Abstract
As the primary producer of extracellular matrix (ECM) proteins in skeletal muscle, fibroblasts play an important role providing structural support to muscle. Skeletal muscle ECM is vital for force transduction from the muscle cells to the tendon and bones to create movement. It is these ECM connections that allow the movement created in muscle to be transmitted to our skeleton. This review discusses how fibroblasts participate in maintaining this healthy ECM within the skeletal muscle. Additionally, from a basic science perspective, we discuss current methods to identify and study skeletal muscle fibroblasts, as this is critical to bettering our understanding of these important cells. Finally, skeletal muscle fibrosis is discussed, which can be a devastating clinical condition characterized by an overproduction of ECM within skeletal muscle. We discuss the role that fibroblasts and other cells play in muscle fibrosis as well as the implications of this work.
Introduction
A majority of skeletal muscle extracellular matrix (ECM) is produced by fibroblasts that reside in the interstitial space between muscle fibers (Archile-Contreras et al., 2010; Gatchalian et al., 1989; Gillies and Lieber, 2011; Kuhl et al., 1984; Sanderson et al., 1986; Sasse et al., 1981). Although fibroblasts make up a small portion of the cells in skeletal muscle, they play an important role in maintaining muscle structure and are implicated in fibrosis. Given the tremendous impact that they have on skeletal muscle health, structure and their contribution to muscle disease, a review of fibroblasts in skeletal muscle is presented.
Although the vast majority of skeletal muscle research focuses on muscle’s active contractile properties, it is evident that skeletal muscle ECM is structurally and functionally important. Specifically, skeletal muscle ECM provides mechanical stability to muscle fibers, blood vessels and nerves (Kjaer, 2004). Additionally, ECM in skeletal muscle is critical for both longitudinal and lateral force transmission from muscle fibers to tendons (Kjaer, 2004; Purslow, 2002; Tidball, 1991). Therefore, skeletal muscle ECM and ECM producing cells are arguably as important as skeletal muscle fibers in providing functionality to muscle.
ECM elements are highly responsive to altered use, disease, and may even prevent rehabilitation of muscle after injury (Kjaer et al., 2006; Sato et al., 2014; Voermans et al., 2008). Since fibroblasts are one of the main producers of ECM proteins, these ECM changes are likely due to alterations in resident muscle fibroblasts. Given fibroblasts’ role in producing muscle ECM in health and disease, molecular identification of fibroblasts represents an important goal for scientists. We review current fibroblast labeling techniques as well as novel techniques that are under development. These new methods have the potential to increase our knowledge of these cells’ role in muscle homeostasis and disease.
Fibrosis, an excess accumulation of ECM, is present in numerous skeletal muscle conditions resulting in significant muscle weakness and decreased passive range of motion (Klingler et al., 2012; Zumstein et al., 2008). Given that fibroblasts produce a majority of the skeletal muscle ECM, we also review processes leading to fibrosis and potential cell targeting therapies to ameliorate this dysfunctional condition.
ECM in skeletal muscle
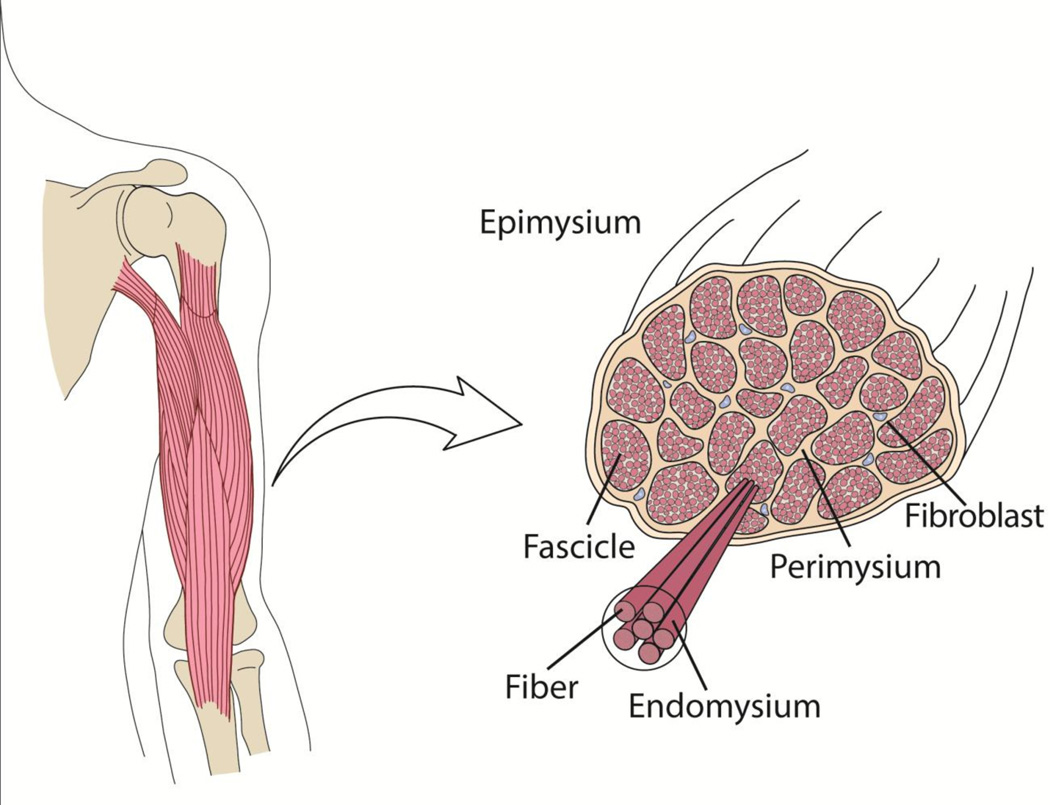
Numerous cell types in skeletal muscle work in concert to create the composite tissue. In addition to myofibers, skeletal muscle is composed of various cell types that play a supportive role in maintaining tissue structure and health. Perhaps the most important of these support cells is the fibroblast, as these cells are responsible for the majority of structural integrity and mechanical linkages provided by the production of ECM proteins (Gillies and Lieber, 2011; Kjaer, 2004). In skeletal muscle, ECM proteins are intricately arranged throughout the skeletal muscle ECM that we traditionally divide into endo-, peri- and epimysium based on anatomical location (Fig. 1). The hierarchical organization of skeletal muscle ECM into these three layers leads to the concept that muscle cells transmit force to the basement membrane and endomysium via costameres and then this force coalesces into a tensile force in the tendon.
Figure 1. Skeletal muscle extracellular matrix structure.
Skeletal muscles are composite and hierarchical tissues with three layers of ECM, the epi-, peri- and endomysium. Skeletal muscle fibroblasts reside in the extracellular space between muscle fibers and fascicles, where they secrete ECM proteins to be incorporated into skeletal muscle ECM. Figure modified from (Mathewson and Lieber, 2015).
The molecular composition of each ECM layer in mature skeletal muscle is primarily dominated by collagen types I and III (Kjaer, 2004; Light and Champion, 1984). These fibrillar molecules bear tremendous stresses within the muscle and provide skeletal muscle ECM with the ability to transmit force to tendons. Collagen IV is also found in skeletal muscle where it is concentrated to the basement membrane (Kjaer, 2004). The basement membrane is unique in the sense that there is a diverse group of ECM proteins, such as laminins and collagen IV, that are not found in fibrillar ECM (Gillies and Lieber, 2011; Grounds et al., 2005; Voermans et al., 2008; Yurchenco and Patton, 2009). These unique molecules bind directly to skeletal muscle protein complexes (e.g. the dystroglycan complex and integrins) in the sarcolemma and serve as an important intermediary between myofibers and the fibrillar ECM (Voermans et al., 2008).
Muscle endomysium is the best understood ECM layer in terms of ultrastructure (Purslow and Trotter, 1994; Trotter and Purslow, 1992). Scanning electron micrographs of series-fibered muscles, in which the fibers do not span the entire length of a muscle, revealed a complex and highly oriented sheath around the muscle. In these muscles the endomysial ECM becomes an important element that transfers tension between overlapping muscle fibers. Through their observations of endomysial collagen fibril deformation during muscle stretch and their use of fibrous composite theory, the authors inferred that force between muscle fibers is transmitted through trans-laminar shear as opposed to in-plane tension.
The perimysium is understood to a lesser extent compared with the endomysium, perhaps due to the fact that the distinction between layers is not clear. Perimysial ECM is contiguous with the tendon, so it is theorized that this is the main mechanism by which force is transmitted from the muscle to the tendons to create movement (Passerieux et al., 2007). Perimysial ECM is dominated by so called perimysium ‘cables’ that are thick bundles primarily composed of tightly packed collagen I and III fibrils (Borg and Caulfield, 1980; Light and Champion, 1984). Recent electron microscopy has imaged these structures in great detail and has even allowed for the creation of 3-dimensional (3D) reconstructions aimed at further understanding the role of perimysium in skeletal muscle (Gillies and Lieber, 2011; Gillies et al., 2014). The location and wavy structure observed in 3D reconstructions of perimysial cables suggests that these cables act as parallel elastic elements within the muscle to bear passive load (Fig. 2). In addition to the highly detailed images produced with electron microscopy, polarized light microscopy has been used to understand ECM density and collagen orientation in cardiac and skeletal muscles (Pierard, 1989; Rich and Whittaker, 2005; Smith and Barton, 2014; Whittaker et al., 1994). Although the resolution of this method is not high enough to image individual collagen cables, an overall assessment of the ECM can be achieved, especially with regard to the orientation of collagen fibers. This imaging technique can be useful in conditions with an over-production of ECM, such as in fibrosis.
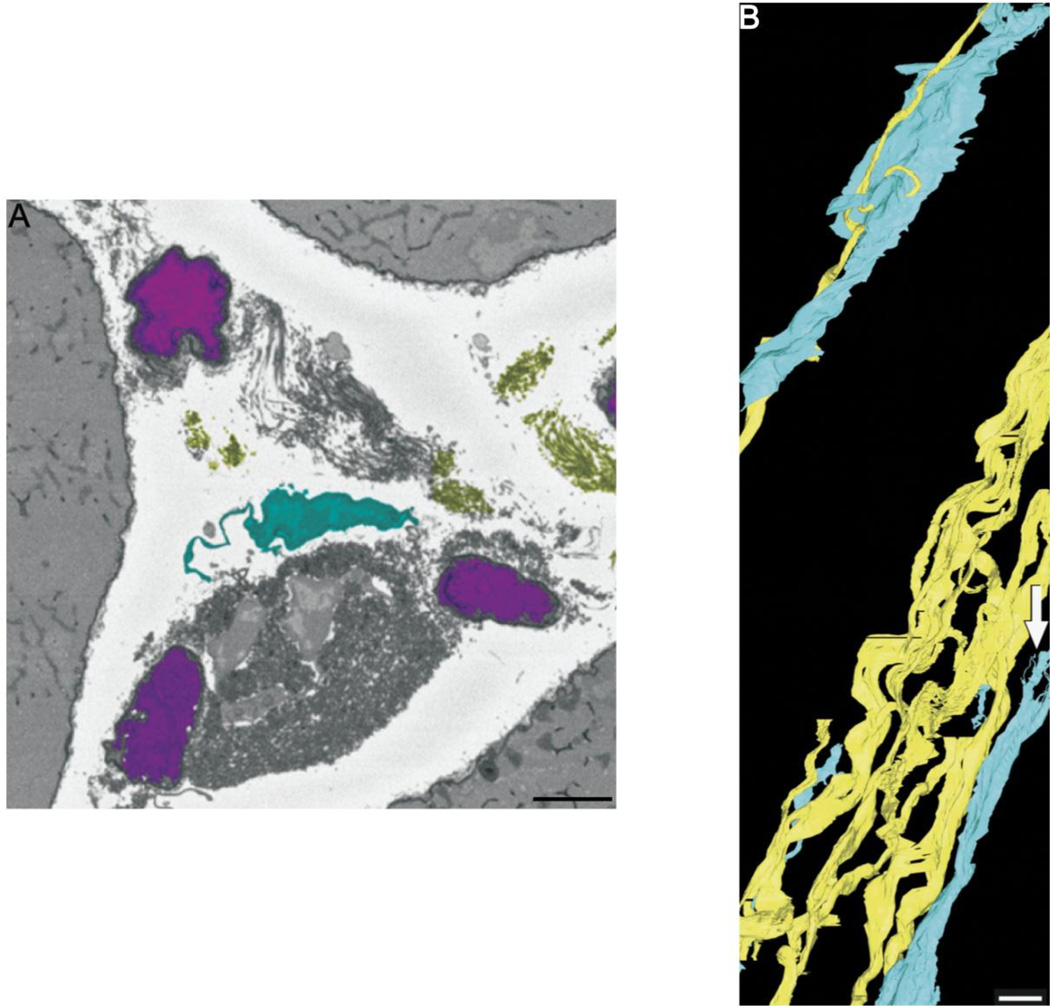
Figure 2. Electron microscopy for examining skeletal muscle ECM ultrastructure.
(A) Scanning electron micrograph of skeletal muscle extracellular space. Capillaries are depicted in magenta, collagen in yellow and fibroblasts in cyan. Scale bar = 5 µm (B) 3-dimensional reconstruction of collagen cables (yellow) and fibroblasts (cyan) from serial block face imaging micrographs. Capillaries have been left out of the reconstruction for clarity. The close relationship between collagen and fibroblasts is evident in this image as fibroblasts processes are wrapped around collagen cables. Scale bar = 5µm (From Gillies et al., 2014).
The epimysium of skeletal muscle surrounds the entire muscle belly and can easily be isolated from the muscle tissue (Gillies et al., 2014). Due to this ease of dissection, epimysial mechanical properties are easily measured and recent studies have described large collagen bundles in the epimysium and well as a finite element model that describes the non-linear nature of epimysial ECM (Gao and Kostrominova, 2008; Gao et al., 2008).
Importance of ECM in healthy skeletal muscle
Skeletal muscle fibers make up about 95% of a muscle’s cross-sectional area and the ECM, only about 1–9% (Light and Champion, 1984). As such, the ECM is much stiffer than muscle fibers as stress becomes concentrated in ECM during muscle contraction. In addition to the well-described active length-tension relationship that is described for skeletal muscle fibers, skeletal muscle has a passive mechanical component that is less understood (Lieber, 2010). Recent experiments demonstrated that the ECM, rather than the muscle fibers themselves, actually bear most of the load in passive muscle (Meyer and Lieber, 2011). This study tested the mechanical stiffness of single skeletal muscle fibers, groups of fibers in which many single fibers were grouped together, and skeletal muscle bundles, which included ECM between the fibers. It was shown that ECM provided a vast majority of skeletal muscle passive stiffness since the bundles had significantly elevated tissue stiffness over the other two groups (Meyer and Lieber, 2011).
While it has been shown that passive load is borne by ECM, it has not been possible to establish a quantitative relationship between ECM components and stiffness. While in skeletal muscle fibrosis, there is an over abundance of ECM, research groups have measured collagen content in the tissue and found little or no correlation with tissue mechanical properties (Chapman et al., 2014; Smith and Barton, 2014; Smith et al., 2011). It was recently reported that collagen crosslinking was found to dictate stiffness in cardiac muscle (López et al., 2012). As such, we measured collagen cross-links in skeletal muscle but no direct relationship between collagen crosslinking and tissue stiffness was found (Chapman et al., 2015). The discrepancies between cardiac and skeletal muscle findings could be due to the functional differences between how these two muscle types contract. Skeletal muscle force production is primarily uniaxial, however the mechanics of heart contractions involve multi-dimensional deformation (Buckberg et al., 2008). Given the difference in how these tissues operate, it is possible that collagen crosslinking alters tissue mechanics in the heart but not skeletal muscle. Furthermore, López, et al. calculated stiffness from cardiac filling pressures and not directly from the cardiac tissue as was done in skeletal muscle. These experimental differences could also explain the discrepancies in collagen crosslinking’s role in tissue stiffness. We suggest that other parameters, such as collagen organization or glycosaminoglycans may dictate stiffness in skeletal muscle.
ECM Production
Fibroblasts produce a large array of ECM proteins, such as collagen, fibronectin, matrix metalloproteinases and proteoglycans. Skeletal muscle ECM is composed primarily of collagen I and III, suggesting that skeletal muscle fibroblasts primarily secrete and assemble these fibrillar collagen isoforms (Bailey et al., 1979; Light and Champion, 1984). Collagen IV is also produced by fibroblasts, which is a major component of the basal lamina in skeletal muscle (Kuhl et al., 1984). Furthermore, fibroblasts also produce and assemble other less common collagen types that are important for muscle function, such as collagen VI, which provides an important link between the basal lamina and fibrillar ECM (Bateman et al., 2009; Pace et al., 2008). When collagen VI assembly is hindered, which can occur in the presence of glycine mutations, collagen VI muscular dystrophies result which are characterized by muscle weakness and joint contractures. Other cell types, such as skeletal muscle myoblasts, have also been shown to produce collagens, but the presence of fibroblasts is crucial for the assembly of this collagen into functional ECM (Kühl et al., 1982; Lipton, 1977a, 1977b).
The molecular mechanisms behind collagen production are well studied, and this has been reviewed in the literature (Ghosh, 2002; Gillies and Lieber, 2011; Lieber and Ward, 2013). In short, collagen production, and in particular collagens I and III, begins with gene activation by certain transcription factors, such as TGF-β, NF-κβ, and TNF-α (see Ghosh et al for a review of the transcription factors involved in collagen I synthesis). After gene activation, the synthesis of pro-α chains occurs followed by various post-translational modifications that allow the α chains to self-assemble into procollagen triple helices (Alberts et al., 2008). Procollagen is then secreted by the cell where pro-peptides are cleaved and these molecules self-assemble into collagen fibrils. In skeletal muscle, these resulting collagen fibrils aggregate to form epi-,peri- and endomysium.
Identification of fibroblasts in skeletal muscle
A precise definition of a fibroblast is difficult. This ambiguity becomes important when attempting to identify fibroblasts experimentally. Various methods are used to identify fibroblasts, such as collagen production, cell morphology, cell location and cellular expression of surface markers. Generally, fibroblasts can be identified as collagen producing, spindle-shaped cells residing in the ECM that are positive for the intermediate filament protein vimentin (Baum and Duffy, 2011; Fernandez-Madrid et al., 1981; Goodpaster et al., 2008). While this is a useful definition it is not entirely specific to fibroblasts. In skeletal muscle, these identifiers would also classify myofibroblasts and pericytes into the same category (Mann et al., 2011; Ricard et al., 2014). Myofibroblasts are defined as ECM-producing, contractile cells that stain positively for α-smooth muscle actin (α-SMA) (Baum and Duffy, 2011; Mayer and Leinwand, 1997). These cells become important in the development of muscle fibrosis, which is discussed below. Pericytes are distinct from connective tissue fibroblasts in that they reside in the microvasculature basement membrane and support vascular development and remodeling (Armulik et al., 2005). However, upon muscle damage, these cells become involved in ECM production when they migrate from their niche to the interstitial ECM (Birbrair et al., 2013). Although fibroblasts, pericytes and myofibroblasts are purported to be distinct cell populations, the ability to distinguish these cells from one another is difficult because each cell type produces ECM proteins, resides in the ECM and is positive for α-SMA and vimentin (Armulik et al., 2005; Mann et al., 2011; Mathew et al., 2011; Ricard et al., 2014). The cross-reactivity of these markers with each of these ECM-producing cells in muscle challenges our understanding of these populations and questions whether they are truly distinct cell populations. It has even been suggested that myofibroblasts may not exist at all in skeletal muscle, instead they could be mature fibroblasts expressing ECM proteins (Mann et al., 2011). Furthermore, it has been found that pericyte subpopulations differentiate into myofibroblasts during muscle fibrosis, as discussed below (Birbrair et al., 2013; Dulauroy et al., 2012). This shared lineage could explain the similarities among these cell populations and the difficulty in positively identifying a homogeneous fibroblast population.
To fully understand and characterize fibroblasts in skeletal muscle, it is important to develop methods for fibroblast identification. As mentioned above, traditional cellular labeling methods have been difficult to employ because of a lack of a definitive fibroblast marker (Mathew et al., 2011). Classically, fibroblasts have been histologically identified by positive staining for the intermediate filament protein vimentin coupled with a spindle-like morphology and negative staining for other mesenchymal cell types (Chang et al., 2002; Goodpaster et al., 2008). Unfortunately, this definition is not specific enough because numerous cell types stain for vimentin making it difficult to differentiate among cell types (Goodpaster et al., 2008). Given the lack of appropriate markers, there is an effort to define specific fibroblast identifiers.
For use in flow cytometry, surface markers are considered to be the most efficient way of labeling and separating cells. However, at the moment, there is a lack of external markers for fibroblasts in skeletal muscle, which has been a roadblock to thoroughly understanding these important cells. Several internal cellular markers have been used, however, and ER/TR7 is used as a label of reticular fibroblasts (Tsujie et al., 2000). Studying these reticular fibroblasts, however, may not be of particular relevance since they primarily produce collagen III, which is less prominent than collagen I in skeletal muscle. A recent study showed that Transcription Factor 4 (Tcf4) is highly expressed in connective tissue fibroblasts and can be used to label the cells (Mathew et al., 2011). However, Tcf4+ cells also stained positive for the pericyte marker, NG2, the myofibroblast marker, α-SMA, and vimentin. Furthermore, Tcf4 expression was found, although to a lower extent, in myoblasts, suggesting this marker may not specifically mark fibroblasts. Furthermore, the use of internal markers, and in particular transcription factors, do not provide good staining due to less efficient labeling and the relatively low expression level of transcription factors. Given this, work is still needed to develop reliable flow cytometry markers for fibroblasts. However, due to the heterogeneous nature of fibroblasts, it may not be a reality to efficiently label all fibroblasts in a particular sample (Fries et al., 1994; Sorrell and Caplan, 2004).
As mentioned previously, the use of advanced imaging modalities, such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are being used to understand the ECM (Gillies et al., 2014; Starborg et al., 2013). Using these novel imaging techniques, cells in the interstitial space were described. Based on cell morphology and placement it is hypothesized that these cells are fibroblasts residing in the extracellular space in skeletal muscle. Using a novel method of serial block face imaging (Denk and Horstmann, 2004), researchers created detailed 3D reconstructions of skeletal muscle ECM as well as fibroblasts. These images and 3D reconstructions depict the close relationship between fibroblasts and the ECM, as well as show that these cells are spread over very long biological distances of ~100 µm ((Gillies et al., 2014); Figs. 2 & 3), suggesting that fibroblasts are potentially involved in the deposition/remodeling of collagen cables. From these data, it is clear that collagen is intimately associate with fibroblasts and that the cells and its processes may extend hundreds of microns along the length of a muscle fiber. This is just the beginning of examining skeletal muscle ECM at this detailed level, and hopefully this work will shed light on an understudied portion of skeletal muscle structure. It should be noted that since these cells have only been identified based on morphology and location, further work is necessary to positively identify them as fibroblasts.
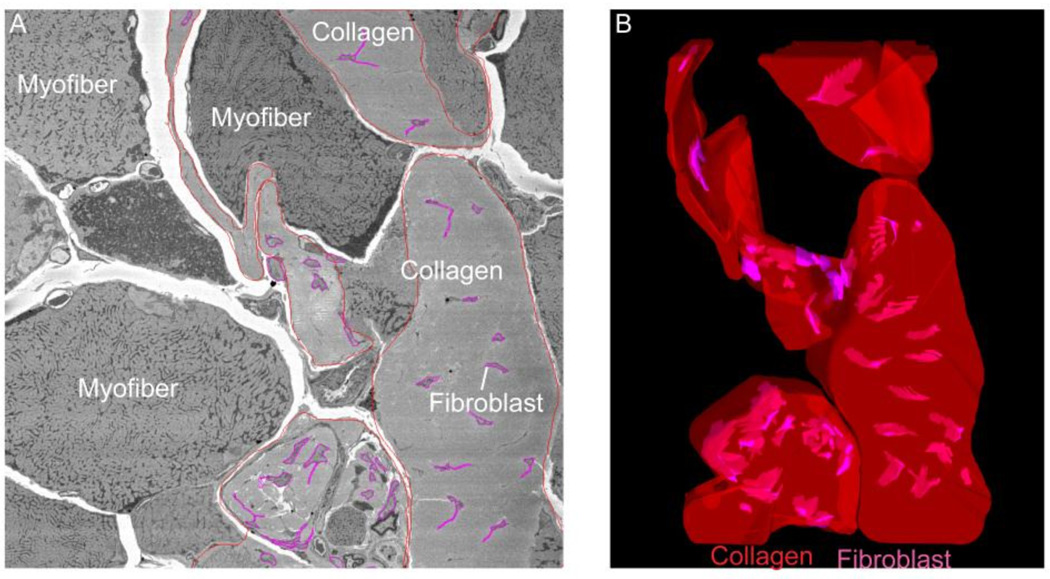
Figure 3. Severely fibrotic tissue with numerous fibroblasts embedded in large deposits of collagen.
(A) Electron micrograph from the tibialis anterior of a nesprin-desmin double knockout mouse with severe fibrosis. This micrograph shows fibroblasts embedded in the large deposits of collagen between muscle fibers. (B) 3-dimensional reconstruction of the extracellular space seen on the right using serial block face imaging (collagen-red, fibroblasts-magenta). With this microscopy technique we are able to observe the 3D distribution of fibroblasts in the ECM of skeletal muscle for the first time. This technique will allow researchers to understand how tissue morphology is altered in disease. (From Meza and Lieber, unpublished data.)
In addition to antibody labeling and SEM/TEM imaging, genetic labeling can be used to label a subset of fibroblasts. Genetic labeling of Tcf4 expressing cells has been successfully used to label connective tissue fibroblasts in muscle with green fluorescent protein (GFP) (Mathew et al., 2011). As previously mentioned, myoblasts also express Tcf4, so the Tcf4GFP/Cre+neo mouse was created to specifically lable muscle connective tissue fibroblasts and not myogenic cells. This was accomplished by causing Cre to only be active in cells with high expression of Tcf4, and since myoblasts have a relatively low expression level of Tcf4, only fibroblasts were genetically labeled with GFP.
Research on liver fibrosis led to the development of a novel mouse model in which all cells producing collagen type I, a major contributor to fibrotic scars in many tissues, also produce GFP (Yata et al., 2003). This is accomplished by inserting a GFP cassette downstream of the collagen 1(α1) promoter. The development of this mouse permits easy identification of the specific cells that contribute to collagen production in liver. As discussed above, fibroblasts produce collagen I in skeletal muscle, so this same mouse model can be used to study skeletal muscle fibroblasts in healthy and fibrotic skeletal muscle.
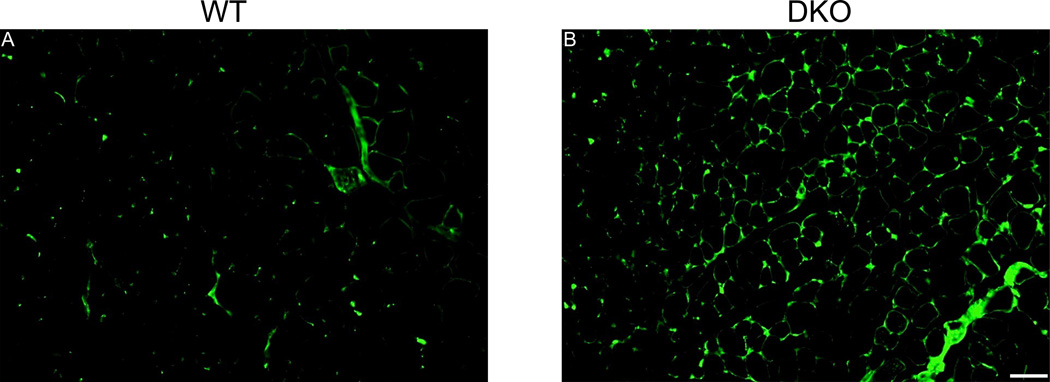
This work is currently underway and we have successfully bred an existing model of skeletal muscle fibrosis (nesprin-desmin double knockout mouse (Chapman et al., 2014)) with collagen-GFP reporter mice (Yata et al., 2003). We found that there is an increased number of collagen producing cells in fibrotic skeletal muscle determined by microscopy (Fig. 4) and fluorescence activated cell sorting (FACS). Interestingly, when performing FACS analysis, we determined that collagen producing cells in skeletal muscle are heterogeneous and consist of fibroblasts, fibro/adipogenic progenitor cells and skeletal muscle progenitor cells. This finding further highlights the fact that the use of a single marker (e.g., collagen I production) is insufficient to specifically label fibroblasts. Furthermore, using this genetic model, we are able to show that all three collagen producing cell populations show a significant increase in numbers, while the collagen-producing cell composition and gene expression generally remained the same between WT and DKO muscle. These results demonstrate the heterogeneity of collagen I producing cells in healthy and fibrotic muscle. This knowledge may lead to development of therapies aimed at suppressing collagen I cell proliferation and collagen I deposition (Chapman et al., 2016).
Figure 4. Collagen producing cells are more abundant in skeletal muscle fibrosis.
Fluorescence micrographs of tibialis anterior sections from wild type (A) and double knockout (DKO) (B) mice. Note the increased GFP signal in DKO mice, which reflects increased type I collagen production by DKO mouse muscle. This cell population is heterogeneous, with a portion being fibroblasts. Scale bar = 50µm. (From (Chapman et al., 2016)).
Fibroblasts in skeletal muscle regeneration
As previously mentioned, it has been shown that, even though myoblasts secrete ECM proteins, fibroblasts are necessary for the proper assembly of those proteins into functional fibrillar ECM. The opposite has also been shown in a recent study demonstrating that fibroblasts are required for the proper regeneration of skeletal muscle by satellite cells (Murphy et al., 2011). In this study, mouse lines were developed in which they were able to inducibly ablate satellite cells by targeting Pax7 producing cells, or all connective tissue fibroblasts by inducibly ablating Tcf4+ cells (Murphy et al., 2011). Perhaps unsurprisingly, when satellite cells were ablated, skeletal muscle regeneration was inhibited. However, when connective tissue fibroblasts were ablated, satellite cell dynamics were also altered, resulting in premature differentiation of satellite cells and poorly regenerated muscle fibers with decreased diameters. The authors propose a mechanism by which the presence of fibroblasts prevents premature differentiation of satellite cells allowing satellite cell expansion, and thus proper muscle regeneration. This dynamic equilibrium between fibroblasts and satellite cells has significant implications since many conditions result in satellite cell depletion (see section ‘Fibroblasts in skeletal muscle fibrosis’).
These findings have implications for the treatment of skeletal muscle pathologies where proper regeneration is hindered, such as muscular dystrophy and cerebral palsy. This work suggests that fibroblasts and satellite cells may exist in some sort of equilibrium which is necessary to maintain proper muscle health. This should be taken into account when new therapies are being developed to combat fibrosis because therapies aimed at suppressing fibrosis by targeting connective tissue fibroblasts may fail and cause reduced tissue regeneration.
Fibroblasts in skeletal muscle fibrosis
Skeletal muscle fibrosis results from both acute (e.g. stroke or muscle trauma) and chronic (e.g. muscular dystrophy and cerebral palsy) injury conditions. With excessive accumulation of ECM in skeletal muscle, patients present with decreased muscle force production and decreased passive range of motion (Klingler et al., 2012; Zumstein et al., 2008). In chronic disease, skeletal muscle fibrosis results as a response to the primary condition. For example, patients suffering from Duchenne Muscular Dystrophy (DMD) lack dystrophin, which weakens sarcolemmal integrity, resulting in contraction-induced myofiber damage (Petrof et al., 1993). In response to this damage, muscle attempts to regenerate, however the regenerated muscle still lacks dystrophin, which ultimately results in myofiber necrosis. The myofibrillar damage that occurs in these patients results in an inflammatory response cascade involving the invasion and activation of inflammatory cells (e.g. macrophages, eosinophils) into the injured tissue (Mann et al., 2011; Wynn, 2008). These inflammatory cells release cytokines such as TGF-β and TNF-α that promote ECM production in fibroblasts. Furthermore, chronic inflammation resulting from muscular dystrophies results in a positive feedback release of these inflammatory cytokines from fibroblasts and additional ECM production (Lieber and Ward, 2013; Mann et al., 2011; Wynn, 2008). The perpetual inflammation results in chronic activation of skeletal muscle fibroblasts which ultimately leads to ECM overproduction in the diseased muscle, causing muscle fibrosis. These ‘activated’ fibroblasts have been termed myofibroblasts, but, as previously discussed, the actual identity of these cells is debated (Baum and Duffy, 2011; Mann et al., 2011; Phan, 2008).
Regardless of whether these cells are myofibroblasts or simply mature fibroblasts, determining their origin is critical for understanding the mechanisms of fibrosis and for developing anti-fibrotic therapies. In an elegant lineage tracing study, researchers demonstrated that a majority of myofibroblasts could be traced to a subset of perivascular cells that express a disintigrin and metalloproteinase 12 (ADAM12) during development (Dulauroy et al., 2012). After skeletal muscle injury, when ADAM12 cells were genetically ablated, a significant reduction in fibrosis was observed, showing that the reduction of ADAM12+ cells and their progeny can ameliorate skeletal muscle fibrosis. Using separate perivascular cell markers, Nestin−/NG2+ perivascular cells were also implicated as a source of skeletal muscle fibrosis in aged mice (Birbrair et al., 2013). Given that a genetic ablation technique for these cells has not yet been developed, it remains to be seen whether fibrosis is prevented in the absence of this cell type.
In addition to pericytes, recent reports identify a skeletal muscle precursor cell that can differentiate into either fibroblasts or adipocytes, referred to as fibro/adipogenic progenitor cells (FAP) (Joe et al., 2010; Uezumi et al., 2011). FAPs are defined by negative staining for CD31 and CD45 and positive staining for CD34 and Sca-1. The authors in both studies cited showed that, upon muscle injury, FAP cells rapidly differentiate and assist in regeneration. The discovery of these cells lends itself to the creation of new therapeutic techniques to combat conditions with an over production of fibrotic and fatty tissue, such as in rotator cuff tears or lumbar spine muscle pathology. Patients with torn rotator cuffs often present with significant levels of fibrotic tissue and fatty infiltration in the muscles of the rotator cuff (Meyer et al., 2004; Ward et al., 2006; Zumstein et al., 2008). With the discovery of FAP cells, it is possible that a therapy could be developed aimed at reducing the ability for FAP cells to enter the cell cycle and produce fibrotic/fatty tissue. In conjunction with surgical techniques, this therapy could improve the strength and flexibility of patients with rotator cuff tears. In developing these therapies, care should be made to only suppress excessive FAP proliferation, since these cells can also promote muscle regeneration.
Creating anti-fibrotic therapies is crucial for attaining muscle regeneration in myopathies with fibrotic phenotypes. This is made even more apparent with the publication of recent studies showing that the muscle microenvironment is important for proper tissue regeneration (Boldrin et al., 2015; Meng et al., 2015). Typically, it is stated that the satellite cell pool becomes ‘exhausted’ in chronic skeletal myopathies and the number of satellite cells participating in tissue regeneration is reduced (Sacco et al., 2010). In addition to this decreased number of regenerating cells, the skeletal muscle microenvironment is no longer able to support proper cellular differentiation, and engraftment becomes less efficient (Boldrin et al., 2015; Meng et al., 2015). This research has concluded that satellite cells residing in dystrophic tissue are capable of tissue regeneration when placed in a healthy microenvironment. Thus, therapies involving satellite cell/myoblast transplantation alone cannot ameliorate the condition. It is important that these treatments be coupled with anti-fibrotic therapies to attain the best outcome. This could be a reason that cell therapy in DMD has resulted in inefficient cell engraftment and little functional benefits (Gussoni et al., 1997; Mendell et al., 1995; Skuk et al., 2007, 2006, 2004).
Anti-fibrotic therapies targeting the inflammatory TGF-β pathway are also being developed to prevent ECM overproduction by fibroblasts in injured muscle (Foster et al., 2003; Zhu et al., 2007). However, when this technique is used in animal models of muscular dystrophy, an increased inflammatory response occurs, questioning its application as a feasible therapy (Andreetta et al., 2006). Given this, research aimed at specific targets within the proinflammatory TGF-β pathway are being developed for more sensitive therapies. One such example is a recently described system where suppressing microRNA-21, an important regulator of the inflammatory plasminogen activator system, is able to ameliorate dystrophy-associated fibrosis in a mouse model (Ardite et al., 2012).
Conclusions
Skeletal muscle fibroblasts play an important role in maintaining muscle mechanical and biological health. However, fibroblasts are also implicated in muscle disease when their over activity results in debilitating fibrosis. This is important to be aware of when designing new therapies to treat muscle disease, because these apparent bystanders in skeletal muscle turn out to be important for proper tissue regeneration. New techniques, such as genetic labeling and advanced electron microscopy, are beginning to shed light on the dual role of fibroblasts in skeletal muscle homeostasis and disease and hold great promise for development of novel cellular and molecular therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Fifth. Garland Science; 2008. [Google Scholar]
- Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P. Immunomodulation of TGF-beta1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response : Implications for antifibrotic therapy. J. Neuroimmunol. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Archile-Contreras aC, Mandell IB, Purslow PP. Phenotypic differences in matrix metalloproteinase 2 activity between fibroblasts from 3 bovine muscles. J. Anim. Sci. 2010;88:4006–4015. doi: 10.2527/jas.2010-3060. [DOI] [PubMed] [Google Scholar]
- Ardite E, Perdiguero E, Vidal B, Gutarra S, Serrano AL, Muñoz-Cánoves P. PAI-1-regulated miR-21 defines a novel age-associated fibrogenic pathway in muscular dystrophy. J. Cell Biol. 2012;196:163–175. doi: 10.1083/jcb.201105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Bailey AJ, Restall DJ, Sims TJ, Duance VC. Meat tenderness: Immunofluorescent localisation of the isomorphic forms of collagen in bovine muscles of varying textureNo Title. J. Sci. Food Agric. 1979;30:203–210. [Google Scholar]
- Bateman JF, Boot-Handford RP, Lamandé SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat. Rev. Genet. 2009;10:173–183. doi: 10.1038/nrg2520. [DOI] [PubMed] [Google Scholar]
- Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J. Cardiovasc. Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang Z-M, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am. J. Physiol. Cell Physiol. 2013;305:C1098–C1113. doi: 10.1152/ajpcell.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L, Zammit PS, Morgan JE. Satellite cells from dystrophic muscle retain regenerative capacity. Stem Cell Res. 2015;14:20–29. doi: 10.1016/j.scr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg K, Caulfield B. MORPHOLOGY OF CONNECTIVE SKELETAL MUSCLE TISSUE. Tissue Cell. 1980;12:197–207. doi: 10.1016/0040-8166(80)90061-0. [DOI] [PubMed] [Google Scholar]
- Buckberg G, Hoffman JIE, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–2587. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- Chang HY, Chi J-TJ, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO, Rijn M, Van De, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MA, Mukund K, Subramaniam S, Brenner D, Lieber R. Three Distinct Skeletal Muscle Cell Types Express Collagen for Different Niches During Fibrosis. 2016 Submitted. [Google Scholar]
- Chapman MA, Pichika R, Lieber RL. Collagen crosslinking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J. Biomech. 2015;48:375–378. doi: 10.1016/j.jbiomech.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MA, Zhang J, Banerjee I, Guo LT, Zhang Z, Shelton GD, Ouyang K, Lieber RL, Chen J. Disruption of both nesprin 1 and desmin results in nuclear anchorage defects and fibrosis in skeletal muscle. Hum. Mol. Gen. 2014;23:5879–5892. doi: 10.1093/hmg/ddu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2 doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- Fernandez-Madrid F, Noonan S, Riddle J. “spindle-shaped” body in fibroblasts: intracellular collagen fibrils. J. Anat. 1981;132:157–166. [PMC free article] [PubMed] [Google Scholar]
- Foster W, Somogyi G, Huard J. Gamma interferon as an antifibrosis agent in skeletal muscle. J. Orthop. Res. 2003;21:798–804. doi: 10.1016/S0736-0266(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, Phipps RP. Evidence of Fibroblast Heterogeneity and the Role of Fibroblast Subpopulations in Fibrosis. Clin. Immunol. Immunopathol. 1994;72:283–292. doi: 10.1006/clin.1994.1144. [DOI] [PubMed] [Google Scholar]
- Gao Y, Kostrominova T. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J. …. 2008;41:465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Waas AM, Faulkner Ja, Kostrominova TY, Wineman AS. Micromechanical modeling of the epimysium of the skeletal muscles. J. Biomech. 2008;41:1–10. doi: 10.1016/j.jbiomech.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Gatchalian CL, Schachner M, Sanes JR. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin(J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J. Cell Biol. 1989;108:1873–1890. doi: 10.1083/jcb.108.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp. Biol. Med. 2002;227:301–314. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- Gillies AR, Bushong Ea, Deerinck TJ, Ellisman MH, Lieber RL. Three-Dimensional Reconstruction of Skeletal Muscle Extracellular Matrix Ultrastructure. Microsc Microanal. 2014:1–6. doi: 10.1017/S1431927614013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–31. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:347–358. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD, Sorokin L, White J. Strength at the extracellular matrix-muscle interface. Scand. J. Med. Sci. Sport. 2005;15:381–391. doi: 10.1111/j.1600-0838.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Blau H, Kunkel L. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki Ma, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12:153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004;84:649–98. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Magnusson P, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise Tendon and collagen synthesis. J. Anat. 2006;208:445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012;31:184–195. [PMC free article] [PubMed] [Google Scholar]
- Kuhl U, Ocalan M, Timpl R, Mayne R, Hay E, von der Mark K. Role of muscle fibroblasts in the deposition of type-IV collagen in the basal lamina of myotubes. Differentiation. 1984;28:164–172. doi: 10.1111/j.1432-0436.1984.tb00279.x. [DOI] [PubMed] [Google Scholar]
- Kühl U, Timpl R, von der Mark K. Synthesis of type IV collagen and laminin in cultures of skeletal muscle cells and their assembly on the surface of myotubes. Dev. Biol. 1982;93:344–354. doi: 10.1016/0012-1606(82)90122-1. [DOI] [PubMed] [Google Scholar]
- Lieber RL. Skeletal muscle structure, function, and plasticity. 3rd. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- Lieber RL, Ward SR. Cellular Mechanisms of Tissue Fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am. J. Physiol. Cell Physiol. 2013;305:C241–C252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light N, Champion AE. Characterization of muscle epimysium, perimysium and endomysium coliagens. Biochem J. 1984;219:1017–1026. doi: 10.1042/bj2191017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton BH. Collagen synthesis by normal and bromodeoxyuridine-modulated cells in myogenic culture. Dev. Biol. 1977a;61:153–165. doi: 10.1016/0012-1606(77)90288-3. [DOI] [PubMed] [Google Scholar]
- Lipton BH. A fine-structural analysis of normal and modulated cells in myogenic cultures. Dev. Biol. 1977b;60:26–47. doi: 10.1016/0012-1606(77)90108-7. [DOI] [PubMed] [Google Scholar]
- López B, Querejeta R, González A, Larman M, Díez J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension. 2012;60:677–683. doi: 10.1161/HYPERTENSIONAHA.112.196113. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson Ja, Hutcheson Da, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson Ma, Lieber RL. Pathophysiology of muscle contractures in cerebral palsy. Phys Med Rehabil Clin N Am. 2015;26:57–67. doi: 10.1016/j.pmr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DCG, Leinwand LA. Sarcomeric gene expression and contractility in myofibroblasts. J. Cell Biol. 1997;139:1477–1484. doi: 10.1083/jcb.139.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J, Kissel J, Amato A, King W, Signore L, Prior T, Sahenk Z, Benson S, McAndrew P, Rice R, Nagaraja H, Stephens R, Lantry L, Morris G, Burghes A. Myoblast transfer in the treatment of duchenne’s muscular dystrophy. N. Engl. J. Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Meng J, Bencze M, Asfahani R, Muntoni F, Morgan JE. The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet. Muscle. 2015;5:11. doi: 10.1186/s13395-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DC, Hoppeler H, Rechenberg B, Von, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J. Orthop. Res. 2004;22:1004–1007. doi: 10.1016/j.orthres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Meyer Ga, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J. Biomech. 2011;44:771–773. doi: 10.1016/j.jbiomech.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Lawson Ja, Mathew SJ, Hutcheson Da, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RA, Peat RA, Baker NL, Zamurs L, Mörgelin M, Irving M, Adams NE, Bateman F, Mowat D, Smith NJC, Lamont PJ, Moore SA, Mathews KD, North KN, Lamandé SR. Collagen VI glycine mutations: perturbed assembly and a spectrum of clinical severity. Ann. Neurol. 2008;64:294–303. doi: 10.1002/ana.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J. Struct. Biol. 2007;159:19–28. doi: 10.1016/j.jsb.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly aM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. PNAS. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan SH. Biology of fibroblasts and myofibroblasts. Proc. Am. Thorac. Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierard GE. Sirius Red Polarization Method is Useful to Visualize the Organization of Connective Tissues but not the Molecular Composition of their Fibrous Polymers. Matrix. 1989;9:68–71. doi: 10.1016/s0934-8832(89)80021-6. [DOI] [PubMed] [Google Scholar]
- Purslow P, Trotter J. The morphology and mechanical properties of endomysium in series-fibred muscles$: variations with muscle length. J. Muscle Res. Cell Motil. 1994;308:299–308. doi: 10.1007/BF00123482. [DOI] [PubMed] [Google Scholar]
- Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. 2002;133:947–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, Dorfmüller P, Humbert M, Guignabert C. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129:1586–1597. doi: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- Rich L, Whittaker P. Collagen and picosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Brazilian J. Morphol. Sci. 2005;22:97–104. [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM. Short Telomeres and Stem Cell Exhaustion Model Duchenne Muscular Dystrophy in mdx / mTR Mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson RD, Fitch JM, Linsenmayer TR, Mayne R. Fibroblasts promote the formation of a continuous basal lamina during myogenesis in vitro. J. Cell Biol. 1986;102:740–747. doi: 10.1083/jcb.102.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse J, von der Mark H, Kühl U, Dessau W, von der Mark K. Origin of collagen types I, III, and V in cultures of avian skeletal muscle. Dev. Biol. 1981;83:79–89. doi: 10.1016/s0012-1606(81)80010-3. [DOI] [PubMed] [Google Scholar]
- Sato EJ, Killian ML, Choi AJ, Lin E, Esparza MC, Galatz LM, Thomopoulos S, Ward SR. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J. Orthop. Res. 2014;32:1111–1116. doi: 10.1002/jor.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard J, Roy R, Dugre FJ, Sylvain M, Lachance J, Desche L. Dystrophin Expression in Muscles of Duchenne Muscular Dystrophy Patients After High-Density Injections of Normal Myogenic Cells. J. Neuropathol. Exp. Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Côté CH, Chapdelaine P, Hogrel J-Y, Paradis M, Bouchard J-P, Sylvain M, Lachance J-G, Tremblay JP. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul. Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulet M, Chapdelaine P, Bouchard J, Roy R, Dugre FJ, Lachance J, Desche L, Sylvain M, Tremblay JP. Dystrophin Expression in Myofibers of Duchenne Muscular Dystrophy Patients Following Intramuscular Injections of Normal Myogenic Cells. Mol. Ther. 2004;9:475–482. doi: 10.1016/j.ymthe.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am. J. Physiol. Cell Physiol. 2014;306:C889–C898. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J. Physiol. 2011;589:2625–2639. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- Starborg T, Kalson NS, Lu Y, Mironov A, Cootes TF, Holmes DF, Kadler KE. Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat. Protoc. 2013;8:1433–1448. doi: 10.1038/nprot.2013.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG. Force transmission across muscle cell membranes. J. Biomech. 1991;24:43–52. doi: 10.1016/0021-9290(91)90376-x. [DOI] [PubMed] [Google Scholar]
- Trotter J, Purslow P. Functional Morphology of the Endomysium in Series Fibered Muscles. J. Morphol. 1992;122:109–122. doi: 10.1002/jmor.1052120203. [DOI] [PubMed] [Google Scholar]
- Tsujie M, Isaka Y, Ando Y, Akagi Y, Kaneda Y, Ueda N, Imai E, Hori M. Gene transfer targeting interstitial fibroblasts by the artificial viral envelope-type hemagglutinating virus of Japan liposome method. Kidney Int. 2000;57:1973–1980. doi: 10.1046/j.1523-1755.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Bönnemann CG, Huijing PA, Hamel BC, van Kuppevelt TH, de Haan A, Schalkwijk J, van Engelen BG, Jenniskens GJ. Clinical and molecular overlap between myopathies and inherited connective tissue diseases. Neuromuscul. Disord. 2008;18:843–856. doi: 10.1016/j.nmd.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns Ka, Fithian DC, Friden J, Lieber RL. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin. Orthop. Relat. Res. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Kloner R, Boughner D, Pickering J. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Patton BL. Developmental and Pathogenic Mechanisms of Basement Membrane Assembly. Curr. Pharm. Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca M, Huard J. Relationships between Transforming Growth Factor-1, Myostatin, and Decorin. J. Biol. Chem. 2007;282:25852–25863. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]
- Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The Clinical and Structural Long-Term Results of Open Repair of Massive Tears of the Rotator Cuff. J. Bone Jt. Surgery. 2008;90:2423–2431. doi: 10.2106/JBJS.G.00677. [DOI] [PubMed] [Google Scholar]