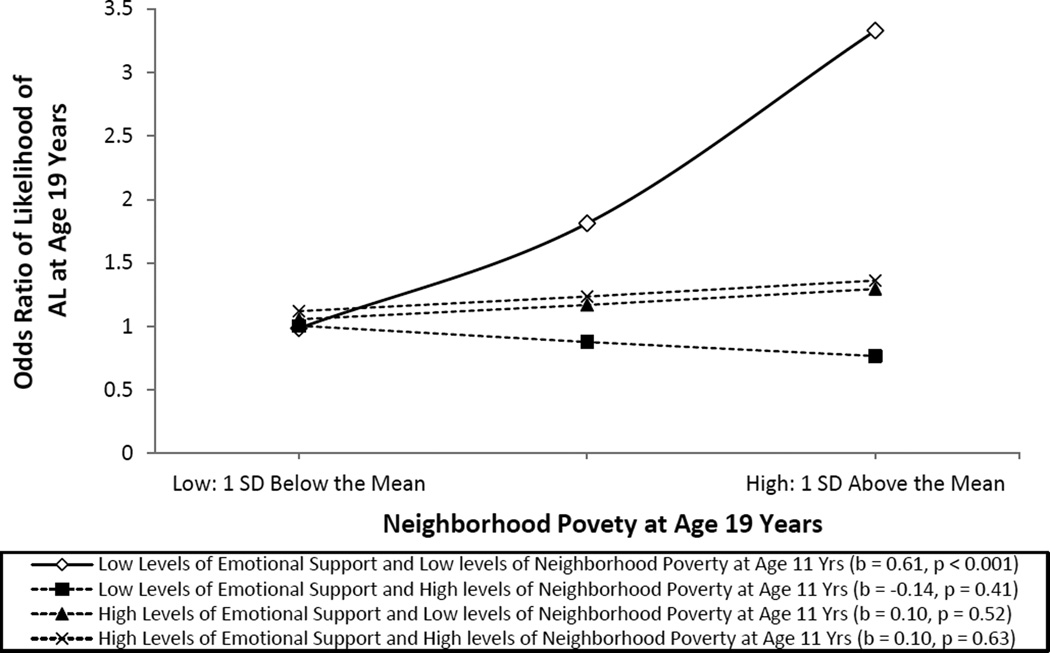
The results revealed that youths who lived in neighborhoods in which poverty levels increased from ages 11 to 19 evinced the highest levels of AL, even after accounting for individual-level covariates. The results also demonstrated the protective effects of emotional support from others in the adolescents’ family and peer networks on ameliorating neighborhood effects on AL. These results are depicted in Figure 3. Taken together, this study added to our proof-of-principle arsenal by illustrating how changes in neighborhood contexts contribute to biological adaptations that may render some youths more vulnerable to CDAs. AL was higher when the neighborhoods in which youths lived grew progressively poorer over time. These findings remained robust after controlling for change over time in family poverty and economic hardship. This association did not emerge, however, when youths received high levels of emotional support, underscoring the importance of relational support to youths’ stress reactivity and health.
Figure 3.
The effect of neighborhood poverty at age 19 years on predicted AL by level of neighborhood poverty at age 11 years and emotional support assessed by using a multilevel Poisson regression model. The analysis controlled for family poverty, gender, diet, smoking, binge drinking, perceived stress, unemployment, and financial stress. The lines represent the regression lines for different levels of neighborhood poverty (low: 1 SD below the mean; high: 1 SD above the mean) and emotional support (low: 1 SD below the mean; high: 1 SD above the mean). Numbers in parentheses refer to simple slope for each group. Adapted from “Neighborhood Poverty and Allostatic Load in African American Youth,” by G. H. Brody, M. K. Lei, E. Chen and G. E. Miller, 2014, Pediatrics, 134, p. e1367. Copyright 2014 by the American Academy of Pediatrics. Adapted with permission.
Studies 4 and 5: The biological weathering effects of racial discrimination and the protective benefits of supportive family environments
The fourth and fifth proof-of-principle studies were designed to test a hypothesis that had been proposed in the health disparities literature but had not yet been examined empirically: Exposure to racial discrimination during childhood and adolescence will have negative effects on the functioning of biological stress regulatory systems and, ultimately, on health later in life (Geronimus et al., 2006; Shonkoff et al., 2009). This hypothesis was based on the premise that the health inequities that African Americans experience arise from more than class disadvantage. Racial discrimination presents daily challenges in the lives of many African American youths and their families (D. R. Williams & Mohammed, 2013). These incidents are demeaning and induce stress, frustration, depression, and anxiety in adults (Pascoe & Smart Richman, 2009) and in adolescents (Priest et al., 2013).
Associations have been found between racial discrimination and health-relevant physiological outcomes. Research conducted primarily with adults has demonstrated that discrimination is associated with a range of health-relevant biomarkers, including neuroendocrine risk markers for poor birth outcomes, elevated glucocorticoids, pro-inflammatory cytokines, C-reactive protein, and other markers of inflammation (Brondolo et al., 2008; Lewis, Cogburn, & Williams, 2015; Pascoe & Smart Richman, 2009). Together, these studies supported our examinations of the biological weathering effects of exposure to racial discrimination during adolescence, a developmental stage in which African American youths become keenly aware of their treatment by others and are particularly cognizant of targeted rejection (Stevenson, 2004).
Our approach to racial discrimination effects focused on exposure to it across adolescence. A review of the research literature led us to believe that African American adolescents’ encounters with racial discrimination would best be characterized by qualitatively different subgroups, with many youths encountering low and increasing exposures to racial discrimination and others encountering consistently frequent exposure (Brody, Miller, Yu, Beach, & Chen, in press). On the basis of the heterogeneity reported in the literature, we hypothesized that adolescents who encountered qualitatively more interpersonal discrimination across time would be at greater risk for biological weathering. This hypothesis was based on the premise that exposure to frequent discrimination across time leads to frequent activations of stress response systems, the hormonal end products of which (cortisol, epinephrine, norepinephrine) contribute to the weathering process and to poor health later in life (Miller, Chen, et al., 2011).
Despite exposure to high levels of discrimination, many rural African Americans will not experience biological weathering. We expected supportive family environments during adolescence to prevent racial discrimination from getting under the skin. For rural African American youths, supportive family environments promote psychological resilience to cumulative contextual stressors (Brody, Kogan, et al., 2012). Recent reports also suggest that family support can buffer metabolic, inflammatory, and cardiovascular reactions that children develop after experiencing adversity. In particular, family support buffers the effects of childhood maltreatment on AL (Carroll et al., 2013), of low childhood SES on adult genomic pro-inflammatory signaling profiles (Chen, Miller, et al., 2011), and on metabolic profiles (Miller, Lachman, et al., 2011). These findings led us to predict that supportive family environments would reduce the impact of exposure to high levels of racial discrimination on biological weathering.
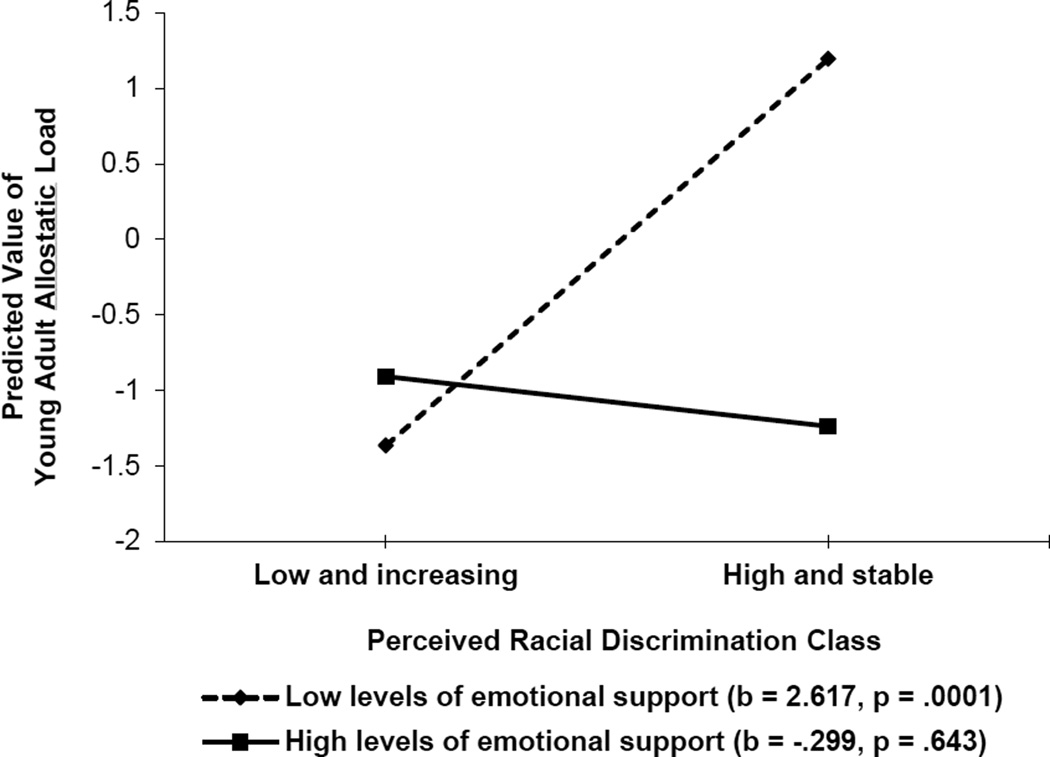
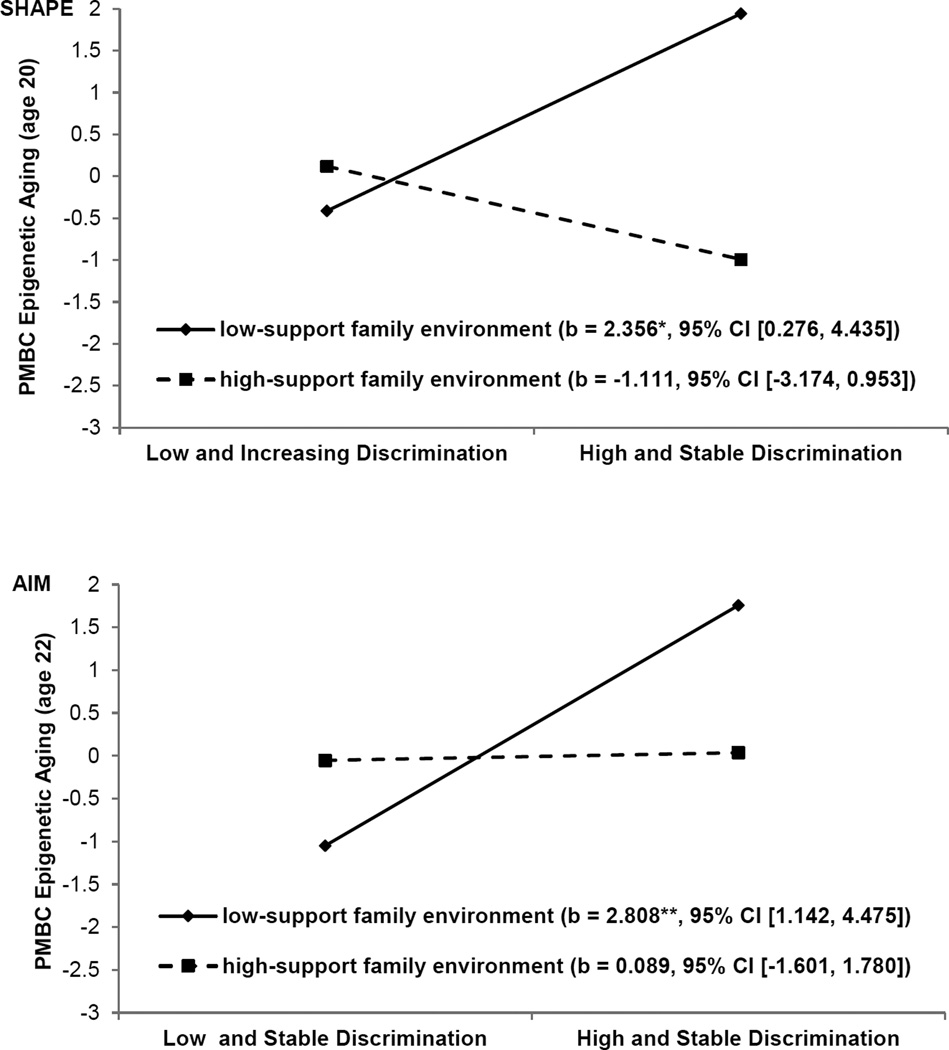
We conducted two studies to test the hypothesis that supportive family environments would reduce the likelihood of biological weathering among youths who frequently encounter discrimination. In the first study, conducted with a sample of 331 African Americans, we obtained assessments of racial discrimination from ages 16 to 18 years. When youths were 18 years of age, caregivers reported parental emotional support (Brody, Lei, Chae, et al., 2014). AL and potential confounder variables were assessed when youths were 20 years of age. Latent growth mixture models identified two classes of exposure to discrimination across adolescence: high and stable, and low and increasing. A main effect of exposure to racial discrimination and an interaction between exposure to discrimination and receipt of family support emerged even with confounder variables controlled. As expected, the prospective racial discrimination to AL link was not significant for young adults who received high levels of family emotional support. These findings are depicted in Figure 4.
Figure 4.
The effect of perceived racial discrimination on young adults’ allostatic load by level of emotional support. The lines represent the regression lines for different levels of emotional support (low: 1 SD below the mean; high: 1 SD above the mean). Numbers in parentheses refer to simple slopes. Adapted from “Perceived Discrimination Among African American Adolescents and Allostatic Load: A Longitudinal Analysis With Buffering Effects,” by G. H. Brody, M. K. Lei, D. H. Chae, T. Yu, S. M. Kogan, and S. R. H. Beach, 2014, Child Development, 85, p. 997. Copyright 2014 by the authors.
The second study was designed to extend the buffering effects hypothesis in two ways. First, the prospective contribution of racial discrimination to epigenetic aging was examined as an indicator of weathering. Second, two independent longitudinal samples were studied, allowing us to determine whether our findings could be replicated (Brody et al., in press). Participants in the Strong African American Healthy Adult Project (SHAPE; N = 322) and the Adults in the Making project (AIM; N = 294) took part in this study. Racial discrimination and supportive family environments, along with confounder variables, were assessed when youths in SHAPE were 16 to 18 years of age and those in AIM were ages 17 to 19 years. Participants in each cohort provided antecubital blood samples (at age 20 in SHAPE and age 22 in AIM) that were used to assess epigenetic aging. Gender, cumulative SES risk, perceived life stress, depressive symptoms, and BMI were controlled in the analyses. The results are illustrated in Figure 5. Among youths in supportive family environments, exposure to high levels of racial discrimination did not forecast epigenetic aging. Among youths in less supportive family environments, exposure to high levels of racial discrimination forecast accelerated epigenetic aging. The results did not change when confounder variables were included in the data analyses, and the results were replicated across cohorts. Taken together, the results of both studies support the hypothesis that exposure to racial discrimination during early stages of development, particularly for youths without countervailing protective resources, can weather physiological systems, resulting in the premature aging of cells and perhaps a shorter life expectancy.
Figure 5.
The effect of racial discrimination on youths’ epigenetic aging by supportive family environments for SHAPE (top) and AIM (bottom). The lines represent the regression lines for different levels of support from the family environment (low: 1 SD below the mean; high: 1 SD above the mean). Numbers in parentheses refer to simple slopes. For the outcome, higher values reflect immune cells that are epigenetically older than expected based on chronological age. Adapted from “Supportive Family Environments Ameliorates the Link Between Racial Discrimination and Epigenetic Aging: A Replication Across Two Longitudinal Cohorts,” by G. H. Brody, G. E. Miller, T. Yu, S. R. Beach, and E. Chen, in press, Psychological Science. Copyright 2016 by the authors.
What is the “take-away” message of the proof-of-principal studies?
The proof-of-principal studies were based on research suggesting that children and youths who experience chronic stressors are vulnerable to a wide range of health problems across the lifespan (Miller, Chen, et al., 2011). They show heightened prevalence of CDAs such as metabolic syndrome, coronary heart disease, some cancers, and autoimmune conditions as they age (Shonkoff et al., 2009). The proof-of-principal studies support the hypothesis that changes in biological processes and the weathering of young bodies that foreshadow CDAs are presaged by economic hardship in families and neighborhoods, and by race-related stressors. This work is also consistent with extant literature that has identified supportive family processes that protect youths exposed to adversity from developing markers of biological weathering, such as AL and epigenetic aging. We recognize that these results are derived from longitudinal studies that cannot, by themselves, distinguish among causes, effects, and confounds. The results, however, converge with animal experiments that document long-term health consequences of early stress exposure (Avitsur, Hunzeker, & Sheridan, 2006; Lupien, McEwen, Gunnar, & Heim, 2009). Considered together, the proof-of-principal studies suggest that the economic hardship and race-related stressors with which some children and adolescents contend contribute causally to health problems later in life.
Is Resilience Only Skin Deep? Can Striving for Success Cost African American Young Adults Their Health?
The resilience literature is characterized by a widespread assumption that, if children and youths who faced major adversities are doing well as indexed by external behaviors—for example, if they excel academically and evince markers of psychosocial adjustment—they have successfully negotiated those adversities. This is true for many African American children, youths, and young adults whom we have been following in our research program. They do so in part by cultivating a kind of determined persistence similar to the high-effort coping strategy termed “John Henryism” (James, 1994). Often, with nurturing from a parent, relative, or mentor, they set goals for the future, work diligently toward them, navigate setbacks, stay focused on the long term, and resist temptations that might deter them from reaching their goals.
We began studying these resilient young people to find out if their self-control, planfulness, success at school, and determination to succeed also translated into physical health benefits. We reasoned that, if youths from rural low-SES backgrounds were succeeding academically and emotionally, they might also be protected from health problems that were more common in lower-income youths. We found, however, the exact opposite to be true. These young people were achieving success by all conventional markers: doing well academically, staying out of trouble, making friends, and developing a positive sense of self. Underneath, however, their physical health was deteriorating. Next, we describe the studies that led us to this conclusion.
Psychosocial Competence and Self-Control as a “Double-Edged Sword” for Physical Health among Low-SES Rural African American Youths
Our first hints of this pattern came from a study of 489 rural African American youths in Georgia, whom we had been following for 15 years (Brody, Yu, Chen, Miller, et al., 2013). Most of them came from families who were working but poor. In 2010, their average family annual income was about $12,000; about half lived below the poverty line. We found a subgroup of resilient children who, despite these obstacles, were rated at age 11 by their teachers as being diligent, focused, planful, academically successful, and strong in social skills.
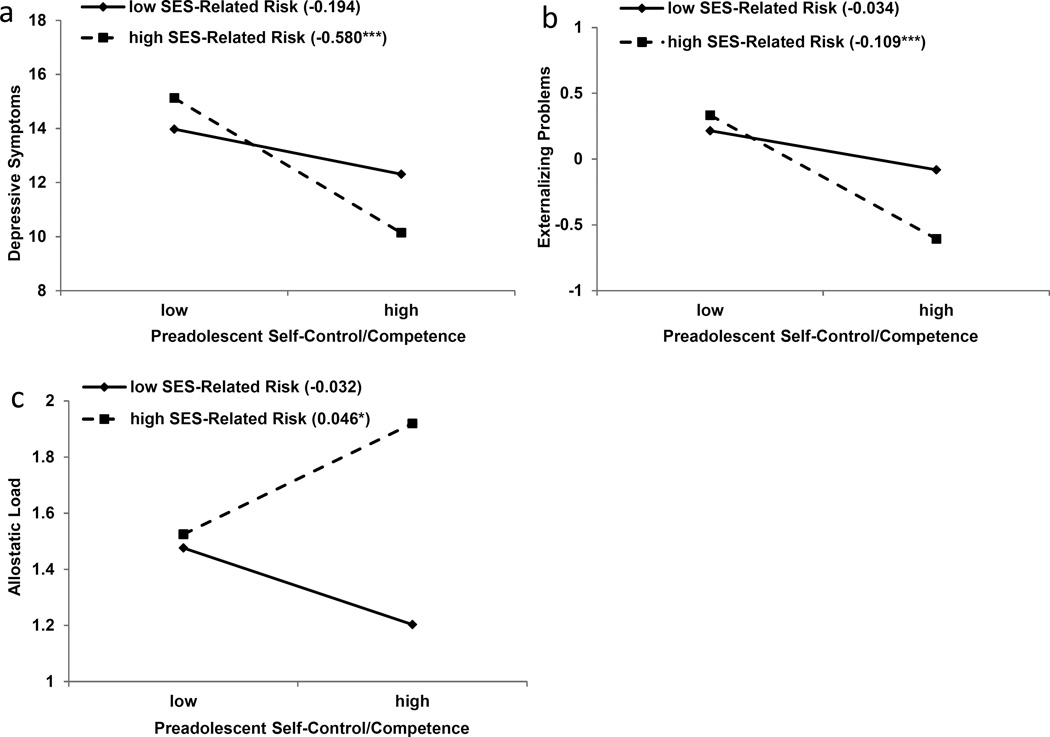
This sample was followed over time, and when the youths reached age 19 years, we assessed depression, externalizing behavior, and AL. As in past studies, those who were rated positively at age 11 by their teachers had relatively few problems with depression or externalizing behavior. When we looked beneath their skin at their AL levels, though, these apparently resilient young people were not faring well; they evinced higher AL levels compared with others in the study. Remarkably, their AL was even worse than peers who, at age 11, had been rated by their teachers as having poorer self-control and psychosocial competence. They were at substantial risk for developing diabetes and hypertension in the future. The results are depicted for depressive symptoms and externalizing behavior in Figure 6a and Figure 6b and for AL in Figure 6c.
Figure 6.
Young adults’ (a) depressive symptoms, (b) externalizing problems, and (c) allostatic load as a function of their socioeconomic status (SES)-related risk and self-control/competence in preadolescence (low = 1 SD below the mean; high = 1 SD above the mean). The lines represent the results of regression analyses at low and high levels of SES-related risk, and the numbers in parentheses refer to the simple slopes (***p < .001, *p < .05). Adapted from “Is Resilience Only Skin Deep? Rural African Americans’ Socioeconomic Status-Related Risk and Competence in Preadolescence and Psychological Adjustment and Allostatic Load at Age 19,” by G. H. Brody, T. Yu, E. Chen, G. E. Miller, S. M. Kogan, and S. R. H. Beach, 2013, Psychological Science, 24, p. 1290. Copyright 2013 by the authors.
Neighborhood poverty, college attendance, and skin-deep resilience
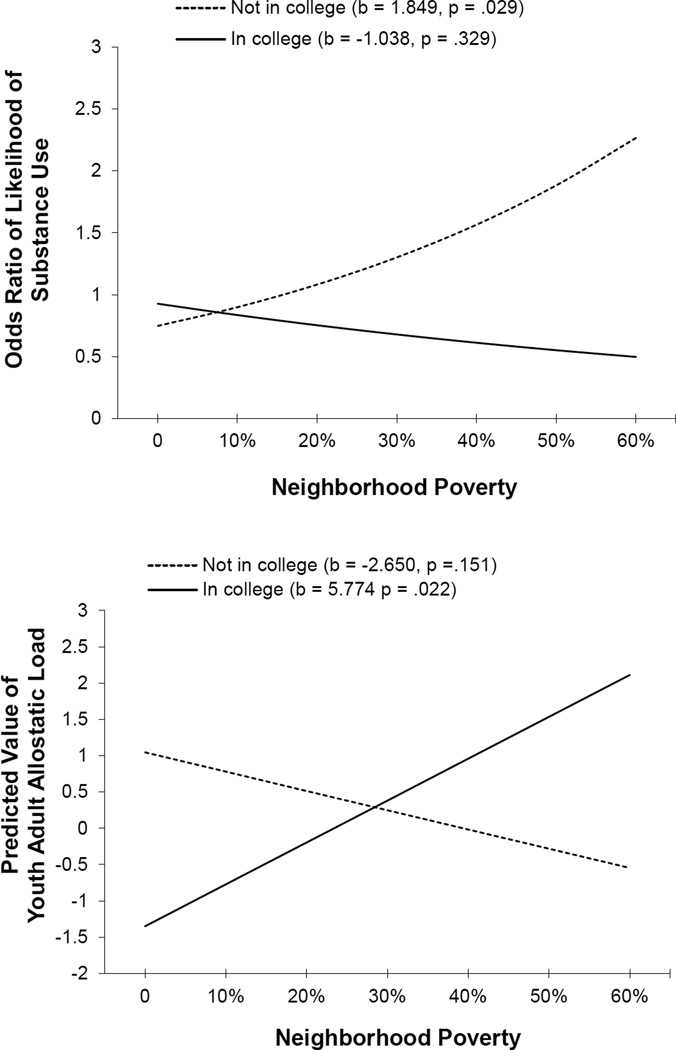
We continued studying the youths described in the preceding section as they transitioned into young adulthood. In this analysis, Chen et al. (2015) focused on a subgroup of participants who would normatively be viewed as resilient. These individuals maintained academic success—they had graduated from high school and were attending college—despite living in challenging neighborhoods with concentrated poverty. Compared with other participants, this cohort had lower rates of cigarette, alcohol, and marijuana use at 20 years of age. This resilience, however, was just skin deep. Despite their academic success and healthful lifestyles, these youths showed relatively poor health as indexed by higher levels of AL. Figure 7 depicts these results. These results and those of our first study in this research program were interpreted from the perspective of John Henryism (JH) theory (James, 1994). The JH construct takes its name from, and is inspired by, the legend of John Henry, “the steel-driving man.” According to the story (B. Williams, 1983), John Henry was an African American railroad worker in the late 1800s whose fame was derived from his participation in a steel driving contest in which he defeated a steam-powered drill. John Henry was forced to harness his great strength to overpower the mechanical drill, but afterward died of exhaustion. For James (1994), the fabled actions of John Henry illuminate associations among high-effort coping, chronic sympathetic nervous system (SNS) arousal, and health problems, including hypertension. The construct comprises three main characteristics: efficacious mental and physical vigor, a strong commitment to hard work, and a single-minded determination to succeed. According to this theory, many rural African Americans should resemble John Henry. While growing up in a high-risk context, they are highly goal oriented, hard-working, and focused persistently on success; they avoid behaviors that could derail their pursuit of success; and, eventually, they manifest indicators of physiological stress. These findings suggest that resilience is multidimensional; hidden indicators, such as AL, of compromised physiological health may accompany observable competence and positive adjustment. In such cases, resilience may indeed be only skin deep.
Figure 7.
Effect of neighborhood poverty on substance use (top) and allostatic load (bottom) by college attendance. Adapted from “Neighborhood Poverty, College Attendance, and Diverging Profiles of Substance Use and Allostatic Load in Rural African American Youth,” by E. Chen, G. E. Miller, G. H. Brody, and M. K. Lei, 2015, Clinical Psychological Science, 3, p. 681. Copyright 2014 by the authors.
Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youths
The development of self-control played a prominent role in the two studies just described. A growing research literature indicates that youths who exhibit greater self-control go on to perform better in school, earn higher salaries, remain more stably employed, and save more money. These youths are less likely to use drugs, be arrested for and convicted of crimes, and develop psychiatric disorders. In early adulthood, these youths also show better physical health (Moffitt et al., 2011). The research previously reported, however, added a caveat to the role of self-control for rural African American youths by showing that, despite academic success and healthful lifestyles, youths from a low SES background evinced relatively poor cardiometabolic health as reflected in obesity, high blood pressure, and stress hormones. These findings suggested that self-control may act as a double-edged sword, facilitating academic success and psychosocial adjustment while at the same time undermining cardiometabolic health.
To extend this research program, Miller, Yu, Chen, & Brody (2015) tested the hypothesis that the self-regulatory demands that are described in JH theory as being necessary for African American youths to attain normatively favorable life outcomes would prematurely age bodily tissues through a process known as weathering (Geronimus et al., 2006). This hypothesis was tested with a new sample of rural African American youths who were followed across the transition from adolescence to adulthood. To clarify the mechanisms by which skin-deep resilience develops, this study focused on the aging of immune system cells using an epigenetic biomarker derived from DNA methylation. This marker was described previously in the Chen et al. (2015) and Brody et al. (in press) proof-of-principal studies. A hypothesis was examined with 292 participants from the AIM study. From 17 to 20 years of age, we annually assessed SES, self-control, depressive symptoms, substance use, aggressive behaviors, and internalizing problems. At age 22 years, we obtained DNA methylation profiles of PBMC. These data were used to measure epigenetic aging, a methylation-derived biomarker reflecting the disparity between biological and chronological aging.
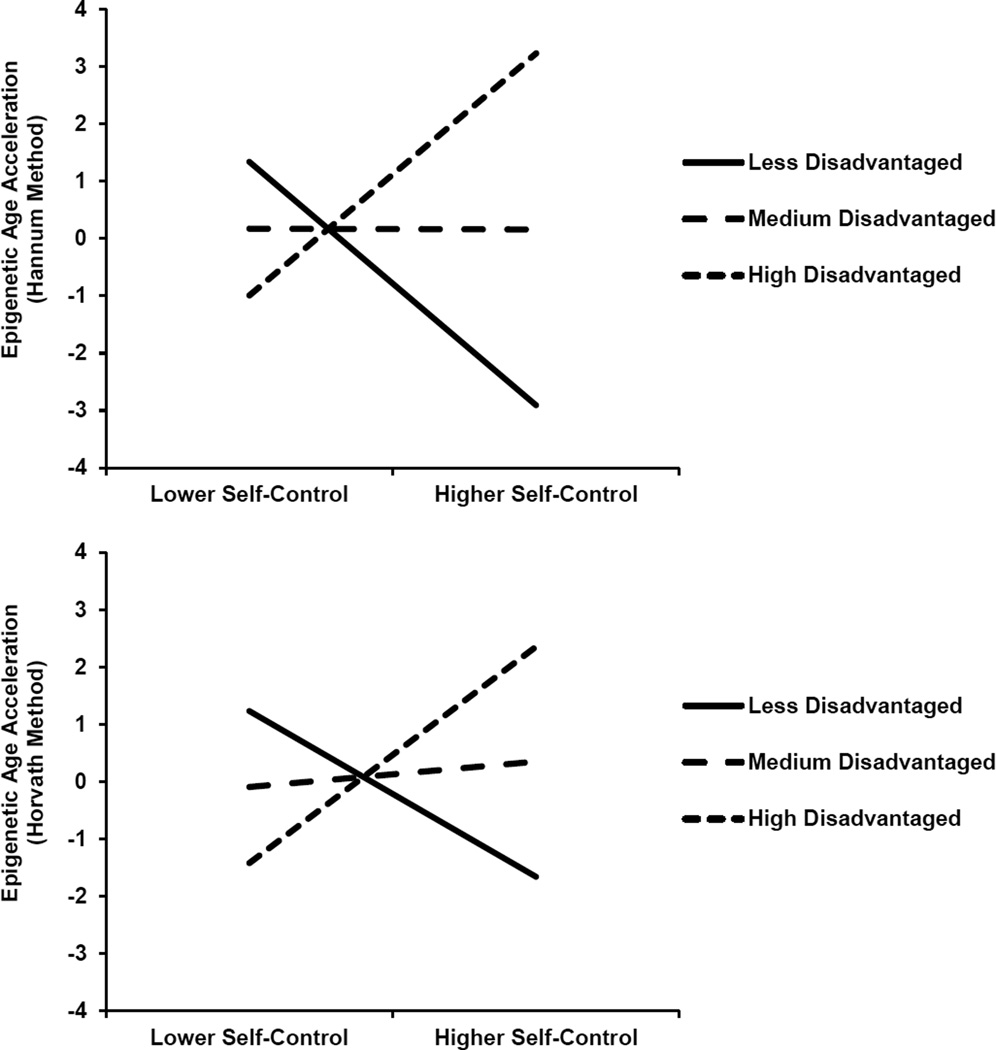
Among relatively higher SES youths, better mid-adolescent self-control presaged favorable psychological and epigenetic aging outcomes. Among low-SES youths, however, self-control displayed divergent associations with these outcomes. Self-control forecast lower rates of depressive symptoms and other internalizing problems, substance use, and aggressive behavior, but faster epigenetic aging. The results for epigenetic aging are presented in Figure 8. These patterns again suggest that, for low-SES youths, resilience is a skin-deep phenomenon wherein outward indicators of success can mask emerging physical health problems. The patterns in this study are consistent with evidence indicating that relentlessly pursuing goals can detract from physical health (Wrosch, Scheier, & Miller, 2013), particularly when structural forces impede progress toward those goals. As rural African Americans strive for favorable life outcomes, they have substantial barriers to overcome and competing demands to balance, including resource-deprived schools, family obligations, and both interpersonal and structural discrimination (Johnson, Richeson, & Finkel, 2011). Navigating these challenges requires intense and persistent self-control (Vohs 2013), which is demanding to maintain and, as our studies show, forecast health costs in the forms of cardiometabolic risks and rapid cell aging.
Figure 8.
Self-control’s association with epigenetic age acceleration varies according to SES. Depiction of estimated Hannum values (top) and Horvath values (bottom) at lower (−1.5 SD) and higher (+1.5 SD) levels of self-control and socioeconomic disadvantage. Adapted from “Self-Control Forecasts Better Psychosocial Outcomes But Faster Epigenetic Aging in Low-SES Youth,” by G. E. Miller, T. Yu, E. Chen, and G. H. Brody, 2015, Proceedings of the National Academy of Sciences of the United States of America, 112, pp. 10326–10327. Copyright 2015 by the authors.
What can be done to mitigate these negative health effects? To begin, schools and colleges that serve lower-income African American students could provide health education, screenings, and check-ups as a part of their curricula and student services programs. This would allow the detection and correction of incipient health problems before they become serious. Second, prevention programs could be developed to help young people learn ways of coping other than doubling down on their self-regulatory strengths and becoming even more determined to succeed. One such coping style, “shift and persist,” could be encouraged. “Shifting” entails accepting one’s life circumstances and adapting to them, and “persisting” involves enduring those circumstances by holding on to meaning and optimism. Together, these strategies mitigate the health impact of stressors that many low-SES youths face. Research shows that low-SES youths who use shift and persist strategies have profiles of low inflammation similar to those of their high-SES peers (Chen, Lee, Cavey, & Ho, 2013; Chen, Strunk, et al., 2011). Other prevention approaches could equip college-bound youths with (a) cognitive behavioral management skills to reduce negative and increase positive perseverative cognitions (Antoni et al., 2001), (b) mindfulness training that can help youths to focus on the present and reduce ruminative stress-generating cognitions (Bishop, 2002), and (c) social networks of college-aged and older mentors who have navigated similar life challenges and can offer “real-world” guidance and support. Mentors and other trusted confidants could help temper feelings of social isolation and provide ways to cope with and address both explicit and subtle racial discrimination.
The Health Benefits of Family-Centered Drug Use Prevention Programs
To date, we have focused particularly on resilience in our research program. Our proof-of-principal studies demonstrated that, even in populations who experience high levels of SES risk or race-related stressors, only a minority experience indicators of biological weathering. Resources such as supportive family environments functioned as stress buffers for these youths. To increase confidence in our findings that supportive relationships offset biological weathering associated with SES and race-related stressors, we conducted secondary analyses of two randomized prevention trials. Both trials were designed to enhance parenting and strengthen family relationships among rural African American preadolescents and young adults. The Strong African American Families (SAAF) program (Brody et al., 2004) was designed to enhance parenting, strengthen family relationships, foster youth competencies, and deter drug use among rural African American preadolescents. Its efficacy in enhancing protective parenting processes and deterring drug use has been well documented (Brody, Kogan, et al., 2012). The second prevention trial was the AIM project (Brody, Yu, Chen, Kogan, & Smith, 2012). AIM has been shown to enhance family support and deter drug use and the problems it brings, particularly among young adults confronting high levels of contextual risks (Brody, Yu, Beach, & Philibert, 2015; Brody, Yu, et al., 2012). To test the potential health benefits of these prevention programs, we collected biomarkers from youths who took part in these trials to determine whether participation in these family-centered interventions also reduced indicators of biological weathering.
SAAF and reductions in inflammation
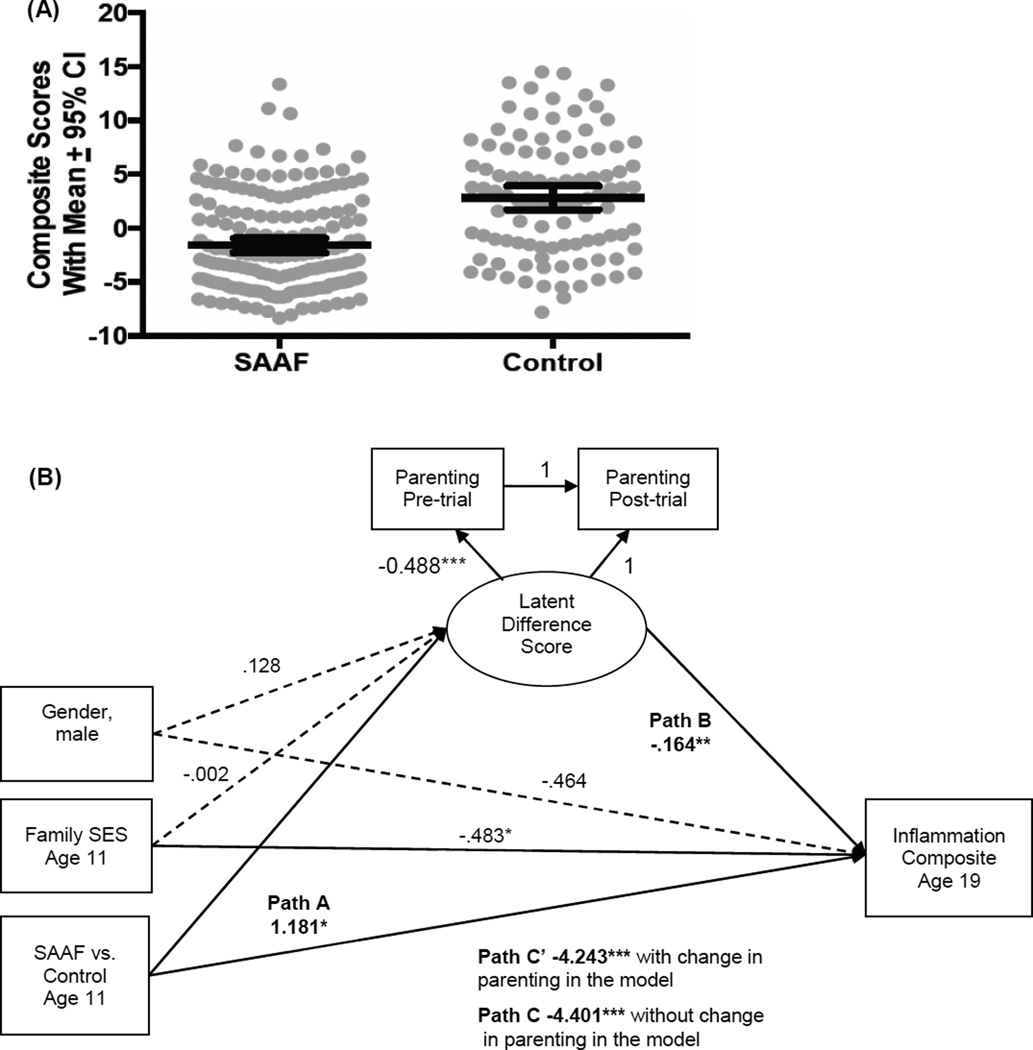
In this analysis, we sought to determine whether participation in SAAF at age 11 could reduce markers of low-grade inflammation measured when the participants were 19 years of age (Miller, Brody, Yu, & Chen, 2014). Low-grade inflammation has clinical significance for African Americans because it is a common pathogenic mechanism in the progression of cardiovascular disease, diabetes, stroke, and hypertension, all of which are highly prevalent among African Americans residing in the Black Belt. When youths reached age 20 years, peripheral blood was collected to measure six cytokines that orchestrate inflammation. Youths who participated in SAAF had significantly less inflammation on all six cytokine indicators than did members of the control group. These results are depicted in Figure 9A. We also tested the hypothesis that SAAF-induced increases in nurturant parenting, combined with decreases in harsh parenting, would account for the intervention’s effects on cytokines. Mediational analyses, presented in Figure 9B, suggested that improved parenting was partially responsible for SAAF’s benefits. Inflammation was lowest among youths who, as a consequence of the intervention, received more nurturant-involved parenting and less harsh-inconsistent parenting. This study provided quasi-experimental evidence that a family-centered intervention can reduce inflammation in youths, in part by improving the quality of the parenting they receive. To the extent that they are substantiated in future research, these results may provide another strategy for combating racial and social disparities.
Figure 9.
(A): Youth whose families participated in SAAF had less inflammation than did controls. The endpoint is a composite indicator of inflammation, formed by summing each subject’s z-scored values for interleukins-1β, 6, 8, and 10, plus tumor necrosis factor-α and IFN-γ. Dots represent individual data points. Within each group, the wide horizontal bar is the mean composite score, and the error bars reflect 95% confidence intervals. (B): Results are consistent with the hypothesis that SAAF’s ability to reduce inflammation is partially attributable to improved parenting. The figure shows results from a mediation model with latent difference scores. Solid and dashed lines reflect significant and nonsignificant paths, respectively. Unstandardized coefficients are shown. *p < 0.05; **p < 0.01; ***p < 0.001. Adapted from “A Family-Oriented Psychosocial Intervention Reduces Inflammation in Low-SES African American Youth,” by G. E. Miller, G. H. Brody, T. Yu, and E. Chen, 2014, Proceedings of the National Academy of Sciences of the United States of America, 111, pp. 11289–11290. Copyright 2014 by the authors.
SAAF ameliorates the longitudinal association between risky family processes and epigenetic aging
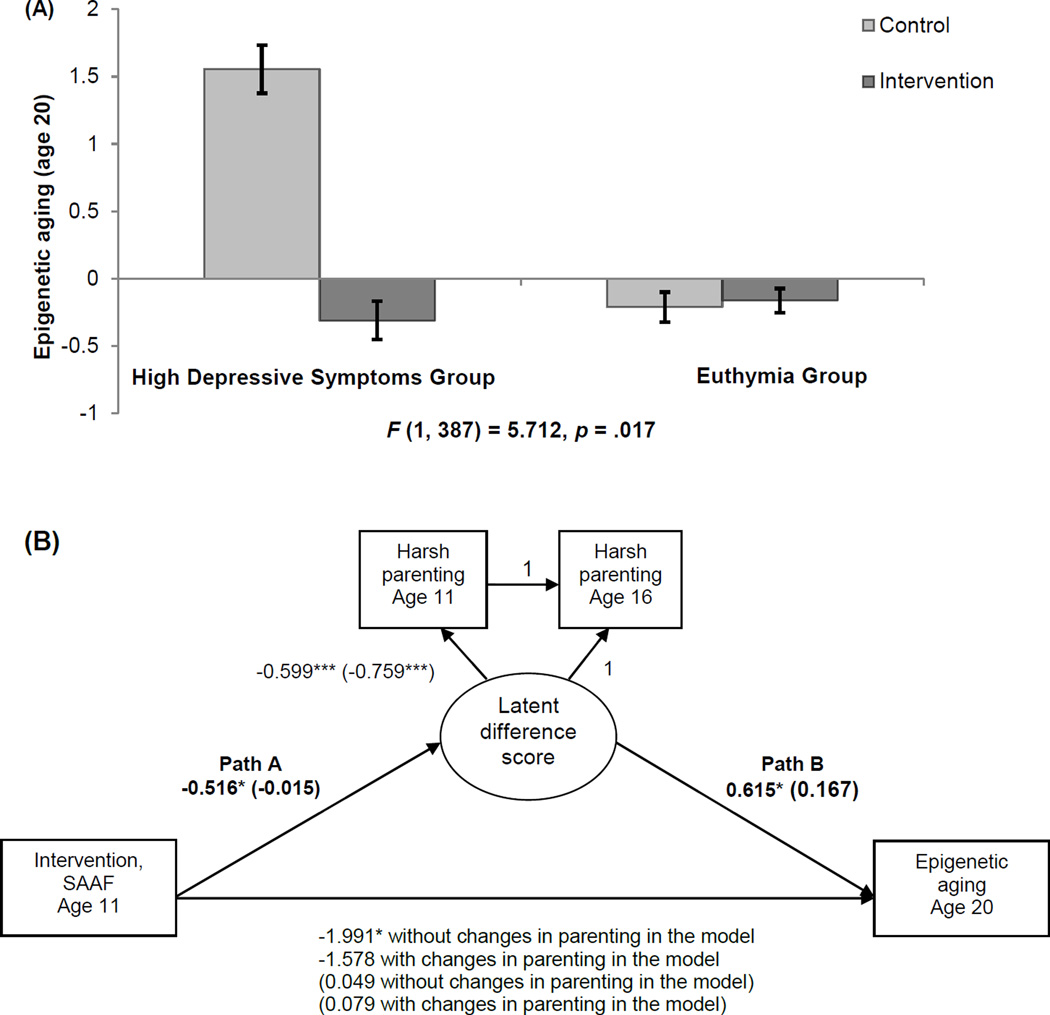
A growing body of research hints at the possibility that emotionally cold and harsh interactions with one’s parents during childhood contribute not only to psychosocial and psychiatric problems but also to vulnerability to chronic diseases later in life (Miller, Chen, et al., 2011). The risky family model (Repetti, Taylor, & Seeman, 2002) posits that home environments headed by a primary caregiver who displays elevated levels of depressive symptoms and uses harsh parenting practices triggers a cascade of psychological and biological vulnerabilities that can weather physiological systems, resulting in premature aging of cells and a shortened life expectancy (Geronimus et al., 2006). This study was designed to further understanding of the ways in which risky family processes generate negative consequences for health. We tested three hypotheses: (a) elevated levels of parental depressive symptoms when children are 11 years of age will forecast accelerated epigenetic aging 9 years later at age 20; (b) participation in SAAF will ameliorate the association between parental depression and epigenetic aging; and (c) a moderation-mediation hypothesis will show that prevention-induced reductions in harsh parenting will account for SAAF effects in reducing accelerated epigenetic aging for youths living with a parent who reports elevated depressive symptoms (Brody, Yu, Chen, Beach, & Miller, 2015). To test these hypotheses, parents reported their own depressive symptoms when their children were 11 years of age, and both youths and parents reported youth exposure to harsh parenting at ages 11 and 16 years. Blood was drawn at age 20 years to measure epigenetic aging. The results revealed that elevated parental depressive symptoms forecast accelerated epigenetic aging among youths in the control condition, but not among SAAF participants. These results are depicted in Figure 10A. Moderated-mediation analyses confirmed that reductions in harsh parenting accounted for SAAF’s protective effects against epigenetic aging. This analysis is presented in Figure 10B. The results supported the study hypotheses and demonstrated that intervention-induced reductions in harsh parenting accounted for the association between parental depression levels and slowed epigenetic aging. Embedding the assessment of epigenetic aging in SAAF increased confidence in the causal nature of the hypothesized linkage between parenting and epigenetic aging.
Figure 10.
(A): Means of epigenetic aging at age 20 for the control and intervention groups by parent-reported depression status. High depressive symptoms group, n = 111: control group = 42, SAAF group = 69. Euthymia group, n = 288: control group = 115, SAAF group = 173. Error bars = ±1 standard error. (B): A moderated-mediation model of intervention status, changes in harsh parenting from age 11 to age 16, and epigenetic aging at age 20 for the high depressive symptoms group versus the euthymic group. Family socioeconomic-related risk, gender, BMI, smoking, unhealthful behaviors, and batch assignment were controlled (not shown). Unstandardized coefficients are presented. Numbers in parentheses refer to coefficients for the euthymic group. *p < .05, two-tailed. ***p < .001, two-tailed. Adapted from “Family-Centered Prevention Ameliorates the Longitudinal Association Between Risky Family Processes and Epigenetic Aging,” by G. H. Brody, T. Yu, E. Chen, S. R. H. Beach, and G. E. Miller, 2015, Journal of Child Psychology and Psychiatry, DOI:10.1111/jcpp.12495. Copyright 2015 by the Association for Child and Adolescent Mental Health. Adapted with permission.
AIM moderates the association between nonsupportive parenting and diminished telomere length
To understand how social environments and psychological processes impact health and the aging process, researchers have sought biomarkers that provide a window into social environments’ associations with aging. Telomere length (TL), like epigenetic aging, appears to be such a marker. Telomeres, the protective caps at the tips of chromosomes, shorten with age. This shortening predicts both disease and longevity (Blackburn, 2005; Epel, 2009). TL may be viewed from a lifespan approach because it reflects, in part, the cumulative number of cell divisions that have occurred and the long-term biochemical environment. Many studies find a negative association between age and TL (Monaghan & Haussmann, 2006), but substantial variation in TL exists among age-matched individuals. Reduction in TL has been associated contemporaneously with the presence of subclinical cardiovascular disease, stroke, diabetes, and autoimmune disease (D'Mello et al., 2015; Haycock et al., 2014). Although in these conditions TL could be only an indicator of ongoing disease processes, other evidence implicates TL as a predictor of the likelihood of future disease states despite current health status. Prospective studies have indicated that short TL predicts the occurrence of cancer (Weischer et al., 2013), hypertension (Yang et al., 2009), and all-cause mortality (Rode, Bojesen, & Nordestgaard, 2015).
A growing body of evidence has demonstrated that telomeres shorten with exposure to chronic stress via oxidative, endocrine, and inflammatory processes, along with other forms of biological stress (Shalev, Entringer, et al., 2013). Studies have found chronic psychological stress (Epel et al., 2004), caregiving strain (Kiecolt-Glaser et al., 2011), social deprivation (Drury et al., 2012), family violence (Drury et al., 2014), and exposure to extrafamilial violence (Shalev, Moffitt, et al., 2013) to be associated with diminished TL. Together, these data suggested that TL would be useful as another marker of the ways in which the stress associated with risky family environments forecasts biological aging at the cellular level.
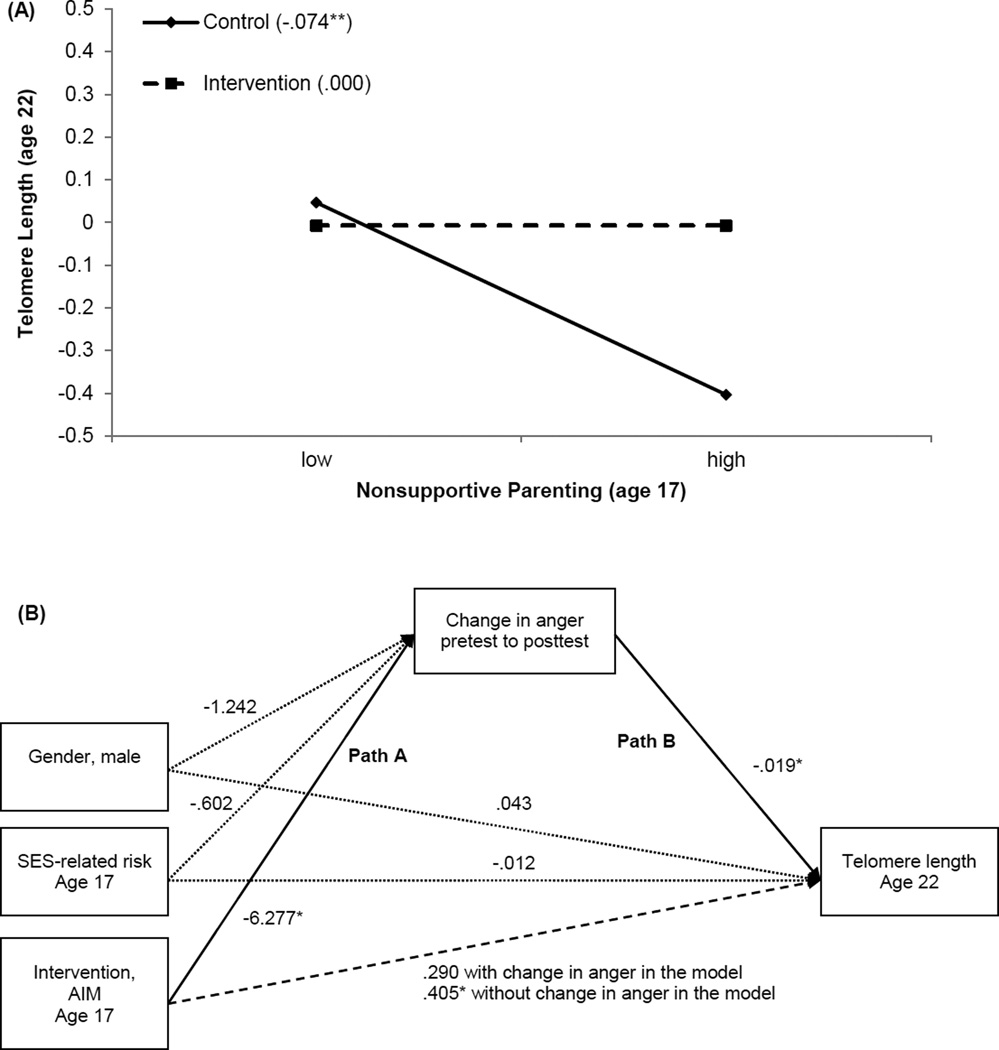
In this study, we tested hypotheses about the association between risky family processes and subsequent TL involving participants in the AIM prevention trial (Brody et al., 2015). The purposes of the study were (a) to determine whether exposure to nonsupportive parenting, defined by high levels of conflict and rancor with low levels of warmth and emotional support, at age 17 years would forecast TL 5 years later; and (b) to determine whether participation in AIM could ameliorate these associations. An exploratory moderation-mediation hypothesis was also tested. We proposed that AIM-induced reductions in anger (a byproduct of nonsupportive parenting; Brody, Yu, et al., 2014) among adolescents who received high levels of nonsupportive parenting would account for AIM’s effects on TL. Rural African American adolescents (N = 216) were assigned randomly to participation in the AIM program or a control condition. Primary caregivers provided data on nonsupportive parenting at baseline, when adolescents were 17 years of age. Adolescents provided data on anger at the pretest and a posttest administered 7 months later. When the participants were 22 years of age, TL was assayed from a blood draw.
The results indicated that heightened nonsupportive parenting forecast diminished TL among young adults in the control condition but not among those who took part in AIM. SES risk, life stress, and drug use at age 17 years, and BMI and blood pressure at age 22 years, were controlled in this analysis. These results are displayed in Figure 11A. In addition, the exploratory moderated-mediation analysis shown in Figure 11B suggested that AIM-induced reduction in adolescents’ anger served as a mediator connecting group assignment to TL. Presumably, anger sponsors frequent activations of the hypothalamic-pituitary-adrenal (HPA) axis and SNS, the end products of which weather cells and tissues, helping to shorten TL. Taken together, this study’s demonstration of program-induced maintenance of TL for young adults who, during adolescence, experienced high levels of parental conflict and rancor along with low levels of warmth, are important. From a theoretical viewpoint, the results show that the developmental progression from a parenting risk factor to TL is not immutable. From a public health perspective, this study, along with the others described in this section, show that developmentally appropriate prevention programs designed to enhance supportive parenting can ameliorate the effects of contextual risk factors on health-relevant outcomes that are evident at the cellular level.
Figure 11.
(A): The contribution of nonsupportive parenting to youths’ telomere length by intervention status. Numbers in parentheses refer to simple slopes for control group and intervention group. **p < 0.01. (B): A moderated-mediation model of intervention status, change in anger from pretest to posttest, and telomere length at age 22 with socioeconomic-related risk and gender controlled. Unstandardized coefficients are presented; n = 65. *p < 0.05. Adapted from “Prevention Effects Ameliorate the Prospective Association Between Nonsupportive Parenting and Diminished Telomere Length,” by G. H. Brody, T. Yu, S. R. H. Beach, and R. A. Philibert, 2015, Prevention Science, 16, pp. 176–177. Copyright 2014 by the Society for Prevention Research. Adapted with permission.
Some Concluding Remarks
Understanding the origins of health disparities and ways of preventing them requires a new, developmentally focused approach to research. Accordingly, our initial work was designed to determine whether and how the socio-contextual environments in which rural African Americans grow up presage biological residues that signal dysregulations in biological stress regulatory systems, immunological functioning, and cellular aging. This effort, using longitudinal, epidemiological research designs and randomized prevention trials, also showed how exposure to resilience-promoting environments functions as a stress buffer for youths. These studies further our understanding of the reasons why only a minority of youths who contend with economic hardship and race-related stressors go on to develop health problems (Chen & Miller, 2012).
The biological mechanisms underlying the studies described in this paper remain unclear. Navigating the challenges posed by economic hardship and life in rural contexts can be metabolically and behaviorally demanding. These experiences seem likely to cause persistent activation of stress-response systems, in particular the HPA axis and SNS. The hormonal end products of these systems can have downstream effects on immune functions, epigenetic aging, and TL. A second generation of research is needed that includes multiple waves of psychosocial, hormonal, and other biomarker data to pinpoint the neuroendocrine mechanisms involved in the processes through which stress gets under young people’s skin. More importantly, such a research program would reveal the ways in which close relationships and certain coping styles interrupt this process to foster in youths resilience both above and below the skin. Some of this new generation of research should focus on processes beginning before birth, as the uterine environment is affected by the mother’s environmental exposures and physiological responses. As we mentioned previously, early risks associated with low SES are linked not only to childhood health problems but also to adult health status regardless of one’s SES in adulthood. Finally, our findings to date suggest ways to design more effective interventions that buffer the pathways by which economic hardship and SES-related stressors undermine health. Our research suggest that developmentally appropriate family-centered interventions designed to enhance parenting and strengthen families can buffer the biological residues of growing up in challenging contexts.
Acknowledgments
The research reported in this article was supported by Grant Number P30 DA027827 from the National Institute on Drug Abuse and Grant Number R01 HD030588 from the National Center of Child Health and Human Development.
References
- Adams RE, Santo JB, Bukowski WM. The presence of a best friend buffers the effects of negative experiences. Developmental Psychology. 2012;47:1786–1791. doi: 10.1037/a0025401. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lehman JM, Klibourn KM, Boyers AE, Culver JL, Alferi SM, Carver CS. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology. 2001;20:20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain, Behavior, and Immunity. 2006;20:339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Bishop SR. What do we really know about Mindfulness-Based Stress Reduction? Psychosomatic Medicine. 2002;64:71–83. doi: 10.1097/00006842-200201000-00010. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Letters. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: A systematic review. American Journal of Preventive Medicine. 2010;39:263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Boatright SR. The Georgia county guide. 24th. Athens: Center for Agribusiness and Economic Development; 2005. [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health. 2010;100(S1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Egerter S. Overcoming obstacles to health. Princeton, NJ: Robert Wood Johnson Foundation; 2008. [Google Scholar]
- Brody GH, Kogan SM, Grange CM. Translating longitudinal, developmental research with rural African American families into prevention programs for rural African American youth. In: King RB, Maholmes V, editors. The Oxford handbook of poverty and child development. New York, NY: Oxford University Press-USA; 2012. pp. 553–570. [Google Scholar]
- Brody GH, Lei M-K, Chae DH, Yu T, Kogan SM, Beach SRH. Perceived discrimination among African American adolescents and allostatic load: A longitudinal analysis with buffering effects. Child Development. 2014;85:989–1002. doi: 10.1111/cdev.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Lei M-K, Chen E, Miller GE. Neighborhood poverty and allostatic load in African American youth. Pediatrics. 2014;134:e1362–e1368. doi: 10.1542/peds.2014-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Miller GE, Yu T, Beach SRH, Chen E. Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: A replication across two longitudinal cohorts. Psychological Science. doi: 10.1177/0956797615626703. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, Neubaum-Carlan E. The Strong African American Families program: Translating research into prevention programming. Child Development. 2004;75:900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Neubaum E, Boyd GM, Dufour M. Health consequences of alcohol use in rural America. In: Robertson EB, Sloboda Z, Boyd GM, Beatty L, Kozel NJ, editors. Rural substance abuse: State of knowledge and issues. NIDA Research Monograph 168. Rockville, MD: U. S. Department of Health and Human Services; 1997. pp. 137–174. [PubMed] [Google Scholar]
- Brody GH, Yu T, Beach SRH, Kogan SM, Windle M, Philibert RA. Harsh parenting and adolescent health: A longitudinal analysis with genetic moderation. Health Psychology. 2014;33:401–409. doi: 10.1037/a0032686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Beach SRH, Philibert RA. Prevention effects ameliorate the prospective association between nonsupportive parenting and diminished telomere length. Prevention Science. 2015;16:171–180. doi: 10.1007/s11121-014-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Beach SRH, Miller GE. Family-centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. Journal of Child Psychology and Psychiatry. 2015 Dec 17; doi: 10.1111/jcpp.12495. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Miller GE, Kogan SM, Beach SRH. Is resilience only skin deep? Rural African Americans’ preadolescent socioeconomic status-related risk and competence and age 19 psychological adjustment and allostatic load. Psychological Science. 2013;24:1285–1293. doi: 10.1177/0956797612471954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen Y-f, Kogan SM, Evans GW, Beach SRH, Philibert RA. Cumulative socioeconomic status risk, allostatic load, and adjustment: A prospective latent profile analysis with contextual and genetic protective factors. Developmental Psychology. 2013;49:913–927. doi: 10.1037/a0028847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen Y-f, Kogan SM, Smith K. The Adults in the Making program: Long-term protective stabilizing effects on alcohol use and substance use problems for rural African American emerging adults. Journal of Consulting and Clinical Psychology. 2012;80:17–28. doi: 10.1037/a0026592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo E, Brady N, Thompson S, Tobin JN, Cassells A, Sweeney M, Contrada RJ. Perceived racism and negative affect: Analyses of trait and state measures of affect in a community sample. Journal of Social and Clinical Psychology. 2008;27:150–173. doi: 10.1521/jscp.2008.27.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proceedings of the National Academy of Sciences of the USA. 2013 Oct 15;110:17149–17153. doi: 10.1073/pnas.1315458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Lee WK, Cavey L, Ho A. Role models and the psychological characteristics that buffer low-socioeconomic-status youth from cardiovascular risk. Child Development. 2013;84:1241–1252. doi: 10.1111/cdev.12037. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. “Shift-and-persist” strategies: Why low socioeconomic status isn’t always bad for health. Perspectives on Psychological Science. 2012;7:135–158. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Yu T, Brody GH. The Great Recession and health risks in African American youth. Brain, Behavior, and Immunity. 2015 Dec 21; doi: 10.1016/j.bbi.2015.12.015. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Strunk RC, Trethewey A, Schreier HMC, Maharaj N, Miller GE. Resilience in low-socioeconomic-status children with asthma: Adaptations to stress. Journal of Allergy and Clinical Immunology. 2011;128:970–976. doi: 10.1016/j.jaci.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of Economic Advisors. Economic Report of the President. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- D'Mello MJJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circulation: Cardiovascular Genetics. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP. The association of telomere length with family violence and disruption. Pediatrics. 2014;134:e128–e137. doi: 10.1542/peds.2013-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Theall KP, Gleason MM, Smyke AT, De Vivo I, Wong JYY, Nelson CA. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Molecular Psychiatry. 2012;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Telomeres in a life-span perspective: A new “psychobiomarker”? Current Directions in Psychological Science. 2009;18:6–10. [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the USA. 2004 Dec 7;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiologic Reviews. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. "Weathering" and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E, Daniels SR, Dolan LM. Socioeconomic disparities in insulin resistance: Results from the Princeton School District Study. Psychosomatic Medicine. 2007;69:61–67. doi: 10.1097/01.psy.0000249732.96753.8f. [DOI] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. British Medical Journal. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schönfels W, Ahrens M, Hampe J. Obesity accelerates epigenetic aging of human liver. Proceedings of the National Academy of Sciences of the USA. 2014 Oct 28;111:15538–15543. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SA. John Henryism and the health of African-Americans. Culture, Medicine and Psychiatry. 1994;18:163–182. doi: 10.1007/BF01379448. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Richeson JA, Finkel EJ. Middle class and marginal? Socioeconomic status, stigma, and self-regulation at an elite university. Journal of Personality and Social Psychology. 2011;100:838–852. doi: 10.1037/a0021956. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur Studies of Successful Aging. Psychosomatic Medicine. 2006;68:500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Weng N-P, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, Lynch JW, House JS, Lepkowski JM, Mero RP, Musick MA, Williams DR. Socioeconomic disparities in health change in a longitudinal study of US adults: The role of health-risk behaviors. Social Science and Medicine. 2001;53:29–40. doi: 10.1016/s0277-9536(00)00319-1. [DOI] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology. 2015;11:407–440. doi: 10.1146/annurev-clinpsy-032814-112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 3. Risk, disorder, and adaptation. 2nd. Hoboken, NJ: Wiley; 2006. pp. 739–795. [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Deary IJ. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biology. 2015;16:25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly MJ, Bean JA. Issue Brief no. 18. Durham, NH: Carsey Institute; 2010. The unequal distribution of child poverty: Highest rates among young Blacks and children of single mothers in rural America. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998 Jan 15;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005 Mar 15;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proceedings of the National Academy of Sciences of the USA. 2014 Aug 5;111:11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science. 2011;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Yu T, Chen E, Brody GH. Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proceedings of the National Academy of Sciences of the USA. 2015;112:10325–10330. doi: 10.1073/pnas.1505063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky DW, Dickson N, Hancox RJ, Harrington H, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the USA. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, Haussmann MF. Do telomere dynamics link lifestyle and lifespan? Trends in Ecology and Evolution. 2006;21:47–53. doi: 10.1016/j.tree.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Pascoe EA, Smart Richman L. Perceived discrimination and health: A meta-analytic review. Psychological Bulletin. 2009;135:531–554. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest N, Paradies Y, Trenerry B, Truong M, Karlsen S, Kelly Y. A systematic review of studies examining the relationship between reported racism and health and wellbeing for children and young people. Social Science and Medicine. 2013;95:115–127. doi: 10.1016/j.socscimed.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Probst JC, Samuels ME, Jespersen KP, Willert K, Swann RS, McDuffie JA. Minorities in rural America: An overview of population characteristics. Columbia, SC: University of South Carolina, Norman J. Arnold School of Public Health; 2002. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–336. [PubMed] [Google Scholar]
- Rode L, Bojesen SE, Nordestgaard BG. Peripheral blood leukocyte telomere length and mortality among 64 637 individuals from the general population. Journal of the National Cancer Institute. 2015;107:djv074. doi: 10.1093/jnci/djv074. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Epel E, Gruenewald T, Karlamangla AS, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: McArthur studies of successful aging. Proceedings of the National Academy of Sciences of the USA. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—Allostatic load and its health consequences: MacArthur Studies of Successful Aging. Archives of Internal Medicine. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: A longitudinal study. Molecular Psychiatry. 2013;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009 Jun 3;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Simpkin AJ, Gemani G, Suderman M, Gaunt TR, Lyttleton O, McArdle WL, Smith GD. Prenatal and early life influences on epigenetic age in children: A study of mother-offspring pairs from two cohort studies. Human Molecular Genetics. doi: 10.1093/hmg/ddv456. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Siahpush M, Kogan MD. Rising social inequalities in US childhood obesity, 2003–2007. Annals of Epidemiology. 2010;20:40–52. doi: 10.1016/j.annepidem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Stevenson HC. Racial socialization. In: Jones RL, editor. Black psychology. 4th. Hampton, VA: Cobb and Henry; 2004. pp. 176–189. [Google Scholar]
- Vohs KD. Psychology. The poor’s poor mental power. Science. 2013;341:969–970. doi: 10.1126/science.1244172. [DOI] [PubMed] [Google Scholar]
- Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. Journal of the National Cancer Institute. 2013;105:459–468. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- Williams B. John Henry: A bio-bibliography. Westport, CT: Greenwood Press; 1983. [Google Scholar]
- Williams DR, Mohammed SA. Racism and health I: Pathways and scientific evidence. American Behavioral Scientist. 2013;57:1152–1173. doi: 10.1177/0002764213487340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly RC, Morris LV. The Southern Black Belt: A national perspective. Lexington, KY: TVA Rural Studies Press; 1997. [Google Scholar]
- Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(5 Suppl):757S–769S. doi: 10.1378/chest.07-1904. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Scheier MF, Miller GE. Goal adjustment capacities, subjective well-being, and physical health. Social and Personality Psychology Compass. 2013;7:847–860. doi: 10.1111/spc3.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Huang X, Jiang H, Zhang Y, Liu H, Qin C, Ju Z. Short telomeres and prognosis of hypertension in a Chinese population. Hypertension. 2009;53:639–645. doi: 10.1161/HYPERTENSIONAHA.108.123752. [DOI] [PMC free article] [PubMed] [Google Scholar]