Abstract
(1S,2E,4S,6R,7E,11E)-2,7,11-cembratriene-4,6-diol (1) and its 4-epi-analog (2) are the cembranoid precursors to several key flavor ingredients in most Nicotiana (tobacco) species. Nearly 40–60% of 1 and 2 are purposely degraded during the commercial tobacco fermentation. However, 1 and 2 display promising bioactivities, including anticancer. Breast cancer is the most diagnosed cancer in women and ranked second female disease killer. The receptor tyrosine kinase c-Met correlates with aggressiveness of certain breast cancer phenotypes and thus considered a valid therapeutic target. This study reports the discovery and optimization of the tobacco-based cembranoid 1 as a novel c-Met inhibitory scaffold using combined structure- and ligand-based approaches. 1 displayed antiproliferative, anti-migratory and anti-invasive effects against the c-Met overexpressing MDA-MB-231 breast cancer cells at moderate μM concentrations. The Z′-LYTE kinase platform and Western blot analysis identified c-Met as a potential macromolecular target. Rationally designed carbamate analogs were proposed to probe additional targeted c-Met interactions and improve the cellular potency. The 6-phenyl carbamate 3 showed enhanced c-Met inhibitory activity. Structure-activity relationships of different substituents on the 3’s phenyl moiety were studied. The most active analog 20 showed potent in vitro anticancer activities against the MDA-MB-231 breast cancer cells at low μM concentrations, with minimal toxicity on the non-tumorigenic MCF-10A mammary epithelial cells. Cembranoid 20 potently inhibited the c-Met catalytic activity in Z′-LYTE kinase assay and various cellular c-Met-driven signaling pathways. Furthermore, 20 displayed a robust antitumor activity in a breast cancer xenograft athymic mouse model and thus promoted to the lead rank. Cembranoids are novel c-Met inhibitors appropriate for future use to control c-Met dependent malignancies.
Keywords: Breast cancer, c-Met, Hit-to-lead, Rational design, Semisynthesis, Tobacco cembranoids 1.
Graphical abstract

Introdcution
Tobacco (Nicotiana tabaccum L.) is one of the most economically important agricultural crops.1 Tobacco smoke contains harmful ingredients like nicotine, N-nitroso amines, and others, but it also contains some compounds of prospective highly beneficial uses. Beside the notorious psychoactive nicotine, more than four thousands of other diverse secondary metabolites have been identified in tobacco leaf, smoke, and flower.2 Isoprenoids are among the prominent classes of tobacco-driven secondary metabolites. Isoprenoids are biosynthesized in the glandular heads of trichomes covering tobacco leaves and flowers and extensively present in the gummy exudates.3,4 The tobacco isoprenoid family comprises three major classes, carotenoids, labdanoids and cembranoids. The 14-membered macrocyclic diterpene cembranoids have been granted much attention due to their biological activities. Cembranoids are prone to biodegradation during cure fermentation or flue curing of tobacco leaf, mostly by rupture of the double bonds in the main skeleton, to a variety of volatile and low molecular weight products having from 8 to 19 carbon skeleton that impart the characteristic aroma of the commercial tobacco.5,6
The epimeric diols (1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (1) and (1S,2E,4R,7E,11E)-2,7,11-cembratriene-4,6-diol (2) (Table 1) are the most abundant tobacco cembranoids.7 Nevertheless, the two metabolites differ only in the configuration of C-4, they exhibited epimer-specific pharmacological activities. For instance, only 2 was proven to bind to the nicotinic acetylcholine receptors (nAChR’s) and display neuroprotective and nicotine anti-addictive properties.8 In addition, 2 exerted a protective effect against the neuronal death and brain inflammation in rats exposed to organophosphate-based insecticides.9 Moreover, the first pharmacokinetics and metabolism study of 2 in rats was recently reported.10 On the other hand, most of the reported bioactivities for 1 were related to oncology. For instance, 1 was identified as a tumor promotion inhibitor of the 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-mediated induction of Epstein–Barr virus early antigen (EBV-EA) in vitro model in lymphoblastoid Raji cells.11 Moreover, 1 displayed a higher potency over 2 in inhibiting 7,12-dimethylbenz[a]anthracene (DMBA)-initiated TPA-promotion in a mouse skin tumor model.12 In these initiation-promotion experiments, 1 was much more active than its epimer 2. Application of 3.3 μM of 1 40 minutes prior to TPA treatment resulted in a 53% reduction in the incident of papilomas in mice.12 A dose of 50 μM of 1 was able to inhibit 28.4% of 32Pi incorporation into the phospholipids of TPA-stimulated Hela cells without any interaction with calmodulin, even at a dose of 100 μM, unlike many other anticancer agents and no cytotoxic activity was observed at this dose level.12 However, 1 treatment was not found to inhibit the specific binding of 3H-TPA to mouse epidermal particulate fraction even at doses up to 1 mM in vitro, and 10 mg in vivo.12 Moreover, 1 was found to induce a dose-responsive inhibition of TPA-stimulated phosphorylation of the 47K-Da human platelet protein, and displayed an IC50 value of 100 μM.12 These results clearly suggested that 1 does not directly compete with nor modulate TPA binding on the protein kinase C (PKC) receptor.12 Thus, 1 was suggested to inhibit PKC activity by some other unknown mechanism of action and not by directly antagonizing TPA binding to this kinase.12 Earlier, our group reported the antiproliferative activity of 1 and its biotransformation and semisynthetic derivatives against the highly malignant +SA mouse mammary epithelial cells.13 Despite multiple reported promising anticancer activities, the potential mechanistic target(s) mediating the anticancer effects of cembranoid 1 remains unknown.
Table 1.
Chemical structure of cembranoids 1 and 2 and analogs 3-20.

| |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 |
| 3 | H | H | H | H | H |
| 5 | H | H | Ph | H | H |
| 7 | H | H | C2H5 | H | H |
| 8 | H | H | Cl | H | H |
| 9 | H | CH3 | H | H | H |
| 10 | H | Cl | H | H | H |
| 11 | Cl | H | H | H | H |
| 12 | F | H | H | H | H |
| 13 | H | H | F | H | H |
| 14 | H | Cl | H | Cl | H |
| 15 | Cl | H | H | H | Cl |
| 16 | Cl | H | Cl | H | H |
| 17 | H | Cl | Cl | H | H |
| 18 | H | CF3 | H | H | H |
| 19 | H | H | CF3 | H | H |
| 20 | H | CF3 | H | CF3 | H |
Tobacco cembranoids have been subjected to diverse biocatalytic and semisynthetic transformations to improve their pharmacological activities. However, previously reported optimizations were solely ligand-based, due to lack of validated molecular targets. For instance, biocatalysis of 2 using marine Bacilli and synthesis of its carbamate analogs were associated with a significant enhancement of anti-invasive activity against the prostate cancer PC-3M-CT cell line.14 In addition, biocatalytic and semisynthetic analogs of 1 showed a remarkable improvement of antiproliferative activity against the highly malignant +SA mouse mammary epithelial cells.13 In aggregate, structural modifications of tobacco cembranoids framework through biocatalysis and semisynthesis led to enhancement of different bioactivities, however, no rational design semisynthestic reports of tobacco cembranoids due to the lack of knowledge of specific valid molecular target(s).
Breast cancer ranks the second leading cause of death among women worldwide.15 The advancement in early detection techniques and development of targeted therapies resulted in a significant decline in the disease mortality rate over the past two decades. Nevertheless, only in the U.S., more than 246,000 new cases are estimated to be diagnosed with breast cancer and more than 40,000 are expected to die from the disease complications in 2016.16 Clinically, the characterization of breast cancer is extensively relying on the molecular analyses of different protein biomarkers or gene expression profiles.17 For instance, the expression levels of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) are primarily used for clinical classification and are taken in account to determine the optimal therapeutic approaches. The ER+ breast cancers are best responsive to the hormonal therapy, including selective estrogen receptor modulators (SERMs), aromatase inhibitors, estrogen receptor downregulators (ERDs) and luteinizing hormone releasing hormone agents (LHRHs). Meanwhile, HER2 overexpressing breast cancers are best treated with the monoclonal antibody trastuzumab. Moreover, breast cancer lacking the expression of the three receptors (ER, PR and HER2) is described as triple-negative (TNBC). The management of TNBC is extremely challenging because it lacks targeted therapy and has aggressive phenotype and relative poor prognosis.18
Tyrosine kinases (TKs) are frequently dysregulated in breast malignancies.19 In particular, the receptor tyrosine kinase c-Met is overexpressed in TNBC and associated with the aggressive metastatic progression of the disease.20,21 Structurally, c-Met comprises an extracellular α-subunit connected to a transmembrane β-subunit through a disulfide linkage. The extracellular portion incorporates four IPT (immuonoglobulin-like fold shared by plexins and transcription factors) domains, to which the highly specific ligand hepatocyte growth factor (HGF, also known as scatter factor) binds and sparks the c-Met signaling pathway.22 The binding of HGF to c-Met triggers receptor homodimerization and activation of the intracellular intrinsic tyrosine kinase catalytic activity. Consequently, Tyr1234 and 1235 residues of the kinase domain become phosphorylated, leading to another phosphorylation of C-terminal docking Tyr1349 and Y1356 residues. The later creates a docking site for many adaptor molecules to bind to the intracellular domain of c-Met.22 These adaptors mediate diverse downstream signaling pathways, including PI3K/Akt/mTOR, Ras/Raf/MAPK and Paxillin/FAK, which ultimately regulate cell survival, proliferation, migration and invasion.23–25 Consequently, c-Met has been validated as a therapeutic target for controlling various malignancies with aberrant c-Met signaling as evidenced by the FDA approval of two c-Met inhibitors, including cabozantinib (dual c-Met-VEGFR2 inhibitor) and crizitonib (dual c-Met-ALK inhibitor) that were approved for medullary thyroid and ALK-driven lung cancers, respectively.26,27 Currently, there are seventy-nine ongoing clinical trials related to c-Met inhibitors.28 These studies are clearly translating the potential role of c-Met inhibitors as valid cancer-targeting therapies.
Herein, we report the discovery and optimization of the tobacco-based cembranoid diterpene (1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (1) as a novel c-Met inhibitory scaffold. 1 was evaluated in proliferation, migration and invasion assays against multiple breast cancer cell lines. Rationally designed carbamate analogs were proposed based on the favorable bind pose of 1 at the c-Met kinase domain, to probe additional molecular binding interactions with nearby amino acids. The structure-activity relationship (SAR) of the parent phenyl carbamate analog 3 was then optimized using a ligand-based strategy. The most active carbamate analog 20 was tested against the TNBC and c-Met overexpressing MDA-MB-231 cells in various in vitro and in vivo assays.
2. Results and discussion
The cembranoid (1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (1) was obtained by extracting fresh tobacco leaves blend composed of Virginia, Oriental, and Burley tobacco (1:1:1) using CH2Cl2. This extract was subjected to vacuum liquid chromatography on Si gel 60 using successive amounts of n-hexanes-ethyl acetate, followed by chromatographic purification over C-18 reversed phase Si gel using gradient H2O-MeCN elution. The chemical identity of 1 was confirmed by NMR spectroscopic analysis and comparing with literature.3–5
The anticancer effects of cembranoids 1, 2, and their analogs against breast and prostate cancers were previously reported.13,14 Neither of these studies identified specific molecular target(s) mediating, at least in part, these cembranoids anticancer effects. This encouraged the investigation of 1’s activity against a selected panel of human breast cancer cell lines and possible identification of its potential mechanistic target(s). This panel incorporates breast cancer cells with diverse phenotypic and molecular profiles. Each cell line harbors one or more dysregulated oncogenic pathways that may provide preliminary clues for possible mode of action. For instance, MCF-7 and T-47D cells are estrogen-dependent cells (ER+), BT-474 cells overexpress HER2 and ER+, while SK-BR-3 are HER2 overexpressing, but lacking ER. The MDA-MB-231 and MDA-MB-468 cells represent a triple-negative subtype that overexpresses the oncogenic RTK c-Met. Thus, sensitivity of one or more of these cell lines should propose potential cell-specific oncogenic pathway target for subsequent investigations. Results showed as dose-responsive cell proliferation inhibition upon treatment with 1, where the TNBC cells were relatively more sensitive (Figure 1, Table 2).
Figure 1.
Effect of cembranoid 1 and 20 on the proliferation of various breast cancer cells. Data represent the dose-dependent inhibition of percent cell proliferation (mean ± SEM) at indicated concentrations. * Indicates statistical significance at P <0.05.
Table 2.
IC50 of cembranoid 1 and 20 in proliferation, migration, and invasion assays against multiple breast cancer cell lines.a
| Assay IC50 (mean ± SEM) μM | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Proliferation | Migration | Invasion | ||||
| Cell line/cpd. | 1 | 20 | 1 | 20 | 1 | 20 |
| MCF-7 | 61.2 ± 4.6 | 7.2 ± 1.1 | ND | ND | ND | ND |
| T-47D | 61.9 ± 4.1 | 11.2 ± 1.3 | ND | ND | ND | ND |
| MDA-MB-231 | 34.3 ± 2.5 | 1.3 ± 0.2 | 28.6 ± 2.1 | 1.4 ± 0.2 | 37.6 ± 2.3 | 1.7 ± 0.3 |
| MDA-MB-468 | 39.3 ± 2.7 | 2.1 ± 0.2 | ND | ND | ND | ND |
| SK-BR-3 | 48.9 ± 3.1 | 4.7 ±0.9 | ND | ND | ND | ND |
| BT-474 | 52.6 ± 3.5 | 3.9 ±0.7 | ND | ND | ND | ND |
ND: Not determined.
Breast cancer mortality correlates well with the metastatic degree of the primary breast tumor. The wound healing scratch assay (WHA) is the most common assay for assessment of antimigratory activity. Thus, the c-Met overexpressing and TNBC MDA-MB-231 cells were treated with various concentrations of 1 and stimulated by HGF to spark the HGF/c-Met motility-signaling axis. Results revealed a dose-responsive inhibition of cell migration upon treatment with 1 (Figure 2A). Approximately, 23 h post treatment, the HGF-treated control cells showed a remarkable migration and nearly closed the wound unlike 1’s treatments, which resulted in a significant cell migration inhibition, with a calculated IC50 28.6 μM (Figure 2B, Table 2).
Figure 2.
Effect of cembranoid 1 and 20 on the migration of the TNBC MDA-MB-231 cells in wound healing assay. (A) Dose-dependent migration inhibition of cells treated with 1 and 20. Data represent mean percent cell migration (± SEM) at indicated concentrations. The olive phenolic oleocanthal (OC)42 was used as antimigratory positive control. *Indicates a statistical significance at P< 0.05. (B) Microscopic images (40x) of wounds at zero time and 23 h post-treatment with vehicle, 1 and 20.
Malignant cell invasion constitutes a crucial stage of the subsequent metastatic cascade.29 To assess the anti-invasive activity of 1, the highly metastatic and TNBC MDA-MB-231 cells were treated with various concentrations of 1 and the effect was monitored using the CultreCoat® cell invasion kit. Cembranoid 1 showed a significant dose-dependent invasion inhibition through the basement membrane extract (BME) lining the base of the invasion chambers, with an IC50 of 37.6 μM (Figure 3A). Figure 3B shows the ability of 1’s 40 μM treatment to remarkably inhibit the trans-well cell invasion, indicated by the relatively higher cell-density at upper surface of invasion chamber compared to vehicle control wells, where most of cells invaded to the lower surface.
Figure 3.
Effect of cembranoid 1 and 20 on the invasion of the TNBC MDA-MB-231 cells in Cultrcoat® cell invasion assay. (A) Dose-dependent invasion inhibition of cells treated with 1 and 20. Data represent mean percent cell invasion (± SEM) at indicated concentrations. The olive oil phenolic oleocanthal (OC)42 was used as a positive control. * Indicates a statistical significance at P< 0.05. (B) Microscopic images (40X) of invasion chambers in DMSO, 1 and 20-treated cells after 24 h of incubation.
The good activity of 1 in multiple anticancer bioassays and relative sensitivity of the TNBC cells motivated the evaluation of its activity against the proto-oncogenic receptor tyrosine kinase c-Met that is known to be overexpressed in TNBC cell lines and represents the main driving signaling pathway for oncogenic growth, motility, migration, and invasion.30 Therefore, the Z′-LYTE kinase platform was used to assess the ability of 1 to inhibit the in vitro c-Met catalytic activity. This cell-free assay has the advantages of excluding cellular barriers and plausible cellular biotransformation because the tested compound directly brought to interact with the macromolecular target in a solution state. Results showed that 1 optimally inhibited the c-Met kinase catalytic activity with a calculated IC50 22.6 μM (Table 3).
Table 3.
IC50 of cembranoid 1 and the most active semisynthetic analogs in Z′-LYTE kinase assay.
| Compound | IC50 (μM) |
|---|---|
| 1 | 22.6 |
| 10 | 3.4 |
| 14 | 2.9 |
| 18 | 1.8 |
| 20 | 1.1 |
The c-Met catalytic inhibition by 1 was further validated at the cellular level by Western blotting analysis. The MDA-MB-231 cells were treated with different concentrations of 1, lysed and analyzed by Western blot to assess its effects on c-Met phosphorylation (activation) levels. 1 treatments showed a significant concentration-dependent downregulation of p-Met but had no effect on the total c-Met level (Figure 4A). Promisingly, these results suggest that anticancer effects displayed by 1 in different anticancer assays against c-Met overexpressing breast cancer cells are correlated, at least in part, with the c-Met phosphorylation inhibition. However, the cellular potency still needs further optimization.
Figure 4.
Effects of cembranoid 1 and 20 on the c-Met and downstream signaling axis in the TNBC MDA-MB-231 cells. (A) Immunoblots show the inhibitory effect of 1 on phosphorylated levels of c-Met. (B) Immunoblots show a dose-dependent downregulation of p-Met and various downstream effectors in cells treated with 20 compared to untreated cells.
Biological assays disclosed diverse anticancer activities of 1 at moderate μM concentrations and revealed the proto-oncogenic c-Met as a potential macromolecular target. Therefore, 1 was subjected to a rational semisynthetic optimization to enhance its c-Met inhibitory activity and cellular potency. The molecular structure of 1 was docked at the kinase domain of the human c-Met crystal structures to predict potential binding modes and explore protein-ligand interactions. The docking analysis of 1 within the ATP-binding cleft revealed an in-pocket orientation of the cembrene scaffold (Figure 5A). The macrocycle was positioned towards the phenolic side-chain of the activation loop Tyr1230 and Ala1108 and Val1092 side-chains, probably for hydrophobic interactions. Meanwhile, the polar epitopes (C-4 OH and C-6 OH) faced the kinase hinge region, where the C-4 OH created a hydrogen bond interaction with the backbone amide carbonyl oxygen of the critical hinge region Met1160. These structural insights further guided the subsequent hit optimization.
Figure 5.
Docking of cembranoids 1 and 20 at the kinase domain of the human c-Met crystal structure PDB 4R1V.33 (A) Binding mode of 1 shows H-bonding interaction of its C-6 OH with backbone amide hydrogen of the hinge Met1160. Its cembrene scaffold is imbedded within a hydrophobic pocket lined with the side chains of Ala1108, Val1092 and Tyr1230. The arrow indicates the perspective molecular extension towards His1162. (B) Binding mode of 20 shows similar binding interactions as 1, in addition to the π-π stacking of its phenyl moiety with the imidazole side-chain of the targeted His1162.
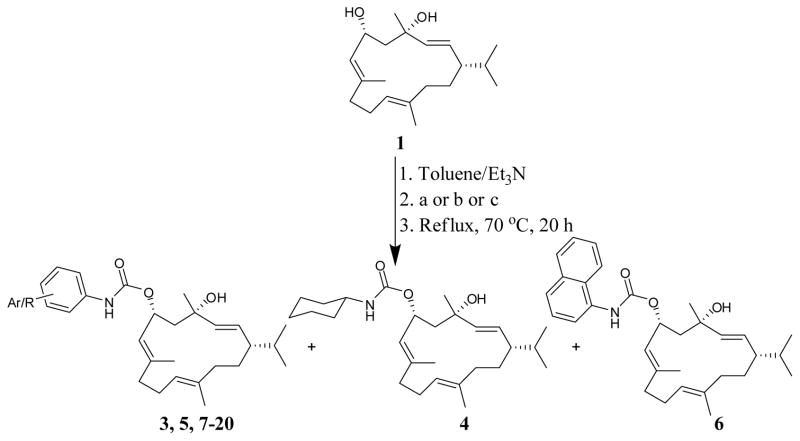
The first optimization cycle aimed to extend the parent cembrene scaffold with additional structural moieties to probe new interactions with the nearby amino acids. This would be expected to improve the c-Met binding interactions and subsequent cellular potency of the deigned analogs. The secondary alcoholic group (6-OH) represented an extension point for synthesizing the proposed analogs. Exploring the amino acids residues in the close vicinity to the C-6 OH suggested the nearby (7.87 Å) imidazole side-chain of the hinge His1162 for a possible stacking with aromatic moieties extended from C-6 OH. Previous studies proposed the positive impact of targeting residues at the ribose binding motif, which encloses His1162, on c-Met binding affinity.31,32 Moreover, the hydrogen bonding networks make this particular external part of the active site a highly conserved region and thus it is more preferred than other flexible regions for the SAR studies of small molecule c-Met inhibitors.32 Additionally, the hydrophobicity was a proposed favorable chemical feature for substituents occupying this region.32 Furthermore, Cho and colleagues have reported 3-(benzo[d]oxazol-2-yl) pyridine-2-amines as orally active c-Met inhibitors, with focused efforts on structural modification of substituents that can bind at the external region of the c-Met active site.33 Consequently, this study aimed at coupling of 1 with aromatic isocyanates to furnish the corresponding carbamate analogs, with aromatic moieties expected to π-π stack with the His1162’s imidazole side-chain in the c-Met external region active site. To validate this virtual hypothesis, 1’s phenyl carbamate analog 3 was first synthesized (Scheme 1) and tested in an MTT proliferation assay. The TNBC MDA-MB-231 and MDA-MB-468 cells were chosen to monitor the biological activity during the optimization process. As expected, 3 exhibited a remarkable cellular potency enhancement, with IC50 values of 19.8 and 22.7 μM against MDA-MB-231 and MDA-MB-468 cells, respectively (Table 4). The proposed stacking hypothesis was evaluated through synthesizing and testing the cyclohexyl carbamate analog 4. Interestingly, 4 was only weakly active with calculated IC50 of 38.2 and 39.6 μM against MDA-MB-231 and MDA-MB-468 cells, respectively. This clearly validated the important role of C-6 extension with a phenyl moiety for stacking with the His1162 imidazole side-chain and thus improving c-Met inhibitory activity and overall cellular potency.
Scheme 1.
Semisynthesis of the cembranoid carbamate analogs 3-20.
a. Phenyl and substituted phenyl isocyanates; b. Cyclohexyl isocyanate; c. 1-Naphthyl isocyanate
Table 4.
Antiproliferative activity of cembranoids 3-20 against the TNBC cell lines MDA-MB-231 and MDA-MB-468 in MTT assay.
| Cell line IC50 (± SEM) | Cell line IC50 (± SEM) | ||||
|---|---|---|---|---|---|
|
| |||||
| Compound | MDA-MB-231 | MDA-MB-468 | Compound | MDA-MB-231 | MDA-MB-468 |
| 3 | 19.8 ± 1.5 | 22.7 ± 1.1 | 12 | 16.1 ± 1.2 | 17.8 ± 0.7 |
| 4 | 38.2 ± 1.5 | 39.6 ± 1.9 | 13 | 16.2 ± 1.1 | 19.5 ± 0.8 |
| 5 | >50 | >50 | 14 | 5.3 ± 0.5 | 6.6 ± 0.3 |
| 6 | >50 | >50 | 15 | 10.8 ± 0.6 | 12.3 ± 0.8 |
| 7 | 26.3 ± 1.1 | 25.7 ± 1.3 | 16 | 10.4 ± 0.3 | 15.4 ± 0.8 |
| 8 | 13.4 ± 1.1 | 12.8 ± 0.9 | 17 | 8.5 ± 0.3 | 10.7 ± 0.5 |
| 9 | 27.6 ± 1.2 | 27.9 ± 1.5 | 18 | 4.9 ± 0.5 | 5.8 ± 0.4 |
| 10 | 9.1 ± 0.8 | 10.6 ± 0.5 | 19 | 8.9 ± 0.5 | 11.6 ± 0.6 |
| 11 | 13.7 ± 0.9 | 14.5 ± 0.7 | 20 | 1.3 ± 0.2 | 2.1 ± 0.2 |
The second optimization round was focused on testing whether or not the kinase pocket could accommodate further extension of the phenyl moiety. Therefore, 5 (4-biphenyl carbamate) and 6 (1-naphthyl carbamate) were proposed, synthesized and tested. The cellular proliferation results (Table 4) indicated that both new analogs were significantly less potent than 3, indicating the optimal size of the phenyl moiety for activity at that point. Steric clashes and/or disruption of original binding pose were hypothesized, at least in part, to justify the observed cellular potency reduction in each case. This was based on docking studies of 5 and 6 at the c-Met kinase domain, where both analogs were oriented differentially when compared to the original hypothesized pose of 1 and thus none of them showed the stacking interaction with His1162’s imidazole side chain (Supplementary Figure 1).
The substituent electronic effect on the phenyl carbamate moiety was assessed through the synthesis and biological testing of 7 (p-ethylphenyl carbamate) and 8 (p-chlorophenyl carbamate). The results (Table 3) indicated that an electron-withdrawing substituent would have a positive impact on the antiproliferative activity unlike the electron-donating counterpart. Additionally, the enhanced bioactivity observed for the m-chlorophenyl (10) versus the m-tolyl (9) augmented the preferential role of the electron-withdrawing substituents for improving the bioactivity (Table 4). The positional effect of the chloro substitution on the phenyl ring was further assessed through the synthesis and biological evaluation of the o-chlorophenyl carbamate 11.
Carbamate 10 exhibited the most enhanced cell proliferation inhibition versus 8 and 11 (Table 4), thus indicating the preference of the meta-position for the chloro substituents on the phenyl ring for improving the antiproliferative potency. Synchronously, 10 inhibited c-Met catalytic activity in the Z′-LYTE kinase assay with an IC50 3.4 μM (Table 3). This clearly indicated the significant c-Met inhibition improvement, compared to the parent 1 and correlated the observed enhancement of cellular potency, at least in part, with the c-Met inhibition.
The impact of the electron-withdrawing substituent hydrophobicity was then investigated through synthesizing and testing of the fluorinated analogs 12 and 13. Both analogs were relatively less potent than the chlorinated counterparts (Table 4); thus, adding an additional important role of the substituent hydrophobicity for activity enhancing.
The dicholro analogs were then devised for expected activity enhancement. The dichlorinated analogs 14 (m,m-dichloro), 15 (o,o-dichloro), 16 (o,p-dichloro), and 17 (m,p-dichloro) were proposed, synthesized, and tested. 14 was the most potent, with calculated IC50 values of 5.3 and 6.6 μM against MDA-MB-231 and MDA-MB-468 cells, respectively (Table 4). This result was consistent with the earlier conclusion of the preferential m-chloro substitution for the activity improvement. 14 inhibited the c-Met catalytic activity with a calculated IC50 2.9 μM in the Z′-LYTE kinase assay (Table 3).
It was concluded earlier that the substituent-hydrophobicity would enhance the antiproliferative activity; therefore, the strong electron-withdrawing and hydrophobic trifluoromethyl group was suggested as an optimal functionality to fulfill both criteria. Thus, 18 (m-CF3) and 19 (p-CF3) were prepared and tested. Results revealed that 18 exhibited a significant activity improvement compared to 19, with a potency nearly comparable to the m,m-dichloro analog 14. Cembranoid 18 inhibited the c-Met catalytic activity in the Z′-LYTE kinase assay with an IC50 value of 1.8 μM (Table 3). This further validated the hypothesis that c-Met inhibition enhancement would also enhance the cellular potency.
The m,m-di-CF3 analog 20 was then devised, synthesized and tested. Expectedly, 20 exhibited the most potent cell proliferation inhibition against the TNBC MDA-MB-231 and MDA-MB-468 cells, with IC50 1.3 and 2.1 μM, respectively. Additionally, 20 inhibited c-Met kinase catalytic activity in a concentration dependent manner, with a calculated IC50 of 1.1 μM in the Z′-LYTE kinase assay (Figure 6). This clearly indicated the successful optimization strategy for enhancing c-Met kinase inhibition and improving the overall antiproliferative cellular potency. Moreover, 20 attenuated the proliferation of other breast cancer cells, however, at relatively higher concentrations, comparing to TNBC cell lines (Table 2). The docking study of 20 in the c-Met kinase domain indicated that it maintained the original binding interactions of the parent 1 at the ATP-binding cleft, in addition to the π-π stacking of its phenyl moiety with the His1162 imidazole side-chain (Figure 5B). These promising results further augmented the rational design hypothesis for c-Met-targeted enhanced binding. In addition, 20 showed similar interactions with Met1160 and His1162 of the kinase domain when its structure was docked into another c-Met crystal structure PDB4R1Y, confirming the reproducibility of the molecular extension for additional targeted π-π stacking activity enhancement hypothesis (Supplementary Figure 2).
Figure 6.

Effect of 20 on c-Met catalytic activity using the Z′-LYTE™ kinase assay. Data represent mean percent c-Met phosphorylation (± SEM) at indicated concentrations. Oleocanthal (OC)42 was used as a positive c-Met inhibitor control. * Indicates a statistical significance at P< 0.05.
Most small-molecule kinase inhibitors interact with multiple members of the protein kinase family; achieving selective inhibition of specific protein kinases is challenging.34 However, clinical outcomes and growing understanding of kinase biology proved that hits with a broader pattern of inhibitory activities can also be effective.35,36 Nevertheless, the selectivity of such compounds still need to be well-defined. Currently, it has become a common practice to assess the inhibitory activity of new inhibitors against a panel of protein kinases.37 The low μM potency of compound 20 in the cell-free assay prompted the investigation of its inhibitory effects against other structurally related, oncogenically relevant TKs. A panel of 10 TKs was selected including: Abelson murine leukemia viral oncogene homolog 1 (ABL1), anaplastic lymphoma receptor tyrosine kinase (ALK), AXL receptor tyrosine kinase, epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), fibroblast growth factor receptor1 (FGFR1), insulin-like growth factor 1 receptor (IGF1R), c-Met, macrophage stimulating 1 receptor (MST1R), and v-ros avian UR2 sarcoma virus oncogene homolog 1 (ROS1), was selected for profiling. The calculated percent inhibition at 10 μM (Table 5) revealed the c-Met as relatively utmost sensitive kinase with 84% average inhibition, however, 20 showed some degrees of inhibition of other kinases. For instance, 20 showed 48.7% FGFR1 inhibition. Meanwhile, 20 also displayed 30–35% range of inhibition to ABL1, IGFR and MSTR at the same concentration. HER2 was relatively less sensitive with 23% inhibition (Table 5). These promising data emphasize the enhanced c-Met inhibition selectivity of the optimized cembranoid 20 and encourage further future medicinal chemistry efforts to consider cembranoid aromatic carbamate as a novel c-Met inhibitory entity.
Table 5.
Initial TK selectivity profile of the cembranoid carbamate 20.
| Kinase | Mean % inhibition at 10 μM |
|---|---|
| ABL1 | 31 |
| ALK | 10 |
| AXL | 11 |
| EGFR (ErbB1) | 9 |
| ERBB2 (HER2) | 22 |
| FGGR1 | 49 |
| IGF1R | 30 |
| MET | 84 |
| MST1R (RON) | 31 |
| ROS1 | 3 |
The antimigratory activity of 20 was also evaluated using the wound healing platform against the HGF-stimulated TNBC MDA-MB-231cells. Treatment with 20 resulted in a concentration-dependent cell migration inhibition, with a calculated IC50 1.4 μM (Figure 2, Table 2). These results interesting clearly indicated the significant improvement of antimigratory activity of 20, compared to the parent 1 (vide supra).
The anti-invasive activity of 20 was assessed using the Cultrecoat® cell invasion kit. Treatment with 20 significantly reduced the invasion of MDA-MB-231 cells in a concentration-responsive manner (Figure 3A) compared to vehicle control. The calculated IC50 was 1.7 μM, compared to 37.6 μM of parent 1 (Table 2). Figure 3B shows the ability 20 at 2 μM to significantly impede tumor cell invasion, indicated by higher cell density at the upper surface of the invasion chamber, compared to untreated cells. These results obviously indicated the significant anti-invasive activity enhancement of 20, compared to the parent 1.
The significant improvement c-Met catalytic inhibition and cellular potency displayed by Carbamate 10 exhibited the most potent enhanced cell proliferation inhibition versus 8 and 11 (Table 4), thus indicating the preference of the meta-position for the chloro substituents on the phenyl ring for enhancing improving the antiproliferative potency. Synchronously, 10 inhibited the c-Met catalytic activity in the Z′-LYTE kinase assay with an IC50 3.4 μM (Table 3). This clearly indicated the significant c-Met inhibition improvement, compared to the parent 1 and correlated the observed enhancement of cellular potency, at least in part, with the c-Met inhibition. the most active analog 20 were further validated by Western blot analysis of the cell lysates from TNBC. The TNBC MDA-MB-231 cells treated with different concentrations of 20. Immunoblots showed a significant concentration-dependent downregulation of activated p-c-Met (Figure 4B). c-Met inhibition would invariably block the stimulation of diverse c-Met-driven downstream signaling pathways. Further immunoblotting data disclosed significant reductions of the levels of activated forms of survival oncogenic protein p-Akt, mammalian target of rapamycin (p-mTOR) kinase,38 and focal adhesion kinase (p-FAK) phosphorylation, with 20 effective treatments (Figure 4B). Thus, the observed in vitro cell motility and invasion inhibition would be correlated, in part, with the downregulation of c-Met/FAK signaling axis. In addition, FAK inhibition was concomitantly accompanied with phosphorylation reduction of its well-recognized downstream and the critical adaptor-protein, paxillin. Furthermore, a dose-dependent downregulation of the significant mitogenic kinase p-MAPK was observed compared to untreated cells. Altogether, these results strongly supported the effective 20-mediated c-Met inhibition and correlated, at least in part, the observed in vitro cell proliferation, migration and invasion inhibition with effective c-Met blockade.
Apoptosis and necrosis are the two major forms of cellular death.39 In apoptosis, cells shrink and condense the cellular components in apoptotic bodies, which are rapidly engulfed by neighboring cells or macrophages. Meanwhile, necrotic cell death is distinguished by disruption of cell membrane permeability and release of intracellular components to the surrounding microenvironment and thus stimulates inflammatory responses. Thus, detection of intracellular components in culture media can be used as a marker for cell necrosis. To determine whether the treatment of MDA-MB-231 cells with 20 results in a cellular necrosis, the release of intracellular LDH enzyme was quantified in the culture media after 24 h of incubation. Interestingly, effective anticancer concentrations of 20 resulted only in a minimal LDH release, which indicated the mechanistic anticancer activity of 20 rather than induction of a nonselective necrotic death (Supplementary Figure 3).
The human mammary epithelial MCF-10A cells were implemented as a non-tumorigenic breast cell model for in vitro assessment of selectivity of 20. Cells were incubated with various concentrations of 20 for 24 h and the cell survival was determined by MTT. Results showed that treatment with 20 at effective doses didn’t induce a significant cellular death. Interestingly, 15 μM of 20 (~>7-fold of its IC50 in different anticancer assays) resulted only in 8.3% reduction in cell viability (Supplementary Figure 4). These results suggest the tolerability of MCF-A10 cells to 20, at the indicated anticancer concentrations.
The promising in vitro anticancer activities of 20 prompted the evaluation of its in vivo efficacy in an athymic nude mouse model xenografted with MDA-MB-231/GFP tumor cells. After implantation, tumors were allowed to develop to an average size of 50 mm3 before starting i.p. dosing of 20 at 10 mg/kg, 3X/week. Administration of 20 significantly attenuated the tumor growth in treatment group compared to vehicle-treated control animals (Figures 7A and B). This was obvious by the average tumors volume at the end of the study, where control tumors reached an average size of 2048 mm3, while 716 mm3 was the average tumors size of 20-treated group, representing a 66.7% tumor growth inhibition. Additionally, animals’ body weights were regularly monitored and the average was used as a marker for vehicle or treatment toxicities. The average body weight in both groups was non-significant throughout the study (Figure 7C), indicating possible tolerability of 20 in nude mice at the used dose regimen and rout of administration.
Figure 7.
Antitumor activity of 20 against breast cancer in a nude mouse model. (A) Effect of 20 on the in vivo growth of MD-MB-231/GFP cells. Data represent mean tumor volume (± SEM) at indicated times. (B) Representative photographs of mice (i) and excised tumors (ii) from the vehicle and treatment groups at end of the study. (C) Effect of 20 on animal’s body weights. Data represent mean (± SEM) at indicated times. (D) Western blot analysis of the c-Met and p-Met in tumor samples excised from control and treatremt groups. * indicates statistical significance at P< 0.05.
Tumor samples from control and treatment groups were analyzed to prove c-Met inhibition, at least in part, as a mechanistic basis for the observed antitumor activity. The excised malignant tissues from each group were homogenized, lysed and analyzed by Western blotting to assess the phosphorylated levels of c-Met. Immunoblotting data (Figure 7D) disclosed the significant decrease in the level of p-c-Met in tumors excised from treatment group animals, in comparison to its level in vehicle-treated control group. Consequently, these results strongly supported the 20-mediated c-Met inhibition, in part, as a potential for the observed tumor growth inhibition in this animal model.
3. Conclusions
Targeting HGF/c-Met signaling axis represents an interesting approach to control invasive c-Met-dependent breast malignancies. Interference with c-Met kinase activity by small molecule inhibitors should impair multiple downstream oncogenic cascades and thus attenuates tumor cell proliferation, migration and invasion. Natural products remain and will continue to be the top most successful and innovative resource for discovery of unique anticancer scaffolds. Tobacco cembranoids is a novel class of selective c-Met inhibitors appropriate for future optimizations to control c-Met-dependent malignancies. Unfortunately, most of the natural bioactive cembranoids are purposely degraded during the commercial tobacco fermentation. Identifying fresh tobacco as a source for bioactive natural products is adding a pharmaceutical value to this important agricultural crop.
Experimental section
3.1. General
1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively, in CD3Cl or (CD3)2CO, using the residual solvent peaks as a internal reference, on a JEOL Eclipse ECS-400 NMR spectrometer (JEOL Inc., Peabody, MA, USA). The ESI-MS experiments were conducted using a 3200 Q-trap LC/MS/MS system (Applied Biosystems, Foster City, CA, USA) using Analyst version 1.4.1 software (MDS Sciex, Toronto, Canada). Thin layer chromatography (TLC) analysis conducted on pre-coated Si gel 60 F254 500 μm TLC plates (EMD Millipore, Billerica, MA, USA ), using 1% p-anisaldehyde in 10% H2SO4/MeOH as a chemical visualizing reagent. Si gel 60 (Natland International Corporation, 230–400 μm) was used for column chromatography with gradient n-hexanes-EtOAc mixtures as mobile phases. Generally, 1:100 ratio of mixtures to be chromatographed versus Si gel 60 was applied in all liquid chromatographic purifications.
3.2. Chemicals and reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated. Organic solvents were purchased from VWR (Suwanee, GA, USA), dried by standard procedures, packaged under nitrogen in Sure/Seal bottles and stored over 4 Å molecular sieves. HGF growth factor, used in cell assays, was acquired from PeproTech Inc. (Rocky Hill, NJ, USA). Recombinant human c-Met proto-oncogene was purchased from Life Technologies, Inc. (Grand Island, NY, USA). Unless otherwise indicated, cell culture reagents were obtained from Life Technologies, Inc.
3.3. Extraction and isolation of cembranoids
Tobacco cembranoids 1 and 2 were isolated from fresh tobacco leaf powder (Nicotiana tabaccum, Solanaceae) purchased from Custom Blends, NY, Blend #28, Batch Number TP-TN-15001, containing Virginia, Oriental, and Burley tobacco (1:1:1) by initial extraction with CH2Cl2 (25 lb × 20 L X3). This CH2Cl2 dry extract was fractionated on normal phase and finally on C-18 RP Si gel as previously described.13 Identity of 1 was based on comparing its NMR data (Supplementary Table 1) with literature.3–5
3.4. Synthesis of analogs 3-20
To stirring solutions of 1 (50 mg, 0.16 mmole) in 10 mL toluene, 300 μL of Et3N were added and the mixture was then left to stir for 15 mins at 70 °C. Different isocyanates (0.16 mmole) were then added to each stirring mixture. Reaction mixtures were then left for 24 h under reflux and periodically monitored by TLC, using n-hexanes and EtOAc (6:4) as mobile phase. After reactions completion, solvents were then evaporated under reduced pressure, and the residues partitioned between 2% aq. HCl and EtOAc (10 mL, each). Organic layers were pooled, washed with brine and finally dried over anhyd Na2SO4. Concentrated organic layers were loaded on celite and then purified over Si gel columns using mixtures of n-hexanes and EtOAc to yield pure analogs.
6-O-[(N-phenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (3). Yield: 23.9 mg (35.1%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 448.4 [M+Na]+ (calcd for C27H39NO3).
6-O-[(N-cyclohexyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (4). Yield: 25.1 mg (36.4%), yellowish viscous solid. 1H NMR and 13C NMR Supplementary Table 1. ESIMS m/z 454.2 [M+Na]+ (calcd for C27H45NO3).
6-O-[(N-4-biphenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (5). Yield: 31.5 mg (39.3%), yellowish amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 524.2 [M+Na]+ (calcd for C33H43NO3).
6-O-[(N-1-naphthyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (6). Yield: 29.2 mg (38.4%), yellowish amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 498.4 [M+Na]+ (calcd for C31H41NO3).
6-O-[(N-4-ethylphenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (7). Yield: 10.6 mg (14.6%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 476.2 [M+Na]+ (calcd for C29H43NO3).
6-O-[(N-4-chlorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (8). Yield: 17.5 mg (23.8%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 482.3 [M+Na]+ (calcd for C27H38ClNO3).
6-O-[(N-3-tolyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (9). Yield: 15.4 mg (21.9%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 462.2 [M+Na]+ (calcd for C28H41NO3).
6-O-[(N-3-chlorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (10). Yield: 24.0 mg (32.7%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 482.3 [M+Na]+ (calcd for C27H38ClNO3).
6-O-[(N-2-chlorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (11). Yield: 35.0 mg (47.6%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 482.3 [M+Na]+ (calcd for C27H38ClNO3).
6-O-[(N-2-fluorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (12). Yield: 19.6 mg (27.6%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 466.4 [M+Na]+ (calcd for C27H38FNO3).
6-O-[(N-4-fluorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (13). Yield: 17.8 mg (25.1%), yellowish viscous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 466.4 [M+Na]+ (calcd for C27H38FNO3).
6-O-[(N-(3,5-dichlorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (14). Yield: 21.7 mg (27.5%), white amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 516.3 [M+Na]+ (calcd for C27H37Cl2NO3).
6-O-[(N-(2,6-dichlorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (15). Yield: 21.3 mg (27.0%), yellowish amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 516.3 [M+Na]+ (calcd for C27H37Cl2NO3).
6-O-[(N-(2,4-dichlorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (16). Yield: 15.2 mg (19.3%), yellowish amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 516.3 [M+Na]+ (calcd for C27H37Cl2NO3).
6-O-[(N-(3,4-dichlorophenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (17). Yield: 11.1 mg (14.1%), yellowish amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 516.3 [M+Na]+ (calcd for C27H37Cl2NO3).
6-O-[(N-(3-trifluoromethylphenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (18). Yield: 13.5 mg (17.5%), yellowish amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 504.3 [M+Na]+ (calcd for C28H38F3NO3).
6-O-[(N-(4-trifluoromethylphenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (19). Yield: 22.8 mg (29.6%), yellowish amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 504.4 [M+Na]+ (calcd for C28H38F3NO3).
6-O-[(N-(3,5-bis-(trifluoromethyl)phenyl)carbamoyl)]-(1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol (20). Yield: 16.4 mg (18.3%), white amorphous solid. 1H NMR and 13C NMR see Supplementary Table 1. ESIMS m/z 584.2 [M+Na]+ (calcd for C29H37F6NO3).
3.5. Molecular modeling
The in silico experiments were carried out using Schrödinger molecular modeling software package installed on an iMac 27-inch Z0PG workstation with a 3.5 GHz Quad-core Intel Core i7, Turbo Boost up to 3.9 GHz, processor and 16 GB RAM (Apple, Cupertino, CA, USA).
3.6. Protein structure preparation
The X-ray crystal structure of huamn c-Met kinase domain (PBD code: 4R1V)40 was retrieved from the Protein Data Bank.41 The Protein Preparation Wizard of Schrödinger suite was used to prepare the kinase domain. The protein was reprocessed by assigning bond orders, adding hydrogens, creating disulfide bonds and optimizing H-bonding networks using PROPKA (Jensen Research Group, Copenhagen, Denmark). Finally, energy minimization with a root mean square deviation (RMSD) value of 0.30 Å was applied using an Optimized Potentials for Liquid Simulation (OPLS_2005, Schrödinger, New York, NY) force field.
3.7. Ligand preparation
The 2D structures of test compounds were sketched in the Maestro 9.3 panel (Maestro, version 9.3, 2012, Schrödinger, New York, NY). The LigPrep 2.3 module (LigPrep, version 2.3) of the Schrödinger suite was utilized to generate 3D structures and to search for different conformers. The Optimized Potentials for Liquid Simulation (OPLS_2005) force field was applied to geometrically optimize the ligands and to compute partial atomic charges. Finally, at most 32 poses per ligand were generated with different spatial features for subsequent docking studies.
3.8. Docking
The prepared human c-Met kinase domain was employed to generate the energy grids using the default value of the protein atomic scale (1.0 Å) within the cubic box centered on experimental cocrystallized ligand. After receptor grid generation, structures were docked using Glide 5.8 module (Glide, version 5.8, 2012, Schrödinger, New York, NY, USA).
3.9. Biology
3.9.1. Cell lines and culture conditions
Human breast cancer cell lines MDA-MB-231, MDA-MB-468, MCF-7, T-47D, BT-474, SK-BR-3, and the non-tumorigenic mammary epithelial MCF-10A cell line, were all obtained from the American Type Culture Collection (Manassas, VA, USA). Breast cancer MDA-MB-231/Green Florescent Protein-tagged (MDA-MB-231/GFP) cell line was purchased from Cell Biolabs (San Diego, CA, USA). Cancerous cells, except MCF-7, were cultured and maintained in RPMI-1640 medium (Corning, Manassas, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Valley Biomedical, Winchester, VA, USA) in a 5% CO2 humidified incubator at 37 °C. MCF-7 cells were maintained in DMEM (Gibco® by Life Technologies, Grand Island, NY, USA) supplemented with 10% hyclone fetal bovine serum (GE Healthcare Life Sciences, Pittsburgh, PA, USA). The immortalized mammary epithelial MCF-10A cells were maintained in DME/high glucose medium (Life Technologies, Grand Island, NY, USA) supplemented with 10 % horse serum (Gibco® by Life Technologies, Grand Island, NY, USA), 20 ng/mL EGF (Peprotech, Rocky Hill, NJ, USA), 0.5 mg/mL hydrocortisone, 100 ng/mL cholera toxin and 10 μg/mL insulin.
3.9.2. Cell proliferation assay
Cells were seeded into 96-well plates at a density of 5 × 103 cells/well in 100 μL of culture medium, and incubated overnight at 37 °C in a 5% CO2 humidified incubator so that the cells could recover and attach. Media were then removed, and cells were washed with PBS. Compounds were prepared as stock solutions (all were 10 mM, except 1 was 50 mM) in DMSO, and immediately added to culture medium (supplemented with 100 ng/mL HGF, for c-Met expressing cells) to prepare the final working concentrations. Treatment media (100 μL) were added, in triplicates, and cells were incubated for 72 h. At the end of incubation period, media were gently aspirated, and cells were rinsed with PBS. Fresh media (100 μL) and MTT solution (50 μL) were then added to each well, and cells were incubated for an additional 4 h to allow the formation of insoluble formazan crystals. Supernatants were then carefully removed, and crystals were dissolved in 100 μL DMSO. The plate was then incubated for 5 minutes and with gentle shaking, to ensure solubilization of the crystals, prior measuring the absorbance at 570 nm using a Synergy 2 Microplate Reader (BioTek, Winooski, VT, USA). Cell numbers were derived from a standard curve executed at the beginning of each experiment. IC50 values were calculated using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA, USA).
3.9.3. Cell migration assay
MDA-MB-231 cells were seeded into a 24-well plate at a density of 1 × 105 cells/well, and incubated at 37 °C in a 5% CO2 humidified incubator overnight, so the cells could recover and attach. Wounds were inflicted in cell monolayers using sterile 200 μL pipette tips. Cells were then washed with PBS and re-incubated in serum-free media for 5 h. After then, media were replaced with fresh ones supplemented with HGF (100 ng/mL), and containing different concentrations of test compounds or DMSO as vehicle control. The olive oil phenolic oleocanthal (10 μM) was used as positive control antimigratory compound.42 Wounds were photographed at zero time and then monitored for closing up to 24 h. When wounds were about to close, media were gently aspirated, and cells were rinsed with PBS and fixed with cold methanol for 15 minutes at 4 °C. Finally, wounds were photographed for treatments, including the vehicle-treated control wells for comparison. Percentages cell migration were calculated using the following formula:
Where, T0 is the average wound thickness at zero time, Tdmso is the average wound thickness in DMSO-treated control wells, and Tt is the average wound thickness in treatment wells.43 IC50 were calculated using GraphPad Prism version 5.01 (GraphPad Software, CA, USA).
3.9.4. Cell invasion assay
The experiment was performed according to manufacturer procedures (Cultrecoat® invasion kit) after optimizing the number of MDA-MB-231 cells per well. The 96-well invasion chamber was equilibrated at rt for 1 h prior to use. Inserts were rehydrated by adding 25 μL of warm RPMI-1640 medim and incubation at 37 °C for 1h. MDA-MB-231 cells in culture plates were serum-starved for 16 h in serum-free medium prior to the assay. Cells were then harvested, resuspended, and counted to prepare a working concentration of 1 × 106 cells/mL in RPMI-1640 culture medium. Cells (2.5 × 104 cells) were added to the top hydrated inserts, and 150 μL of serum-free media, supplemented with HGF (100 ng/mL) and containing either test compounds or DMSO, were added to the bottom chamber. The olive oil phenolic oleocanthal (15 μM) was used a positive control anti-invasive natural product.35 Plates were then assembled and incubated at 37 °C in a 5% CO2 humidified incubator for 24 h. After incubation, the top chamber was inverted to remove the media, and then transferred to a black receiver plate. Wells of the top chamber were then washed with 100 μL of warm washing buffer, 100 μL of cell dissociation solution/calcein AM were added to each well of the lower chamber, and plates were incubated for an additional 1 h. Finally, the top chamber was removed, and fluorescence in lower plate wells was measured at 485 nm excitation, 520 nm emission using a Synergy 2 microplate reader (BioTek, Winooski, VT, USA). Relative fluorescence units (RFU) were used to calculate number of invaded cells in control and treatment wells, using a standard curve constructed prior start of the experiment. Mean percent invasion values were calculated relative to the vehicle-treated control wells. IC50 values were calculated using GraphPad Prism version 5.01 (GraphPad Software, CA, USA).
3.9.5. Lactate dehydrogenase (LDH) release assay
LDH cytotoxicity assay kit (Cayman, Ann Arbor, MI, USA) was used to measure cell death in response to various concentrations of the test compound. The assay followed the manufacturer procedure after optimization regarding number of cells per well and serum concentration. Briefly, MDA-MB-231 cells were seeded into 96-well plate at a density 3 × 104 cells/well in 200 μL culture media, while three wells were left without cells as background control. After cell recovery and attachment, media were removed and cells were treated with 200 μL culture media supplemented with 5% FBS and containing the test compound at indicated concentrations. Triton X-100 (20 μL) was added to three wells containing the cells (as maximum release) and 20 μL of assay buffer to other three cell-free wells (as spontaneous release). The cells were incubated at 37 °C in a 5% CO2 humidified incubator for 24 h. After incubation, the plate was centrifuged at 400× g for 5 minutes and 100 μL of supernatant were then transferred to a new 96-well plate. Reaction buffer (100 μL of NAD+, lactic acid, INT, reconstituted diaphorase) was added to each well and plate was incubated with gentle shaking on an orbital shaker for 30 min at rt. Finally, absorbance was measured at 490 nm using Synergy 2 Microplate Reader (BioTek, Winooski, VT, USA). The percent cytotoxicity was calculated as following:
3.9.6. Cytotoxicity assay
The human non-tumorigenic MCF-10A breast epithelial cells were seeded into a 96-well plate at density of 3 × 104 cells/well. Cells were incubated overnight at 37 °C in a 5% CO2 humidified incubator to allow them to recover and attach. Media were carefully removed, and cells were washed with PBS. Various concentrations of the test compound in serum-free media were added, in triplicates, while vehicle control wells were treated with media containing the maximum amount of DMSO added in treatment sets. Doxorubicin (1 μM) was used as a positive cytotoxic control drug. Cells were then incubated for 24 h. At the end of incubation period, media were removed, and cells were washed with PBS and fresh culture media (100 μL) and of MTT (50 μL) were added to each well, and the cells were re-incubated for 4 h and checked periodically for the formation of formazan crystals. Supernatants were carefully removed, and formazan crystals were dissolved in 100 μL DMSO after crystals were fully grown. The plate was incubated in the dark for 5 minutes, and gently swirled before measuring the absorbance at 570 nm using Synergy 2 microplate reader (BioTek, Winooski, VT, USA). Average values from triplicate readings were calculated and subtracted from the mean value obtained for blank wells. Cell numbers were deduced from a standard curve executed at the beginning of the experiment. Percent cell viability was calculated by comparing numbers of cells in treatment wells to the mean for DMSO-treated control wells.
3.9.7. Biochemical c-Met kinase assay
Invitrogen Z′-LYTE™ Kinase Assay-Tyr6 Peptide kit (Thermo Fisher Scientific, Madison, WI, USA) was used to evaluate c-Met inhibitory activity of the test compound. Briefly, 20 μL/well reactions were set in 96-well plate containing kinase buffer, 200 μM ATP, 4 μM Z′-LYTE™ Tyr6 Peptide substrate, 2500 ng/mL c-Met kinase domain and various concentrations of norstictic acid. The olive oil phenolic oleocanthal (5 μM) was used a standard c-Met inhibitor positive control.35 Reaction plate was incubated for 1 h at rt, after which 10 μL of the development solution containing site-specific protease were added to each well and plate was incubated for additional 1 h. The reaction was then stopped, and the fluorescence signal ratio of 445 nm (coumarin)/520 nm (fluorescein) was determined using FLx800™ plate reader (BioTek, Winooski, VT), which reflects the peptide substrate cleavage status and/or the kinase inhibition in the reaction. The IC50 was calculated by nonlinear regression of log concentration versus the % phosphorylation, implemented in GraphPad Prism version 5.01 (GraphPad Software, CA, USA).
3.9.8. Protein extraction and immunoblotting
The human breast cancer MDA-MB-231 cells were seeded at a density of 5 × 105 cells/100 mm culture dish and incubated overnight to recover and attach. Cells were treated either with test compound or DMSO as vehicle control for 72 h in serum-reduced media (supplemented with 10 ng/ml HGF for the c-Met dependent MDA-MB-231 cells). Total cellular protein contents were obtained using RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, Madison, WI, USA) supplemented with mammalian protease arrest (G-Biosciences, St. Louis, MO, USA). Samples were diluted in Laemmli buffer (BIO-RAD, Hercules, CA, USA) containing 5% β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) prior loading on gels. Cell lysates (30 μg) were electrophoresed on Mini-PROTEAN® TGX™ precast polyacrylamide gels (BIO-RAD, Hercules, CA, USA) using Tris/Glycine/SDS running buffer, and then transferred to Immu-Blot® PVDF membranes (BIO-RAD, Hercules, CA, USA). Blotted membranes were then blocked with 5% BSA (Cell Signaling Technology, Beverly, MA, USA) in TBST (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20) for 2 h with gentle agitation at rt. Immunoblots were incubated overnight at 4 °C with appropriate primary antibodies (Cell Signaling Technology, Beverly, MA, USA). After incubation, membranes were washed with TBST and then incubated with HRP-labeled secondary antibodies (Cell Signaling Technology, Beverly, MA, USA) for 1 h with agitation at rt. Chemiluminescence detection was performed using the Supersignal West Pico kit (Thermo Fisher Scientific, Madison, WI, USA) and G. BOX imaging system with high-resolution 100m pixel camera (Syngene, Fredrick, MD, USA).
3.9.9. In vivo study of breast cancer xenograft in nude mice
Female athymic nude mice (5–6 weeks old) were purchased from Harlan Laboratories (Cumberland, IN, USA). Animals were housed at the Animal Facility (University of Louisiana at Monroe, School of Pharmacy) and maintained under clean conditions in sterile filter-top cages, at temperature of 24 ± 2 °C, 50 ± 10% relative humidity and 12:12 h artificial light-dark cycle. Mice received mouse chow and water ad libitum. All procedures were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and approval by the Institutional Animal Care and Use Committee (IACUC).
MDA-MB-231 Green Florescent Protein-tagged (MDA-MB-231/GFP) cells were harvested, washed with PBS, and resuspended in RPMI-1640 medium. Cells (1 × 106 cells/25 μL) were injected into the mammary fat pad of each nude mouse, using a 29G hypodermic needle. Animals were observed daily for the growth of palpable tumors at the site of injection. Ten days post-implantation, tumors became visible with an approximate average size of 50 mm3. Mice were then randomized and allocated to control and treatment groups (5 mice/group). Compound 20 was prepared as a stock solution in DMSO, diluted with sterile PBS containing 0.1% Tween 80, and then injected i.p. at a dose regimen of 10 mg/kg body weight, three times per week, for the indicated times in each set of experiments. Animals in the control groups received the same volume of vehicle, following the same treatment protocol. Tumor dimensions were monitored using a digital caliper (VWR, Radnor, PA, USA). Animals were monitored daily for any signs of treatment- or vehicle-associated toxicity. Tumor volume was calculated using the well-established formula: tumor volume (mm3) = [(length × width2)/2]. Animals were killed at the indicated times, unless they appeared to be moribund or a tumor showed signs of necrosis. At termination, tumors were excised from the connective tissues and snap-frozen for subsequent analysis.
3.9.10. Kinase profiling
Compound 20 was screened at 10 μM against the following kinases: ABL1, ALK, AXL receptor tyrosine kinase, EGFR, HER2, FGFR1, IGF1R, c-Met, MST1R, and ROS1, using SelectScreen® Kinase Profiling Selectivity Testing Services (Life Sciences, Carlsbad, CA). Percent inhibition for each kinase was determined using a time-resolved fluorescence resonance energy transfer assay. Protocols are available at https://www.thermofisher.com/us/en/home/products-and-services/services/custom-services/screening-and-profiling-services/selectscreen-profiling-service/selectscreen-kinase-profiling-service.html.
3.9.11. Statistical analyses
All in vitro experiments were performed in triplicate. Pooled data were subjected to statistical analyses using GraphPad Prism version 5.01 (GraphPad Software, CA, USA). Differences between means from two different groups were subjected to student’s ‘t’ test, whereas one-way analysis of variance (ANOVA) was used to test for significant differences between three or more groups. The in vivo tumor growth data were subjected to two-tailed student’s ‘t’ tests. Results were considered to be significantly different when P < 0.05, indicated by * symbol.
Supplementary Material
Acknowledgments
The Egyptian Ministry of Higher Education is acknowledged for supporting H. Y. E.’s fellowship. Research reported in this publication was supported in-part by the National Cancer Institute of the National Institutes of Health under Award Number R15CA167475-01.
Footnotes
Supplementary data associated with this article, including: overlay of binding pose of cembranoid 1 with 5 and 6 at the c-Met kinase domain; binding mode of 20 at c-Met kinase domain PDB 4R1Y; effect of compound 20 on LDH release in MDA-MB-231 cells; effect of compound 20 on the viability of MCF-10A (non-tumorigenic mammary epithelial cells), and 1H and 13C NMR data of cembranoid 1 and analogs 3-20 can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.World Health Organization. [accessed 3.13.16];The tobacco atlas. http://www.who.int/tobacco/statistics/tobacco_atlas/en/print.html.
- 2.Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. 2. CRC Press; Boca Raton, Florida, US: 1994. pp. 120–121. [Google Scholar]
- 3.Wahlberg I, Enzell CR. Tobacco cembranoids. Beitrage zur Tabakforschung International. 1984;12:93. [Google Scholar]
- 4.Wahlberg I, Eklund AM. In: Progress in the Chemistry of Organic Natural Products. Herz W, Kirby GW, Moore RE, Steglich W, Tamm CH, editors. Vol. 59. Springer-Verlag Wien; New York: 1992. pp. 142–293. [Google Scholar]
- 5.Wahlberg I, Eklund AM. In: Trends in Flavour Research. Maarse H, Heji V, editors. Elsevier Science B.V; Oxford, UK: 1994. pp. 449–462. [Google Scholar]
- 6.Wahlberg I, Olsson E, Berg JE. Progress in Flavour Precursor Studies. In: Schreier P, Winterhalter P, editors. Proceedings of Int. Conf; Carol Stream, IL: Allured Publishing Corporation; 1993. pp. 83–95. [Google Scholar]
- 7.Wahlberg I, Enzell CR. Nat Prod Rep. 1987;4:237–276. doi: 10.1039/np9870400237. [DOI] [PubMed] [Google Scholar]
- 8.Ferchmin PA, Pagán OR, Ulrich H, Szeto AC, Hann RM, Eterović VA. Toxicon. 2009;54:1174–1182. doi: 10.1016/j.toxicon.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferchmin PA, Andino M, Reyes Salaman R, Alves J, Velez-Roman J, Cuadrado B, Carrasco M, Torres-Rivera W, Segarra A, Martins AH, Lee JE, Eterovic VA. Neurotoxicology. 2014;44:80–90. doi: 10.1016/j.neuro.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vélez-Carrasco W, Green CE, Catz P, Furimsky A, O’Loughlin K, Eterović VA, Ferchmin PA. PLoS One. 2015;10:e0121540. doi: 10.1371/journal.pone.0121540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito Y, Nishino H, Yoshida D, Mizusaki S, Ohnishi A. Oncology. 1988;45:122–126. doi: 10.1159/000226545. [DOI] [PubMed] [Google Scholar]
- 12.Saito Y, Tsujino Y, Kaneko H, Yoshida D, Mizusaki S. Agric Biol Chem. 1987;51:941–943. [Google Scholar]
- 13.El Sayed KA, Laphookhieo S, Yousaf M, Prestridge JA, Shirode AB, Wali VB, Sylvester PW. J Nat Prod. 2008;71:117–122. doi: 10.1021/np0704351. [DOI] [PubMed] [Google Scholar]
- 14.El Sayed KA, Laphookhieo S, Baraka HN, Yousaf M, Hebert A, Bagaley D, Rainey FA, Muralidharan A, Thomas S, Shah GV. Bioorg Med Chem. 2008;16:2886–2893. doi: 10.1016/j.bmc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. [Accessed 04.05.16]; http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/index.
- 17.Leong AS, Zhuang Z. Pathobiology. 2011;78:99–114. doi: 10.1159/000292644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav BS, Sharma SC, Chanana P, Jhamb S. World J Clin Oncol. 2014;5:125–133. doi: 10.5306/wjco.v5.i2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes NE. Breast Cancer Res. 2000;2:154–157. doi: 10.1186/bcr48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolgay Ocal I, Dolled-Filhart M, D’Aquila TG, Camp RL, Rimm DL. Cancer. 2003;97:1841–1848. doi: 10.1002/cncr.11335. [DOI] [PubMed] [Google Scholar]
- 21.Lengyel E, Prechtel D, Resau JH, Gauger K, Welk A, Lindemann K, Salanti G, Richter T, Knudsen B, Vande Woude GF, Harbeck N. Int J Cancer. 2005;113:678–682. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 22.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 23.Ma PC, Maulik G, Christensen J, Salgia R. Cancer Metastasis Rev. 2003;22:309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 24.Gao CF, Vande Woude GF. Cell Res. 2005;15:49–51. doi: 10.1038/sj.cr.7290264. [DOI] [PubMed] [Google Scholar]
- 25.Organ SL, Tsao MS. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [accessed 05.04.16];FDA approves Cometriq to treat rare type of thyroid cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm330143.htm.
- 27. [accessed 05.04.16];FDA Approves Crizotinib. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm376058.htm.
- 28.ClinicalTrials.gov. [accessed on 5.17.16]; https://clinicaltrials.gov/ct2/results?term=c-Met+inhibitor&Search=Search.
- 29.van Zij F, Krupitza G, Mikulits W. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parr C, Jiang WG. Int J Oncol. 2001;19:857–863. [PubMed] [Google Scholar]
- 31.Caballero J, Quiliano M, Alzate-Morales JH, Zimic M, Deharo E. J Comput Aided Mol Des. 2011;25:349–369. doi: 10.1007/s10822-011-9425-1. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Lee K, Kim HR, Chae CH. Bull Korean Chem Soc. 2013;34:3553–3558. [Google Scholar]
- 33.Cho SY, Han SY, Ha JD, Ryu JW, Lee CO, Jung HJ, Kang NS, Kim HR, Koh JS, Lee JK. Bioorg Med Chem Lett. 2010;20:4223–4227. doi: 10.1016/j.bmcl.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Harrison C. Nat Rev Drug Discov. 2012;11:21. doi: 10.1038/nrd3891. [DOI] [PubMed] [Google Scholar]
- 35.Krug M, Hilgeroth A. Mini-Rev Med Chem. 2008;8:1312–1327. doi: 10.2174/138955708786369591. [DOI] [PubMed] [Google Scholar]
- 36.Petrelli A, Giordano S. Curr Med Chem. 2008;15:422–432. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 37.Lynette A, Smyth LA, Collins I. J Chem Biol. 2009;2:131–151. doi: 10.1007/s12154-009-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conn CS, Qian SB. Cell Cycle. 2011;10:1940–1947. doi: 10.4161/cc.10.12.15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan FK, Moriwaki K, De Rosa MJ. Methods Mol Biol. 2013;979:65–70. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorsch D, Schadt O, Stieber F, Meyring M, Grädler U, Bladt F, Friese-Hamim M, Knühl C, Pehl U, Blaukat A. Bioorg Med Chem Lett. 2015;25:1597–1602. doi: 10.1016/j.bmcl.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Protein Data Bank. [accessed 02.05.16]; http://www.rcsb.org/pdb/home/home.do.
- 42.Akl MR, Ayoub NM, Mohyeldin MM, Busnena BA, Foudah AI, Liu YY, El Sayed KA. PLoS One. 2014;9:e97622. doi: 10.1371/journal.pone.0097622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebrahim HY, Elsayed HE, Mohyeldin MM, Akl MR, Bhattacharjee J, Egbert S, El Sayed KA. Phytother Res. 2016;30:557–566. doi: 10.1002/ptr.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.