Abstract
Background and Purpose
In acute arterial occlusion, FLAIR vascular hyperintensity (FVH) has been linked to slow flow in leptomeningeal collaterals and cerebral hypoperfusion, but the impact on clinical outcome is still controversial. In this study, we aimed to investigate the association between FVH topography or FVH-ASPECTS pattern and outcome in acute M1-MCA occlusion patients with endovascular treatment.
Methods
We included acute M1-MCA occlusion patients treated with endovascular therapy. All patients had DWI and FLAIR before endovascular therapy. Distal FVH ASPECT score was evaluated according to distal MCA-ASPECT area (M1–M6) and acute DWI lesion was also reviewed. Presence of FVH inside and outside DWI positive lesions was separately analyzed. Clinical outcome after endovascular therapy was analyzed with respect to different distal FVH-ASPECTS topography.
Results
Among 101 patients that met inclusion criteria for the study, mean age was 66.2±17.8 and median NIHSS was 17.0 (IQR 12.0–21.0). FVH-ASPECTS measured outside of the DWI lesion was significantly higher in patients with good outcome (mRS 0–2), (8.0 vs 4.0, p<0.001). Logistic regression demonstrated that FVH-ASPECTS outside of the DWI lesion was independently associated with clinical outcome of these patients (OR 1.3; 95% CI, 1.06 to 1.68; p=0.013). FVH-ASPECTS inside the DWI lesion was associated with hemorrhagic transformation (OR 1.3; 95% CI, 1.04 to 1.51; p=0.019).
Conclusions
Higher FVH-ASPECTS measured outside the DWI lesion is associated with good clinical outcomes in patients undergoing endovascular therapy. FVH-ASPECTS measured inside the DWI lesion was predictive of hemorrhagic transformation. The FVH pattern, not number, can serve as an imaging selection marker for endovascular therapy in acute middle cerebral artery occlusion.
Keywords: ischemic stroke, revascularization, imaging, magnetic resonance imaging
Introduction
Advanced imaging method was an essential component of recently clinical trials’ success in support of mechanical thrombectomy for acute ischemic stroke with large artery occlusion1, 2. ASPECT scoring is a validated method for assessing tissue status with either CT or MR imaging3 and improved collateral flow was associated with improved imaging and clinical outcomes in patients undergoing acute endovascular therapy4. Then, finding an easy and reproducible ASPECT collateral scoring that reflects both tissue and vascular status can extend this opportunity to more patients at greatest risk of long-term disability.
FLAIR vascular hyperintensity (FVH), which represents slow blood flow in leptomeningeal collaterals5, 6, can provide a direct and non-invasive visualization of collateral pathways7–9. As to the prognostic value of FVHs, the results of different studies7, 10–12 lack consensus. These discrepancies might be explained by difference among populations, end points, and FVH classifications13. However, we found that all these studies focused solely on the presence or number of FVHs, rather than the topography or pattern of FVHs. Furthermore, the juxtaposition of FVH relative to established DWI lesions indicative of core infarction may be linked with subsequent imaging and clinical outcomes.
The purpose of this study was to prospectively assess the association between FVH-ASPECTs pattern or topography and the outcome of patients with acute middle cerebral artery (MCA) occlusion.
Patients and Methods
Patients and Clinical Assessment
We prospectively evaluated consecutive patients who received endovascular therapy (ET, intra-arterial thrombolytic therapy or mechanical thrombectomy) for acute cerebral ischemia between September 2004 and December 2014. Patients were included in our study if they had initial imaging demonstrating occlusion of proximal M1 segment of the MCA, and underwent conventional angiography for consideration of ET. The patients were excluded if they didn’t undergo MRI scan or the imaging couldn’t be analyzed. Patients were also excluded if tandem vessel occlusions (ie, ICA+MCA) were identified. Finally, 101 patients were included in the analysis. Demographic, clinical and laboratory data were retrieved from a prospectively maintained, single center dataset of consecutive cases. The following stroke risks factors were identified: age, sex, hypertension, diabetes, hyperlipidemia, previous stroke/ transient ischemic attack (TIA), coronary artery disease (CAD), chronic heart failure (CHF), and arterial fibrillation (AF). Baseline characteristic, including NIHSS, systolic blood pressure (SBP), diastolic blood pressure (DBP), blood glucose, cholesterol and previous medications were also collected from these patients.
Imaging Analysis
All patients’ imaging data were reviewed at UCLA Neurovascular Imaging Research Core by two authors (D.L and W.S). All MRI studies included diffusion-weighted imaging (DWI) and FLAIR. DWI lesion volume measurement was performed by one of the authors (F. S) blinded to the clinical information using a computer-assisted volumetric analysis program (Olea Medical, La Ciotat, France). Diffusion was measured at three values of b (b=0, 500, 1000 s/mm2), and average apparent diffusion coefficient (ADC) maps were generated. DWI volumes were quantified from analysis of isotropic b1000 images and ADC maps with threshold of ADC<600. Determination of M1 occlusion was made by review of angiographic images. Hemorrhagic transformation (HT) was defined as a new hyperattenuated region identified on any follow-up CT scan before patient discharge, as previously described14. In all cases, angiography was performed subsequent to the initial MRI study.
FVH were defined as focal, tubular, or serpentine hyperintensities in the subarachnoid space relative to CSF and corresponding to the typical arterial course5. As shown in Figure 1, Total FVH-Alberta Stroke Program Early CT Score (FVH-T-ASPECTS) was assessed according to distal MCA-ASPECTS area (M1–M6). FVH in every distal MCA-ASPECTS area was separately evaluated. For example, no FVH in M1 area was recorded as 0, less than M1 half area was recorded as 1, more than M1 half area was recoded as 2. Those distal MCA-ASPECTS areas were defined as DWI-positive area if there was acute infarction lesion in the areas. FVH-ASPECTS score outside DWI-positive area (FVH-O-ASPECTS) and FVH-ASPECTS inside DWI-positive area (FVH-I-ASPECTS) were separately analyzed. Then, FVH-I-ASPECTS was graded as subtle FVH-I-ASPECTS (0–2 points) and prominent FVH-I-ASPECTS (>2 points). Similarly to that, FVH-O-ASPECTS was graded as subtle FVH-O-ASPECTS (0–4 points) and prominent FVH-O-ASPECTS (>4 points).
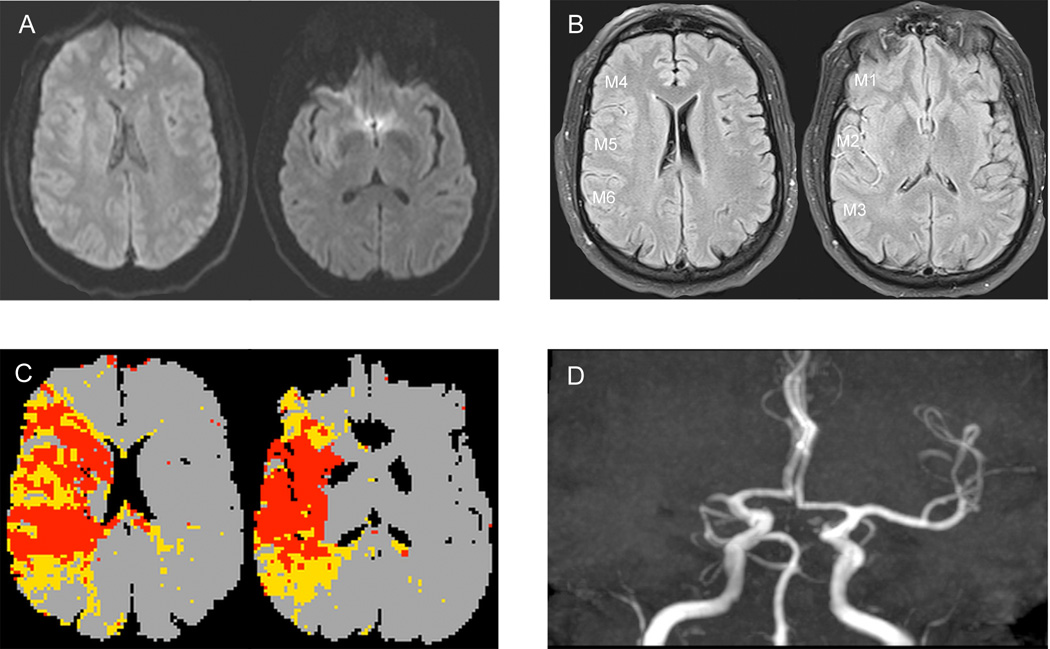
Figure 1.
Illustrative case of FVH-ASPECTS evaluation in a patient with a right MCA occlusion (A–D). No hyperintense lesions are visible in the right MCA territory (A). FVH-T-ASPECT score was 11 (M1=1, M2=2, M3=2, M4=2, M5=2, M6=2), FVH-O-ASPECTS was 11 and FVH-I-ASPECTS was 0 (B). PWI showed that mismatch on the Tmax map was congruent with the FVHs distribution(C).
Abbreviations: FVH-T-ASPECTS=total FVH-ASPECT score, FVH-O-ASPECTS=FVH-ASPECT score outside DWI-positive area, FVH-I-ASPECTS=FVH-ASPECT score inside DWI-positive area
Angiographic collateral grade was evaluated with the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) Collateral Flow Grading System on baseline angiography. Collateral rating was performed by one author (D.S.L) with extensive experience in angiographic interpretation in acute stroke, blinded to the clinical presentation and outcomes. Dichotomization was then performed by dividing into groups of 0–2 vs 3–4. Assessment of vascular recanalization was based on the Thrombolysis in Cerebral Infarction (TICI) scale.
Statistical analysis
Continuous variables with a normal distribution were described as mean±SD and non-normally distributed variables were described as median and interquartile range. We compared continuous variables using the Student t test or Mann-Whitney U test, as appropriate. Categorical variables were compared using Pearson χ2 or Fisher exact test, as appropriate. Logistic regression analyses were done to determine the independent predictors of favorable clinical outcome and HT. All covariates with a P value≤0.1 in a univariate analysis were entered into this logistic regression model and a value of P<0.05 was used to indicate statistical significance.
Results
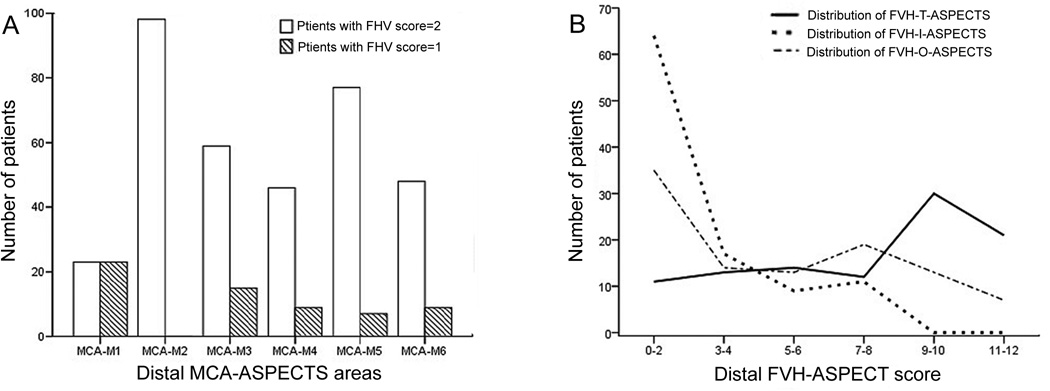
The main baseline characteristics of the patients are summarized in Table 1 and Figure 2. During this period, 101 acute ischemic stroke patients with MCA-M1 occlusion were included in our study. Among these patients, 29 (28.7%) patients were male and the average age was 66.2±17.8 years. 6 patients were treated with IA tPA, 49 patients were treated with Mechanical Embolus Removal in Cerebral Ischemia (MERCI) device, 20 patients were treated with Solitaire device, 26 patients were treated with other methods (including Penumbra device, mechanical disruption, angioplasty and all complex methods). Median baseline NIHSS score was 17 [range 12–21] and median FVH-T-ASPECTS score was 9 [range 5–10]. 98 (98/101, 97.0%) patients have FVH on the FLAIR imaging. FVH were facing the M2, M5, M3, M6, M4, and M1 ASPECTS regions in 97%, 83%, 73%, 56%, 54%, and 46% of patients, respectively (average of 2 readers). The distribution of FVH-T-ASPECTS score in different ASPECT areas was also shown in Figure 2.
Table 1.
Baseline characteristics in patients with different FHV-ASPECTS
| Characteristics | Subtle FVH-O- ASPECTS (n=49) |
Prominent FVH-O- ASPECTS (n=52) |
p Value | Subtle FVH-I-ASPCTS (n=64) |
Prominent FVH-I- ASPECTS (n=37) |
p Value |
|---|---|---|---|---|---|---|
| Age, (mean±SD) | 68.2±17.3 | 64.3±18.2 | 0.269 | 66.0±18.6 | 66.4±16.7 | 0.925 |
| Male, n(%) | 13(26.5) | 16(30.8) | 0.638 | 17(26.6) | 12(32.4) | 0.530 |
| Risk factors | ||||||
| Hypertension, n(%) | 35(71.4) | 35(67.3) | 0.654 | 44(68.8) | 26(70.3) | 0.873 |
| Diabetes, n(%) | 10(20.4) | 10(19.2) | 0.882 | 12(18.8) | 8(21.6) | 0.727 |
| Hyperlipidemia, n(%) | 16(32.7) | 19(36.5) | 0.682 | 21(32.8) | 14(37.8) | 0.609 |
| Stroke/TIA, n(%) | 7(14.3) | 2(3.8) | 0.086 | 7(10.9) | 2(5.4) | 0.347 |
| CAD, n(%) | 8(16.3) | 9(17.3) | 0.895 | 12(18.8) | 5(13.5) | 0.498 |
| CHF, n(%) | 7(14.3) | 3(5.8) | 0.152 | 8(12.5) | 2(5.4) | 0.250 |
| AF, n(%) | 23(46.9) | 23(44.2) | 0.785 | 30(46.9) | 16(43.2) | 0.724 |
| Initial NIHSS | 19.0(13.0–23.0) | 14.5(11.0–19.8) | 0.016* | 13.5(10.0–19.8) | 20.0(15.5–23.0) | 0.001* |
| SBP mmHg, median (IQR) | 156.4±35.5 | 151.9±32.2 | 0.511 | 154.2±33.5 | 153.8±34.7 | 0.960 |
| DBP mmHg, median (IQR) | 89.0±22.9 | 84.9±17.4 | 0.314 | 87.3±20.2 | 86.3±20.6 | 0.815 |
| Blood glucose, mM, median (IQR) |
132.5±52.1 | 132.3±38.9 | 0.983 | 127.4±39.9 | 140.9±53.5 | 0.154 |
| Cholesterol, mM, median (IQR) | 149.1±38.1 | 167.0±48.9 | 0.043* | 164.7±45.5 | 147.2±41.6 | 0.059 |
| Previous medications | ||||||
| Antiplatelet therapy, n(%) | 13(26.5) | 10(19.2) | 0.382 | 14(21.9) | 9(24.3) | 0.777 |
| Anticoagulation therapy, n(%) | 6(12.2) | 7(13.5) | 0.855 | 11(17.2) | 2(5.4) | 0.088 |
| Right side | 23(46.9) | 28(53.8) | 0.488 | 34(53.1) | 17(45.9) | 0.487 |
| TOTAL FVH -ASPECTS | 5.0(3.0–8.0) | 10.0(9.0–11.0) | <0.001* | 7.5(4.0–10.0) | 9(7.0–12.0) | 0.001* |
| Infarct Volume | 46.5(14.6–89.6) | 13.7(6.3–33.7) | 0.001* | 13.0(5.9–23.7) | 65.0(30.5–97.5) | <0.001* |
| ASITN (0–2) | 36(73.5) | 25(48.1) | 0.009* | 33(51.6) | 28(75.7) | 0.017 |
| oTICI (0–2a) | 36(73.5) | 26(50.0) | 0.015 | 39(60.9) | 23(62.2) | 0.903 |
| IV t-PA | 23(46.9) | 20(38.5) | 0.389 | 28(43.8) | 15(40.5) | 0.753 |
| ET methods | 0.789 | 0.818 | ||||
| IA tPA | 3(6.1) | 3(5.8) | 3(4.7) | 3(8.1) | ||
| MERCI | 26(53.1) | 23(44.2) | 33(51.6) | 16(43.2) | ||
| Solitaire | 8(16.3) | 12(23.1) | 12(18.8) | 8(21.6) | ||
| Other methods | 12(24.5) | 14(26.9) | 16(25.0) | 10(27.0) | ||
| HT | 19(38.8) | 13(25.0) | 0.137 | 15(23.4) | 17(45.9) | 0.019* |
| Discharge mRS≤2 | 3(6.1) | 16(30.8) | 0.002* | 17(26.6) | 2(5.4) | 0.009* |
Abbreviations: FVH-O-ASPECTS=FVH-ASPECTS score outside DWI-positive area; FVH-I-ASPECTS= FVH-ASPECTS inside DWI-positive area; TIA= transient ischemic attack; CAD= coronary artery disease; CHF= Chronic heart failure; AF=arterial fibrillation; SBP=systolic blood pressure; DBP= diastolic blood pressure; ET= Endovascular treatment; HT= hemorrhagic transformation.
Figure 2.
Location and frequencies of FVH according to the surface of distal ASPECT territories.
A. Number of patients with FVHs in different ASPECT area.
B. Number of patients according to different FVH distribution.
FVH-O-ASPECTS score and FVH-I-ASPECTS score
The interobserver agreement for FVH-T-ASPECTS was k = 0.72 (95% CI, 0.62–0.81), for FVH-O-ASPECTS was k = 0.71 (95%, CI 0.61–0.80) and for FVH-I-ASPECTS was k = 0.74 (95% CI, 0.65–0.83). In our study, subtle FVH-O-ASPECTS score were observed in 49 patients (48.5%) and prominent FVH-O-ASPECTS score were observed in 52 patients (51.5%). As shown in Table-1, subtle FVH-O-ASPECTS has a higher baseline NIHSS score (19.0 vs 14.5, p=0.016), lower baseline cholesterol (149.1±38.1 vs 167.0±48.9, p=0.043), larger infarct volume (46.5 vs 13.7, p=0.001), and more patients with lower ASITN score (73.5% vs 48.1%, p=0.009). The number of patients with a favorable clinical outcome was also smaller in subtle FVH-O-ASPECTS group than prominent FVH-O-ASPECTS group (6.1% vs 30.8%, p=0.002). As to HT, there was no difference in these two groups (38.8% vs 25.0%, p=0.137).
As shown in the details of Table 1, 101 patients were graded as subtle FVH-I-ASPECTS group (64/101, 63.4%) and prominent FVH-I-ASPECTS group (37/101, 36.6%). Compared to prominent FVH-I-ASPECTS group, subtle FVH-I-ASPECTS group has a lower baseline NIHSS score (13.5 vs 20.0, p=0.001), smaller infarct volume (13.0 vs 65.0, p<0.001), and less patients with low ASITN score (51.6% vs 75.7%, p=0.017). More patients in subtle FVH-I-ASPECTS group had a good clinical outcome than prominent FVH-I-ASPECTS group (26.6% vs 5.4%, p=0.009). Patient in subtle FVH-I-ASPECTS group also had less hemorrhagic transformation after endovascular therapy (23.4% vs 45.9%, p=0.019).
Clinical and Imaging Outcomes
Variables associated with a favorable clinical outcome are shown in Table 2. Patients’ age (p=0.013), history of diabetes (p=0.078), baseline NIHSS score (p<0.001), FVH-O-ASPECT score (p<0.001), FVH-I-ASPECT score (p=0.009), infarct volume (p=0.006), oTICI score (p=0.015), and HT (p=0.006) were included in our analysis. After adjusting for representative variables, higher FVH-O-ASPECTS (OR 1.3; 95% CI, 1.06 to 1.68; p=0.013) appeared as independent predictors of favorable outcome (shown in Figure 3).
Table 2.
Logistic regression analysis of factors associated with clinical and radiological outcome
| Characteristics | mRS≤2 (n=19) | mRS>2 (n=82) |
p Value | OR | 95% CI | P value | Without HT (n=69) |
With HT (n=32) |
P value | OR | 95% CI | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FVH-T-ASPECTS | 9.0(7.0–11.0) | 8.0(4.0–10.0) | 0.187 | 8.0(4.0– 10.0) |
9.0(5.0– 10.0) |
0.450 | ||||||
| FVH-O-ASPECTS | 8.0(6.0–10.0) | 4.0(2.0–8.0) | <0.001 | 1.335 | 1.063– 1.677 |
0.013 | 5.0(2.0– 8.0) |
4.0(2.0– 8.0) |
0.293 | |||
| FVH-I-ASPECTS | 0(0–2.0) | 2(0–4.25) | 0.009 | 0.925 | 0.561– 1.524 |
0.759 | 1.0(0– 3.5) |
3.0(0.25– 6.0) |
0.016 | 1.250 | 1.037–1.506 | 0.019 |
Abbreviations: FVH-T-ASPECTS = Total FVH-ASPECTS score; FVH-O-ASPECTS=FVH-ASPECTS score outside DWI-positive area; FVH-I-ASPECTS = FVH-ASPECTS inside DWI-positive area; HT= hemorrhagic transformation.
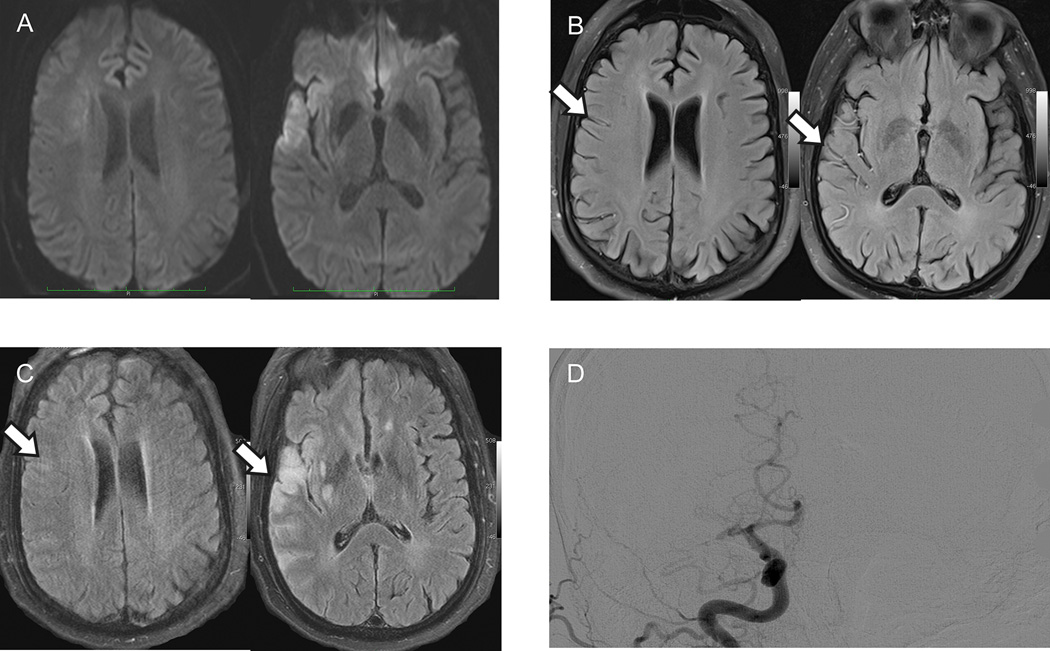
Figure 3.
Representative case of prominent FVH-O-ASPECTS.
A 46-years-old man with a right MCA occlusion and NIHSS of 13 on admission (A–D). Baseline total FVH-ASPECT score (B) was 11 (FVH-O-ASPECTS=9 and FVH-I-ASPECTS=2). After recanalization of the right MCA, FVH-ASPECTS decreased to 2 and no hemorrhagic transformation was found on the images (arrows on B and C). Discharge mRS of this patient was 1.
As shown in Table 2 and Figure 4, 32 patients (32/101, 31.7%) had hemorrhagic transformation after endovascular therapy. Although higher baseline NIHSS score (p=0.012), higher blood glucose (p=0.016), history of anticoagulation therapy (p=0.066), higher FVH-I-ASPECTS (p=0.016), infarct volume (p=0.010), and lower ASITN score (p=0.041) were related to hemorrhagic transformation in univariate analysis, in the multivariate analysis, only history of anticoagulation therapy (OR 4.7; 95% CI, 1.26–17.12; p=0.021) and higher FVH-I-ASPECTS (OR 1.3; 95% CI, 1.04 to 1.51; p=0.019) emerged as independent predictors of hemorrhagic transformation.
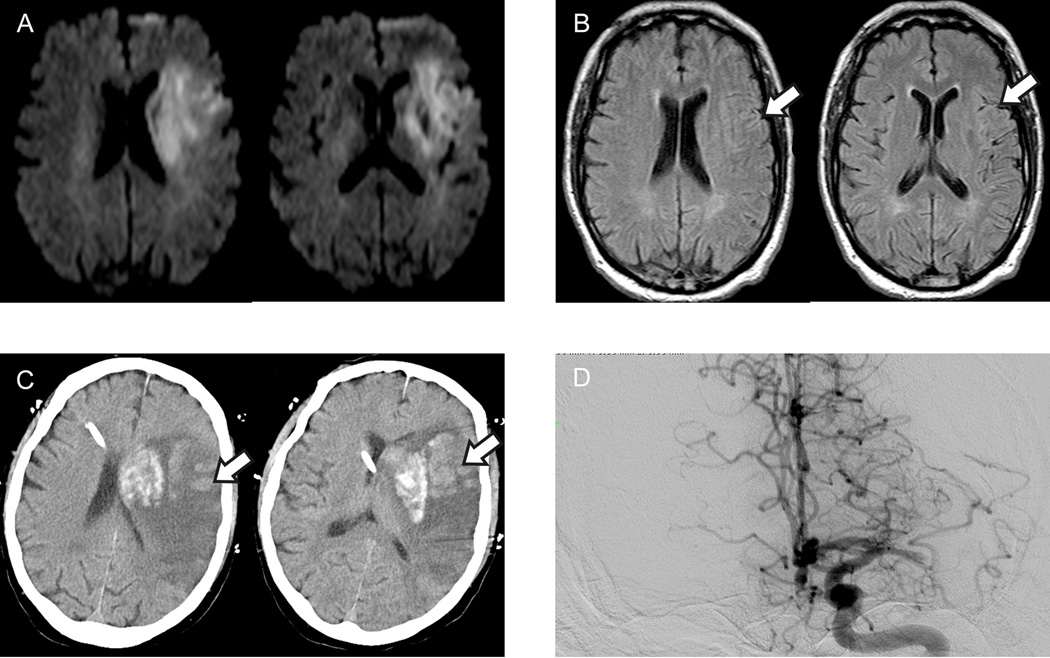
Figure 4.
Representative case of prominent FVH-I-ASPECTS.
A 70-years-old man with a left MCA occlusion and NIHSS of 30 on admission (A–D). Baseline total FVH-ASPECT score (B) was 10 (FVH-O-ASPECTS=5 and FVH-I-ASPECTS=5). Hemorrhagic transformation was found on the follow-up CT after recanalization (C). The hematoma shape was similar to FVH-I-ASPECTS distribution (arrows on B and C) and this patient died 4 days after admission.
Discussion
Our novel results demonstrate that the number of distal FVH-ASPECTS have no prognostic value in acute MCA occlusion patients undergoing endovascular therapy, yet FVH topography is key. Distal FVH-ASPECTS measured outside the DWI lesion is associated with good clinical outcomes. In addition, distal FVH-ASPECTS measured inside the DWI lesion is predictive of hemorrhage transformation. FVH topography and juxtaposition to DWI lesions, not number, can serve as an imaging selection marker for endovascular therapy in acute middle cerebral artery occlusion.
FVH is related with a higher grader of collateral circulation distal to large-vessel stenosis or occlusion6, and the presence of FVH ranges from 45% to 100% in stroke patients with intracranial arterial occlusion7, 8. In the present study, we included patients with proximal MCA occlusion and all of them were imaged within a short time, which can explain the high prevalence of FVH (97%). In addition to the high prevalence of FVHs, the distribution of the FVHs in our study is also similar to the previous study13. As we know, the underlying mechanism involved in the FVH presence is a slow blood flow through the leptomeningeal collaterals5, 15, 16. After proximal MCA occlusion, leptomeningeal collaterals from ACA or PCA open and the blood pressure falls when moving from borderzone areas (M1, M6) to more proximal areas (M2, M5). Then, it may be the reason why more FVHs were observed in M2 area than M1 area in our study, which is also proved by the previous studies13, 17.
The prognostic value of FVHs has been widely investigated by previous studies7, 8, 18, and discrepancies have been attributed to differences among populations, imaging time and FVH classifications. Similar to the previous study10, our study got the result that total FVHs within MCA territory had no prognostic value. However, we found that FVHs outside DWI lesion had a good prognostic value in patients with acute proximal MCA occlusion. Extent of FVHs was associated with the presence of a PWI-DWI mismatch19, 20, and FVHs beyond the DWI lesion represent markedly impaired hemodynamics13. More FVHs outside DWI lesion mean larger amounts of tissue at risk of infarct expansion. Then, after acute MCA occlusion, patients with more FVHs beyond DWI lesion may benefit better clinical recovery because recanalization of occlusion artery can save at-risk tissue.
Hemorrhagic transformation, the major complication of endovascular therapy, is associated with increased stroke morbidity and mortality21. Several brain imaging approaches, including MRI enhancement patterns, T2*-permeability MRI22, apparent diffusion coefficient23 and very low cerebral blood volume24, have been evaluated to predict HT after stroke. Furthermore, collateral circulation has also been associated with HT in patients where recanalization occurs25. In the present study, we found that FVHs inside DWI lesion was associated with HT in acute MCA occlusion. As we know, ROS and blood-derived factors MMP-9 have emerged as important mediators in early HT21. Within 30minutes of focal cerebral ischemia, ischemic stroke elicits a robust activation of the immune system and circulating leukocytes adhere to vascular endothelial cells26. More FVHs inside DWI lesion means that more circulating leukocytes can move to ischemic area through leptomeningeal collaterals. Then, leukocytes adhesion and migration across the vasculature can activate a number of signaling cascades that increase the BBB permeability26.
The present study has some limitations. First, this is a hospital-based clinical study of moderate size. However, to our knowledge, this is the first study that analyzes FVHs pattern involving FVHs distribute inside DWI lesion. Further multicenter studies are needed to confirm our findings. Second, based on the consideration of patient homogeneity, we only included patients with acute MCA occlusion. Acute MCA stroke without MCA occlusion are not analyzed in the present study, which can be analyzed in the future study. Third, perfusion imaging was not available for all these patients. However, previous study has demonstrated that FVH beyond the DWI lesion represent the ischemic penumbra. Then, more FVHs distributing outside the DWI lesion means that more tissue at risk can be saved by endovascular therapy.
Conclusions
Acute MCA occlusion patients with more FVHs (FVH-ASPECTs >4) outside DWI lesion and less FVHs (FVH-ASPECTs ≤2) inside DWI lesion have better outcome after endovascular therapy. Then, FVHs pattern can provide as a novel imaging criteria for patient selection of endovascular therapy.
Acknowledgments
None
Sources of Funding
This study was supported by grants from NIH-NINDS (Liebeskind - K24NS072272), AHA (Scalzo - 16BGIA27760152), National Natural Science Foundation of China (grant no.81220108008 and no.81000501), Jinling Hospital Medical Scientific Research Projects (2012003), and Clinical Medical Center Program of Jiangsu Province (Neurology Center).
Footnotes
Disclosures
The abstract of this paper has been presented as a poster on International Stroke Conference 2016.
References
- 1.Tansy AP, Hinman JD, Ng KL, Calderon-Arnulphi M, Modir R, Chatfield F, et al. Image more to save more. Frontiers in neurology. 2015;6:156. doi: 10.3389/fneur.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palaniswami M, Yan B. Mechanical thrombectomy is now the gold standard for acute ischemic stroke: Implications for routine clinical practice. Interventional neurology. 2015;4:18–29. doi: 10.1159/000438774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turc G, Apoil M, Naggara O, Calvet D, Lamy C, Tataru AM, et al. Magnetic resonance imaging-dragon score: 3-month outcome prediction after intravenous thrombolysis for anterior circulation stroke. Stroke; a journal of cerebral circulation. 2013;44:1323–1328. doi: 10.1161/STROKEAHA.111.000127. [DOI] [PubMed] [Google Scholar]
- 4.Sheth SA, Sanossian N, Hao Q, Starkman S, Ali LK, Kim D, et al. Collateral flow as causative of good outcomes in endovascular stroke therapy. Journal of neurointerventional surgery. 2016;8:2–7. doi: 10.1136/neurintsurg-2014-011438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azizyan A, Sanossian N, Mogensen MA, Liebeskind DS. Fluid-attenuated inversion recovery vascular hyperintensities: An important imaging marker for cerebrovascular disease. AJNR. American journal of neuroradiology. 2011;32:1771–1775. doi: 10.3174/ajnr.A2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanossian N, Saver JL, Alger JR, Kim D, Duckwiler GR, Jahan R, et al. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR. American journal of neuroradiology. 2009;30:564–568. doi: 10.3174/ajnr.A1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebinger M, Kufner A, Galinovic I, Brunecker P, Malzahn U, Nolte CH, et al. Fluid-attenuated inversion recovery images and stroke outcome after thrombolysis. Stroke; a journal of cerebral circulation. 2012;43:539–542. doi: 10.1161/STROKEAHA.111.632026. [DOI] [PubMed] [Google Scholar]
- 8.Cheng B, Ebinger M, Kufner A, Kohrmann M, Wu O, Kang DW, et al. Hyperintense vessels on acute stroke fluid-attenuated inversion recovery imaging: Associations with clinical and other mri findings. Stroke; a journal of cerebral circulation. 2012;43:2957–2961. doi: 10.1161/STROKEAHA.112.658906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka K, Ishibashi S, Shiraishi A, Yokota T, Mizusawa H. Distal hyperintense vessels on flair images predict large-artery stenosis in patients with transient ischemic attack. Neuroradiology. 2013;55:165–169. doi: 10.1007/s00234-012-1092-y. [DOI] [PubMed] [Google Scholar]
- 10.Schellinger PD, Chalela JA, Kang DW, Latour LL, Warach S. Diagnostic and prognostic value of early mr imaging vessel signs in hyperacute stroke patients imaged<3 hours and treated with recombinant tissue plasminogen activator. AJNR. American journal of neuroradiology. 2005;26:618–624. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on flair: An mri marker for collateral circulation in acute stroke? Neurology. 2009;72:1134–1139. doi: 10.1212/01.wnl.0000345360.80382.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez de la Ossa N, Hernandez-Perez M, Domenech S, Cuadras P, Massuet A, Millan M, et al. Hyperintensity of distal vessels on flair is associated with slow progression of the infarction in acute ischemic stroke. Cerebrovascular diseases (Basel, Switzerland) 2012;34:376–384. doi: 10.1159/000343658. [DOI] [PubMed] [Google Scholar]
- 13.Legrand L, Tisserand M, Turc G, Naggara O, Edjlali M, Mellerio C, et al. Do flair vascular hyperintensities beyond the dwi lesion represent the ischemic penumbra? AJNR. American journal of neuroradiology. 2015;36:269–274. doi: 10.3174/ajnr.A4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-pa acute stroke study group. AJNR. American journal of neuroradiology. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Kamran S, Bates V, Bakshi R, Wright P, Kinkel W, Miletich R. Significance of hyperintense vessels on flair mri in acute stroke. Neurology. 2000;55:265–269. doi: 10.1212/wnl.55.2.265. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Xu G, Yue X, Wang X, Ma M, Zhang R, et al. Hyperintense vessels on flair: A useful non-invasive method for assessing intracerebral collaterals. European journal of radiology. 2011;80:786–791. doi: 10.1016/j.ejrad.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Hohenhaus M, Schmidt WU, Brunecker P, Xu C, Hotter B, Rozanski M, et al. Flair vascular hyperintensities in acute ica and mca infarction: A marker for mismatch and stroke severity? Cerebrovascular diseases (Basel, Switzerland) 2012;34:63–69. doi: 10.1159/000339012. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Liu W, Zhu W, Ni G, Sun W, Ma M, et al. Distal hyperintense vessels on flair: A prognostic indicator of acute ischemic stroke. European neurology. 2012;68:214–220. doi: 10.1159/000340021. [DOI] [PubMed] [Google Scholar]
- 19.Haussen DC, Koch S, Saraf-Lavi E, Shang T, Dharmadhikari S, Yavagal DR. Flair distal hyperintense vessels as a marker of perfusion-diffusion mismatch in acute stroke. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2013;23:397–400. doi: 10.1111/j.1552-6569.2012.00784.x. [DOI] [PubMed] [Google Scholar]
- 20.Gawlitza M, Gragert J, Quaschling U, Hoffmann KT. Flair-hyperintense vessel sign, diffusion-perfusion mismatch and infarct growth in acute ischemic stroke without vascular recanalisation therapy. Journal of neuroradiology. Journal de neuroradiologie. 2014;41:227–233. doi: 10.1016/j.neurad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bang OY, Buck BH, Saver JL, Alger JR, Yoon SR, Starkman S, et al. Prediction of hemorrhagic transformation after recanalization therapy using t2*-permeability magnetic resonance imaging. Ann Neurol. 2007;62:170–176. doi: 10.1002/ana.21174. [DOI] [PubMed] [Google Scholar]
- 23.Tong DC, Adami A, Moseley ME, Marks MP. Relationship between apparent diffusion coefficient and subsequent hemorrhagic transformation following acute ischemic stroke. Stroke; a journal of cerebral circulation. 2000;31:2378–2384. doi: 10.1161/01.str.31.10.2378. [DOI] [PubMed] [Google Scholar]
- 24.Campbell BC, Christensen S, Parsons MW, Churilov L, Desmond PM, Barber PA, et al. Advanced imaging improves prediction of hemorrhage after stroke thrombolysis. Ann Neurol. 2013;73:510–519. doi: 10.1002/ana.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christoforidis GA, Karakasis C, Mohammad Y, Caragine LP, Yang M, Slivka AP. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: The role of pial collateral formation. AJNR. American journal of neuroradiology. 2009;30:165–170. doi: 10.3174/ajnr.A1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiology of disease. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]