Abstract
Objective
To develop a risk score to identify women with vesicovaginal fistula at high risk of residual urinary incontinence after surgical repair.
Methods
We conducted a prospective cohort study among 401 women undergoing their first vesicovaginal fistula repair at a referral fistula repair center in Lilongwe, Malawi, between September 2011 and December 2014, who returned for follow-up within 120 days of surgery. We used logistic regression to develop a risk score to identify women with high likelihood of residual urinary incontinence, defined as incontinence grade 2-5 within 120 days of vesicovaginal fistula repair, based on preoperative clinical and demographic characteristics (age, number of years with fistula, HIV status, body mass index, previous repair surgery at an outside facility, revised Goh Classification, Goh vesicovaginal fistula size, circumferential fistula, vaginal scaring, bladder size, and urethral length). The sensitivity, specificity, positive and negative predictive values of the risk score at each cut-point were assessed.
Results
Overall, 11 (3%) women had unsuccessful fistula closure. Of those with successful fistula closure (n=372), 85 (23%) experienced residual incontinence. A risk score cut-point of 20 had sensitivity 82% (95% CI 72%, 89%) and specificity 63% (95% CI 57%, 69%) to potentially identify women with residual incontinence. In our population, the positive predictive value for a risk score cut-point of _20 or higher was 43% (95% CI 36%, 51%) and the negative predictive value was 91% (95% CI 86%, 94%). Forty-eight percent of our study population had a risk score ≥20 and therefore, would have been identified for further intervention.
Conclusions
A risk score 20 or higher was associated with an increased likelihood of residual incontinence, with satisfactory sensitivity and specificity. If validated in alternative settings, the risk score could be used to refer women with high likelihood of postoperative incontinence to more experienced surgeons.
Introduction
Obstetric fistula is a debilitating condition that affects an estimated 1-2 million women globally.(1, 2) Vesicovaginal fistula is an abnormal communication between the bladder and vagina, which typically occurs as a result of prolonged obstructed labor during childbirth.(3) Vesicovaginal fistula leaves women incontinent of urine and has devastating physical and emotional consequences.(4)
Surgical repair remains the primary treatment for vesicovaginal fistula. Successful surgical repair is defined anatomically as closing the fistula and functionally as the absence of urinary and/or fecal incontinence after surgery.(5-7) Surgical success based on anatomical closure varies from 80%-95%.(5) However, up to 20% (8) of women with successful fistula closure continue to suffer from residual urinary incontinence after surgery. Overall, between 5% (9) and nearly 40% of women experience residual incontinence after surgery.(10) Given the devastating consequences of residual incontinence for women's health and quality of life, a greater understanding of prognostic factors for residual urinary incontinence is needed.(11)
The objective of our study was to develop a risk score to identify women with vesicovaginal fistula at high risk of residual urinary incontinence after surgical repair. The risk score could be used by physicians prior to surgery to identify cases which may benefit from referral to a more experienced surgeon or additional intervention to increase the likelihood of post-operative continence. Determining a risk score cut-point which could be used to identify women at high risk of residual incontinence may lead to more efficient use of healthcare resources, and ultimately improved surgical outcomes for women with vesicovaginal fistula.
Materials and Methods
We conducted a cohort study of women seeking surgical repair for vesicovaginal fistula at the Freedom from Fistula Care Centre at Bwaila Hospital in Lilongwe, Malawi. Women are referred to the Fistula Centre from throughout Malawi, western Mozambique, and eastern Zambia. Referred patients undergo a comprehensive clinical history and physical examination upon admission to the Fistula Care Centre. In addition, all women complete a 30-minute survey with detailed demographic, obstetric, and reproductive information upon admission. Surveys were conducted in either the local language (Chichewa) or English by staff members trained in data collection.
Prior to surgery, all patients were screened for HIV infection, underwent a physical examination and nutritional assessment, and fistula staging was documented. Fitness for surgery was determined by the head fistula surgeon. Women who test positive for HIV had their CD4 count evaluated and initiated combinational antiretroviral therapy treatment in accordance with national HIV treatment guidelines.(12) After starting HIV treatment, women were re-evaluated after 3-6 months to determine fitness for surgery. Women with poor nutrition, based on weight and mean upper arm circumference, were given nutritional supplementation preoperatively whenever possible and surgery was delayed until their nutrition status improved. All patients received a dose of prophylactic antibiotics prior to surgery.
Fistula cases referred to the Fistula Care Centre are often complex and damage that involves the bladder neck and proximal urethra is common. Women with fistulas that involve the urethral closure mechanism are at high risk of post-operative urinary incontinence.(8) To help improve the changes of regaining continence after surgery a pubococcygeal sling or other sling-like techniques (13) are used in all women with a short urethra (approximately ≤2 cm) as a preventative measure. After surgery, women received antibiotics on a case by case basis based on risk factors during surgery or post-operative risk assessment. Estrogen cream is typically not available in Malawi, but was used as needed post-operatively in post-menopausal women, when available. Patients remained in the hospital ward with an indwelling catheter for a variable period of time at the discretion of the surgeon (typically 10-21 days), based on the nature and difficulty of the surgical repair.
Women were included in the present analysis if they were diagnosed with vesicovaginal fistula, underwent their first repair surgery at the Fistula Care Center between September 2011 and December 2014, completed a baseline survey, and returned for their first follow-up assessment within 120 days of their repair. Women who had undergone previous fistula repair surgeries at an outside facility were not excluded. Women who did not have a successful fistula closure, as determined by post-operative dye test (on average at 14 days post-operative), were also not excluded. However, women without fistula and those with non-vesicovaginal causes of fistula were excluded. Information on malignancies was not available in our data. Ethical approval was obtained from the National Health Sciences Research Committee of Malawi (Protocol #929) and the University of North Carolina School of Medicine Institutional Review Board (#11-2345). Women over 18 gave informed consent, while those under 18 underwent parental consent and pediatric assent. Trained research assistants double-entered and compared the data using REDCap (Research Electronic Data Capture, NC).(14)
The primary outcome was residual urinary incontinence, defined as incontinence grade 2-5 irrespective of fistula closure status,(15) within 120 days of fistula repair surgery. Women were asked to return for follow-up within 30 days of discharge (typically 14-30 days after repair) to assess surgical outcomes. However, due to the rural nature of much of Malawi and the fact that the Fistula Care Center draws women from around the East Africa region, women included in this analysis were allowed up to 120 days from time of surgery to return for follow-up. We chose incontinence, rather than fistula closure, as our primary outcome since 20% (8) of women with a successful closure may remain incontinent.(5-7)
Both clinical and demographic predictors of residual incontinence within 120 days of surgery were considered. Candidate predictors were selected based on literature review and clinical consideration. However, we prioritized information likely to be readily available to physicians prior to performing a fistula surgery in settings like Malawi. We considered the following predictors: age as a proxy for menopausal status,(16, 17) number of years with fistula,(16-18) HIV status,(19) body mass index, previous repair surgery at an outside facility,(8, 20) revised Goh Classification,(21) Goh vesicovaginal fistula size,(22) circumferential fistula,(8, 23) vaginal scaring,(8, 23) bladder size,(8, 17) and urethral length.(8, 23)
In order to develop a risk score that could easily be calculated by hand by clinical staff working in resource-limited settings, all candidate predictors were coded as binary or categorical variables. Variables were categorized based on clinical considerations and to allow enough variation across the data (e.g. to avoid categories with a small number of observations).
The primary goal of our analysis was to develop an easy-to-use clinical tool to identify women with vesicovaginal fistula who are at high risk of residual urinary incontinence after surgical repair. The analysis comprised two steps. First, we developed a predictive model for residual urinary incontinence within 120 days of fistula repair surgery and used the beta coefficients from the model to develop a risk score. We then internally validated the risk score by bootstrapping.(24)
To develop the risk score, we used logistic regression to estimate unadjusted (bivariable) odds ratios (ORs) and 95% confidence intervals (CIs) for all candidate predictors. Predictors associated with residual incontinence within 120 days of surgery with a p-value ≤ 0.25 were identified and included in the full multivariable model. To reduce the full model to a more parsimonious final model we used manual backward elimination based on likelihood ratio tests. After the elimination of each variable, the area under the curve (AUC) was compared with the full model to determine whether the two models had comparable predictive ability. Multi-collinearity was assessed using Spearman correlations. For variables that were collinear, the variable with the strongest predictive power (e.g. largest coefficient) was retained in the final model. Variable elimination stopped when all predictors remaining in the multivariable model had at least one category with a p-value ≤ 0.10, in an effort to balance including potentially important predictors with parsimony. We used the Hosmer-Lemeshow test to assess model fit and assessed outliers and influential observations using Pregibon leverage and deviance residuals.
To calculate the final risk score, beta coefficients from the final logistic regression model were multiplied by 10 and rounded to the nearest integer. The values associated with each person's covariates were summed to calculate a risk score for each person. Next, the sensitivity and specificity of each risk score cut-point to identify women with residual incontinence was assessed in the entire study population. Based on clinical considerations, a risk score cut-point with sensitivity > 80% and specificity >60% was specified as optimal a priori. We selected these thresholds for sensitivity and specificity in order to prioritize identifying the women with a high likelihood of residual incontinence (higher sensitivity), while preserving an acceptable ability to identify women likely to be continent after surgery (specificity).
We internally validated the risk score by bootstrapping the original data set (n=1,000) and comparing the distributions of sensitivity and specificity in the bootstrapped and observed data.(24). All statistical analyses were performed using Stata version 13 (StataCorp, College Station, Texas).
Results
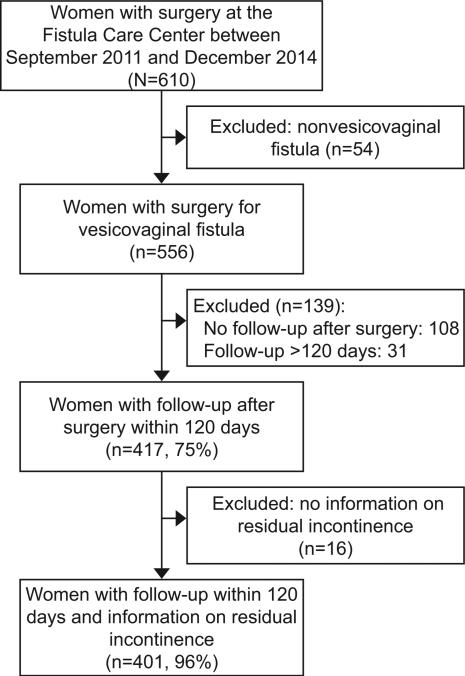
A total of 401 women who underwent a vesicovaginal fistula repair who returned to the Fistula Care Centre within 120 days of their surgery and had information on residual incontinence at that time were included in our analysis (Figure 1). Of 383 women with post-operative dye tests, 372 (97%) were negative indicating successful fistula closure. Of those with successful fistula closure (n=372), 85 (23%) experienced residual incontinence. Overall, 97 (24%) women had residual urinary incontinence within 120 days of surgery. The majority of women (84%) were < 50 years of age, which was considered a proxy for being premenopausal. The prevalence of HIV was lower (7%) than reported for women in the general population in Malawi (13%).(25) A high proportion of women had been living with a fistula for 6 or more years (42%) or 0 to 5 years (45%). Nearly a quarter of patients (24%) had undergone a previous operation at an outside facility. Clinical characteristics indicating complicated fistulas were common: 26% of women had a fistula >3cm in size, 29% had circumferential fistula and 56% had vaginal scaring that was moderate to obliterated (Table 1).
Figure 1.
Flowchart of study population of women with vesicovaginal and a measure of urinary incontinence within 120 days of fistula repair surgery.
Table 1.
Pre-operative characteristics of 401 undergoing vesicovaginal fistula repair, by incontinence status within 120 days of surgery.
| Pre-operative characteristic | Continent N=304 (75.8) |
Residual Incontinence N=97 (24.2) |
P-value* |
|---|---|---|---|
| Age | <0.01 | ||
| <50 | 266 (88.1) | 67 (69.8) | |
| ≥50 | 36 (11.9) | 29 (30.2) | |
| Missing, n | 2 | 1 | |
| Number of years with fistula | 0.01 | ||
| 0-5 years | 127 (49.0) | 23 (29.9) | |
| 6-20 years | 104 (40.2) | 38 (49.4) | |
| >20 years | 28 (10.8) | 16 (20.8) | |
| Missing, n | 45 | 20 | |
| HIV status | 0.56 | ||
| HIV − | 280 (93.0) | 90 (94.7) | |
| HIV + | 21 (7.0) | 5 (5.3) | |
| Missing, n | 3 | 2 | |
| Body mass index, kg/m2 | 0.94 | ||
| < 25.0 | 217 (78.9) | 69 (79.3) | |
| ≥ 25.0 | 58 (21.1) | 18 (20.7) | |
| Missing, n | 29 | 10 | |
| Number of prior fistula surgeries (at outside facility) | 0.02 | ||
| 0 | 240 (79.2) | 65 (67.7) | |
| ≥ 1 | 63 (20.8) | 31 (32.3) | |
| Missing, n | 1 | 1 | |
| Revised Goh classification | <0.01 | ||
| Type 1 | 138 (48.1) | 7 (7.5) | |
| Type 2 | 66 (23.0) | 21 (22.3) | |
| Type 3 | 66 (23.0) | 45 (47.9) | |
| Type 4 | 17 (5.9) | 21 (22.3) | |
| Missing, n | 17 | 3 | |
| Goh vesicovaginal fistula size | <0.01 | ||
| <1.5 cm | 115 (40.2) | 20 (21.3) | |
| 1.5-3.0 cm | 108 (37.8) | 39 (41.5) | |
| >3.0 cm | 63 (22.0) | 35 (37.2) | |
| Missing, n | 18 | 3 | |
| Circumferential fistula | <0.01 | ||
| No | 241 (29.3) | 42 (43.3) | |
| Yes | 63 (20.7) | 55 (56.7) | |
| Missing, n | 0 | 0 | |
| Vaginal scarring | <0.01 | ||
| None/Mild | 150 (51.2) | 20 (20.6) | |
| Moderate/Severe/Obliterated | 143 (48.8) | 77 (79.4) | |
| Missing, n | 11 | 0 | |
| Bladder size, measured from urethra | 0.02 | ||
| Large (>6 cm) | 118 (43.7) | 28 (29.8) | |
| Small/moderate (≤ 6 cm) | 152 (56.3) | 66 (70.2) | |
| Missing, n | 34 | 3 | |
| Urethral length | <0.01 | ||
| >1.5 cm | 245 (89.4) | 60 (62.5) | |
| ≤1.5 cm | 29 (10.6) | 36 (37.5) | |
| Missing, n | 30 | 1 |
P-values based on chi-square tests.
In the bivariable analysis, age, number of years with fistula, prior surgery at an outside facility, Goh vesicovaginal fistula size, circumferential fistula, vaginal scarring, bladder size, and urethral length were identified as predictors of residual urinary incontinence and included in the full multivariable model. Revised Goh classification was also predictive of residual incontinence, but due to limited sample size in some categories and strong collinearity with urethral length and circumferential fistula, it was not included in the full multivariable model. The final multivariable model included all from the full multivariable model, with the exception of number of years with a fistula and bladder size. These two variables were removed based on likelihood ratio tests (Table 2). The final multivariable model had good predictive ability, with an area under the curve of 0.82 (95% CI 0.77, 0.86).
Table 2.
Bivariable and Multivariable Analyses to develop a Risk Score to Identify Women at High Risk of Residual Urinary Incontinence.
| Bivariable Analysis | Multivariable Analysis - Full Model | Multivariable Analysis - Final Model |
Risk Score Value |
||||
|---|---|---|---|---|---|---|---|
| Pre-operative Characteristic | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value* | |
| Age | |||||||
| ≤ 50 years | 1.00 | 1.00 | 1.00 | 0 | |||
| >50 years | 3.20 (1.83-5.59) | <0.01 | 9.25 (2.23-38.38) | <0.01 | 5.66 (2.72-11.74) | <0.01 | 17 |
| Number of years with fistula | |||||||
| ≤5 years | 1.00 | 1.00 | -- | -- | |||
| 6-20 years | 2.02 (1.13-3.60) | 0.02 | 1.63 (0.80-3.32) | 0.17 | -- | -- | |
| >20 years | 3.16 (1.48-6.73) | <0.01 | 0.78 (0.17-3.51) | 0.75 | -- | -- | |
| HIV status | |||||||
| HIV-uninfected | 1.00 | -- | -- | -- | -- | ||
| HIV-infected | 0.74 (0.27-2.02) | 0.56 | -- | -- | -- | -- | |
| Body mass index, kg/m2 | |||||||
| < 25.0 | 1.00 | -- | -- | -- | -- | ||
| ≥ 25.0 | 0.98 (0.54-1.77) | 0.94 | -- | -- | -- | -- | |
| Number of prior fistula surgeries (at outside facility) | |||||||
| 0 | 1.00 | 1.00 | 1.00 | 0 | |||
| ≥1 | 1.51 (0.84-2.71) | 0.17 | 2.14 (0.95-4.81) | 0.07 | 3.31 (1.61-6.82) | <0.01 | 12 |
| Revised Goh classification | |||||||
| Type 1 | 1.00 | -- | -- | -- | -- | ||
| Type 2 | 6.27 (2.54-15.49) | <0.01 | -- | -- | -- | -- | |
| Type 3 | 13.44 (5.75-31.41) | <0.01 | -- | -- | -- | -- | |
| Type 4 | 24.35 (9.02-65.72) | <0.01 | -- | -- | -- | -- | |
| Goh vesicovaginal fistula size | |||||||
| <1.5 cm | 1.00 | 1.00 | 1.00 | 0 | |||
| 1.5-3.0 cm | 2.08 (1.14-3.78) | 0.02 | 2.71 (1.12-6.56) | 0.03 | 2.12 (0.96-4.69) | 0.06 | 8 |
| >3.0 cm | 3.19 (1.70-5.99) | <0.01 | 1.67 (0.63-4.43) | 0.31 | 2.02 (0.86-4.77) | 0.11 | 7 |
| Circumferential fistula | |||||||
| No | 1.00 | 1.00 | 1.00 | 0 | |||
| Yes | 5.01 (3.07-8.16) | <0.01 | 3.16 (1.45-6.89) | <0.01 | 4.51 (2.21-9.22) | <0.01 | 15 |
| Vaginal scarring | |||||||
| None/Mild | 1.00 | 1.00 | 1.00 | 0 | |||
| Moderate/Severe/Obliterated | 4.04 (2.35-6.95) | <0.01 | 3.32 (1.15-4.71) | 0.02 | 2.07 (1.09-3.94) | 0.03 | 7 |
| Bladder size | |||||||
| Large (>6 cm) | 1.00 | 1.00 | -- | -- | |||
| Small/moderate (≤6cm) | 1.83 (1.11-3.03) | 0.02 | 1.47 (0.75-2.88) | 0.26 | -- | -- | |
| Urethral length | |||||||
| >1.5 cm | 1.00 | 1.00 | 1.00 | 0 | |||
| ≤ 1.5 cm | 5.07 (2.88-8.92) | <0.01 | 2.12 (0.95-4.75) | 0.07 | 1.86 (0.91-3.81) | 0.09 | 6 |
OR= Odds ratio.
Odds ratios and p-values estimated using logistic regression.
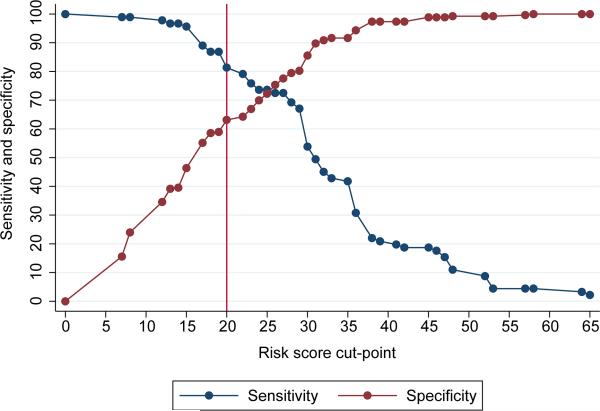
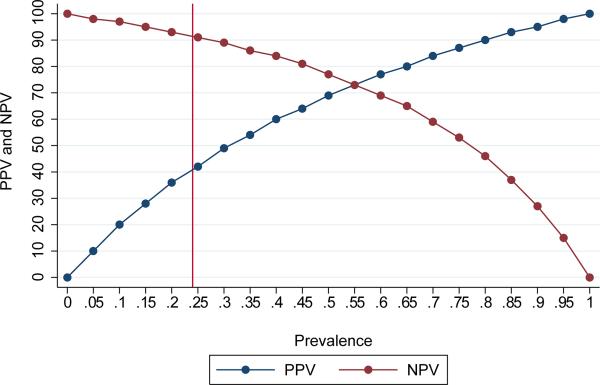
The final multivariable model included 354 women with complete data. Individual risk scores ranged from 0 to 65. A risk score cut-point of >_20 had sensitivity 82% (95% CI 72%, 89%) and specificity 63% (95% CI 57%, 69%) to identify women with residual urinary incontinence within 120 days of surgery and met our a priori criteria for satisfactory sensitivity (>80%) and specificity (>60%) (Figure 2). In our population, the overall prevalence of residual incontinence was 24%. This resulted in a positive predictive value for a risk score cut-point of 20 of 43% (95% CI 36%, 51%) and the negative predictive value of 91% (95% CI 86%, 94%) (Figure 3). Using a risk score cut-point of 20 to identify women who may benefit from a more experienced surgeon or other additional intervention would identify 48% of the population for referral. A risk score cut-point of 20 identified 27% of the population who did not develop residual incontinence as high risk for residual incontinence, and did not identify 4% of the population who did develop residual incontinence (Appendix 1, available online at http://links.lww.com/xxx). Bootstrapped values of sensitivity and specificity were very similar to those observed in the data, indicating good internal validity of the risk score.
Figure 2.
Sensitivity and specificity at each risk score cut-point. The reference line at 20 indicates the risk score cut-point with sensitivity of >80% and specificity >60%.
Figure 3.
Positive predictive value and negative predictive value for a risk score cut-point of 20, by prevalence. The reference line at 24% indicates the prevalence of residual incontinence in the study population.
Discussion
Obstetric fistula remains one of the most debilitating conditions a woman can experience. Surgical repair remains the best hope for regaining urinary continence, but the need for greater agreement on prognostic factors for urinary incontinence has been discussed(11, 26)). Our analysis attempts to address this issue by developing a risk score to identify women with vesicovaginal fistula who are at high risk of residual urinary incontinence within 120 days of repair based on a range of prognostic factors. In our setting, a risk score cut-point of ≥ 20 had satisfactory sensitivity and specificity, low positive predictive value and high negative predictive value to identify women with a high likelihood of residual incontinence. As with all risk scores, external validation of the risk score with an independent dataset is required before use in alternative settings.
Our risk score was developed using data from a referral fistula center in sub-Saharan Africa. Many of the women included in our analysis had complicated fistulas and surgical repair was performed by experienced surgeons. However, the decision about whether to refer women to more advanced surgeons typically takes place at district or community hospitals. Prior to surgery, physicians could easily calculate a risk score (see Appendix 2, available online at http://links.lww.com/xxx) for all patients based on their clinical characteristics. If validated in these alternative settings, the risk score may provide a useful diagnostic for distinguishing women most likely to benefit from specialized care and those likely to have good surgical outcome with lower-level fistula surgeons. Interventions such as pre and postoperative physical therapy and health education have been shown to reduce residual incontinence after surgery.(27) Preventative surgical measures, such as the pubococcygeal sling or traditional fascial sling, may also reduce the changes of residual incontinence after surgery.(13)
In our setting the negative predictive of using a risk score cut-point of ≥ 20 was 91%, indicating a high probability of continence after surgery for women with a risk score <20. However, because the prevalence of residual incontinence can vary widely by population, it is important to remember that the positive and negative predictive values of the risk score at any cut-point will vary based on the prevalence. In settings with less experienced surgeons, the prevalence of residual incontinence after surgery would be expected to be higher, and therefore the negative predictive value lower.
The overall prevalence of residual urinary incontinence within 120 days of repair surgery was 24% in our study population. This proportion is similar to what has been reported in other sub-Saharan African settings.(28) However, there is no agreed upon time after surgery for reporting the prevalence of residual incontinence and estimates vary widely by study, from 5% (9) to nearly 40%.(10) The somewhat higher prevalence of residual incontinence in our study likely reflects the fact that complicated cases or cases where previous surgery has failed (24% in our study population) are frequently referred to our center.
Our study had a number of important strengths. Our analysis draws on one of the largest databases of obstetric fistula patients in sub-Saharan Africa with a high proportion of post-operative follow-up (75%) to develop a standardized clinical tool that may identify women at high risk of residual incontinence after fistula repair surgery. Given our large and diverse population, we were able to evaluate a number of pre-operative characteristic as potential predictors of residual urinary incontinence in our analysis.
Our study also has important limitations. Data in our analysis come from a large fistula care referral center which draws patients, many with complicated fistula, from throughout the East Africa region. A majority of surgeries were performed by an experienced fistula surgeon with over 15 years of experience. Thus, our risk score may not be generalizable to clinics or health centers with less experienced surgeons, where the need to triage women based on their prognosis for surgical success may be greatest, and would need to be validated before use. Our risk score also may not be generalizable to resource-rich settings where the prevalence of vesicovaginal fistula is extremely low and fistulas that do occur are likely to be less complicated and not related to obstructed labor. Finally, even with our large sample of vesicovaginal fistula patients, we had to categorize some variables, such as urethral length and bladder size, more coarsely than would be clinically optimal due to limited sample size.
Supplementary Material
Acknowledgments
Supported by the Freedom from Fistula Foundation (FfFF), the UNC Department of OB-GYN, the Doris Duke Charitable Foundation, and the University of North Carolina, Johns Hopkins, Morehouse School of Medicine, and Tulane University NIH Fogarty International Center Grant [5R25TW009340]. Support for use of the REDCap database was funded by grant [1UL1TR001111] from the North Carolina Clinical and Translational Science Award program of the Division of Research Resources at the University of Chapel Hill. Dr. Kopp's research efforts were supported by NICHD training grant [5T32 HD075731-01] to the University of North Carolina-Chapel Hill.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Organization WH. Obstetric fistula: guiding principles for clinical management and programme development. WHO; Geneva, Switzerland: 2006. [DOI] [PubMed] [Google Scholar]
- 2.Adler AJ, Ronsmans C, Calvert C, Filippi V. Estimating the prevalence of obstetric fistula: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2013;13:246. doi: 10.1186/1471-2393-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrowsmith S, Hamlin EC, Wall LL. Obstructed labor injury complex: obstetric fistula formation and the multifaceted morbidity of maternal birth trauma in the developing world. Obstet Gynecol Surv. 1996;51(9):568–74. doi: 10.1097/00006254-199609000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Wall LL. Obstetric vesicovaginal fistula as an international public-health problem. Lancet. 2006;368(9542):1201–9. doi: 10.1016/S0140-6736(06)69476-2. [DOI] [PubMed] [Google Scholar]
- 5.Wall LL, Arrowsmith SD. The “continence gap”: a critical concept in obstetric fistula repair. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(8):843–4. doi: 10.1007/s00192-007-0367-z. [DOI] [PubMed] [Google Scholar]
- 6.Arrowsmith SD, Ruminjo J, Landry EG. Current practices in treatment of female genital fistula: a cross sectional study. BMC Pregnancy Childbirth. 2010;10:73. doi: 10.1186/1471-2393-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutman RE, Dodson JL, Mostwin JL. Complications of treatment of obstetric fistula in the developing world: gynatresia, urinary incontinence, and urinary diversion. Int J Gynaecol Obstet. 2007;99(Suppl 1):S57–64. doi: 10.1016/j.ijgo.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Barone MA, Frajzyngier V, Ruminjo J, Asiimwe F, Barry TH, Bello A, et al. Determinants of postoperative outcomes of female genital fistula repair surgery. Obstet Gynecol. 2012;120(3):524–31. doi: 10.1097/AOG.0b013e31826579e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessessew A, Mesfin M. Genitourinary and rectovaginal fistulae in Adigrat Zonal Hospital, Tigray, north Ethiopia. Ethiop Med J. 2003;41(2):123–30. [PubMed] [Google Scholar]
- 10.Siddle K, Vieren L, Fiander A. Characterising women with obstetric fistula and urogenital tract injuries in Tanzania. Int Urogynecol J. 2014;25(2):249–55. doi: 10.1007/s00192-013-2185-9. [DOI] [PubMed] [Google Scholar]
- 11.Frajzyngier V, Ruminjo J, Barone MA. Factors influencing urinary fistula repair outcomes in developing countries: a systematic review. Am J Obstet Gynecol. 2012;207(4):248–58. doi: 10.1016/j.ajog.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Ministry of Health M . Malawi Integrated Guidelines for HIV Services. The Ministry of Health, Malawi; Malawi: 2011. Clinical Management of HIV in Children and Adults. [Google Scholar]
- 13.Browning A. Prevention of residual urinary incontinence following successful repair of obstetric vesico-vaginal fistula using a fibro-muscular sling. Bjog. 2004;111(4):357–61. doi: 10.1111/j.1471-0528.2004.00080.x. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browning A, Menber B. Women with obstetric fistula in Ethiopia: a 6-month follow up after surgical treatment. BJOG. 2008;115(12):1564–9. doi: 10.1111/j.1471-0528.2008.01900.x. [DOI] [PubMed] [Google Scholar]
- 16.Raassen TJ, Verdaasdonk EG, Vierhout ME. Prospective results after first-time surgery for obstetric fistulas in East African women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):73–9. doi: 10.1007/s00192-007-0389-6. [DOI] [PubMed] [Google Scholar]
- 17.Browning A. Risk factors for developing residual urinary incontinence after obstetric fistula repair. BJOG. 2006;113(4):482–5. doi: 10.1111/j.1471-0528.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 18.Melah GS, El-Nafaty AU, Bukar M. Early versus late closure of vesicovaginal fistulas. Int J Gynaecol Obstet. 2006;93(3):252–3. doi: 10.1016/j.ijgo.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Tebeu PM, Maninzou SD, Takam D, Nguefack-Tsague G, Fomulu JN, Rochat CH. Surgical outcome following treatment of obstetric vesicovaginal fistula among HIV-positive and HIV-negative patients in Cameroon. Int J Gynaecol Obstet. 2014;125(2):168–9. doi: 10.1016/j.ijgo.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Sjoveian S, Vangen S, Mukwege D, Onsrud M. Surgical outcome of obstetric fistula: a retrospective analysis of 595 patients. Acta Obstetricia et Gynecologica Scandinavica. 2011;90(7):753–60. doi: 10.1111/j.1600-0412.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 21.Goh JT. A new classification for female genital tract fistula. Aust N Z J Obstet Gynaecol. 2004;44(6):502–4. doi: 10.1111/j.1479-828X.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 22.Goh JT, Browning A, Berhan B, Chang A. Predicting the risk of failure of closure of obstetric fistula and residual urinary incontinence using a classification system. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1659–62. doi: 10.1007/s00192-008-0693-9. [DOI] [PubMed] [Google Scholar]
- 23.Nardos R, Browning A, Chen CC. Risk factors that predict failure after vaginal repair of obstetric vesicovaginal fistulae. Am J Obstet Gynecol. 2009;200(5):578 e1–4. doi: 10.1016/j.ajog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE. Regression Model Strategies. 1st ed. Springer; New York, New York: 2002. p. 572. [Google Scholar]
- 25.National Statistical Office (NSO), ICF Macro . Malawi Demographic and Health Survey 2010. NSO and ICF Macro; Zomba, Malawi, and Calverton, Maryland, USA: 2011. [Google Scholar]
- 26.Arrowsmith SD, Barone MA, Ruminjo J. Outcomes in obstetric fistula care: a literature review. Curr Opin Obstet Gynecol. 2013;25(5):399–403. doi: 10.1097/GCO.0b013e3283648d60. [DOI] [PubMed] [Google Scholar]
- 27.Castille YJ, Avocetien C, Zaongo D, Colas JM, Peabody JO, Rochat CH. Impact of a program of physiotherapy and health education on the outcome of obstetric fistula surgery. Int J Gynaecol Obstet. 2014;124(1):77–80. doi: 10.1016/j.ijgo.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Tayler-Smith K, Zachariah R, Manzi M, van den Boogaard W, Vandeborne A, Bishinga A, et al. Obstetric fistula in Burundi: a comprehensive approach to managing women with this neglected disease. BMC Pregnancy Childbirth. 2013;13:164. doi: 10.1186/1471-2393-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.