Abstract
Neurofilaments are uniquely complex among classes of intermediate filaments in being composed of four subunits (NFL, NFM, NFH and alpha-internexin in the CNS) that differ in structure, regulation, and function. Although neurofilaments have been traditionally viewed as axonal structural components, recent evidence has revealed that distinctive assemblies of neurofilament subunits are integral components of synapses, especially at postsynaptic sites. Within the synaptic compartment, the individual subunits differentially modulate neurotransmission and behavior through interactions with specific neurotransmitter receptors. These newly uncovered functions suggest that alterations of neurofilament proteins not only underlie axonopathy in various neurological disorders but also may play vital roles in cognition and neuropsychiatric diseases. Here, we review evidence that synaptic neurofilament proteins are a sizable population in the CNS and we advance the concept that changes in the levels or post-translational modification of individual NF subunits contribute to synaptic and behavioral dysfunction in certain neuropsychiatric conditions.
Keywords: Neurofilament subunit, synapse, dendritic spine, neuropsychiatric disease
1. Introduction
Neurofilaments (NFs), the intermediate filaments of mature neurons, are among the most abundant proteins in brain. Unlike the intermediate filaments of other cell types, which are usually homopolymers, NFs in the CNS are hetero-polymers composed of NFL, NFM, NFH and alpha-internexin subunits [187]. Although structurally distinctive, these four NF subunits share a basic tripartite domain structure consisting of a conserved central α-helical rod region, a short variable head domain at the amino-terminal end and a tail of highly variable length at the C-terminal end. The short head domain is rich in serine and threonine residues and contains consensus sites for O-linked glycosylation and phosphorylation [188]. The central rod domain, which is relatively conserved among intermediate filament family members, contains long stretches of hydrophobic heptad repeats favoring formation of α-helical coiled-coil dimers. The C-terminal domains contain glutamic- and lysine-rich stretches of varying length that mainly establish the size range (58–200 kDa on SDS gels) of the four NF subunits. Although all intermediate filament types serve roles as structural scaffolds, NF subunit heterogeneity also confers specialized structural properties to NF, in axons where the filaments are extremely long and are often arranged in parallel with uniform spacing conferred by the long C-terminal tails of NFM and NFH extending perpendicularly from the filament core [141, 142]. These unique space filling properties of NF facilitate their well-established role in caliber expansion of large-diameter myelinated axons of peripheral nerves, which is critical for effective nerve conduction [198]. NFs also extensively cross-link with other cytoskeletal elements along axons to form a large metabolically stable stationary NF network [126, 185, 190] that is critical to axon caliber expansion [66, 84, 129] and organelle distribution along axons [143].
Besides these unique structural and functional features, NF subunits are distinguished from other intermediate filament proteins by the complex regulation of their head and tail domains by phosphorylation, especially those of NFM and NFH, which involves actions of multiple protein kinases and phosphatases at many polypeptide sites [133]. Although certain phosphorylation events are known to control tail extension and subunit assembly and slow turnover, the purpose of such complex and dynamically changing phosphate topography on NF subunits [49, 127, 133] has remained puzzling, given the mainly static structural support roles ascribed to NF. Both the complex hetero-polymeric structure and dynamically changing phosphate topography of NF proteins suggests that individual NF proteins might serve additional biological roles although there has been relatively little exploration of this issue until recently.
NF gene mutations are well recognized as causes of several neurological disorders mainly involving degeneration of peripheral nerve fibers in accordance with the prominent function of NF in supporting large-diameter myelinated axons [26]. Notably, however, NF proteins are abundant in grey matter CNS regions as well as white matter [33] but influence caliber expansion much less dramatically in most populations of CNS axons [55]. These observations and evidence that NF subunits can be axonally transported in various minimally assembled forms (including as heterodimers) suggest a broader distribution and range of assembly forms of NF subunits within CNS neurons, including substantial populations in synapses. In light of these findings, alterations of a particular NF subunit as seen in specific brain regions in psychiatric and neuropsychiatric disorders [30, 40, 69] may reflect an alteration within synapses which influences the clinical phenotype.
Psychiatric diseases, affecting an estimated 54 million Americans yearly, cause mild to severe disturbances in thought or behavior usually in the absence of known changes in axonal integrity. Nevertheless, changes in levels and phosphorylation of NF subunits have consistently been noted in certain psychiatric disorders although the location of these changes within neurons is poorly understood. Psychiatric diseases prominently involve alterations of synaptic transmission. The highly specialized composition of synapses includes not only the well characterized vesicular and protein receptor machinery supporting neurotransmission but also a specialized cytoskeleton important for delivering, inserting, and recycling synaptic components. Like other domains in a neuron, the cytoskeleton in synapses is composed of microtubules, actin filaments, the spectrin-rich membrane skeleton, and as recently shown, NF assemblies [191, 193]. Evidence is also emerging that the cytoskeleton, and especially the NF scaffold, acts as a docking platform to organize the topography of organelles within different neuronal compartments. Rearrangements of this topography are dynamically coordinated at least in part by cellular signals regulating the phosphorylation state of the binding partners [137, 143, 163, 193]. Such evidence suggests a range of possibilities for understanding the newly recognized roles of individual NF subunits in modulating synaptic function. In this review, we consider the properties of synaptic NF assemblies in the CNS and, in this context, raise the possibility that certain changes in levels or phosphorylation of NF subunits reported in neuropsychiatric disorders (Table 1) disrupt synaptic signaling or, in some cases, reflect adaptive or maladaptive responses to these synaptic disruptions.
Table 1.
Neurofilament Subunit Expression in Human and Rodent Models of Psychiatric Disease
| Disease | NF subunit (gene symbol) |
Model system |
Regulation | ||||
|---|---|---|---|---|---|---|---|
| Protein level |
Phosphorylation | Brain region |
Method | Reference | |||
| Schizophrenia | NEFL | Human | Decrease | DLPFC | WB | [100] | |
| Human | Decrease | ACC white matter |
Proteomic | [39] | |||
| Human | Decrease | CC | Proteomic | [157] | |||
| Human | Decrease | DLPFC | Proteomic | [135] | |||
| Human | Decrease | DLPFC white matter |
Proteomic | [58] | |||
| NEFM | Human | Decrease | CC | Proteomic | [157] | ||
| NEFH | Human | Decrease | DLPFC white matter |
Proteomic | [58] | ||
| INA | Human | Increase | ACC white matter |
Proteomic | [39] | ||
| Human | Increase | CC | Proteomic | [157] | |||
| Bipolar disorder |
NEFL | Human | Decrease | DLPFC | Prpteomic | [135] | |
| Human | Decrease | DLPFC white matter |
Proteomic | [58] | |||
| NEFM | Human | Increase | DLPFC | Proteomic | [135] | ||
| Human | Increase | DLPFC white matter |
[58] | ||||
| NEFH | Human | Decrease | DLPFC white matter |
Proteomic | [58] | ||
| INA | Human | Decrease | DLPFC | Proteomic | [135] | ||
| Drug addiction |
NEFL | Rat | Decrease | VTA | WB | [16] | |
| Rat | Decrease | VTA | IHC | [28] | |||
| Human | Decrease | Frontal cortex |
WB | [69] | |||
| Neuronal culture |
Decrease | Hippocampus | IHC | [149] | |||
| NEFM | Rat | Decrease | Increase | VTA | WB | [16] | |
| Rat | Decrease | VTA | IHC | [28] | |||
| Neuronal culture |
Decrease | Hippocampus | IHC | [149] | |||
| NEFH | Rat | Decrease | Increase | VTA | WB | [16] | |
| Rat | Decrease | VTA | IHC | [28] | |||
| Neuronal culture |
Decrease | Hippocampus | IHC | [149] | |||
| Alzheimer disease |
NEFL | Human | Decrease | Occipital cortex |
WB | [9] | |
| NEFM | Human | Decrease | Decrease | Sciatic nerve |
WB | [85] | |
| Human | Increase | Hippocampus | IHC | [160] | |||
| Human | Increase | Hippocampus | WB | [179] | |||
| Human | Increase | Frontal cortex |
WB, IHC & iTRAQ |
[146] | |||
| NEFH | Human | Decrease | Decrease | Sciatic nerve |
WB | [85] | |
| Human | Increase | Hippocampus | IHC | [160] | |||
| Human | Increase | Hippocampus | WB | [179] | |||
| Human | Increase | Frontal cortex |
WB, IHC & iTRAQ |
[146] | |||
| Human | Increase | Temporal cortex |
IHC | [170] | |||
| INA | Human | Increase | Cortex | Proteomic & WB |
[197] | ||
Note: DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; CC, corpus callosum; WB, Western blotting; IHC, immunohistochemistry; iTRAQ, isobaric peptide tags for relative and absolute quantification;
2. NF proteins in synapses
2.1 Distinctive assemblies of NF subunits in synapses
Synapses have long been considered to be degradative sites for NF reaching terminals by axonal transport [145]. When NF proteins have been detected in synaptic fractions and bound to synaptic proteins in vitro, they have previously been viewed as contaminating axonal NF proteins [112] and their possible role in synapses has rarely been entertained. Using multiple independent approaches, however, Yuan et al. recently provided definitive evidence that all four CNS NF subunits are integral resident proteins of synapses and have distinct roles in synaptic function and behavior (Figure 1)[191, 193]. The NF assemblies isolated from synapses are distinctive as compared to those in other parts of the neuron in terms of their morphology and in having higher proportions of alpha-internexin, a lowered phosphorylation state of NFM, and a higher NFH phosphorylations state. This unique population of synaptic NF proteins is more abundant in the postsynaptic area than in adjacent dendritic areas or presynaptic terminals and exhibits a different response to subunit perturbation than the axonal population. For example, when NFM is deleted from mice, NFH phosphorylation and the ratio of NFH to NFL subunit increase significantly in the synaptic pool in comparison to the total NF pool [193]. Because NF subunit monomers are inefficiently transported along axons [186, 187, 193], NF proteins in synapses are at least oligomeric in form and can be transported as such in axons, providing one possible basis for their synaptic location. It is also possible that some NF protein in postsynaptic boutons is synthesized locally because mRNAs of NFL [45, 134] and alpha-internexin [180] are reported to be present in dendrites and polyribosomes are preferentially localized under the base of dendritic spines [161]. In this regard, evidence suggests that NF proteins identified in nerve terminals isolated from squid brain are produced by local protein synthesis [46, 110].In light of these findings, aforementioned reports noting the presence of NF in synaptic fractions and interactions of NF proteins with known synaptic proteins can be viewed as further support for a substantial synaptic pool of NF proteins in the brain.
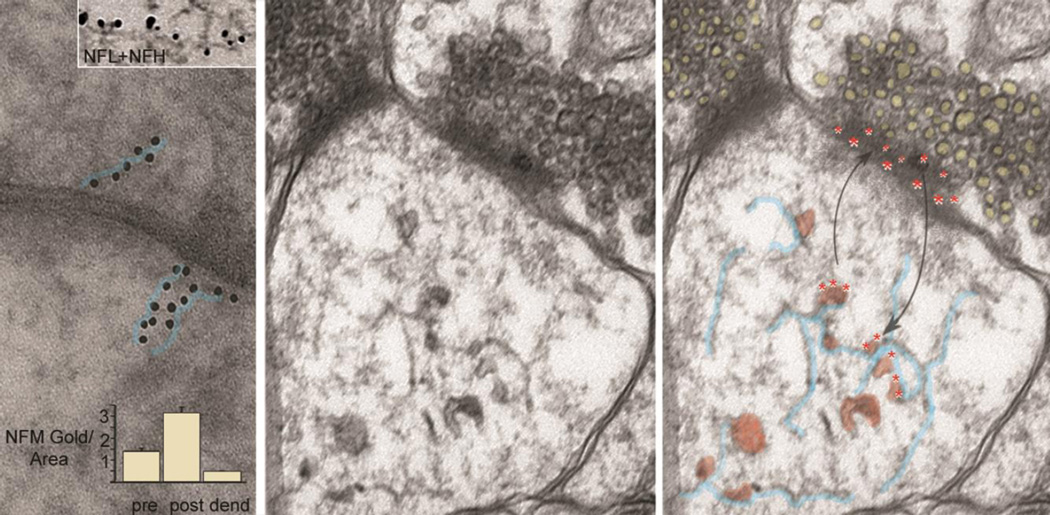
Figure 1. Functional NF subunit assemblies in synapses.
Left panel: Immunogold labeled antibodies against the NFM subunit decorating synaptic structures in a linear pattern (immunogold particles outlined in blue) suggesting the presence of short NFs and protofilament /protofibril or unit length filament assemblies. In the upper inset, a filament within a postsynaptic bouton is decorated by immunogold antibodies to both NFL (large gold dots) and NFH (small gold dots). Morphometric analysis indicates a higher density of immunogold labeling in postsynaptic boutons than in preterminal dendrites or presynaptic terminals (graph inset). Middle panel: Ultrastructural image of a human synapse depicts membranous vesicles, many of which appear to be associated with a loose network of short 10nm filaments in the post-synaptic region. Right panel: Evidence supports a biological mechanism whereby D1 dopamine receptors internalized on endosomes from the postsynaptic surface (red asterisks) dock on synaptic NF subunit assemblies (outlined in blue) where they are readily available to recycle on endosomes to the surface in response to ligand stimulation (adapted from Yuan et al. Mol Psychiatry 2015; 20:915 with permission).
Remarkably, previous proteomic analyses of synaptic fractions have consistently identified NF proteins but these findings were not followed up with additional proof of a synaptic localization or function. During the study of activity-regulated proteins in postsynaptic density fractions by two-dimensional gel electrophoresis and mass spectrometry, Satoh et al. identified a total of 90 spots containing 47 different protein species in the PSD fractions isolated from mouse forebrain [148]. Among these spots, alpha-internexin and NFM accounted for 8 and 4 spots, respectively. With an improved proteomic method involving nanoflow HPL coupled to electrospray tandem mass spectrometry (LC-MS/MS), Jordan et al. identified all four CNS NF proteins (NFL, NFM, NFH and alpha-internexin) as abundant components of biochemically purified PSD fractions from rat or mouse brain[92]. A subsequent phospho-proteomic analysis of PSD proteins, identified 42 phosphoproteins in PSD preparations from mouse brains [172]. Out of 90 phosphorylated peptides derived from these 42 phosphoproteins, NF proteins accounted for 23 (NFL 1, NFM 5 and NFH 17), indicating the considerable abundance of NF proteins in the PSD. NF proteins are also glycosylated and in a glyco-proteomic analysis of PSD preparations from mouse brain, NFL, NFM and alpha-internexin were among 18 identified GlcNAc-modified proteins [181]. A limited idea of how NF proteins are organized within the PSD has been achieved using a novel solid phase and chemical crosslinking approach to distinguish proteins that reside either at the surface of the PSD or in its interior [108]. The analysis suggested that NFM, NFH and alpha-internexin together with tubulin subunits reside in the interior of the PSD while actin resides primarily on the surface. Recently, Moczulska et al. performed precise quantification of the mouse synaptosomal proteome during postnatal maturation (from 3 – 8 weeks of age) using peptide-based iTRAQ labeling and high-resolution two-dimensional peptide fractionation [119]. Among the 3422 identified proteins with complete quantifications, NFL, NFM, NFH and alpha-internexin were established to be components of the synaptosomes and their levels increased during brain postnatal maturation, a period when synapse formation sharply increases [2]. Alpha-internexin had long been believed to form a separate filament system from NF triplet proteins until it was shown to be the fourth subunit of NFs in the mature CNS [187] although somewhat over-represented among the other NF subunits present in synapses [193]. Benson et al. detected alpha-internexin immunoreactivity in dendrites extending into dendritic spines of cultured hippocampal neurons [17]. This immunoreactivity receded from spines and remained at the base of dendritic protrusions when cells were treated with latrunculin A to disrupt actin filaments [195].
Besides their association with the PSD, NF subunits have been noted to interact with other synaptic proteins. The synapse-specific PSD95-associated protein SAPAP interacts via its N-terminal region with all 4 NF subunits, co-immunoprecipitates with NF proteins from rat brain, and co-localizes with NFs in transfected COS cells [80]. Spinophilin, a synaptic protein highly enriched in dendritic spines [3], regulates the formation and function of the spines [59]. Using a shotgun proteomic approach, Baucum et al. showed that spinophilin interacts via its N-terminal domain with NFL, NFM, and alpha-internexin in mouse striatum [13, 14, 79]. Moreover, the association increased significantly during mouse brain maturation [14] during the period of synaptogenesis [2] and was dependent on the phosphorylation state of either spinophilin or NF protein [79]. The NFL subunit also directly binds to tau [118], a protein initially considered to localize in dendritic spines only under pathological conditions [86], but is not believed to have a physiological role in dendritic functioning [65, 90]. Knockdown of tau using specific shRNA significantly decreased the spine density of cultured hippocampal neurons [36] and deletion of tau in mice abolished long-term depression [95]. Thus the interaction between NFL and tau in dendritic spine could be important for normal synaptic transmission.
NF polymers are highly abundant in myelinated axons as estimated by conventional electron microscopy but 10nm filaments have also been noted in the perikarya and dendrites of neurons, including ones lacking a myelinated axon [55, 81, 98, 196]. In 1977, Blomberg et al. reported 10 nm filaments as major constituents of the partially broken up PSD isolated from dog cerebral cortex and assumed that they were composed of NF proteins [21]. Using a rapid-freezing technique, Landis and Reese described infrequent 9–10nm filaments in dendritic spines of mouse brain but interpreted these structures as actin filaments due to their periodicity [104]. Yuan et al. also identified in some synapses that short 10 nm filaments were decorated by immunogold antibodies to different NF subunits indicating that at least some synaptic NF subunits exist in polymerized form (Yuan et al. 2015b)[193]. Although NF proteins are present in both pre- and postsynaptic areas, 10nm NF polymers are not abundant in synaptic areas compared to myelinated axons, estimated by conventional electron microscopy. The infrequency of conventional long intermediate-sized polymers in synapses indicates that most NF subunits in synapses are probably in the form of oligomeric structures (protofilaments or protofibrils). Consistent with this interpretation, transport of NFM in non-filamentous, but oligomeric form, has been reported [166, 184, 186]. The organization of oligomeric NF assemblies in synapses, quite different from those parallel arrays with spacing controlled by C-terminal extensions of NFH/MFM in axons, might be closely associated with the synaptic actin network since disruption of such actin filaments also remarkably affects the distribution of NF assemblies [195]. The relative abundance of NF proteins in synapses is synapse type-dependent, e.g., NF proteins are not present at the postsynaptic sites of neuromuscular junctions. Instead, another intermediate filament protein desmin is highly concentrated near the AChR-rich crests of the junctional folds at the neuromuscular junctions [154].
2.2 NF subunit-specific modulation of synaptic functions and behavior
N-methyl-D-aspartate (NMDA) receptors are excitatory neurotransmitter receptors critical for synaptic plasticity and neuronal development in the mammalian brains [107]. These receptors are highly concentrated in postsynaptic membranes of glutamatergic synapses. Yeast-two-hybrid screening revealed that NFL interacts through its rod domain directly with the cytoplasmic C-terminal domain of NR1 [56] suggesting that NFs may anchor or localize NMDA receptors within the neuronal plasma membrane. Co-expression of NFL with either NR1 or NR2B subunits in HEK293 cells did not increase surface expression of these subunits whereas expression of all three components increased surface abundance of NR1 by 20% and concomitantly increased NMDA-mediated cytotoxicity [144]. The NR1 subunit is ubiquitinated in HEK293 cells and the co-expression with NFL antagonizes this ubiquitination process, suggesting another way that the interaction of NFL with NR1 may stabilize the NMDA receptor. NFL also binds to protein phosphatase-I (PP1), a protein /serine /threonine phosphatase in the PSD [168]. NFL-bound PP1 could regulate the phosphorylation states of NF subunits and NMDA NR1 receptors the cellular distribution of which is regulated by the phosphorylation of specific serines in the C1 exon cassette [57]. On the one hand, NFL may modulate synaptic plasticity through interactions with NR1, PP1 or 14-3-3 [56, 115, 168]. On the other hand, the phosphorylation state of NFL is regulated by synaptic plasticity events such as long-term potentiation (LTP) [78] and long-term potentiation depression (LTD) [77]. NFL selectively influences dendritic spine and glutamate receptor function since NFL-null mice not only have abnormal spines but also dysfunction of hippocampal-dependent spatial memory, NMDA receptor protein expression and synaptic plasticity (Yuan, Veeranna et al. unpublished data). LTP is a persistent increase in synaptic strength following high-frequency stimulation of a chemical synapse and is generally considered one of the major cellular mechanisms that underlie learning and memory [20]. Yuan et al. recently determined LTP in the Schaffer collateral pathway of the hippocampal slices prepared from wild-type control and IHL-TKO mice lacking alpha-internexin, NFH and NFL subunits [192]. The tetanic stimulation evoked a typical LTP of fEPSP in slices from wild-type mice. These responses were stable for 120 min. However, tetanic stimulation evoked a significantly reduced fEPSP slopes in IHL-TKO mice, indicating NF proteins are, therefore, required for synaptic plasticity. Because LTP in the Schaffer collateral pathway of the hippocampus is NMDA receptor-dependent, altered LTP may be associated with reduced levels of NMDA-NR1 receptor (Yuan, Veeranna et al. unpublished data). Yuan et al. also conducted a 5-trial social memory assay to determine if IHL-TKO mice have a social memory defect. In this test, the subject mouse was given four brief exposures (trials 1–4) in its home cage to the same stimulus mouse (intruder). In the 5th trial, the subject mouse encountered an entirely novel stimulus mouse (novel intruder). Wild-type control mice displayed normal social memory, as demonstrated by a marked habituation (decreased exploration) during the first 4 trials and a striking dishabituation (increased exploration) on the presentation of a novel animal on the 5th trial. In contrast, IHL-TKO mice showed no significant habituation during the 4 exposures to the stimulus mouse or dishabituation to the novel stimulus mouse. Consistent with reduced LTP, IHL-TKO showed social interaction deficits, indicating that mice lacking NF proteins failed to develop normal social memory.
Dopamine receptors are G-protein-coupled receptors that mediate functions of dopamine ranging from voluntary movement and reward to hormonal regulation and hypertension [72]. These receptors are highly concentrated in the postsynaptic membrane of dopaminergic synapses. Yeast-two-hybrid screening has shown that the C-terminal tail of NFM directly interacts with the cytoplasmic loop of dopamine D1receptor [94] and that their co-expression in HEK-293 cells resulted in more than 50% reduction of receptor binding. Recently, the relevance of this interaction at synapses was established in genetically modified mice. Deletion of NFM, but not deletion of any of the other NF subunits, greatly amplified dopamine D1-receptor-mediated motor responses to cocaine while redistributing postsynaptic D1-receptors from endosomes to the plasma membrane (Figure 2)[193]. In wild-type mice, NFM colocalized with the D1 receptor in synaptic boutons by immunogold electron microscopy. Deletion of NFM also significantly increased D1R–stimulated hippocampal LTP further indicating an in vivo interaction between NFM and D1R [193]. Depressed hippocampal LTP induction is NF subunit-selective: maintenance of LTP is deficient in NFH-null mice while basal neurotransmission and induction of LTP are normal in NFM-null mice. Eliminating all NF proteins from the CNS profoundly disrupted synaptic plasticity and social memory without altering the structural integrity of synapses [193].
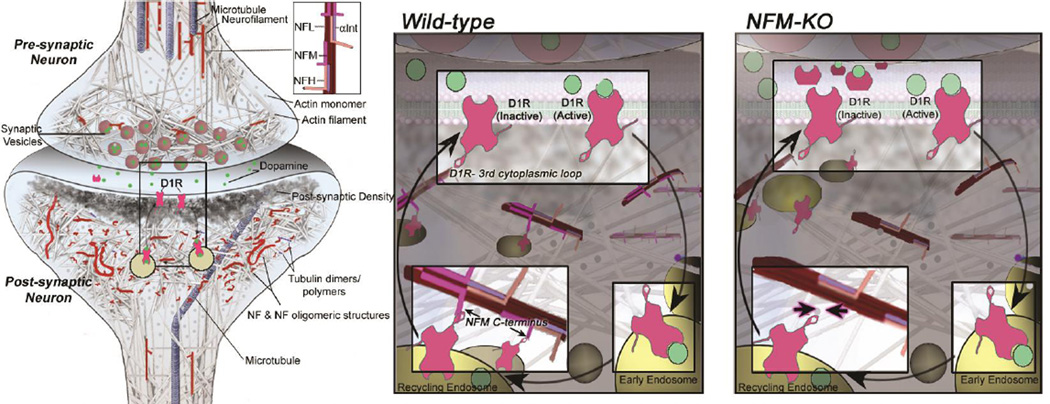
Figure 2. Model of D1R–containing endosomes anchored on NFM-containing cytoskeletal assemblies.
Based on collective findings on NF scaffolding functions and our D1R data on NF subunit null mice, we propose a model by which NFM acts in synaptic terminals to anchor D1R–containing endosomes formed after agonist-induced internalization of membrane D1R. Retention of D1R in a readily available internal pool within the synapse would favor desensitization to D1R stimulation: in the absence of NFM, the greater recycling back to the plasma membrane surface would favor hypersensitivity to D1R agonists, as observed in our in vivo studies (adapted from Yuan et al. Mol Psychiatry 2016; 20:986–994 with permission).
Peripherin, a subunit of peripheral nerve NFs, is almost exclusively expressed in the neurons of PNS and in the CNS only in defined subsets of neurons directly projecting to the periphery [25, 189]. However, brain injuries such as stab lesions and focal ischemia can trigger peripherin expression in CNS neurons normally silent for this gene [15]. Although definitive evidence for the presence of peripherin in synapses in the CNS is lacking, electrophysiological studies revealed synaptic plasticity was markedly altered in transgenic mice over-expressing the normal peripherin gene under its own promoter [101]. LTP was 50% greater in CA3 and 50% diminished in CA1 of hippocampus of the mutant mice than in wild-type controls. These data indicate a role for peripherin as a mediator of plasticity of hippocampal synapses potentially relevant to cognitive diseases. Peripherin was recently shown to be a strong binding partner of Rab-7A mutations of which are capable of altering the ratio of soluble and insoluble peripherin in vitro and are causative of CMT2B [42], indicating that peripherin may also have a role in vesicular transport. More recent studies involving yeast two-hybrid screens established interactions of peripherin and SNAP25-interacting protein SIP30 through coiled-coil domains [71] further suggesting a potential important role of peripherin in regulating vesicular trafficking at the synapses.
3. Alterations of NF proteins in psychiatric disorders
3.1. Schizophrenia and bipolar disorder
Schizophrenia, a severe chronic brain disorder affecting ∼1% of the population [136], is characterized by abnormal interpretation of reality and social behavior. The behavioral syndrome reflects a combination of positive symptoms (eg. hallucinations, delusions, and disordered thinking and behavior), negative symptoms (eg. reduced expression, feelings, and speech production), and cognitive deficits (eg. poor executive functioning, working memory, and attention). The causes of schizophrenia are unknown. Various alterations of neuronal and glial functions have been reported although loss of neurons or neurodegenerative change is not considered fundamental to disease development. Postmortem brains from individuals with schizophrenia are usually characterized by reduced dendritic spine density [70, 73, 165].
Pharmacological and biochemical evidence supports an important role of NMDA receptor hypofunction in the pathophysiology of schizophrenia [11, 44]. Many genetic risk factors for schizophrenia have been reported, including inherited common single nucleotide polymorphisms, copy number variants, rare single nucleotide variants and rare de novo variants. De novo genomic copy number variants known to substantially increase susceptibility to schizophrenia are enriched for members of the NMDA receptor postsynaptic signaling complex and are significantly more frequent in individuals with schizophrenia than in controls [96]. Fromer et al. confirmed these findings and further implicated abnormalities of synaptic cytoskeleton regulation and glutamatergic neurotransmission [67]. The fact that NFL, NFM and NFH genes map to chromosomal regions (8p21, 8p22 and 22q12, respectively) that are strongly implicated in schizophrenia suggests the involvement of NF proteins in this disease [8, 106]. As earlier noted, NFL in synapses [193] binds the NR1 receptor and may stabilize its presence in the cell membrane by preventing its ubiquitination and subsequent degradation [56, 144]. Besides binding NR1 to influence NMDA receptor function, NFL has also been shown to interact in a phosphorylation-dependent manner with 14-3-3 gamma [115], another protein implicated in schizophrenia [117, 171].
Of further potential relevance to NMDA receptor dysfunction in schizophrenia is a consistent body of evidence that points to selectively reduced levels of NFL subunits in brain regions essential for the cognitive and behavior functions affected in schizophrenia [100]. In dorsolateral prefrontal cortex (DLPFC), NFL transcript expression is significantly increased across all cortical isodense layers in DLPFC from elderly individuals with schizophrenia (mean patient age 80y) while levels of NFL protein are decreased about 50%, collectively suggesting possibly that turnover of NFL is abnormally high. Proteomic analysis of the DLPFC in schizophrenia revealed synaptic and metabolic abnormalities, including significantly reduced NFL (1.5-fold) and NFM levels (1.2-fold) without changes in NFH and alpha-internexin proteins [135]. Reduced NFL and NFM levels were also found in study of DLPFC deep white matter in schizophrenia (English et al.) and in the ACC (anterior cingulate cortex) white matter [39] where a 3-fold increase (p<0.05) in alpha-internexin was also measured. Similar changes in NFL and alpha-internexin levels were seen in another proteomic analysis of schizophrenic corpus callosum [157]. Although NFL transcription was significantly decreased in the thalamus of younger adult schizophrenics (mean age 43y), it was increased 25%-30% relative to controls, along with NFM levels, in elderly schizophrenics (mean age 70y) [41]. Collectively, these results implicate regional abnormalities of NFL transcription and /or accelerated NFL degradation in schizophrenia. NFL protein has been shown to be more vulnerable than NFM and NFH subunits to calcium-dependent proteases [199]. The changes observed in NFs may be part of the dynamic cytoskeletal remodeling in the pathogenesis of schizophrenia. Dramatic changes in the levels of NF proteins were also observed in axotomized motor and sensory neurons [84, 169, 183]. In light of evidence linking NFL to the functional activity of the NMDA receptor, the lowered levels of NFL could be a potential contributor to the NMDA hypofunction proposed to underlie various phenotypic features of schizophrenia.
Bipolar disorder is a brain disease that causes extreme mood swings that include emotional highs and lows. The exact cause of bipolar is unknown. Imaging and cellular studies have identified dysfunction of the dorsolateral prefrontal cortex in bipolar disease [54, 140, 162]. NFL transcripts are reported significantly reduced, along with NFM and alpha-internexin proteins [135], in adults with bipolar disorder (mean age 42y), but not in major depressive disorder [41]. Examining the differential protein expression in the deep white matter from DLPFC from individuals with bipolar disorder and controls, English et al. identified differences in 15% of proteins classified as cytoskeletal proteins which included increased NFM (1.66 fold, p= 0.03)[58].
Given that most studies of psychiatric disorders focusing on cytoskeletal changes analyzed brain areas enriched in white matter axons and the alterations of NF proteins may well reflect disturbed function of the axonal compartment. Given the ubiquity and abundance of synapses in CNS, it is also possible that even white matter analyses capture alterations of the significant synaptic NF protein pool in neuropsychiatric disorders. It is also reasonable to expect that future deeper analyses that include terminal regions of these same vulnerable brain regions and target additional relevant grey matter regions enriched with synaptic boutons, may identify dysfunction of the synaptic cytoskeletal network, including NF subunit interactions, more sensitively.
3.2. Drug addiction
Drug addiction is a chronic brain disorder that causes compulsive drug seeking and use despite adverse consequences. The mesolimbic pathway, which connects the ventral tegmental area (VTA) to the nucleus accumbens, has been implicated in common mechanisms of drug addition [139]. The mesolimbic pathway releases dopamine into the nucleus accumbens, where it affects motivation for rewarding stimuli. Drugs abused by humans such as morphine, cocaine, ethanol and nicotine have very different chemical structures but all preferentially increase synaptic dopamine concentrations in the mesolimbic system [53]. This is believed to be the neural substrate mediating the reinforcing properties of drugs of abuse. Given their diverse primary sites of action on cell surface receptors, these addictive drugs have to act indirectly on intracellular proteins as a convergence point to exert similar effects on mesolimbic dopamine function. Beitner-Johnson et al. first reported that NF proteins NFL, NFM and NFH were decreased 15–50% in the VTA by chronic administration of morphine or cocaine in rats [16]. The lowering of NF protein levels by these drugs was selective since the levels of 8 other major cytoskeletal or cytoskeletal-associated proteins did not change. Chronic exposure to psychotropic drugs lacking reinforcing properties did not alter NF levels. In separate studies, morphine administration caused a decrease of NFL immunoreactivity in rat cerebral cortex, which was antagonized completely by concurrent administration of naloxone [22]. Similar antagonism was achieved in three separate knockout mice of opioid receptors (mu or delta or kappa) [68], implicating all opioid receptor subtypes in these effects of morphine. Chronic morphine and cocaine also increased phosphorylation of NFH and NFM tail domains, an effect ablated by knockout of cortical mu-opioid receptors [68]. Interestingly, chronic cocaine, but not chronic morphine, lowered alpha-internexin levels in the VTA, hinting at somewhat different mechanisms at play for these two drugs, which are known to act at different receptors [16].
Rats inbred for alcohol preference expressed lower levels of NF subunits, suggesting that NF proteins in the VTA may influence preference for drugs [74, 75]. Examining its influence on NF proteins, GFAP and NMDA receptors, Ortiz et al. reported that chronic ethanol treatment significantly decreased NF protein levels in VTA (by 14%-37%) while increasing GFAP (by 40%) and NMDA NR1 subunit (by 27%) [131]. Similar to other drugs of abuse, nicotine activates the mesolimbic pathway by increasing cell firing of dopaminergic neurons in the VTA via nicotinic receptors [113]. Nicotine microinfusions into the VTA which resulted in sensitization of dopamine release [10], also reduced NFL (by 34%, p<0.05), NFM (by 42%, p<0.01), and NFH levels (by 38%, p<0.05) in the VTA but not in the substantia nigra in rats [28, 150] without altering neuron numbers. The similar reductions of NF proteins in the VTA by the chronic treatment of different drugs of abuse with reinforcing properties suggest that common mechanisms underlie these addictive states.
Other brain targets, such as frontal cortex [29, 156], also show lowered NFL levels (47%, p < 0.001) in brains of addicts dying from opiate overdose [69]. Similar NFL reductions (49%, p<0.001) are also seen in the frontal cortex of rats after chronic morphine treatment. Narayana et al. reported immunohistochemical evidence for a significant decrease in NFH expression in the fimbria and internal capsule of rats after chronic cocaine administration [123]. Analysis of NF phosphorylation in the prefrontal cortex of human opioid addicts uncovered significantly reduced nonphosphorylated forms of NFH, NFM, and NFL and corresponding increases in phosphorylated forms [61, 62]. Similar changes are seen in brain regions of rats chronically treated with cocaine [109]. Acute morphine exposure also induces a marked increase in phosphorylated NFH whereas chronic morphine administration followed by opiate withdrawal results in a time-dependent decline in phosphorylated NFH [32, 91]. A recent study found chronic morphine causes persistent nitration of NFL in cortex and subcortex in mice even during abstinence [132], although its significance remains unclear.
Since PP2A has been shown to be the most effective in the nervous system in dephosphorylating the sites in NFH known to be phosphorylated by cdk5 [177], this phosphatase together with cdk5 were also examined. Surprisingly, the levels of PP2A were found unchanged while the levels of cdk5 and its neuron-specific activator p35 in the prefrontal cortex of human opioid addicts were decreased by 18% and 26–44%, respectively [62]. In these brains, phosphorylated NFH significantly correlated with p35 but not with cdk5. Further studies showed that acute treatment of rats with morphine increased the density of cdk5 by 35% in the cerebral cortex. In contrast to the acute effects, chronic morphine induced a marked decrease in cdk5 by 40% and p35 by 47% in rat brain [62]. It therefore appears that, despite the possible stimulating effect of the deadly opiate overdose on cdk5, the reduced expression of the cdk5/p35 complex in brains of opioid addicts is the net result of a chronic opiate effect. These results indicate that opioid addiction is associated with down regulation of cdk5/p35 levels in the brain and the hyperphosphorylation of NFH is not the result of a reduced dephosphorylation process. Although the abundance of cdk5 was decreased in the brains from chronic opioid abusers, Narita et al. showed that the level of phosphorylated cdk5 (serine-159) immunoreactivity in the frontal cortex was significantly increased by chronic morphine treatment [124] and phosphorylation of cdk5 at serine-159 dramatically increases cdk5 activation [155]. They also demonstrated that the treatment of selective cdk5 inhibitor, roscovitine and a half deletion of the cdk5 gene caused a significant inhibition of the rewarding effect of morphine. In addition, chronic cocaine or morphine administration also activates extracellular signal-regulated kinase (ERK), a known NF protein kinase [176], by promoting its phosphorylation [18, 174]. Altogether, these data indicate the hyperphosphorylation of NF proteins under these conditions may be related to the up-regulation of phosphorylated cdk5 and increased ERK activity.
D1R and NMDA NR1 receptors are assembled as an oligomeric complex and are functionally interdependent [63, 105]. Both receptors bind to NF proteins [56, 94] and are also involved in drug addiction [43, 173]. Cocaine has been shown to reduce the D1R-NR1 physical interaction and may influence intracellular signaling [164]. On the one hand, D1R is a primary target of stimulant drugs and chronic exposure to drugs of abuse lower levels of NF proteins and alter their phosphorylation state [16]. On the other hand, NMDA receptor inhibition increases the phosphorylation state and the solubility of NFM protein [64]. Glutamate has been shown to inhibit NF transport, which can be reversed by NMDA receptor blocker MK-801 [1]. Over-activation of primarily NMDA receptors by glutamate leads to rapid disruption of NF proteins in cultured neurons [37]. The basis for these NF changes and their relationship to synaptic function remain elusive. As discussed above, our recent studies demonstrated that deletion of NFM in mice amplifies D1R–mediated motor responses to cocaine while redistributing postsynaptic D1R from endosomes to the plasma membrane, indicating a role of this NF subunit in drug addiction [193].
4. Alterations of NF proteins in Alzheimer’s disease
Alzheimer’s disease is an irreversible, degenerative brain disorder that progressively destroys memory and other mental functions. Early striking loss of synaptic connections within brain regions involved in memory and thinking skills is followed by loss of neurons of many types. Accompanying neurodegenerative changes is the development of defining neuropathological hallmarks of AD, neuritic plaques, and neurofibrillary tangles, along with extracellular deposition of β-amyloid. The causes of the most common late-onset forms of AD (more than 95% of all cases) are not fully understood: the much less common early-onset familial forms (less than 5% of all cases) are caused by mutations of either of three genes, amyloid precursor protein (APP) and presenilins 1 and 2(PSEN1 and PSEN2) [76, 175]. These causative genes and other risk factors influence the production and clearance of APP metabolites that mediate APP signaling cascades and may also have cytotoxic properties.
An important feature of AD is the disruption of the neuronal cytoskeleton leading to formation of neurofibrillary tangles (NFTs) [27, 102] numbers of which correlate well with neurodegeneration and severity of cognitive decline in AD [5, 19, 23]. The main constituents of tangles are paired helical filaments (PHF), initially believed to be composed of NF proteins [4, 47, 88] and later shown to be mainly fibrillar aggregates of tau protein [99, 103, 128]. Although NF proteins were somewhat ignored as tangle constituents, data has amply confirmed that NF proteins are integral components of the NFTs [138]. Most recently, mass spectrometry analysis by Pant and colleagues confirmed the presence of NFM and NFH in purified NFTs from AD brain independently of antibody cross-reaction between NF protein and tau [147]. The NFTs are far more complex than that of tau - PHFs. They also contain abundant NF proteins, vimentin, phosphorylated MAP1, phosphorylated MAP2, nonphosphorylated MAP1B, nonphosphorylated MAP2, nonphosphorylated MAP4 and nonphosphorylated MAP6. The question of whether the formation of tau-PHF is the initiating event in NFT formation has not been thoroughly addressed and the possibility that another NFT constituent, like NF protein, may initiate the process has not been excluded. In this regard, Morrison and colleagues reported that, certain populations of cortico-cortical pyramidal neurons that contain abundant perikaryal NF in relatively low states of phosphorylation are highly vulnerable to neurodegeneration through NFT formation [82, 83, 121, 178, 179]. More recent studies have provided independent evidence for loss of SMI32 immunopositive neurons in temporal cortical areas of AD brain [170]. The loss of SMI32 immunoreactivity on cortical regions of AD brain is often paralleled by increase in NFTs and AT8-positive tau immunoreactivity in neurons [121], indicating nonphosphorylated NF proteins may play a protective role in preventing the formation of NFTs. In fact, it is suggested that the development of neurofibrillary pathology may begin with abnormal NF accumulations in damaged distal processes followed by reactive perikaryal cytoskeletal changes that ultimately lead to tau hyperphosphorylation and NFT formation [178]. The co-localization of caspase-3 cleaved spectrin with nonphosphorylated NF-immunoreactive neurons in AD suggests that caspase-3 activation may be a pathological event responsible for the loss of these vulnerable neurons [6].
NFL mRNA is significantly reduced in AD cortex [38, 114] [97] and CA1 and CA2 regions of hippocampus [159]. Like NFL, NFM mRNA was also significantly decreased while NFH mRNA was unaltered in AD temporal cortex as compared to controls [97]. Also significantly increased in AD are NFH (by 1.7-fold), NFM (by 1.5-fold) and NFL proteins (by 1.6-fold) and the degree of phosphorylation of NFH (by 1.6–2.7-fold) and NFM (by 1.3–1.9-fold) as compared to Huntington disease [182]. Proteomic studies revealed increases of alpha-internexin and NFM proteins in the postsynaptic densities isolated from AD cortex as compared to controls with the increase of alpha-internexin being validated by Western blots [197]. Using quantitative phosphoproteomic methodology, Pant and colleagues recently reported 13 hyperphosphorylated sites in the C-terminal domain of NFM and 10 hyperphosphorylated sites of that of NFH in the frontal cortex of AD brain and all of these sites are in a higher state of phosphorylation in AD (4–8-fold higher) compared with control brains [146]. Surprisingly, the abundance and number of KSP repeat hyperphosphorylation of NFM are significantly higher compared with NFH, even though potential phosphorylation sites in NFH are more numerous. Based on the NF stoichiometry [4:2:2:1 (NFL:alpha-internexin:NFM:NFH)] [187], there are 2-fold more KSP repeat sites hyperphosphorylated in NFM compared with NFH, indicating that NFM might contribute more to aberrant NF phosphorylation in AD compared with NFH.
Additional abnormalities of NF have also been observed in AD. Significantly decreased O-GlcNAcylation of NFM was observed in AD cortex as compared to controls [51] while carbonyl-related posttranslational modification of NFH protein was also present in the AD NFTs [158]. Chapman et al. showed that sera of AD patients contain antibodies that bind specifically to NFH of Torpedo cholinergic neurons [35]. AD patients also have elevated intrathecal synthesis of anti-NFH antibody [12]. Behavioral tests revealed that rats immunized with cholinergic NFH for 12 month performed significantly worse than controls in in T-maze alternation test [34]. This prolonged immunization of rats with NFH results in cognitive impairment, IgG accumulation in the septum, hippocampus and entorhinal cortex with marked loss of neurons in the septum [130]. Interestingly, this cognitive impairment can be reversed by the acetylcholinesterase inhibitor physostigmine administered 30 min prior to testing [116]. These findings suggest that NFH may play a role in the pathogenesis of AD neurons.
Further support for NF protein involvement in AD pathogenesis comes from genetic studies. Deletion of NFL or NFH subunits in T44 mice overexpressing htau resulted in a dramatic decrease in the total number of tau-positive spheroids in the spinal cord and brainstem, indicating a role of NF proteins in the pathogenesis of neurofibrillary tau pathology [89]. Deletion of NFL in APP/PS1 transgenic mice, unexpectedly, increased neocortical β-amyloid deposition, synapse vulnerability and microgliosis surrounding β-amyloid plaques, suggesting a protective role of this NF subunit in AD [60].
The abundance of NFs in axons and the frequency of axonal injury in neurodegenerative disease has led to the use of NF protein measurements as a clinical index of brain injury in various neurological disorders. Higher than normal levels of NFL and NFH in CSF have been reported in AD [24, 87] and correlate with disease severity in a recent study of frontotemporal dementia [153]. CSF NFL concentration is increased by the early clinical stage of AD, correlates with cognitive deterioration and structural changes over time [194] and may be a useful marker of disease progression in AD [7].
Although amyloid plaques and tau tangles are two hallmarks of Alzheimer’s disease, synaptic loss is more robustly correlated than these pathological lesions with cognitive deficits [50, 167]. Synaptic loss is more pronounced in the hippocampus [152] than in temporal and frontal cortex [48] and disturbance of synaptic integrity occurs very early on in the process of AD [111]. Ultrastructural stereological studies on rapid postmortem autopsy samples showed an 18% synapse loss in the hippocampal CA1 region of MCI patients that progressed to a 55% synapse loss in mild AD [151]. Since oligomeric NF proteins are present in synapses where they interact with synaptic proteins, loss of synapses in early AD could affect the level of this synaptic NF pool and may thus contribute to the increased NF protein levels in CSF and blood [7]. Alterations of NF proteins in early AD could also reflect synaptic reorganization while changes of NF subunits in late AD may suggest reorganization of both synaptic and axonal pools.
5. Conclusion
The etiology of major neuropsychiatric disorders critically involves dysfunction of synapses and, in late-onset dementias, the ultimate loss of synapses early in the disease. Relatively little attention, however, has been paid to the dynamics of cytoskeletal proteins at synaptic terminals and particularly the possible synaptic involvement of NF proteins. Until recently, the functioning of NF protein assemblies within synapses has been unappreciated and the focus has been on their axonal roles (eg. radial growth) and disruption in axonopathies involving mainly neurons with long myelinated axons that contain abundant NFs. In the CNS, however, even axons in major fiber tracts like the corpus callosum change minimally in caliber in NF triple knockout mice nearly completely lacking all 4 CNS NF subunits. This finding underscores growing evidence that NF proteins in the CNS have important functions beyond caliber expansion. Emerging evidence shows that NF proteins are present and functional in synapses. The synaptic population of NF proteins is distinctive in phosphorylation state, likely present in unconventional stoichiometries and assembly forms including oligomeric structures, and possibly more plastic in exchanging subunits and altering form in response to signals. Further evidence that individual subunits differentially modulate neurotransmission and behavior through interactions with specific neurotransmitter receptors heightens expectations that alterations of NF proteins could influence synaptic function in psychiatric disorders and dementias. Despite the very frequent occurrence of NF protein changes in multiple major psychiatric states, most of the analyses have been on white matter tracts and the changes seen, often involving levels of only one or two subunit, have been difficult to interpret mechanistically from a perspective of changes in the axonal NF cytoskeleton. The newly uncovered sizable population of synaptic NF proteins should encourage more directed attention to regional analyses that interrogate this synaptic NF protein pool and will potentially yield valuable new insights into the significance of NF subunits in neuropsychiatric disease.
Highlights.
Distinctive assemblies of neurofilament subunits are integral components of synapses
Synaptic neurofilament proteins are a sizable population in the CNS
NF subunits modulate neurotransmission and behavior via interactions with receptors
Alterations of NF subunits may contribute to neuropsychiatric dysfunction
Acknowledgments
This work was supported by Grant 5R01AG005604 from the National Institutes on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
References
- 1.Ackerley S, Grierson AJ, Brownlees J, Thornhill P, Anderton BH, Leigh PN, Shaw CE, Miller CC. Glutamate slows axonal transport of neurofilaments in transfected neurons. J Cell Biol. 2000;150:165–176. doi: 10.1083/jcb.150.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghajanian GK, Bloom FE. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain research. 1967;6:716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- 3.Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci U S A. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderton BH, Breinburg D, Downes MJ, Green PJ, Tomlinson BE, Ulrich J, Wood JN, Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982;298:84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 6.Ayala-Grosso C, Tam J, Roy S, Xanthoudakis S, Da Costa D, Nicholson DW, Robertson GS. Caspase-3 cleaved spectrin colocalizes with neurofilament-immunoreactive neurons in Alzheimer’s disease. Neuroscience. 2006;141:863–874. doi: 10.1016/j.neuroscience.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 7.Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, Staufenbiel M, Neumann M, Maetzler W, Kuhle J, Jucker M. Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron. 2016 doi: 10.1016/j.neuron.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 9.Bajo M, Yoo BC, Cairns N, Gratzer M, Lubec G. Neurofilament proteins NF-L, NF-M and NF-H in brain of patients with Down syndrome and Alzheimer’s disease. Amino Acids. 2001;21:293–301. doi: 10.1007/s007260170015. [DOI] [PubMed] [Google Scholar]
- 10.Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- 11.Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A. 2013;110:E2400–E2409. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartos A, Fialova L, Svarcova J, Ripova D. Patients with Alzheimer disease have elevated intrathecal synthesis of antibodies against tau protein and heavy neurofilament. J Neuroimmunol. 2012;252:100–105. doi: 10.1016/j.jneuroim.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Baucum AJ, 2nd, Jalan-Sakrikar N, Jiao Y, Gustin RM, Carmody LC, Tabb DL, Ham AJ, Colbran RJ. Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach. Mol Cell Proteomics. 2010;9:1243–1259. doi: 10.1074/mcp.M900387-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baucum AJ, 2nd, Strack S, Colbran RJ. Age-dependent targeting of protein phosphatase 1 to Ca2+/calmodulin-dependent protein kinase II by spinophilin in mouse striatum. PLoS One. 2012;7:e31554. doi: 10.1371/journal.pone.0031554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaulieu JM, Kriz J, Julien JP. Induction of peripherin expression in subsets of brain neurons after lesion injury or cerebral ischemia. Brain research. 2002;946:153–161. doi: 10.1016/s0006-8993(02)02830-5. [DOI] [PubMed] [Google Scholar]
- 16.Beitner-Johnson D, Guitart X, Nestler EJ. Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:2165–2176. doi: 10.1523/JNEUROSCI.12-06-02165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson DL, Mandell JW, Shaw G, Banker G. Compartmentation of alpha-internexin and neurofilament triplet proteins in cultured hippocampal neurons. J Neurocytol. 1996;25:181–196. doi: 10.1007/BF02284795. [DOI] [PubMed] [Google Scholar]
- 18.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 20.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 21.Blomberg F, Cohen RS, Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977;74:204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boronat MA, Garcia-Fuster MJ, Garcia-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001;134:1263–1270. doi: 10.1038/sj.bjp.0704364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiology of aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- 24.Brettschneider J, Petzold A, Schottle D, Claus A, Riepe M, Tumani H. The neurofilament heavy chain (NfH) in the cerebrospinal fluid diagnosis of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:291–295. doi: 10.1159/000091436. [DOI] [PubMed] [Google Scholar]
- 25.Brody BA, Ley CA, Parysek LM. Selective distribution of the 57 kDa neural intermediate filament protein in the rat CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9:2391–2401. doi: 10.1523/JNEUROSCI.09-07-02391.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brownlees J, Ackerley S, Grierson AJ, Jacobsen NJ, Shea K, Anderton BH, Leigh PN, Shaw CE, Miller CC. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum Mol Genet. 2002;11:2837–2844. doi: 10.1093/hmg/11.23.2837. [DOI] [PubMed] [Google Scholar]
- 27.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 28.Bunnemann B, Terron A, Zantedeschi V, Merlo Pich E, Chiamulera C. Chronic nicotine treatment decreases neurofilament immunoreactivity in the rat ventral tegmental area. Eur J Pharmacol. 2000;393:249–253. doi: 10.1016/s0014-2999(00)00104-7. [DOI] [PubMed] [Google Scholar]
- 29.Busquets X, Escriba PV, Sastre M, Garcia-Sevilla JA. Loss of protein kinase C-alpha beta in brain of heroin addicts and morphine-dependent rats. Journal of neurochemistry. 1995;64:247–252. doi: 10.1046/j.1471-4159.1995.64010247.x. [DOI] [PubMed] [Google Scholar]
- 30.Cairns NJ, Perry RH, Jaros E, Burn D, McKeith IG, Lowe JS, Holton J, Rossor MN, Skullerud K, Duyckaerts C, Cruz-Sanchez FF, Lantos PL. Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neurosci Lett. 2003;341:177–180. doi: 10.1016/s0304-3940(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 31.Cairns NJ, Zhukareva V, Uryu K, Zhang B, Bigio E, Mackenzie IR, Gearing M, Duyckaerts C, Yokoo H, Nakazato Y, Jaros E, Perry RH, Lee VM, Trojanowski JQ. alpha-internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol. 2004;164:2153–2161. doi: 10.1016/s0002-9440(10)63773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao MH, JI FT, He HY, Liu FN, Liang JJ, L L. Phosphorylation of tau and neurofilament in prefrontal cortex of rat brain after acute and chronic morphine administration. Chinese Journal of Pathophysiology. 2010;26:1718–1721. [Google Scholar]
- 33.Chan SO, Peng D, Chiu FC. Heterogeneous expression of neurofilament proteins in forebrain and cerebellum during development: clinical implications for spinocerebellar ataxia. Brain research. 1997;775:107–118. doi: 10.1016/s0006-8993(97)00834-2. [DOI] [PubMed] [Google Scholar]
- 34.Chapman J, Alroy G, Weiss Z, Faigon M, Feldon J, Michaelson DM. Anti-neuronal antibodies similar to those found in Alzheimer’s disease induce memory dysfunction in rats. Neuroscience. 1991;40:297–305. doi: 10.1016/0306-4522(91)90121-4. [DOI] [PubMed] [Google Scholar]
- 35.Chapman J, Bachar O, Korczyn AD, Wertman E, Michaelson DM. Alzheimer’s disease antibodies bind specifically to a neurofilament protein in Torpedo cholinergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9:2710–2717. doi: 10.1523/JNEUROSCI.09-08-02710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Q, Zhou Z, Zhang L, Wang Y, Zhang YW, Zhong M, Xu SC, Chen CH, Li L, Yu ZP. Tau protein is involved in morphological plasticity in hippocampal neurons in response to BDNF. Neurochem Int. 2012;60:233–242. doi: 10.1016/j.neuint.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Chung RS, McCormack GH, King AE, West AK, Vickers JC. Glutamate induces rapid loss of axonal neurofilament proteins from cortical neurons in vitro. Exp Neurol. 2005;193:481–488. doi: 10.1016/j.expneurol.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Clark AW, Krekoski CA, Parhad IM, Liston D, Julien JP, Hoar DI. Altered expression of genes for amyloid and cytoskeletal proteins in Alzheimer cortex. Annals of neurology. 1989;25:331–339. doi: 10.1002/ana.410250404. [DOI] [PubMed] [Google Scholar]
- 39.Clark D, Dedova I, Cordwell S, Matsumoto I. Altered proteins of the anterior cingulate cortex white matter proteome in schizophrenia. Proteomics Clin Appl. 2007;1:157–166. doi: 10.1002/prca.200600541. [DOI] [PubMed] [Google Scholar]
- 40.Clinton SM, Abelson S, Haroutunian V, Davis KL, Meador-Woodruff JH. Neurofilament subunit protein abnormalities in the thalamus in schizophrenia. Thalamus & Related System. 2004;4:265–272. [Google Scholar]
- 41.Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA Receptor and Associated Intracellular Molecules in the Thalamus in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. 2004;29:1353–1362. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- 42.Cogli L, Progida C, Thomas CL, Spencer-Dene B, Donno C, Schiavo G, Bucci C. Charcot-Marie-Tooth type 2B disease-causing RAB7A mutant proteins show altered interaction with the neuronal intermediate filament peripherin. Acta neuropathologica. 2013;125:257–272. doi: 10.1007/s00401-012-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comings DE, Gade R, Wu S, Chiu C, Dietz G, Muhleman D, Saucier G, Ferry L, Rosenthal RJ, Lesieur HR, Rugle LJ, MacMurray P. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Mol Psychiatry. 1997;2:44–56. doi: 10.1038/sj.mp.4000207. [DOI] [PubMed] [Google Scholar]
- 44.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crino PB, Eberwine J. Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 46.Crispino M, Capano CP, Kaplan BB, Giuditta A. Neurofilament proteins are synthesized in nerve endings from squid brain. Journal of neurochemistry. 1993;61:1144–1146. doi: 10.1111/j.1471-4159.1993.tb03632.x. [DOI] [PubMed] [Google Scholar]
- 47.Dahl D, Selkoe DJ, Pero RT, Bignami A. Immunostaining of neurofibrillary tangles in Alzheimer’s senile dementia with a neurofilament antiserum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2:113–119. doi: 10.1523/JNEUROSCI.02-01-00113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. Journal of the neurological sciences. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 49.de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- 50.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Annals of neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 51.Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, Gong CX. Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J. 2008;22:138–145. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dequen F, Cairns NJ, Bigio EH, Julien JP. Gigaxonin mutation analysis in patients with NIFID. Neurobiology of aging. 2011;32:1528–1529. doi: 10.1016/j.neurobiolaging.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 55.Dyakin VV, Chen Y, Branch CA, Yuan A, Rao M, Kumar A, Peterhoff CM, Nixon RA. The contributions of myelin and axonal caliber to transverse relaxation time in shiverer and neurofilament-deficient mouse models. Neuroimage. 2010;51:1098–1105. doi: 10.1016/j.neuroimage.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehlers MD, Fung ET, O’Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehlers MD, Tingley WG, Huganir RL. Regulated subcellular distribution of the NR1 subunit of the NMDA receptor. Science. 1995;269:1734–1737. doi: 10.1126/science.7569904. [DOI] [PubMed] [Google Scholar]
- 58.English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009;9:3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- 59.Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez-Martos CM, King AE, Atkinson RA, Woodhouse A, Vickers JC. Neurofilament light gene deletion exacerbates amyloid, dystrophic neurite, and synaptic pathology in the APP/PS1 transgenic model of Alzheimer’s disease. Neurobiology of aging. 2015;36:2757–2767. doi: 10.1016/j.neurobiolaging.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Ferrer-Alcon M, Garcia-Sevilla JA, Jaquet PE, La Harpe R, Riederer BM, Walzer C, Guimon J. Regulation of nonphosphorylated and phosphorylated forms of neurofilament proteins in the prefrontal cortex of human opioid addicts. J Neurosci Res. 2000;61:338–349. doi: 10.1002/1097-4547(20000801)61:3<338::AID-JNR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 62.Ferrer-Alcon M, La Harpe R, Guimon J, Garcia-Sevilla JA. Downregulation of neuronal cdk5/p35 in opioid addicts and opiate-treated rats: relation to neurofilament phosphorylation. Neuropsychopharmacology. 2003;28:947–955. doi: 10.1038/sj.npp.1300095. [DOI] [PubMed] [Google Scholar]
- 63.Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- 64.Fiumelli H, Riederer IM, Martin JL, Riederer BM. Phosphorylation of neurofilament subunit NF-M is regulated by activation of NMDA receptors and modulates cytoskeleton stability and neuronal shape. Cell Motil Cytoskeleton. 2008;65:495–504. doi: 10.1002/cm.20278. [DOI] [PubMed] [Google Scholar]
- 65.Frandemiche ML, De Seranno S, Rush T, Borel E, Elie A, Arnal I, Lante F, Buisson A. Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-beta oligomers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:6084–6097. doi: 10.1523/JNEUROSCI.4261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friede RL, Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970;167:379–387. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- 67.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Sevilla JA, Ferrer-Alcon M, Martin M, Kieffer BL, Maldonado R. Neurofilament proteins and cAMP pathway in brains of mu-, delta- or kappa-opioid receptor gene knock-out mice: effects of chronic morphine administration. Neuropharmacology. 2004;46:519–530. doi: 10.1016/j.neuropharm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Sevilla JA, Ventayol P, Busquets X, La Harpe R, Walzer C, Guimon J. Marked decrease of immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. Neuroreport. 1997;8:1561–1565. doi: 10.1097/00001756-199705060-00003. [DOI] [PubMed] [Google Scholar]
- 70.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gentil BJ, McLean JR, Xiao S, Zhao B, Durham HD, Robertson J. A two-hybrid screen identifies an unconventional role for the intermediate filament peripherin in regulating the subcellular distribution of the SNAP25-interacting protein, SIP30. Journal of neurochemistry. 2014;131:588–601. doi: 10.1111/jnc.12928. [DOI] [PubMed] [Google Scholar]
- 72.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 73.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 74.Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse. 1992;12:242–253. doi: 10.1002/syn.890120310. [DOI] [PubMed] [Google Scholar]
- 75.Guitart X, Lumeng L, Li TK, Nestler EJ. Alcohol-preferring and nonpreferring rats display different levels of neurofilament proteins in the ventral tegmental area. Alcohol Clin Exp Res. 1993;17:580–585. doi: 10.1111/j.1530-0277.1993.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 76.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 77.Hashimoto R, Nakamura Y, Komai S, Kashiwagi Y, Matsumoto N, Shiosaka S, Takeda M. Phosphorylation of neurofilament-L during LTD. Neuroreport. 2000;11:2739–2742. doi: 10.1097/00001756-200008210-00026. [DOI] [PubMed] [Google Scholar]
- 78.Hashimoto R, Nakamura Y, Komai S, Kashiwagi Y, Tamura K, Goto T, Aimoto S, Kaibuchi K, Shiosaka S, Takeda M. Site-specific phosphorylation of neurofilament-L is mediated by calcium/calmodulin-dependent protein kinase II in the apical dendrites during long-term potentiation. Journal of neurochemistry. 2000;75:373–382. doi: 10.1046/j.1471-4159.2000.0750373.x. [DOI] [PubMed] [Google Scholar]
- 79.Hiday AC. Phosphorylation state modulates the interaction between spinophilin and neurofilament medium, in: Biology Department. Purdue University. 2015:96. [Google Scholar]
- 80.Hirao K, Hata Y, Deguchi M, Yao I, Ogura M, Rokukawa C, Kawabe H, Mizoguchi A, Takai Y. Association of synapse-associated protein 90/ postsynaptic density-95-associated protein (SAPAP) with neurofilaments. Genes Cells. 2000;5:203–210. doi: 10.1046/j.1365-2443.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- 81.Hirokawa N, Glicksman MA, Willard MB. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984;98:1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol. 1990;301:44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- 83.Hof PR, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: II. Primary and secondary visual cortex. The Journal of comparative neurology. 1990;301:55–64. doi: 10.1002/cne.903010106. [DOI] [PubMed] [Google Scholar]
- 84.Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987;84:3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holzer M, Holzapfel HP, Krohn K, Gertz HJ, Arendt T. Alterations in content and phosphorylation state of cytoskeletal proteins in the sciatic nerve during ageing and in Alzheimer’s disease. J Neural Transm (Vienna) 1999;106:743–755. doi: 10.1007/s007020050195. [DOI] [PubMed] [Google Scholar]
- 86.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu YY, He SS, Wang XC, Duan QH, Khatoon S, Iqbal K, Grundke-Iqbal I, Wang JZ. Elevated levels of phosphorylated neurofilament proteins in cerebrospinal fluid of Alzheimer disease patients. Neurosci Lett. 2002;320:156–160. doi: 10.1016/s0304-3940(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 88.Ihara Y, Nukina N, Sugita H, Toyokura Y. Staining of Alzheimer’s neurofibrillary tangles with antiserum against 200K component of neurofilament. Proc. Japan Acd. 1981;57:152–156. [Google Scholar]
- 89.Ishihara T, Higuchi M, Zhang B, Yoshiyama Y, Hong M, Trojanowski JQ, Lee VM. Attenuated neurodegenerative disease phenotype in tau transgenic mouse lacking neurofilaments. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6026–6035. doi: 10.1523/JNEUROSCI.21-16-06026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 91.Jaquet PE, Ferrer-Alcon M, Ventayol P, Guimon J, Garcia-Sevilla JA. Acute and chronic effects of morphine and naloxone on the phosphorylation of neurofilament-H proteins in the rat brain. Neurosci Lett. 2001;304:37–40. doi: 10.1016/s0304-3940(01)01729-3. [DOI] [PubMed] [Google Scholar]
- 92.Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- 93.Josephs KA, Holton JL, Rossor MN, Braendgaard H, Ozawa T, Fox NC, Petersen RC, Pearl GS, Ganguly M, Rosa P, Laursen H, Parisi JE, Waldemar G, Quinn NP, Dickson DW, Revesz T. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- 94.Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:5920–5930. doi: 10.1523/JNEUROSCI.22-14-05920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kimura T, Whitcomb DJ, Jo J, Regan P, Piers T, Heo S, Brown C, Hashikawa T, Murayama M, Seok H, Sotiropoulos I, Kim E, Collingridge GL, Takashima A, Cho K. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130144. doi: 10.1098/rstb.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Grozeva D, Fjodorova M, Wollerton R, Rees E, Nikolov I, van de Lagemaat LN, Bayes A, Fernandez E, Olason PI, Bottcher Y, Komiyama NH, Collins MO, Choudhary J, Stefansson K, Stefansson H, Grant SG, Purcell S, Sklar P, O’Donovan MC, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kittur S, Hoh J, Endo H, Tourtellotte W, Weeks BS, Markesbery W, Adler W. Cytoskeletal neurofilament gene expression in brain tissue from Alzheimer’s disease patients. I. Decrease in NF-L and NF-M message. J Geriatr Psychiatry Neurol. 1994;7:153–158. doi: 10.1177/089198879400700305. [DOI] [PubMed] [Google Scholar]
- 98.Kong J, Tung VW, Aghajanian J, Xu Z. Antagonistic roles of neurofilament subunits NF-H and NF-M against NF-L in shaping dendritic arborization in spinal motor neurons. J Cell Biol. 1998;140:1167–1176. doi: 10.1083/jcb.140.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–747. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 101.Kriz J, Beaulieu JM, Julien JP, Krnjevic K. Up-regulation of peripherin is associated with alterations in synaptic plasticity in CA1 and CA3 regions of hippocampus. Neurobiology of disease. 2005;18:409–420. doi: 10.1016/j.nbd.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 102.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 103.Ksiezak-Reding H, Dickson DW, Davies P, Yen SH. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987;84:3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Landis DM, Reese TS. Cytoplasmic organization in cerebellar dendritic spines. J Cell Biol. 1983;97:1169–1178. doi: 10.1083/jcb.97.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- 106.Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O’Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O’Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med. 2009;361:302–303. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu SH, Cheng HH, Huang SY, Yiu PC, Chang YC. Studying the protein organization of the postsynaptic density by a novel solid phase- and chemical cross-linking-based technology. Mol Cell Proteomics. 2006;5:1019–1032. doi: 10.1074/mcp.M500299-MCP200. [DOI] [PubMed] [Google Scholar]
- 109.Liu SJ, Fang ZY, Yang Y, Deng HM, Wang JZ. Alzheimer-like phosphorylation of tau and neurofilament induced by cocaine in vivo. Acta Pharmacol Sin. 2003;24:512–518. [PubMed] [Google Scholar]
- 110.Martin R, Schilling K, Fritz W, Giuditta A. Visualization of differential neurofilament phosphorylation in the pre- and postsynaptic axoplasm of the squid giant synapse: an electron spectroscopic study. Neuroscience. 1990;37:553–562. doi: 10.1016/0306-4522(90)90423-2. [DOI] [PubMed] [Google Scholar]
- 111.Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 112.Matus A, Pehling G, Ackermann M, Maeder J. Brain postsynaptic densities: the relationship to glial and neuronal filaments. J Cell Biol. 1980;87:346–359. doi: 10.1083/jcb.87.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McGehee>McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 114.McLachlan DR, Lukiw WJ, Wong L, Bergeron C, Bech-Hansen NT. Selective messenger RNA reduction in Alzheimer’s disease. Brain research. 1988;427:255–261. doi: 10.1016/0169-328x(88)90048-4. [DOI] [PubMed] [Google Scholar]
- 115.Miao L, Teng J, Lin J, Liao X, Chen J. 14-3-3 proteins interact with neurofilament protein-L and regulate dynamic assembly of neurofilaments. J Cell Sci. 2013;126:427–436. doi: 10.1242/jcs.105817. [DOI] [PubMed] [Google Scholar]
- 116.Michaelson DM, Alroy G, Goldstein D, Chapman J, Feldon J. Characterization of an experimental autoimmune dementia model in the rat. Ann N Y Acad Sci. 1991;640:290–294. doi: 10.1111/j.1749-6632.1991.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 117.Middleton FA, Peng L, Lewis DA, Levitt P, Mirnics K. Altered expression of 14-3-3 genes in the prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology. 2005;30:974–983. doi: 10.1038/sj.npp.1300674. [DOI] [PubMed] [Google Scholar]
- 118.Miyata Y, Hoshi M, Nishida E, Minami Y, Sakai H. Binding of microtubule-associated protein 2 and tau to the intermediate filament reassembled from neurofilament 70-kDa subunit protein. Its regulation by calmodulin. J Biol Chem. 1986;261:13026–13030. [PubMed] [Google Scholar]
- 119.Moczulska KE, Pichler P, Schutzbier M, Schleiffer A, Rumpel S, Mechtler K. Deep and precise quantification of the mouse synaptosomal proteome reveals substantial remodeling during postnatal maturation. J Proteome Res. 2014;13:4310–4324. doi: 10.1021/pr500456t. [DOI] [PubMed] [Google Scholar]
- 120.Momeni P, Cairns NJ, Perry RH, Bigio EH, Gearing M, Singleton AB, Hardy J. Mutation analysis of patients with neuronal intermediate filament inclusion disease (NIFID) Neurobiology of aging. 2006;27:778 e771–778 e776. doi: 10.1016/j.neurobiolaging.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 121.Morrison JH, Lewis DA, Campbell MJ, Huntley GW, Benson DL, Bouras C. A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer’s disease. Brain research. 1987;416:331–336. doi: 10.1016/0006-8993(87)90914-0. [DOI] [PubMed] [Google Scholar]
- 122.Mosaheb S, Thorpe JR, Hashemzadeh-Bonehi L, Bigio EH, Gearing M, Cairns NJ. Neuronal intranuclear inclusions are ultrastructurally and immunologically distinct from cytoplasmic inclusions of neuronal intermediate filament inclusion disease. Acta neuropathologica. 2005;110:360–368. doi: 10.1007/s00401-005-1057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Narayana PA, Herrera JJ, Bockhorst KH, Esparza-Coss E, Xia Y, Steinberg JL, Moeller FG. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 2014;221:220–230. doi: 10.1016/j.pscychresns.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Narita M, Shibasaki M, Nagumo Y, Narita M, Yajima Y, Suzuki T. Implication of cyclin-dependent kinase 5 in the development of psychological dependence on and behavioral sensitization to morphine. Journal of neurochemistry. 2005;93:1463–1468. doi: 10.1111/j.1471-4159.2005.03136.x. [DOI] [PubMed] [Google Scholar]
- 125.Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IR. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta neuropathologica. 2009;118:605–616. doi: 10.1007/s00401-009-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nixon RA, Logvinenko KB. Multiple fates of newly synthesized neurofilament proteins: evidence for a stationary neurofilament network distributed nonuniformly along axons of retinal ganglion cell neurons. J Cell Biol. 1986;102:647–659. doi: 10.1083/jcb.102.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nixon RA, Sihag RK. Neurofilament phosphorylation: a new look at regulation and function. Trends Neurosci. 1991;14:501–506. doi: 10.1016/0166-2236(91)90062-y. [DOI] [PubMed] [Google Scholar]
- 128.Nukina N, Kosik KS, Selkoe DJ. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci U S A. 1987;84:3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ohara O, Gahara Y, Miyake T, Teraoka H, Kitamura T. Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J Cell Biol. 1993;121:387–395. doi: 10.1083/jcb.121.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oron L, Dubovik V, Novitsky L, Eilam D, Michaelson DM. Animal model and in vitro studies of anti neurofilament antibodies mediated neurodegeneration in Alzheimer’s disease. J Neural Transm Suppl. 1997;49:77–84. doi: 10.1007/978-3-7091-6844-8_8. [DOI] [PubMed] [Google Scholar]
- 131.Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- 132.Pal A, Das S. Morphine causes persistent induction of nitrated neurofilaments in cortex and subcortex even during abstinence. Neuroscience. 2015;291:177–188. doi: 10.1016/j.neuroscience.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 133.Pant HC. Veeranna, Neurofilament phosphorylation. Biochem Cell Biol. 1995;73:575–592. doi: 10.1139/o95-063. [DOI] [PubMed] [Google Scholar]
- 134.Paradies MA, Steward O. Multiple subcellular mRNA distribution patterns in neurons: a nonisotopic in situ hybridization analysis. Journal of neurobiology. 1997;33:473–493. doi: 10.1002/(sici)1097-4695(199710)33:4<473::aid-neu10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 135.Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM, Wait R, Dunn MJ, Cotter DR. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13:1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- 136.Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppa T, Harkanen T, Koskinen S, Lonnqvist J. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 137.Perrot R, Julien JP. Real-time imaging reveals defects of fast axonal transport induced by disorganization of intermediate filaments. FASEB J. 2009;23:3213–3225. doi: 10.1096/fj.09-129585. [DOI] [PubMed] [Google Scholar]
- 138.Perry G, Rizzuto N, Autilio-Gambetti L, Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985;82:3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 140.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 141.Rao MV, Campbell J, Yuan A, Kumar A, Gotow T, Uchiyama Y, Nixon RA. The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J Cell Biol. 2003;163:1021–1031. doi: 10.1083/jcb.200308076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rao MV, Garcia ML, Miyazaki Y, Gotow T, Yuan A, Mattina S, Ward CM, Calcutt NA, Uchiyama Y, Nixon RA, Cleveland DW. Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J Cell Biol. 2002;158:681–693. doi: 10.1083/jcb.200202037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rao MV, Mohan PS, Kumar A, Yuan A, Montagna L, Campbell J, Veeranna, Espreafico EM, Julien JP, Nixon RA. The myosin Va head domain binds to the neurofilament-L rod and modulates endoplasmic reticulum (ER) content and distribution within axons. PLoS One. 2011;6:e17087. doi: 10.1371/journal.pone.0017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ratnam J, Teichberg VI. Neurofilament-light increases the cell surface expression of the N-methyl-D-aspartate receptor and prevents its ubiquitination. Journal of neurochemistry. 2005;92:878–885. doi: 10.1111/j.1471-4159.2004.02936.x. [DOI] [PubMed] [Google Scholar]
- 145.Roots BI. Neurofilament accumulation induced in synapses by leupeptin. Science. 1983;221:971–972. doi: 10.1126/science.6192501. [DOI] [PubMed] [Google Scholar]
- 146.Rudrabhatla P, Grant P, Jaffe H, Strong MJ, Pant HC. Quantitative phosphoproteomic analysis of neuronal intermediate filament proteins (NF-M/H) in Alzheimer’s disease by iTRAQ. FASEB J. 2010;24:4396–4407. doi: 10.1096/fj.10-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rudrabhatla P, Jaffe H, Pant HC. Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer’s NFTs. FASEB J. 2011;25:3896–3905. doi: 10.1096/fj.11-181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Satoh K, Takeuchi M, Oda Y, Deguchi-Tawarada M, Sakamoto Y, Matsubara K, Nagasu T, Takai Y. Identification of activity-regulated proteins in the postsynaptic density fraction. Genes Cells. 2002;7:187–197. doi: 10.1046/j.1356-9597.2001.00505.x. [DOI] [PubMed] [Google Scholar]
- 149.Saunders DE, DiCerbo JA, Williams JR, Hannigan JH. Alcohol reduces neurofilament protein levels in primary cultured hippocampal neurons. Alcohol. 1997;14:519–526. doi: 10.1016/s0741-8329(97)00043-8. [DOI] [PubMed] [Google Scholar]
- 150.Sbarbati A, Bunnemann B, Cristofori P, Terron A, Chiamulera C, Merigo F, Benati D, Bernardi P, Osculati F. Chronic nicotine treatment changes the axonal distribution of 68 kDa neurofilaments in the rat ventral tegmental area. Eur J Neurosci. 2002;16:877–882. doi: 10.1046/j.1460-9568.2002.02167.x. [DOI] [PubMed] [Google Scholar]
- 151.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 152.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiology of aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 153.Scherling CS, Hall T, Berisha F, Klepac K, Karydas A, Coppola G, Kramer JH, Rabinovici G, Ahlijanian M, Miller BL, Seeley W, Grinberg LT, Rosen H, Meredith J, Jr, Boxer AL. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Annals of neurology. 2014;75:116–126. doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sealock R, Murnane AA, Paulin D, Froehner SC. Immunochemical identification of desmin in Torpedo postsynaptic membranes and at the rat neuromuscular junction. Synapse. 1989;3:315–324. doi: 10.1002/syn.890030404. [DOI] [PubMed] [Google Scholar]
- 155.Sharma P, Sharma M, Amin ND, Albers RW, Pant HC. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc Natl Acad Sci U S A. 1999;96:11156–11160. doi: 10.1073/pnas.96.20.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Simonato M. The neurochemistry of morphine addiction in the neocortex. Trends Pharmacol Sci. 1996;17:410–415. doi: 10.1016/s0165-6147(96)10047-x. [DOI] [PubMed] [Google Scholar]
- 157.Sivagnanasundaram S, Crossett B, Dedova I, Cordwell S, Matsumoto I. Abnormal pathways in the genu of the corpus callosum in schizophrenia pathogenesis: a proteome study. Proteomics Clin Appl. 2007;1:1291–1305. doi: 10.1002/prca.200700230. [DOI] [PubMed] [Google Scholar]
- 158.Smith MA, Rudnicka-Nawrot M, Richey PL, Praprotnik D, Mulvihill P, Miller CA, Sayre LM, Perry G. Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer’s disease. Journal of neurochemistry. 1995;64:2660–2666. doi: 10.1046/j.1471-4159.1995.64062660.x. [DOI] [PubMed] [Google Scholar]
- 159.Somerville MJ, Percy ME, Bergeron C, Yoong LK, Grima EA, McLachlan DR. Localization and quantitation of 68 kDa neurofilament and superoxide dismutase-1 mRNA in Alzheimer brains. Brain Res Mol Brain Res. 1991;9:1–8. doi: 10.1016/0169-328x(91)90123-f. [DOI] [PubMed] [Google Scholar]
- 160.Sternberger NH, Sternberger LA, Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985;82:4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord. 2002;4:80–88. doi: 10.1034/j.1399-5618.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- 163.Styers ML, Salazar G, Love R, Peden AA, Kowalczyk AP, Faundez V. The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol Biol Cell. 2004;15:5369–5382. doi: 10.1091/mbc.E04-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun WL, Zhou L, Quinones-Jenab V, Jenab S. Cocaine effects on dopamine and NMDA receptors interactions in the striatum of Fischer rats. Brain Res Bull. 2009;80:377–381. doi: 10.1016/j.brainresbull.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Terada S, Nakata T, Peterson AC, Hirokawa N. Visualization of slow axonal transport in vivo. Science. 1996;273:784–788. doi: 10.1126/science.273.5276.784. [DOI] [PubMed] [Google Scholar]
- 167.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Annals of neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 168.Terry-Lorenzo RT, Inoue M, Connor JH, Haystead TA, Armbruster BN, Gupta RP, Oliver CJ, Shenolikar S. Neurofilament-L is a protein phosphatase-1-binding protein associated with neuronal plasma membrane and post-synaptic density. J Biol Chem. 2000;275:2439–2446. doi: 10.1074/jbc.275.4.2439. [DOI] [PubMed] [Google Scholar]
- 169.Tetzlaff W, Leonard C, Krekoski CA, Parhad IM, Bisby MA. Reductions in motoneuronal neurofilament synthesis by successive axotomies: a possible explanation for the conditioning lesion effect on axon regeneration. Experimental neurology. 1996;139:95–106. doi: 10.1006/exnr.1996.0084. [DOI] [PubMed] [Google Scholar]
- 170.Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A. Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in Alzheimer’s disease. Neuroscience. 2009;160:427–433. doi: 10.1016/j.neuroscience.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Toyooka K, Muratake T, Tanaka T, Igarashi S, Watanabe H, Takeuchi H, Hayashi S, Maeda M, Takahashi M, Tsuji S, Kumanishi T, Takahashi Y. 14-3-3 protein eta chain gene (YWHAH) polymorphism and its genetic association with schizophrenia. Am J Med Genet. 1999;88:164–167. [PubMed] [Google Scholar]
- 172.Trinidad JC, Thalhammer A, Specht CG, Schoepfer R, Burlingame AL. Phosphorylation state of postsynaptic density proteins. Journal of neurochemistry. 2005;92:1306–1316. doi: 10.1111/j.1471-4159.2004.02943.x. [DOI] [PubMed] [Google Scholar]
- 173.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 174.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Veeranna, Shetty KT, Link WT, Jaffe H, Wang J, Pant HC. Neuronal cyclin-dependent kinase-5 phosphorylation sites in neurofilament protein (NF-H) are dephosphorylated by protein phosphatase 2A. Journal of neurochemistry. 1995;64:2681–2690. doi: 10.1046/j.1471-4159.1995.64062681.x. [DOI] [PubMed] [Google Scholar]
- 178.Vickers JC, Chin D, Edwards AM, Sampson V, Harper C, Morrison J. Dystrophic neurite formation associated with age-related beta amyloid deposition in the neocortex: clues to the genesis of neurofibrillary pathology. Experimental neurology. 1996;141:1–11. doi: 10.1006/exnr.1996.0133. [DOI] [PubMed] [Google Scholar]
- 179.Vickers JC, Riederer BM, Marugg RA, Buee-Scherrer V, Buee L, Delacourte A, Morrison JH. Alterations in neurofilament protein immunoreactivity in human hippocampal neurons related to normal aging and Alzheimer’s disease. Neuroscience. 1994;62:1–13. doi: 10.1016/0306-4522(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 180.Villace P, Marion RM, Ortin J. The composition of Staufen-containing RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs. Nucleic acids research. 2004;32:2411–2420. doi: 10.1093/nar/gkh552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, Snedecor JO, Guan S, Medzihradszky KF, Maltby DA, Schoepfer R, Burlingame AL. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol Cell Proteomics. 2006;5:923–934. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 182.Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I. Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett. 2001;507:81–87. doi: 10.1016/s0014-5793(01)02944-1. [DOI] [PubMed] [Google Scholar]
- 183.Wong J, Oblinger MM. A comparison of peripheral and central axotomy effects on neurofilament and tubulin gene expression in rat dorsal root ganglion neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:2215–2222. doi: 10.1523/JNEUROSCI.10-07-02215.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yabe JT, Pimenta A, Shea TB. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J Cell Sci. 112(Pt 21)(1999):3799–3814. doi: 10.1242/jcs.112.21.3799. [DOI] [PubMed] [Google Scholar]
- 185.Yuan A, Hassinger L, Rao MV, Julien JP, Miller CC, Nixon RA. Dissociation of Axonal Neurofilament Content from Its Transport Rate. PLoS One. 2015;10:e0133848. doi: 10.1371/journal.pone.0133848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yuan A, Rao MV, Kumar A, Julien JP, Nixon RA. Neurofilament transport in vivo minimally requires hetero-oligomer formation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:9452–9458. doi: 10.1523/JNEUROSCI.23-28-09452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna, Liem RK, Eyer J, Peterson AC, Julien JP, Nixon RA. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:10006–10019. doi: 10.1523/JNEUROSCI.2580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments at a glance. J Cell Sci. 2012;125:3257–3263. doi: 10.1242/jcs.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yuan A, Sasaki T, Kumar A, Peterhoff CM, Rao MV, Liem RK, Julien JP, Nixon RA. Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8501–8508. doi: 10.1523/JNEUROSCI.1081-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yuan A, Sasaki T, Rao MV, Kumar A, Kanumuri V, Dunlop DS, Liem RK, Nixon RA. Neurofilaments form a highly stable stationary cytoskeleton after reaching a critical level in axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11316–11329. doi: 10.1523/JNEUROSCI.1942-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yuan A, Sershen H, Veeranna, Basavarajappa BS, Kumar A, Hashim A, Berg M, Lee JH, Sato Y, Rao MV, Mohan PS, Dyakin V, Julien JP, Lee VM, Nixon RA. Functions of neurofilaments in synapses. Mol Psychiatry. 2015;20:915. doi: 10.1038/mp.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yuan A, Sershen H, Veeranna, Basavarajappa BS, Kumar A, Hashim A, Berg M, Lee JH, Sato Y, Rao MV, Mohan PS, Dyakin V, Julien JP, Lee VM, Nixon RA. Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yuan A, Sershen H, Veeranna, Basavarajappa BS, Kumar A, Hashim A, Berg M, Lee JH, Sato Y, Rao MV, Mohan PS, Dyakin V, Julien JP, Lee VM, Nixon RA. Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry. 2015;20:986–994. doi: 10.1038/mp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K. I. Alzheimer’s Disease Neuroimaging, Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA Neurol. 2015:1–8. [Google Scholar]
- 195.Zhang W, Benson DL. Stages of synapse development defined by dependence on F-actin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5169–5181. doi: 10.1523/JNEUROSCI.21-14-05169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang Z, Casey DM, Julien JP, Xu Z. Normal dendritic arborization in spinal motoneurons requires neurofilament subunit L. J Comp Neurol. 2002;450:144–152. doi: 10.1002/cne.10306. [DOI] [PubMed] [Google Scholar]
- 197.Zhou J, Jones DR, Duong DM, Levey AI, Lah JJ, Peng J. Proteomic analysis of postsynaptic density in Alzheimer’s disease. Clin Chim Acta. 2013;420:62–68. doi: 10.1016/j.cca.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhu Q, Couillard-Despres S, Julien JP. Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Experimental neurology. 1997;148:299–316. doi: 10.1006/exnr.1997.6654. [DOI] [PubMed] [Google Scholar]
- 199.Zimmerman UJ, Schlaepfer WW. Characterization of a brain calcium-activated protease that degrades neurofilament proteins. Biochemistry. 1982;21:3977–3982. doi: 10.1021/bi00260a012. [DOI] [PubMed] [Google Scholar]