Abstract
Objective
Vasomotor symptoms (VMS) may be associated with an increased risk of cardiovascular disease. One candidate mechanism may involve alterations in physiological responses to stress. The current study therefore examined the relationship between self-reported VMS bother and cardiovascular, hemodynamic, neuroendocrine and inflammatory responses to an acute psychosocial stress protocol.
Methods
One hundred and eighty-six women in the menopause transition or early postmenopause (age 45-60) provided the data for this report. Subjective hot flash and night sweat bother was assessed using the Greene Climacteric Scale. Women also underwent a stressor battery involving a speech and a mental arithmetic task while cardiovascular, hemodynamic, neuroendocrine and inflammatory responses were assessed. Repeated measures regression analyses were used to examine the relationship between self-reported VMS and physiologic responses to the stressor.
Results
In multivariate analyses adjusting for potential confounders, self-reported hot flash bother was associated with lower overall cardiac index and stroke volume index and higher overall vascular resistance index and levels of the inflammatory cytokine interleukin-6. Hot flash bother also tended to be associated with higher overall cortisol levels and higher baseline levels of plasma norepinephrine. Night sweat bother, on the other hand, was associated with higher overall cortisol levels and tended to be associated with higher interleukin-6.
Conclusion
Self-reported VMS bother is associated with an unfavorable hemodynamic and neuroendocrine profile characterized by increased hypothalamic-pituitary-adrenal axis and central sympathetic activation, inflammation and vasoconstriction. Further research investigating this profile in relation to VMS, as well as the potential health implications of this association, are warranted.
Keywords: vasomotor symptoms, hot flashes, hot flushes, night sweats, stress reactivity, hemodynamics, catecholamines, cortisol, inflammation, Trier Social Stress Test
Vasomotor symptoms (VMS) are estimated to affect up to 70% of women in the early menopausal years1 and are associated with impaired sleep, cognitive decline and overall decreased quality of life (see 2 for review). Although findings are not entirely consistent, with one study finding VMS to be associated with a decreased risk of cardiovascular disease mortality3, several recent studies suggest that VMS frequency and/or severity may be positively associated with cardiovascular disease risk. In one 14-year longitudinal study of over 11,000 women aged 45-50, women who reported having VMS ‘often’ had a two-fold increased risk of developing coronary heart disease (CHD) over the course of the study when compared to women who reported no VMS4. Furthermore, the Women's Health Initiative Observational Study (WHIOS), involving approximately 60,000 postmenopausal women aged 50 to 79 who were followed for an average of 9.7 years,5 found that VMS, particularly late-onset VMS, were associated with an increased risk of major CHD, stroke and total cardiovascular disease (HRs = 1.30-1.46). A third study of over 10,000 Dutch and Swedish women aged 46-64 followed for a mean of 10 years, night sweats, but not hot flashes, were associated with a moderately increased risk of CHD (HR = 1.33)6.
VMS have also been associated with multiple cardiovascular disease precursors, including indices of subclinical cardiovascular disease. In an ancillary study of the Study of Women's Health Across the Nation (SWAN) including 492 women aged 45-58, hot flashes were associated with reduced flow-mediated dilation, increased coronary artery and aortic calcification7 and higher carotid intima media thickness8. A second cohort of 302 women participating in the Healthy Women Study, a longitudinal study initiated in 1983, found that women reporting a longer history of hot flashes exhibited increased aortic calcification9. Others have obtained similar findings10, 11, though null findings have also been reported12. Additionally, VMS have been associated with other CVD risk factors, including increased body mass index, hypertension, elevated LDL cholesterol and triglycerides (see 13 for review), indicators of insulin resistance 14 and diabetes15.
The mechanisms linking VMS with a potentially increased cardiovascular risk profile are poorly understood. However, a small body of recent research investigating the physiology of hot flashes suggests that disturbances in the autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal (HPA) axis may represent a common link between VMS and cardiovascular disease. High frequency heart rate variability, considered an index of cardiac vagal control, has been found to decrease significantly during hot flashes16-18 while low frequency heart rate variability, in part reflecting sympathetic activation, may be higher during hot flashes18, 19. Some evidence indicating that compounds increasing central sympathetic activation (e.g., yohimbine) may provoke hot flashes while compounds reducing sympathetic activation (e.g., clonidine) ameliorate them20 also seem to implicate the ANS in the etiology of VMS. Though the link between VMS and the HPA axis is much less established, one study suggests that hot flashes are followed by a temporary increase in cortisol21.
Indicators of altered ANS and HPA axis regulation are, in turn, associated with cardiovascular disease and its precursors. For example, exaggerated cardiovascular responses to acute mental stress22, 23 have been shown to be associated with the development of hypertension, subclinical atherosclerosis and cardiovascular events themselves14, 15. Though less well-studied, some evidence also suggests that cortisol responses to acute mental stress are associated with coronary artery calcification24. Repeated exposure to elevated cortisol levels may also, over time, render individuals resistant to the anti-inflammatory effects of glucocorticoids, resulting in overall increased inflammation25, which is itself a risk factor for cardiovascular disease26. In addition, the relative contribution that the myocardium versus the vasculature makes to blood pressure (BP) levels, which is regulated by the ANS and sympathoadrenal medullary system, has been shown to be associated with CVD development27. More specifically, a hemodynamic stress profile characterized by less cardiac output and greater relative vasoconstriction has been observed in groups at increased risk for CVD 28-31. Thus, to the extent that ANS and HPA axis disturbances are positively associated with both VMS and CVD risk, such disturbances may represent a common link between VMS and CVD.
The current study examined the relationship between self-reported VMS and cardiovascular, hemodynamic, endocrine and inflammatory responses at rest and during a well-validated acute mental stress task, the Trier Social Stress Test (TSST) in peri- and early postmenopausal women. This work may shed light on potential stress-responsive cardiovascular and neuroendocrine mechanisms linking VMS and CVD.
Methods
Study Overview
The current manuscript describes the results of a cross-sectional analysis of the pre-randomization data collected as part of the Perimenopausal Estrogen Replacement Therapy (PERT) Study, a 12-month placebo-controlled randomized trial evaluating the mood and cardiovascular benefits of transdermal estradiol in perimenopausal and early postmenopausal women.
Participants first underwent an enrollment visit during which their study eligibility was assessed and informed written consent was obtained. At this time, participants completed questionnaires pertaining to their demographic characteristics, medical history and current menopausal symptoms. One month following the enrollment session, those women who were determined to be eligible for the randomized controlled trial (RCT) completed the first laboratory stress testing session, which included the Trier Social Stress Test (TSST), during which multiple measures of cardiovascular and endocrine reactivity were assessed. Participants were subsequently randomized to receive transdermal estradiol (0.1 mg) and micronized progesterone pills (200 mg/day) or a placebo patch and placebo pills. However, the data included in this report constitutes only the measures collected pre-randomization.
Participants
186 participants included in this report (see Table 1 for participant characteristics) were recruited from the community to participate in the PERT Study. Women were recruited who were aged 45-60 years, medically healthy and perimenopausal or early postmenopausal according to the Stages of Reproductive Aging Workshop (STRAW+10) criteria (early perimenopause, defined as menstrual cycle length 7+ days longer than usual in combination with hot flashes; late perimenopause, defined as ≥ 2 skipped cycles and an interval of amenorrhea ≥60 days but within one year of the last menstrual period; and early postmenopause defined as an interval of amenorrhea between one and two years). Women who had their uterus removed (at least one ovary retained) for whom menstrual bleeding patterns could not be assessed were included in the study if: 1) they were experiencing VMS and their baseline estradiol levels were above postmenopausal concentrations (> 40 pg/ml) (n=17) or, 2) they were not experiencing VMS but had baseline estradiol > 40 pg/ml and baseline FSH levels > 14 IU/l (n=4) (a cutoff of two standard deviations above the mean level obtained from a sample of premenopausal women, consistent with STRAW guidelines)32.
Table 1.
Participant characteristics (n = 186)
| Mean (SD) or % | |
|---|---|
| Age (yrs) | 51.0 (3.1) |
| Gross Household Income (U.S. dollars) | $50,000-79,000 |
| BMI (kgs/m2) | 25.7 (3.8) |
| Race | |
| % White | 73.1% |
| % African American | 21.5% |
| % Other | 5.4% |
| % Postmenopausal | 16.7% |
| % Endorsing hot flashes | 66.1% |
| % Endorsing night sweats | 62.2% |
| % Endorsing physical activity | |
| Light | 88% |
| Moderate | 66% |
| Vigorous | 60% |
| Alcohol consumption (drinks/ week) | 2.0 (1.0) |
| Caffeine consumption (cups/ day) | 2.5 (0.9) |
| Smokers (%) | 4.3% |
| Hours of sleep per night | 6.9 (1.1) |
Exclusion criteria included the following: current psychiatric diagnosis of major depressive disorder or any other current psychiatric diagnosis with severity greater than mild, a Center for Epidemiological Studies Depression Scale (CES-D) score > 16, a history of severe substance use within the past 10 years, a history of suicide attempts, use of psychotropic medication, hormonal preparations, or herbal compounds indicated for menopausal symptoms (e.g. Black Cohosh) or mood (e.g. St. John's Wort), use of statins or antihypertensive agents other than diuretics, regular over-the-counter medication use (e.g., non-steroidal anti-inflammatory agents), blood pressure >160/90 mmHg or any history of CVD. Multiple exclusion criteria aimed at minimizing risk related to hormone therapy also applied and included the following: smoking > 10 cigarettes/day, endometrial hyperplasia, abnormal uterine anatomy, history of thrombophlebitis or thromboembolic disorders, history of estrogen-dependent neoplasias, body mass index > 35. To be eligible for the study, women also must have had a normal mammogram within one year of study enrollment. The study protocol was approved by the UNC Chapel Hill Institutional Review Board. All participants provided informed, written consent prior to participating and received up to $1525 in compensation for participating in the full 12 month RCT.
Measures
Vasomotor Symptoms (VMS)
VMS were measured during the enrollment session using the Greene Climacteric Scale (GCS), a self-report form that asks participants to rate the extent to which they are currently bothered by 21 menopausal symptoms on a 4-point scale from ‘not at all’ to ‘extremely,’ Two of these items included “hot flushes” and “sweating at night,” which were used to respectively assess hot flashes and night sweats in the current study.
Potential Confounders
All potential confounding variables were assessed at the enrollment session. Race was assessed by self-report and included the following possible categories from which the participant could choose: White, African American, Native Hawaiian or Pacific Islander, Native American, Alaska Native, Black of Caribbean, Cuban, European or other descent and Asian. Body mass index was calculated using researcher-assessed height and weight measurements. Light, moderate and vigorous physical activity were assessed using the International Physical Activity Questionnaire (IPAQ)- short form33, which provides examples of commonly-performed activities at all intensities of physical activity and collects information on the duration (time spent) and frequency (number of days) engaged in activities lasting longer than 10 minutes over the course of the week to calculate the amount of Metabolic Equivalents (METs)-minutes associated with each physical activity level of intensity. To assess smoking, participants completed a questionnaire asking “Do you smoke?” and “If yes, approximately, how many cigarettes do you smoke in a day?” Categories included 5 or less, 6-10, 11-15, 16-20, 20+. Average number of hours of sleep per night, assessed using one item from an abbreviated version of the St. Mary's Hospital Sleep Questionnaire (SMHSQ)34, was used as an indicator of sleep. Average number of self-reported cups of caffeinated beverages consumed per day was used as an index of caffeine intake. Average number of self-reported alcoholic beverages consumed per week provided an index of alcohol intake. Menopause status was categorized as postmenopausal or perimenopausal based upon self-reported menstrual cycle information. Postmenopausal status required at least one year of amenorrhea. All other participants were classified as perimenopausal, including hysterectomized women meeting the estradiol and/or FSH study eligibility criteria. Psychological symptoms were assessed using the psychological subscale of the GCS, which consists of 11 items – 5 relating to depression and 6 relating to anxiety.
Laboratory Testing
The sequence of laboratory events were as follows: 1) Instrumentation and intravenous set-up; 2) recovery from venipuncture (20 min); 3) baseline (10 min quiet rest); 4) Trier social stress test (TSST) (15 min); 5) recovery (60 min). These events are described below.
All laboratory sessions began at 1:00 p.m. Upon arrival, participants were instrumented with a blood pressure cuff and an electrocardiogram for later monitoring of cardiovascular stress reactivity. A catheter was then placed into a forearm vein, to be used for blood draws using a non-heparinized, multi-stop-cock system allowing for blood samples to be taken at precise time intervals throughout testing. A curtain was drawn to prevent the participant from viewing the catheter and the blood draws during the stress protocol. Thirty minutes of quiet rest followed the i.v. setup, the first 20 min being recovery from venipuncture and minutes 21 - 30 constituting baseline. Blood pressure and heart rate were taken at minutes 21, 23, 25, 27 and 29 and averaged to yield a mean baseline value. Blood was sampled at minute 30 for baseline cortisol, IL-6, epinephrine, norepinephrine and estradiol concentrations.
The Trier Social Stress Test (TSST)
The TSST, which has been shown to induce a reliable stress response35, involved four components: 1) Pre-task instructions (1 minute) during which participants were introduced to the committee who later listened to their job talk and were given instructions for the mental arithmetic task; 2) Speech preparation period (5 minutes) during which time participants prepared a job talk for which they were to assume the role of a job candidate for their “ideal job” while the selection committee stood in the room; 3) Job speech (5 minutes immediately following the preparation period), during which the participant delivered her job talk aimed at persuading the committee that she is the perfect candidate. If the participant ended her talk before 5 minutes, the selection committee questioned the participant in a systematic fashion to ensure the participant would speak the entire 5 minutes. 4) Serial subtraction task, which involved subtracting a 1-digit number from a 4-digit number as fast and as accurately as possible for 5 minutes. For each mistake, the participant was instructed by a member of the selection committee to restart from the beginning. Participants were video-recorded throughout their performance.
Following the arithmetic task, participants were asked to rate on a scale from 0 to 10 the extent to which the job speech and the arithmetic task caused them anxiety, fear, anger/ hostility and feelings of rejection. Using a similar scale, participants also rated how difficult the tasks were and how much effort they exerted in completing the tasks. Participants then sat quietly while watching a 60-minute travel documentary specifically chosen to include only mundane footage.
Blood pressure and heart rate were obtained at minutes 1, 3 and 5 of the speech preparation period, the job speech and the arithmetic task and at minutes 2, 4, 8, 10, 13, 16, 20, 25, 30, 40, 45, 56 and 60 of the recovery period. These measures were then averaged to obtain a mean preparation, speech task, arithmetic task and recovery value for each measure. Plasma epinephrine and norepinephrine were sampled at minute 1 of the job speech and at minute 1 of the arithmetic task since catecholamines peak within the first two minutes of mental stress36. Plasma cortisol and IL-6 was measured 10, 20, 30, 45 and 60 minutes following the end of the TSST to capture the delayed HPA and IL-6 responses to mental stress37-39.
Physiological Recording Procedures
The SunTech Exercise blood pressure monitor, Model 4240 (SunTech Medical Instruments, Inc., Raleigh, NC) provided blood pressure measurements during baseline and mental stress testing. Five standard stethoscopic and automated blood pressure measurements were taken simultaneously to ensure correct microphone placement and cuff position.
Impedance cardiography was used to noninvasively monitor cardiovascular activity40, including cardiac output, stroke volume, total peripheral resistance and heart rate. A custom-designed impedance cardiograph (HIC-100 Bioimpedance Technology, Inc., Model 100, Chapel Hill, NC, USA) was used in conjunction with a tetrapolar band electrode configuration to record impedance dZ/dt and Zo signals. Impedance and electrocardiogram signals were processed online by specialized computer software (BIT, Chapel Hill, NC) with subsequent manual editing to improve accuracy. For each minute of interest, a 30 second continuous sample of waveforms (obtained concurrently with blood pressure) was processed to generate an ensemble-averaged cardiac cycle, from which SV was determined using the Kubicek et al. (1966)41 equation and heart rate was determined using the mean interbeat interval. Cardiac output and total peripheral resistance for these same minutes were then calculated using standard formulae40. Cardiac output, stroke volume and total peripheral resistance were adjusted for individual variations in body size by using body surface areas to derive cardiac index, stroke volume index and vascular resistance index.
Hormone and Neuroendocrine Assays
Plasma cortisol levels were measured using a competitive coated tube radioimmunoassay (RIA) kit and protocol, commercially available from MP Biomedicals, Orangeburg, NY. The sensitivity of the assay is .07 ug/dL with intra- and inter- assay variations of 4.7% and 7.6%, respectively.
Plasma IL-6 levels were determined using a high-sensitivity ELISA assay kit and protocol from R&D Systems, Minneapolis, MN. The sensitivity of the assay is .039 pg/ml with a standard range of .156 to 10 pg/ml with intra- and inter- assay variations of 7.8% and 7.2%, respectively.
Plasma epinephrine and norepinephrine concentrations were determined (Labcorp) using an isocratic high performance liquid chromatography (HPLC) system and an electrochemical detector from Chromsystems, Germany. The lower limit of quantification for both compounds is 15 ng/l. Intra-assay and the intra-assay and inter- day coefficients of variation are <11% and <13%, respectively.
Statistical Analyses
Sensitivity analyses were used to identify extreme outliers prior to data analysis, defined as values 3 or more interquartile ranges below the first quartile or above the third quartile (SAS Institute Inc., 2011). This resulted in IL-6 data being removed for three participants, cardiac index data being removed for one participant, stroke volume index being removed for one participant, norepinephrine data being removed for one participant and epinephrine data being removed for four participants. While blood pressure and heart rate were normally distributed, cardiac index, vascular resistance index, stroke volume index, epinephrine, norepinephrine, cortisol and IL-6 were log-transformed due to non-normality. Means included in tables and figures have been back-transformed.
For each cardiovascular or neuroendocrine measure, t-tests comparing levels during the baseline period versus during the speech task were used to confirm a significant effect of the TSST on cardiovascular and neuroendocrine measures.
Univariate regression analyses, using a p-value of 0.05 as the criterion for statistical significance, assessed whether hot flashes and night sweats were each associated with a number of potential confounding variables that have been shown to influence responses to mental stress. Those variables found to be significantly associated with VMS were identified as covariates to be included in multivariate analyses examining the association between VMS and cardiovascular and neuroendocrine responses to mental stress.
For each physiologic dependent measure, a multivariate repeated measures general linear regression model, with stress testing task (baseline, preparation, speech, arithmetic, recovery) as the repeated measure was used to examine the effects of hot flashes and night sweats (examined separately) on stress physiology. The Greenhouse-Geisser correction was used whenever appropriate to correct for sphericity.
Results
Participant Characteristics
As reflected in Table 1, the participants were of average socioeconomic status and reflected the racial demographics of our region (73% White, 22% African American, and 5% other). The extent to which participants reported being bothered by hot flashes was as follows: 33.9% endorsed “not at all”, 36.0% endorsed “a little”, 25.3% endorsed “quite a bit” and 4.8% endorsed “extremely”. A similar distribution was seen for night sweats: 37.8% endorsed “not at all”, 34.6% endorsed “a little”, 22.2% endorsed “quite a bit” and 5.4% endorsed “extremely”.
Overall Efficacy of the Stress Protocol
Systolic blood pressure, diastolic blood pressure and heart rate all significantly increased in response to the mental stressors (t(179) = 32.8, 30.0 and 23.9, p<.0001, respectively) as did cardiac index (t(179) = 19.7, p<.0001), stroke volume index (t(180) = 2.5, p =.013), epinephrine (t(137) = 9.6, p<.0001), cortisol (t(169) = 8.7, p<.0001) and IL-6 (t(139) = 2.5, p = .013). Vascular resistance index decreased (t(179) = −8.7, p<.0001) while no effect of the mental stress was seen for plasma norepinephrine (p = .098) (Table 2).
Table 2.
Mean (SD) cardiovascular, impedance-derived hemodynamic and neuroendocrine measures by stress testing task.
| Sy stolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | Heart rate (bpm) | Cardiac index (l/min) | Stroke volume index (ml/beat per M2) | Vascular resistance index (dyne s cm−5 M2) | Epinephrine (pg/ml) | Norepinephrine (pg/ml) | Cortisol (pg/ml) | IL-6 (pg/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 112.1 (14.9) | 70.9 (9.1) | 64.9 (8.0) | 3.3 (0.8) | 53.7 (37.0) | 2221.8 (743.5) | 34.9 (32.0) | 445.0 (162.5) | 7.3 (3.4) | 1.2 (0.5) |
| Preparation | 131.2 (17.0) | 78.5 (9.4) | 78.1 (13.0) | 4.0 (1.6) | 54.9 (44.3) | 2129,9 (775.6) | ||||
| Speech | 142.7 (19.2) | 83.5 (10.0) | 85.9 (14.5) | 4.6 (1.3) | 58.8 (61.6) | 1918.2 (599.4) | 83.6 (70.0) | 428.3 (141.6) | ||
| Subtraction | 140.3 (19.2) | 84.6 (9.8) | 82.8 (14.5) | 4.5 (1.3) | 58.8 (54.3) | 1968.4 (587.4) | 69.7 (51.5) | 447.2 (156.4) | ||
| Recovery (overall) | 116.0 (14.0) | 73.8 (8.1) | 65.5 (8.3) | 3.2 (0.8) | 53.0 (36.6) | 2310.0 (749.0) | ||||
| Recovery min 10 | 10.6 (5.3) | 1.2 (0.5) | ||||||||
| Recovery min 20 | 9.4 (4.8) | 1.2 (0.5) | ||||||||
| Recovery min 30 | 8.5 (4.4) | 1.3 (0.5) | ||||||||
| Recovery min 45 | 7.6 (3.9) | 1.3 (0.5) | ||||||||
| Recovery min 60 | 6.9 (3.8) | 1.3 (0.5) |
Vasomotor Symptoms and Potential Confounding Variables
Neither hot flashes nor night sweats were significantly associated with race (Whites vs. Other, Blacks vs. Other) (ps = .150, .274), age (ps = .332, .654), household income (ps = .492, .803), body mass index (ps = .730, .449), caffeine consumption (ps = .963, .367), alcohol consumption (ps = .753, .628), light physical activity (ps = .579, .622), moderate physical activity (ps = .547, .468), smoking (ps = .438, .655) or estradiol levels on the day of stress testing (ps = .651, .613). Although hot flashes were positively associated with postmenopausal status (relative to perimenopausal status, F(1, 185) = 8.2, p = .005), night sweats were not (p = .232). Hot flashes and night sweats were both positively associated with vigorous physical activity (β(SEM) = 0.00 (0.00), p = .006; β(SEM) = 0.00 (0.00), p = .036) and the psychological subscale of the GCS (β(SEM) = 0.06 (0.02), p = .001; β(SEM) = 0.09 (0.02), p <.001). Night sweats (β(SEM) = −0.14 (0.06), p = .023) but not hot flashes (p = .163) were also negatively associated with average number of hours of sleep. Thus, vigorous physical activity, postmenopausal status and psychological GCS subscale score were identified as appropriate covariates for multivariate analyses involving hot flashes and vigorous physical activity, psychological GCS subscale score while average number of hours of sleep were identified as appropriate covariates for multivariate analyses involving night sweats.
Hot Flashes and Physiologic Responses to Stress
Multivariate analyses examining the effect of hot flashes on stress physiology included average weekly METs of vigorous physical activity, psychological GCS subscale score and menopausal status as covariates.
Blood pressure and heart rate
Hot flashes were not associated with systolic blood pressure (p = .715), diastolic blood pressure (p = .916) or heart rate (p = .971), nor did hot flashes interact with task to influence these variables (ps = .303-.610).
Hemodynamic measures
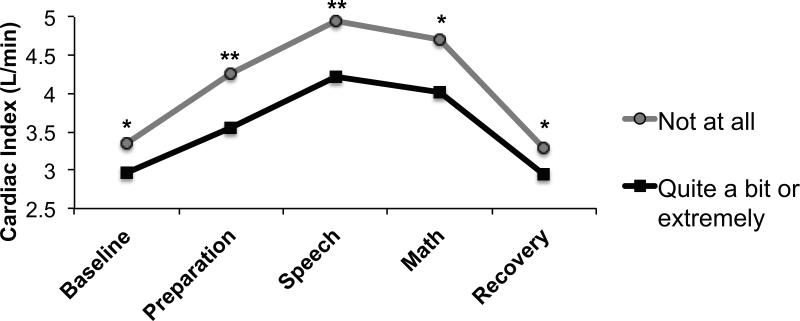
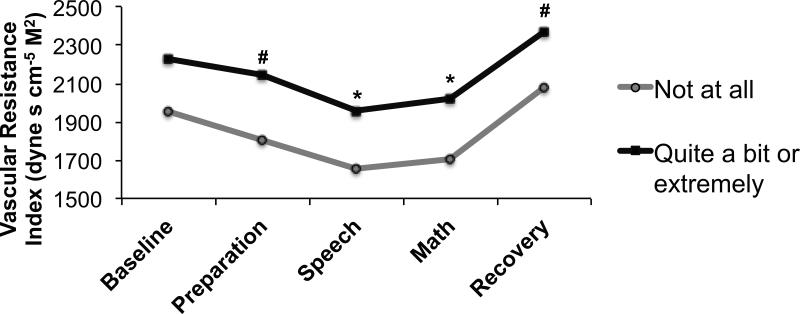
Hot flashes were associated with lower overall cardiac index (F(1, 170) = 7.66, p = .006) (Figure 1) and stroke volume index (F(1, 170) = 7.35, p = .007) and higher overall vascular resistance index (F(1, 169) = 5.72, p = .018) (Figure 2). However, hot flashes did not interact with task to predict cardiac index, stroke volume index or vascular resistance index levels (ps = .499, .528 and .759, respectively).
Figure 1.
Adjusted mean impedance-derived cardiac index in women endorsing being “not at all” (n = 58) versus “quite a bit” or “extremely” (n = 52) bothered by hot flashes. *p<.05; **p<.01
Figure 2.
Adjusted mean impedance-derived vascular resistance index in women endorsing being “not at all” (n = 58) versus “quite a bit” or “extremely” (n = 52) bothered by hot flashes. #p<.10 *p<.05
Norepinephrine
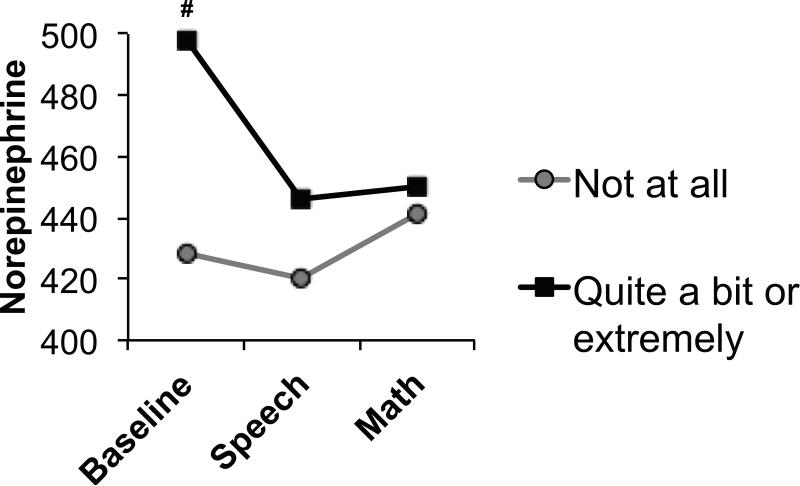
Hot flashes tended to be associated with overall higher norepinephrine (F(1, 130) = 2.80, p = .097) (Figure 3) and tended to interact with task to predict norepinephrine levels (F(2, 260) = 2.51, p = .097) such that the effect of hot flashes on norepinephrine was only significant at baseline (p = .025).
Figure 3.
Adjusted mean norepinephrine in women endorsing being “not at all” (n = 48) versus “quite a bit” or “extremely” (n = 42) bothered by hot flashes. #p<.10
Epinephrine
Hot flashes were not associated with overall epinephrine (p = .168) and did not interact with task to predict epinephrine levels (p = .993).
Cortisol
Hot flashes tended to be associated with overall elevated cortisol levels (F(1, 157) = 2.80, p = .097) (Table 3) but symptoms did not interact with task to predict cortisol levels (p =.395).
Table 3.
Mean (SEM) cardiovascular, impedance-derived hemodynamic and neuroendocrine stress testing levels in women endorsing being “not at all” versus “quite a bit” or “extremely” bothered by vasomotor symptoms (VMS).
| Hot Flash Bother |
Night Sweat Bother |
|||
|---|---|---|---|---|
| ‘Not at all’ (n = 63) | ‘Quite a bit’ or ‘Extremely’ (n = 56) | Not at all (n = 70) | ‘Quite a bit’ or ‘Extremely’ (n = 51) | |
| Systolic blood pressure (mmHg) | 128.0 (122.6-133.5) | 128.3 (123.6-133.0) | 129.9 (125.9-133.9) | 126.9 (122.3-131.6) |
| Diastolic blood pressure (mmHg) | 78.6 (75.3-81.9) | 77.8 (75.0-80.6) | 78.6 (76.2-80.9) | 77.8 (75.1-80.5) |
| heart rate (bpm) | 75.1 (71.3-78.8) | 74.1 (70.9-77.3) | 77.1 (74.3-79.8) | 74.4 (71.3-77.6) |
| cardiac index (l/min) | 4.1 (3.8-4.5) | 3.5 (3.3-3.8)** | 4.0 (3.7-4.3) | 3.7 (3.4-4.0) |
| vascular resistance index (dyne s cm−5 M2) | 1853.1 (1634.5-2100.9) | 2166.1 (1945.7-2411.5)* | 1918.1 (1748.1-2104.6) | 2031.0 (1824.4-2261.1) |
| stroke volume index (ml/beat per M2) | 56.3 (49.8-63.5) | 48.2 (43.4-53.5)* | 53.3 (48.7-58.4) | 51.1 (46.0-56.7) |
| norepinephrine (pg/ml) | 421.0 (374.3-473.4) | 456.2 (411.4-505.8) | 408.5 (373.5-446.7) | 457.6 (413.8-506.0) |
| epinephrine (pg/ml) | 61.7 (48.0-79.3) | 41.7 (33.4-52.1)* | 53.9 (44.2-65.7) | 42.5 (33.9-53.3) |
| Cortisol (pg/ml) | 7.4 (6.4-8.5) | 8.6 (7.6-9.7)# | 6.5 (5.8-7.3) | 8.3 (7.2-9.4)* |
| IL-6 (pg/ml) | 1.1 (0.9-1.2) | 1.2 (1.1-1.4)# | 1.2 (1.0-1.3) | 1.3 (1.1-1.4) |
p<.10
p<.05
p<.01
Although hot flash severity was treated as a continuous variable in the following repeated measures analyses, it has been treated as a dichotomous variable for illustration purposes.
Interleukin-6 (IL-6)
Hot flashes were associated with overall higher IL-6 (F(1, 128) = 4.71, p = .032) and interacted with task to predict IL-6 levels (F(1, 640) = 4.21, p = .002) such that the effect of hot flashes on IL-6 was significant at all time points except 45 and 60 minutes following the end of the TSST.
Night Sweat Severity and Physiologic Responses to Stress
Multivariate analyses examining the effect of night sweats on stress reactivity included average number of hours of sleep per night and average weekly METs of vigorous physical activity and psychological GCS subscale score as covariates.
Blood pressure and heart rate
Night sweats were not associated with systolic blood pressure (p = .900), diastolic blood pressure (p = .510) or heart rate (p = .605), nor did night sweats interact with task to influence these variables (ps = .373-.608).
Hemodynamic measures
Night sweats were not associated with overall cardiac index or vascular resistance index (ps = .125, .123), and did not interact with task to predict cardiac index or vascular resistance index levels (ps = .736 and .967, respectively). No main effect of night sweats or any symptom-by-task interaction effect was observed for stroke volume index (ps = .222, .867).
Norepinephrine
Night sweats were not associated with overall norepinephrine (p = .125) and did not interact with task to predict norepinephrine levels (p = .533).
Epinephrine
Night sweats were not associated with overall epinephrine levels (p = .142) and did not interact with task to predict epinephrine levels (p = .845).
Cortisol
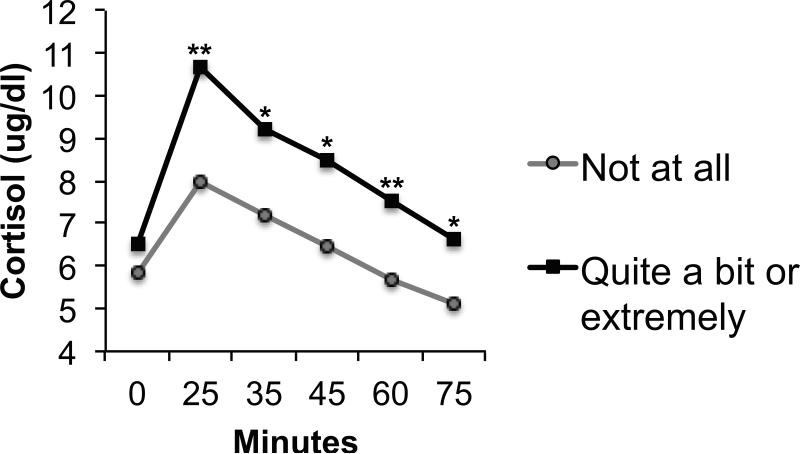
Night sweats were positively associated with cortisol levels overall (F(1, 153) = 4.55, p = .035) but did not interact with task to predict cortisol levels (p = .289) (Figure 4).
Figure 4.
Adjusted mean cortisol in women endorsing being “not at all” (n = 59) versus “quite a bit” or “extremely” (n = 46) bothered by night sweats. *p<.05; **p<.01
Interleukin-6 (IL-6)
Night sweats tended to be associated with higher overall IL-6 levels (F(1, 124) = 3.36, p = .069) (Table 3) but symptoms did not interact with task to predict IL-6 levels (p = .110).
Discussion
The current study investigated the relationship between self-reported VMS and physiological responses to an acute mental stressor battery. Results indicated that although VMS bother was not associated with alterations in stress reactivity, per se, self-reported hot flash bother was associated with lower overall cardiac index, lower overall stroke volume index, higher overall vascular resistance index and higher overall IL-6 levels. Hot flash bother also tended to be associated with elevated baseline norepinephrine levels and higher overall cortisol. Night sweat bother, on the other hand, was only associated with higher overall cortisol levels and tended to be associated with higher overall IL-6 levels.
Plasma norepinephrine is primarily reflective of sympathetic neural activity42 while plasma epinephrine primarily reflects sympathoadrenal medullary activity; the current results therefore suggest that hot flash bother tends to be associated with elevated sympathetic neural activation but unaltered sympathoadrenal activation. An increase in central versus peripheral ANS activity may help explain why hot flashes were found to be associated with overall lower cardiac index, lower stroke volume index and higher vascular resistance index. Plasma norepinephrine, which was higher overall in women reporting being more bothered by hot flashes, binds to β-adrenergic receptors on the heart, leading to increased cardiac output; in the vasculature, however, norepinephrine preferentially binds to α-adrenergic receptors, stimulation of which promotes vasoconstriction and increased vascular resistance43. Thus, a relative profile of increased vasoconstriction coupled with decreased myocardial activity is consistent with the relatively elevated plasma norepinephrine levels observed in women with more bothersome hot flashes.
Our finding that self-reported hot flash bother and, to a lesser extent, night sweat bother, is associated with elevated norepinephrine levels is consistent with the observation that 3-methoxy-4-hydroxyphenylglcol (MHPG), the main metabolite of plasma norepinephrine, is significantly higher in postmenopausal women suffering from VMS compared with asymptomatic women44. It is also in line with several studies implicating the ANS in the pathophysiology of hot flashes themselves. For example, as mentioned above, there is some evidence that increasing central sympathetic output with the use of yohimbine (which blocks the α2 autoreceptor on noradrenergic neurons and therefore increases norepinephrine output) provokes hot flashes while inhibiting central sympathetic activation with clonidine (an α2 autoreceptor agonist) reduces hot flashes (see 20 for review). Studies examining changes in heart rate variability with hot flash onset also suggest that hot flashes are associated with a shift in sympathovagal balance toward sympathetic predominance16-19. However, to our knowledge, the current study is the first to examine VMS in relation to indices of both sympathetic and sympathoadrenal medullary activation within the context of acute mental stress.
It is unclear why daytime hot flashes, but not night sweats, were associated with elevations in central sympathetic activity as well as hemodynamic alterations in cardiac output, stroke volume and total peripheral resistance. These differences will need to be replicated using objective measures of hot flash and night sweat severity to ensure that the observed differences are not driven by less accurate reporting of night sweats relative to daytime hot flashes.
Though the results of this cross-sectional study should be considered preliminary, if confirmed in future research, the association of hot flashes with higher vascular resistance index, lower cardiac index and lower stroke volume index may have important implications for our understanding of the relationship between VMS and CVD. Reduced myocardial activity and greater vasoconstriction in the context of stress is seen in individuals at higher risk for CVD relative to their lower risk counterparts; for example, in postmenopausal relative to premenopausal women 45, in men relative to premenopausal women46, 47, and in Blacks relative to Whites48, 49. Perhaps more relevant to the current study's finding that hot flash severity was associated with overall hemodynamic tone rather than hemodynamic stress reactivity, per say, Blacks have also been shown to have lower resting cardiac index and higher resting vascular resistance index compared with Whites,30 and aging (the primary predictor of cardiovascular disease) is associated with increases in resting vascular resistance index and decreases in resting cardiac index50. Moreover, greater vascular resistance index tone may contribute to left ventricular hypertrophy51, an independent predictor of cardiovascular disease morbidity/mortality52, 53.
Multivariate analyses also suggested a positive association between night sweats and, to a lesser extent, hot flashes, and circulating cortisol levels. These results are consistent with one study linking self-reported VMS bother to increased 24-hour urinary cortisol output54 as well as the observation that cortisol levels increase following an objectively-assessed hot flash21. Given that HPA axis hyperarousal is associated with multiple cardiovascular disease risk factors, including abdominal obesity, hypertension, insulin resistance and diabetes55, 56, a positive association between VMS and circulating cortisol levels could have important health implications for menopausal women.
Hot flash, and to a lesser extent, night sweat bother were also associated with elevated IL-6 levels in multivariate analyses. Though few studies have examined the association between VMS and inflammation, one study did observe elevated IL-6 and IL-8 levels among women reporting more severe hot flashes57. The Study of Women's Health Across the Nation (SWAN) did not, however, find a relationship between VMS frequency and C-reactive protein58 when adjusting for body mass index, the levels of which would be expected to rise in response to elevated levels of IL-6 – further research is therefore needed to settle these inconsistencies. Though the mechanisms by which VMS would be associated with greater inflammation are unknown, one possibility may be that prolonged/ repeated exposure to elevated cortisol, both in the context of hot flashes themselves21 and during stress (as suggested by the current findings) promotes a desensitization to cortisol's anti-inflammatory effects, a process that has been hypothesized to underlie the association between chronic stress and increased inflammation25.
The current study results should be interpreted in light of several limitations, perhaps the most important of which is its use of self-reported VMS bother using two questionnaire items. Assessing objective VMS frequency using skin conductance or even prospective diary would allow us to rule out the possibility that the HPA axis, autonomic and hemodynamic alterations observed in the current study are associated with a greater propensity to be bothered by VMS, rather than being associated with VMS themselves. A related second limitation is that we cannot rule out the possibility that some women reporting more bothersome VMS experienced a hot flash either prior to or during stress testing, which might have influenced some of the measures under investigation. Third, the cross-sectional nature of this study does not allow us to determine the direction of the relationship between VMS severity and the neuroendocrine and hemodynamic alterations observed. Longitudinal studies will clarify whether these physiologic alterations precede or are preceded by the development of VMS in menopause. Such research would provide some insight into whether the observed VMS-related hemodynamic, neuroendocrine and immune/inflammatory alterations are etiologically related to VMS, result from repeated exposure to VMS, or are a by-product of some other process influencing VMS. Fourth, hemodynamic data estimated by impedance cardiography are validated for research purposes only59 and are not intended to reflect clinical indices of cardiovascular function as determined by invasive catheter-based hemodynamic assessments. Hemodynamic data produced by impedance cardiography may also be less precise than catheter-based assessments. Finally, generalizability of the current study's findings may be limited by several aspects of the study design. First, the fact that the women were participants of a randomized clinical trial of hormone therapy disallowed the inclusion of women who may have been at greatest risk for menopausal symptoms for safety reasons (e.g., women with obesity) and also likely resulted in a sample that was healthier than average perimenopausal women. Second, although our sample was racially diverse, there was less diversity in socioeconomic status, which could well influence the nature and frequency of psychosocial stressors that were proximate to the menopause transition. Despite these limitations, the current study fills an important gap in the literature. Though two studies have examined whether mental stress induces VMS in the laboratory17, 60, to our knowledge, this is the first study to examine whether VMS bother is associated with altered physiologic responses to acute stress. Its comprehensive assessment of cardiovascular hemodynamic, catecholamine, HPA, and immune/inflammatory measures is also particularly unique. If confirmed in longitudinal and interventional studies, the current study's findings suggest potential mechanisms by which VMS may be associated with an increased risk of cardiovascular disease.
Conclusions
The current study suggests that self-reported VMS bother is associated with greater HPA and immune/inflammatory activation, both of which have been associated with an increased risk for cardiovascular disease and its precursors. Hot flashes are additionally associated with elevated baseline central sympathetic tone, and an impedance-derived hemodynamic profile characterized by lower cardiac output and stroke volume and greater vasoconstriction, which is also associated with an increased cardiovascular disease risk. Should the current study's results be replicated using a state of the art measure of VMS, they may have important implications for understanding potential relationship between VMS and indices of cardiovascular disease risk and well as the physiology of VMS themselves.
Acknowledgments
Sources of Financial Support: This research was supported by NIH grant RO1-MH087619. Dr. Gordon is also the recipient of a Postdoctoral Fellowship of the Fonds de la Recherche du Québec – Santé (FRQS).
Conflicts of Interest/ Financial Disclosures: Dr. Rubinow serves on the editorial board of Servier Laboratories, is a consultant for Sage Therapeutics Inc. and has received grant funding from the Foundation of Hope. Both Drs. Rubinow and Girdler have also received grant funding from NIH.
References
- 1.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. Journal of general internal medicine. 2008;23(9):1507–13. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health and Quality of Life outcomes. 2005;3(1):47. doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svartberg J, von Mühlen D, Kritz-Silverstein D, Barrett-Connor E. Vasomotor symptoms and mortality: the Rancho Bernardo Study. Menopause. 2009;16(5):888–91. doi: 10.1097/gme.0b013e3181a4866b. [DOI] [PubMed] [Google Scholar]
- 4.Herber-Gast G, Brown WJ, Mishra GD. Hot flushes and night sweats are associated with coronary heart disease risk in midlife: a longitudinal study. BJOG. 2015;122:1560–1567. doi: 10.1111/1471-0528.13163. [DOI] [PubMed] [Google Scholar]
- 5.Szmuilowicz ED, Manson JE, Rossouw JE, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause (New York, NY) 2011;18(6):603. doi: 10.1097/gme.0b013e3182014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gast G-CM, Pop VJ, Samsioe GN, et al. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011;18(2):146–51. doi: 10.1097/gme.0b013e3181f464fb. [DOI] [PubMed] [Google Scholar]
- 7.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease findings from the Study of Women's Health Across the Nation Heart Study. Circulation. 2008;118(12):1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause (New York, NY) 2011;18(4):352. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurston RC, Kuller LH, Edmundowicz D, Matthews KA. History of hot flashes and aortic calcification among postmenopausal women. Menopause (New York, NY) 2010;17(2):256. doi: 10.1097/gme.0b013e3181c1ad3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bechlioulis A, Kalantaridou SN, Naka KK, et al. Endothelial function, but not carotid intima-media thickness, is affected early in menopause and is associated with severity of hot flushes. The Journal of Clinical Endocrinology & Metabolism. 2010;95(3):1199–206. doi: 10.1210/jc.2009-2262. [DOI] [PubMed] [Google Scholar]
- 11.Özkaya E, Cakir E, Kara F, et al. Impact of hot flashes and night sweats on carotid intima–media thickness and bone mineral density among postmenopausal women. International Journal of Gynecology & Obstetrics. 2011;113(3):235–8. doi: 10.1016/j.ijgo.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Wolff EF, He Y, Black DM, et al. Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the Kronos early estrogen prevention study (KEEPS). Fertility and sterility. 2013;99(5):1385–91. doi: 10.1016/j.fertnstert.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco OH, Muka T, Colpani V, et al. Vasomotor symptoms in women and cardiovascular risk markers: Systematic review and meta-analysis. Maturitas. 2015 doi: 10.1016/j.maturitas.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Vasomotor symptoms and insulin resistance in the study of women's health across the nation. The Journal of Clinical Endocrinology & Metabolism. 2012;97(10):3487–94. doi: 10.1210/jc.2012-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herber-Gast G-CM, Mishra GD. Early severe vasomotor menopausal symptoms are associated with diabetes. Menopause. 2014;21(8):855–60. doi: 10.1097/GME.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 16.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control during women's daily lives. Menopause (New York, NY) 2012;19(4):406. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause (New York, NY) 2010;17(3):456. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoikkala H, Haapalahti P, Viitasalo M, et al. Association between vasomotor hot flashes and heart rate variability in recently postmenopausal women. Menopause. 2010;17(2):315–20. doi: 10.1097/gme.0b013e3181c2bb6d. [DOI] [PubMed] [Google Scholar]
- 19.Freedman RR, Kruger ML, Wasson SL. Heart rate variability in menopausal hot flashes during sleep. Menopause (New York, NY) 2011;18(8):897. doi: 10.1097/gme.0b013e31820ac941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman RR. Electrophysiology and Psychophysiology in Psychiatry and Psychopharmacology. Springer; 2014. Postmenopausal Physiological Changes. pp. 245–56. [DOI] [PubMed] [Google Scholar]
- 21.Meldrum DR, Defazio JD, Erlik Y, et al. Pituitary hormones during the menopausal hot flash. Obstetrics & Gynecology. 1984;64(6):752–6. [PubMed] [Google Scholar]
- 22.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 23.Kamarck TW, Everson SA, Kaplan GA, et al. Exaggerated blood pressure responses during mental stress are associated with enhanced carotid atherosclerosis in middle-aged Finnish men findings from the Kuopio Ischemic Heart Disease Study. Circulation. 1997;96(11):3842–8. doi: 10.1161/01.cir.96.11.3842. [DOI] [PubMed] [Google Scholar]
- 24.Hamer M, O'Donnell K, Lahiri A, Steptoe A. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. European heart journal. 2010;31(4):424–9. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- 25.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychology. 2002;21(6):531. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 27.Manuck SB, Kasprowicz AL, Muldoon MF. Behaviorally-evoked cardiovascular reactivity and hypertension: Conceptual issues and potential associations. Annals of Behavioral Medicine. 1990;12:17–29. [Google Scholar]
- 28.Allen MT, Stoney CM, Owens JF, Matthews KA. Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosomatic Medicine. 1993;55(6):505–17. doi: 10.1097/00006842-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Girdler SS, Turner JR, Sherwood A, Light KC. Gender differences in blood pressure control during a variety of behavioral stressors. Psychosomatic Medicine. 1990;52(5):571–91. doi: 10.1097/00006842-199009000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Light KC, Turner JR, Hinderliter AL, Sherwood A. Race and gender comparisons: I. Hemodynamic responses to a series of stressors. Health Psychology. 1993;12(5):354. doi: 10.1037//0278-6133.12.5.354. [DOI] [PubMed] [Google Scholar]
- 31.Treiber FA, Jackson RW, Davis H, et al. Racial differences in endothelin-1 at rest and in response to acute stress in adolescent males. Hypertension. 2000;35(3):722–5. doi: 10.1161/01.hyp.35.3.722. [DOI] [PubMed] [Google Scholar]
- 32.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop+ 10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 2012;15(2):105–14. doi: 10.3109/13697137.2011.650656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth ML, Ainsworth BE, Pratt M, et al. International physical activity questionnaire: 12-country reliability and validity. Med sci sports Exerc. 2003;195(9131/03):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 34.Ellis B, Johns M, Lancaster R, Raptopoulos P, Angelopoulos N, Priest R. The St. Mary's Hospital sleep questionnaire: a study of reliability. Sleep. 1980;4(1):93–7. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- 35.Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1-2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 36.Dimsdale J, Ziegler M. What do plasma and urinary measures of catecholamines tell us about human response to stressors? Circulation. 1991;83(4 Suppl):II36–42. [PubMed] [Google Scholar]
- 37.Kirschbaum C, Klauer T, Filipp S-H, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic medicine. 1995;57(1):23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Kirschbaum C, Prussner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic medicine. 1995;57(5):468–74. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 39.von Känel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain, Behavior, and Immunity. 2006;20(1):40–8. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 41.Kubicek W, Karnegis J, Patterson R, Witsoe D, Mattson R. Development and evaluation of an impedance cardiac output system. Aerospace medicine. 1966;37(12):1208–12. [PubMed] [Google Scholar]
- 42.Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983;5(4):552–9. doi: 10.1161/01.hyp.5.4.552. [DOI] [PubMed] [Google Scholar]
- 43.Obrist PA. Cardiovascular psychophysiology. Springer Science & Business Media; 1981. [Google Scholar]
- 44.Freedman RR, Woodward S. Elevated α 2-adrenergic responsiveness in menopausal hot flushes: pharmacologic and biochemical studies. Thermoregulation: the pathophysiological basis of clinical disorders Basel: Karger. 1992:6–9. [Google Scholar]
- 45.Sherwood A, Park SB, Hughes JW, et al. Cardiovascular hemodynamics during stress in premenopausal versus postmenopausal women. Menopause. 2010;17(2):403–9. doi: 10.1097/gme.0b013e3181b9b061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen MT, Stoney CM, Owens JF, Matthews KA. Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosomatic Medicine. 1993;55(6):505–17. doi: 10.1097/00006842-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Girdler SS, Turner JR, Sherwood A, Light KC. Gender differences in blood pressure control during a variety of behavioral stressors. Psychosomatic Medicine. 1990;52(5):571–91. doi: 10.1097/00006842-199009000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Treiber FA, Jackson RW, Davis H, et al. Racial differences in endothelin-1 at rest and in response to acute stress in adolescent males. Hypertension. 2000;35(3):722–5. doi: 10.1161/01.hyp.35.3.722. [DOI] [PubMed] [Google Scholar]
- 49.Light KC, Turner JR, Hinderliter AL, Sherwood A. Race and gender comparisons: I. Hemodynamic responses to a series of stressors. Health Psychology. 1993;12(5):354–65. doi: 10.1037//0278-6133.12.5.354. [DOI] [PubMed] [Google Scholar]
- 50.Uchino BN, Holt-Lunstad J, Bloor LE, Campo RA. Aging and cardiovascular reactivity to stress: longitudinal evidence for changes in stress reactivity. Psychology and aging. 2005;20(1):134. doi: 10.1037/0882-7974.20.1.134. [DOI] [PubMed] [Google Scholar]
- 51.Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Annals of Internal Medicine. 1988;108(1):7–13. doi: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 52.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Annals of Internal Medicine. 1991;114(5):345–52. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 53.Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Annals of Internal Medicine. 1992;117(10):831–6. doi: 10.7326/0003-4819-117-10-831. [DOI] [PubMed] [Google Scholar]
- 54.Cagnacci A, Cannoletta M, Caretto S, Zanin R, Xholli A, Volpe A. Increased cortisol level: a possible link between climacteric symptoms and cardiovascular risk factors. Menopause. 2011;18(3):273–8. doi: 10.1097/gme.0b013e3181f31947. [DOI] [PubMed] [Google Scholar]
- 55.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. The Journal of Clinical Endocrinology & Metabolism. 2009;94(8):2692–701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 56.Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vascular health and risk management. 2005;1(4):291. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yasui T, Uemura H, Tomita J, et al. Association of interleukin-8 with hot flashes in premenopausal, perimenopausal, and postmenopausal women and bilateral oophorectomized women. The Journal of Clinical Endocrinology & Metabolism. 2006;91(12):4805–8. doi: 10.1210/jc.2006-1100. [DOI] [PubMed] [Google Scholar]
- 58.Thurston RC, El Khoudary SR, Sutton-Tyrrell K, et al. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the Study of Women's Health Across the Nation. Menopause (New York, NY) 2011;18(10):1044. doi: 10.1097/gme.0b013e31821f5d39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherwood A. Use of impedance cardiography in cardiovascular reactivity research. In: Blascovich JJ, Katkin ES, editors. Cardiovascular reactivity to psychological stress & disease. American Psychological Association; Washington, DC: 1993. pp. 157–199. [Google Scholar]
- 60.Swartzman LC, Edelberg R, Kemmann E. Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychology. 1990;9(5):529. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]