Abstract
Objective
This study examined cancer knowledge in adolescent and young adult (AYA) survivors and pilot tested a web-based resource to provide individually tailored information regarding cancer treatment history, late effects risk, and resources.
Methods
Fifty-two survivors (15–28 years old) who completed cancer treatment were recruited from the University of Minnesota oncology clinics. Participants were randomly assigned to receive access to personalized health history, late effects information, and resources via a password-protected web portal or to standard of care (physician counseling) only. Participants completed surveys measuring cancer knowledge, health locus of control, and psychosocial well-being prior to randomization and approximately 1 year later.
Results
Overall, few participants accurately reported their chemotherapy history with detail (19% at baseline and 33% at follow-up), and many did not recognize that previous cancer treatments could impact future health (60% at baseline and 54% at follow-up). Among those randomized to the receive access to the website, utilization was very low, making it difficult to draw conclusions about efficacy. Nonetheless, these data suggest that offering tailored information through the web was not more effective than standard of care at improving cancer knowledge. Anxiety and health beliefs were associated with cancer knowledge, including knowledge of steps survivors could take to mitigate late effects risks (p < 01).
Conclusions
Knowledge gaps exist among AYA survivors regarding important aspects of their treatment histories and ongoing health risks. Offering purely educational information (either in person by providers or via the web) does not appear to be enough to close this gap.
Keywords: cancer, oncology, knowledge, late effects, web-based
Introduction
The transition of care after cancer treatment is an important milestone that often requires survivors to communicate essential information about their past diagnostic and treatment histories to primary care physicians and others who will play a role in their ongoing health[1, 2]. However, many survivors are not knowledgeable about their cancer history, nor are they aware of continuing health risks which stem from this history. In a previous study of a large cohort of long-term childhood cancer survivors, only 35% recognized that they could have serious health problems related to their treatment[3]. Recently, a study involving a comparatively smaller cohort of adult survivors of childhood cancer found that those attending a long-term follow-up (LTFU) clinic were not more knowledgeable about their treatment histories or late effect risk profiles than survivors who did not attend a LTFU clinic[4]. Specifically, few accurately recalled their chemotherapy history with detail, and all underestimated their late effects risks, suggesting that survivors continue to need further education about follow-up care and ongoing health risks beyond the current standard of care.
Continued follow-up care, matched to survivors’ risks and ongoing health needs, is important for survivors of childhood cancer. Medical late effects may include dysfunction of major organ systems (e.g., cardiac), infertility, and second malignancies [5–7], for which surveillance and interventions are often indicated. Having a poor or inaccurate understanding of one’s health history and late effects risks may prevent survivors from effectively communicating such information to new providers, which could have implications for the quality of care they receive [8, 9]. Poor cancer knowledge may also be a barrier to survivors taking important steps to mitigate health risks in their daily lives (e.g., sun protection, smoking cessation, physical activity, diet). Additionally, survivors of childhood cancers face neurocognitive and psychosocial sequelae of their cancer and treatment (e.g., anxiety, problems in social/family relationships, difficulties in attention or memory) [10–13] which may impact their engagement in medical care, or make it challenging for them to understand their health history and risk profile [14, 15]. For some, ongoing follow-up care and medical testing for late effects are associated with worry and distress[16] [17]. In particular, anxiety (both dispositional (trait) and current (state) anxiety and health beliefs have the potential to interfere with survivors’ interest in and receptivity to health education information[18, 19]. Few studies, however, have investigated factors that may influence cancer-knowledge, and fewer still have examined the efficacy of interventions to improve cancer knowledge in randomized controlled trials. While some have suggested that survivors’ self-perceptions, health beliefs and level of psychological distress are factors that appear relevant to their willingness to participate in follow-up care[11, 20, 21] it is not known whether such factors play a role in survivors’ knowledge about their cancer history and understanding of their risk for late effects. The possible contribution of psychological factors to cancer knowledge would be of particular importance, given that there are known avenues for treating distress/anxiety and changing maladaptive health beliefs[22, 23].
Adolescent and young adult (AYA) cancer survivors are an important group to target for interventions to promote cancer knowledge given that they are in a developmental period associated with increased autonomy and decision making with regards to their health[24] and are vulnerable to poor medical outcomes[25, 26]. The primary aims of this study were to: (i) measure cancer knowledge and understanding of late effects risks among AYA survivors and (ii) develop and pilot test a HIPAA-compliant, web-based resource to provide AYA survivors with individually tailored information regarding cancer therapy, ongoing health risks, and relevant health-related information and resources in a randomized controlled trial. It was hypothesized that AYA survivors would show low rates of knowledge of their chemotherapy history and late effects risks, similar to prior reports of older adult survivors of childhood cancers. It was further hypothesized that those randomized to receive access to the web-based intervention would show greater improvements in cancer knowledge at follow-up 1 year later, compared to those who received standard of care (physician counseling) only. Secondary analyses sought to determine whether the intervention had an impact on AYA’s self-reported anxiety and health locus of control. To identify possible barriers to engagement in the intervention and illuminate additional avenues for interventions to promote cancer knowledge, we explored associations between AYA’s anxiety (both state and trait) and health locus of control with website utilization and cancer knowledge.
Methods
Design
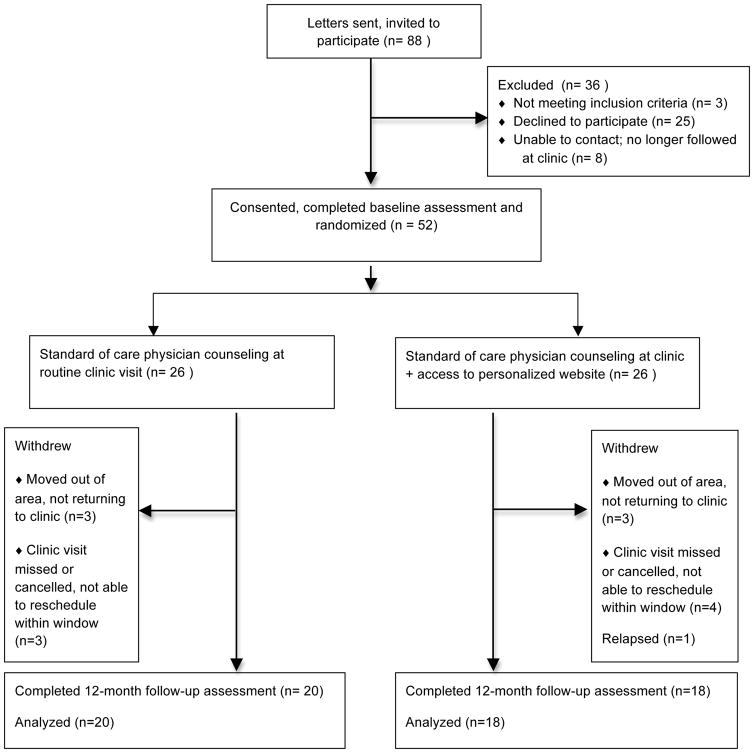
This was a randomized, pre/post-test experimental trial in which participants were randomly assigned to receive access to a secure, HIPAA-compliant website containing individually tailored information pertaining to the participant’s diagnosis, treatments, and treatment-related health risks (intervention group), or a standard of care control (depicted in Figure 1). The randomization sequence was computer-generated in blocks of four and maintained by one of the investigators (AKB) who was not involved in recruitment or data collection. All outcomes were self-reported. Participants completed questionnaires at the time of enrollment (T1) and again approximately 12 months later (T2). These assessments were timed to occur alongside regularly scheduled patient visits for follow-up care to eliminate the need for additional trips to the study center. The Institutional Review Board approved the protocol and study documents. Informed consent, and assent if indicated, was obtained from all participants in accordance with the Declaration of Helsinki. This study is registered with ClinicalTrials.gov (NCT01593618).
Figure 1.
CONSORT diagram
Procedure
Eligible participants were between 15–29 years of age at recruitment, with a history of hematologic malignancy or malignant neoplasm, and were off treatment and in first remission. We were particularly interested in engaging individuals within this age range, as they have been underrepresented in clinical trials and others have previously documented their unique medical and psychosocial needs[27]. Eligible participants were also English-speaking and reported having access to a computer with Internet access, and were without significant visual or neurologic/cognitive impairments which in the judgment of their oncologist would restrict their ability to see and understand website content and complete survey measures. Individuals meeting these criteria were selected from the University of Minnesota’s Oncology database. Letters describing the study were mailed to prospective participants (n=88) with follow-up phone calls to gauge interest. Fifty-two individuals (59%) responded to recruitment materials and provided informed consent (and assent for those < 18 years of age) at the time of their upcoming clinic visit. Participants did not differ significantly from those who declined study participation (n=25) in regards to their demographic background (i.e., age, sex, and race/ethnicity, all p values > .05). Common reasons offered for refusal were lack of time and lack of interest. Participants completed surveys (described below) prior to meeting with their medical provider in clinic at pre-randomization (baseline) visit and again at follow-up. Randomization assignment occurred after completion of their clinic and baseline surveys. Baseline data were collected over a 12-month period between January 2010 and January 2011, with follow up evaluations occurring approximately 12 months later.
Intervention
The intervention group received access to a HIPAA-compliant, password-protected website that contained the following components: (1) the participant’s treatment summary (i.e., diagnosis and treatments with layman language and definitions), (2) hyperlinks from the individual’s treatment summary to user-friendly information about health and treatment-related late effects, adapted with permission for this age group from the Children’s Oncology Group guidelines[28, 29] (3) optional fields that allowed participants to enter other aspects of their health history, (4) tips for communicating with healthcare providers and for getting the most out of their medical follow-up visits, (5) an e-journal to jot notes or to be used as a personal journal of experiences and questions, (6) a “contact us” messaging system which allowed them to connect with our medical team if participants had questions about their health history or risks, and (7) links to relevant local and national resources (e.g., college scholarships, employment rights, insurance) and supportive on-line communities for cancer survivors (e.g., American Cancer Society, LiveStrong). Individuals randomized to the intervention condition had continuous access to their personalized website throughout the duration of the study.
At baseline, after survey completion and after their clinic visit, participants who had been randomly assigned to the intervention group were given a one-on-one tutorial of a mock website by the project coordinator, which included i) basic components of use (i.e., login, password changes, navigating links to access specific information), ii) an overview of the type of information provided on the website (e.g., treatment summary, hyperlinks to specific treatments received), and iii) a discussion of how to get the most out of the website (using a structured online tutorial). Participants’ cancer diagnosis, treatment history, and care plans were abstracted from the medical record, reviewed by their primary oncologist, and uploaded to the password protected, HIPAA-compliant website for the participant. Participants were sent a letter in the mail and a duplicate copy by email within 7–10 days of their baseline visit containing their unique weblink, username, and temporary password for log in.
Primary Outcome
Cancer Knowledge was measured through a self-report survey tapping knowledge about their cancer diagnosis and treatment history. Several cancer knowledge questions previously used in phone interviews with adult survivors by Kadan-Lottick and colleagues[3] were adapted to be used in a paper-and-pencil self-report survey (Appendix A). Participants were asked to their specific cancer diagnosis. They were asked if they received chemotherapy, radiation therapy, and/or surgery as part of their cancer treatment. In open-ended questions, they were further asked to name the chemotherapy agents administered, the sites of radiation therapy and types of surgical procedures received. Responses to these questions were independently coded for accuracy based on the participant’s medical history by two of the investigators (JPN, AKB). Chemotherapy accuracy was categorized as accurate with details (i.e., accurately listing the chemotherapy agents received), accurate without details of therapy (e.g., aware they received chemotherapy but not able to list the specific chemotherapy received), or inaccurate. Participants were also asked: “Do you feel that previous treatment you received for cancer could cause serious future health problems?” with response options of “yes”, “no”, and “don’t know.” Additionally, participants were asked to rate the extent to which they agreed or disagreed with this statement: “I know what I can do to reduce my risk for certain kinds of complications from my cancer treatment” on a 1 (strongly disagree) to 6 (strongly agree) Likert scale. Responses were dichotomized such that ratings of 4–6 were categorized as “agree” and 1–3 were categorized as “disagree.”
Secondary Outcomes
State Trait Anxiety Inventory (STAI)[30] is a 40-item self-report scale that assesses anxiety in regards to both current symptoms (state) and personality (trait). Test-retest reliability is low for the State scale and high for the Trait scale, as would be predicted. The STAI has high internal consistency (0.85 to 0.95) and adequate construct and discriminative validity across diverse clinical and non-clinical samples. Higher scores reflect more anxiety.
Multidimensional Health Locus of Control (HLC)[31] is an 18-item questionnaire comprised of one Internal scale, measuring the extent to which an individual feels they personally have power over their health, and two External scales, measuring the extent to which individuals feel an external control of their health held by an important person (Powerful Others) or that their health outcomes are left up to fate (Chance). Each scale contains 6 items scored on a 6-point scale ranging from “strongly agree to “strongly disagree.” This survey tool is appropriate for the age range specified in the current study, and has been widely used in adolescent and adult health behavior research [32–34].
Computer knowledge, website use, and satisfaction
Participants completed the Brinkerhoff’s Computer Self-Efficacy scale[35] to measure perceived self-confidence in their computer skill. This survey asked participants to respond to statements about their comfort and confidence as a computer user (e.g., I am very confident in my ability to use computers; I consider myself a skilled computer user) on a 6-point Likert scale ranging from 1 (strongly disagree) to 6 (strongly agree); 10 items were reverse scored. Total mean score was used to ascertain baseline computer self-efficacy as a possible barrier to website use. For those randomized to the intervention group, actual site use was tracked electronically, including number of log-ins and number of minutes of use. These participants also completed a brief survey rating their level of satisfaction, ease of use, and usefulness of the website on a 1–5 Likert scale, where higher scores reflected a higher level of satisfaction, ease of use, and usefulness of information.
Results
Participants
Participant characteristics are presented by intervention arm in Table 1. The majority were leukemia survivors, followed by bone tumors, and survivors of Hodgkins lymphoma. There were slightly more males than females and the majority were white/non-Hispanic. Mean age at diagnosis was 12 years, with a range from 4 months to 23 years of age. At baseline, there were no significant differences in demographic characteristics or self-reported survey ratings between those randomly assigned to the intervention and control conditions. Mean self-rated computer efficacy was also consistent across groups and reflected a high level of comfort and self-efficacy with computers. The average time between baseline and follow-up survey was 14 months (range 9–16 months). Thirty-eight of 52 participants (73%) completed at least partial follow-up questionnaires, and rates of attrition did not differ significantly between groups (Figure 1).
Table 1.
Characteristics of participants at baseline
| Total Sample M (SD) n= 52 | Control M (SD) N= 26 | Intervention M (SD) n= 26 | Test Statistics | |
|---|---|---|---|---|
| Age at diagnosis (years) Range: 3 months – 21 years |
12.2 (6.13) | 12.8 (5.3) | 11.6 (6.9) | t(50) = .73 |
| Age at baseline survey (years) Range: 15–29 years |
21.3 (3.9) | 21.1 (4.0) | 21.5 (3.8) | t(50) = −.41 |
| Time since diagnosis (years) Range: 4 months – 23 years |
7.0 (5.5) | 6.2 (5.5) | 7.9 (5.4) | t(50) = −.96 |
| Number of prior visits to LTFUb clinic Range: 0–5 |
1.3 (1.4) | .92 (1.2) | 1.6 (1.6) | t(50) = −1.70 |
| Self-rated computer self-efficacy Range: 1–6 |
5.40 (.93) | 5.37 (.90) | 5.44 (.96) | t(50) = −.70 |
| Sex (% female) | 46% | 42% | 50% | X2(1) = .31 |
| Race/Ethnicity (% white/non-Hispanic) | 90% | 92% | 89% | X2 (1) = .22 |
| Diagnostic Group | % | % | % | X2(8) = 4.22 |
| ALL | 30.8 | 35 | 27 | |
| AML/CML | 5.8 | 3.8 | 7.7 | |
| Hodgkin/Other Lymphoma | 15.4 | 11.5 | 19.2 | |
| Soft Tissue Sarcoma | 7.7 | 7.7 | 7.7 | |
| Wilms Tumor | 3.8 | 3.8 | 3.8 | |
| CNS Tumor | 5.8 | 3.8 | 7.7 | |
| Bone Tumor | 21.2 | 26.9 | 15.4 | |
| Otherc | 9.6 | 7.7 | 11.5 |
Note.
Test statistics reflect evaluation of differences between intervention and control groups; all p values > .05);
LTFU = Long-Term Follow-Up Clinic;
Neuroblastoma, colon cancer, testicular, uterine, liver cancer
All participants had up-to-date address/contact information on file and had been seen by an oncology provider at the University of Minnesota within the past two years. A little more than half of participants (57%) had at least one visit to the Survivorship/Long-Term Follow-Up Clinic at the University of Minnesota prior to their participation in this study, by virtue of their length of time since completing treatment (i.e., > 2 years) and thus had received a written summary of their treatment history, including detailed written description of late effects risks and follow-up recommendations, which is part of the standard of care in this clinic. As shown in Table 1, the number of previous survivorship clinic visits was evenly distributed across intervention and control groups. At baseline, those who had a previous visit to the Survivorship clinic were not more likely to be accurate in their knowledge of their chemotherapy history (X2(1) = 2.65, p > .05) nor were they more likely to agree with the statement that previous treatments they had received for cancer could cause future health risks (X2(1) = 2.72, p > .05) when compared to those who had not yet been seen in the Survivorship clinic.
Accuracy of cancer knowledge in AYAs
At baseline, the vast majority of participants (92%) reported their childhood cancer diagnosis accurately with detail, 79% accurately recalled their history of cancer-related surgeries, and 81% reported their radiation therapy history accurately. However, only 19% of participants accurately reported their chemotherapy histories with detail. Baseline accuracy did not differ between those randomized to the intervention versus control groups (chi-square = 0.429, 0.435, and 0.495, respectively, p < .05). Sixty percent of participants did not believe or did not know whether previous treatments they received for cancer could impact their future health. Nonetheless, most (80%) agreed with the statement that they “know what to do” to reduce their risk for late effects. No differences were found in cancer knowledge between those who were closer to having completed treatment (i.e., within 5 years of completing treatment, n = 23) versus long-term survivors (greater than 5 years from having completed treatment, n = 29).
Compared with those who reported that previous treatments they received for cancer could cause serious future health problems, those who did not believe or did not know whether previous treatment could impact future health were more likely to say that their health is left up to chance (HLC: Chance: t(50)=−2.186, p =.03). Among the 20% of survivors who responded that they do not know what they can do to reduce their risk for certain kinds of complications from cancer treatments, these individuals reported higher state (t(50)=−3.22, p = .002) and trait anxiety (t(50)= −3.15, p = .003) and were less likely to report confidence in the role physicians/others play in their health (HLC: Powerful : t(50)=2.86, p = .006) when compared to survivors who reported that they know what to do to reduce their risks for future complications.
Intervention effects on cancer knowledge, anxiety, and health locus of control
The proportion of participants accurately reporting their chemotherapy history and understanding of late effects risk at the time of follow-up survey was examined between intervention and control groups using logistic regression. The proportion of participants who were accurate with detail about their chemotherapy histories at follow-up assessment (33%) did not differ significantly by group (intervention or control) after adjusting for baseline knowledge accuracy and there was no significant interaction (OR 3.92, 95% CI 0.69–12.16, p = .12). At follow-up assessment, 46% of the total sample agreed with the statement that previous treatments they received for cancer could impact their future health, and there were no significant differences between treatment and control groups, after adjusting for baseline agreement (OR 0.97, 95% CI 0.26–3.53, p = .91). At follow-up, the vast majority of participants (82%) continued to agree with the statement that they “know what to do” to reduce their risk of late effects, which also did not differ between groups after adjusting for baseline response (OR 0.87, 95% CI 0.09–8.43, p = .90).
Mixed design MANOVAs were used to examine whether the two groups (web-intervention or standard of care) differed over time (between baseline and post-intervention) on anxiety (state and trait) and health locus of control (internalizing, chance, and powerful others). Means and standard deviations for survey measures of anxiety and health locus of control scales are reported in Table 2. At both time points, mean scores for both state and trait anxiety were within the average range, consistent with population norms. The results showed no significant time by group interaction, reflecting no differences between intervention and control groups on anxiety (F(2,35) = 0.67, p = .52, η2 = .037) or health locus of control scales (F(3,34) = 0.23, p = .88 , η2 = .020). Univariate tests (reported in Table 2) also indicated there was no intervention effect on any specific anxiety or health locus of control scale.
Table 2.
Pre-Post Survey Measurements: Intervention and Control Groups
| Intervention | Control | ||||
|---|---|---|---|---|---|
| Baseline M (SD) | Follow-Up M (SD) | Baseline M (SD) | Follow-Up M (SD) | Statistical Test | |
| STAI: State | 43.78 (10.1) | 42.28 (8.67) | 44.10 (7.97) | 45.95 (13.1) | F(1,36) = 1.36 p=.26, η2 =.04 |
| STAI: Trait | 44.17 (8.99) | 43.94 (8.36) | 47.65 (9.32) | 49.45 (12.24) | F(1,36) = 0.72 p=.40, η2 =.02 |
| HLC: Internal | 25.24 (5.64) | 24.78 (5.35) | 25.41 (5.67) | 24.40 (4.55) | F(1,36) = 0.18, p=.68, η2 =.005 |
| HLC: Chance | 18.17 (5.50) | 16.78 (5.14) | 18.11 (4.14) | 17.6 (4.42) | F(1,36) = 0.38 p=.54, η2 =.01 |
| HLC: Powerful | 18.72 (5.03) | 17.61 (6.52) | 18.9 (3.93) | 18.12 (5.76) | F(1,36) = 0.04 p=.84, η2 =.001 |
Note. STAI = State Trait Anxiety Inventory; HLC = Health Locus of Control
As this was a pilot and feasibility trial, we were not powered to detect small changes in cancer knowledge or psychological constructs as a result of the intervention. A full-scale trial would need 120 participants (60 in each group) for statistical power of 0.80 with alpha at 0.05 for this two-group, repeated measures design. Nonetheless, the examination of patterns of cancer knowledge, anxiety, and health locus of control between intervention and control groups over the duration of the RCT, combined with information regarding participation rates and intervention engagement (described below), are important components of evaluating the feasibility and potential efficacy of this intervention.
Intervention engagement (website use and satisfaction)
Only forty six percent of the sample randomized to receive access to the website logged in. Of those who did, 1/3 used the site more than once. Average length of total time spent on the website was 13 minutes (range: 1–69 minutes). Within the intervention group, there were also no differences in actual website use or user-reported satisfaction between those who were closer to (within 5 years of having completed treatment) or further from (greater than 5 years) treatment (p > .05). To identify possible barriers to engagement in the intervention and understand who chose to use the website, we examined baseline anxiety and health locus of control ratings of users and non-users. Results revealed that there were no differences in baseline state or trait anxiety (t(16) = 0.33 and 1.56, respectively, p > .05). However, ratings of those who chose not use the website were somewhat higher on a health locus of control scale reflecting the perceived important role powerful others play in one’s health (t(16) = 2.83, p = .05). User satisfaction rates were high; 100% reported the site was “easy” or “very easy” to use and 71% reported being “satisfied” or “very satisfied” with the site. The treatment summary was the most visited part of the website followed by links to other outside sites for cancer survivors and then links to specific health-related information.
Discussion
An understanding of the potential for late effects of cancer and its treatment is important, as survivors require ongoing medical follow-up care matched to their unique risks and health needs. While prior studies have documented deficits in cancer knowledge and understanding of late effects risks in long-term adult survivors of childhood cancer, knowledge in AYA survivors has been infrequently examined. A number of educational modalities exist to potentially close this knowledge gap in childhood cancer survivors (e.g., written material, audio-visual material, computer-delivered material) but very few have been empirically examined in randomized controlled trials[36]. In this study we examined cancer knowledge in AYA survivors and developed and pilot tested a HIPAA-compliant, password protected website to provide individually tailored information regarding late effects risks and resources to AYA cancer survivors in a small randomized controlled trial.
Regardless of randomization arm, less than half of our participants recognized that previous cancer treatments could impact their future health and the majority failed to learn important details of their chemotherapy history that have been linked to late cardiac risks over the one year duration of the study. Although this sample’s knowledge of late health risks stemming from cancer treatment (46% accurate at follow-up across intervention and control groups) is somewhat better than previous studies would suggest (e.g., 35%[3]), there continue to be a substantial number of survivors who have not yet made the connection between their history of cancer treatment and future health despite routine participation in follow-up medical care and access to this web-based resource.
Across the sample as a whole, we found that anxiety and health beliefs were associated with survivors’ knowledge about cancer, including knowledge of steps survivors could take to mitigate risks for late effects. Specifically, those who did not believe that previous treatments they received for cancer could cause serious future health problems or did not know whether previous treatment could impact future health were more likely to say that their health is left up to chance. Among the 20% of survivors at baseline who responded that they do not know what they can do to reduce their risk for certain kinds of complications from their cancer treatments, these individuals reported higher state and trait anxiety and were less likely to report confidence in the role physicians and others play in their health when compared to survivors who reported that they know what to do to reduce their risks for future complications. Similarly, having a poor or inaccurate understanding of one’s cancer treatment history and late effects risk may also contribute to anxiety.
Regarding intervention efficacy, less than half of the participants randomized to receive access to the website used this resource. This low utilization rate over the duration of the study, combined with our already small sample size, is a major limitation and makes it difficult to draw conclusions regarding the potential for this web-based resource to improve cancer knowledge. Nonetheless, it is clear that merely making this personalized web resource available to AYA survivors and orienting them to how to use and learn from this resource did not sufficiently activate them to engage in this learning process independently. Participation could perhaps have been enhanced through periodic text or email messages to encourage survivors to log in to the site, and while offering such reminders may improve use, it may not be sufficient to overcome anxiety enhance motivation or receptivity for learning such information. Future studies using a supportive accountability approach[37] (an approach that offers periodic support from clinicians or coaches via telephone, email or chat rooms accompanying an eHealth intervention) and incorporates motivational interviewing techniques to resolve cognitive dissonance and address anxiety may result in improved use and engagement in the website and thus a better test of efficacy. It is also possible that tailoring the mode of information delivery (e.g., auditory/visual) to the specific preferences of individual participants could have improved their engagement in and use of the website. Perhaps most importantly, the information provided through the website may be most relevant to survivors when they encounter a medical problem or visit a provider who is not aware of their cancer history; thus the duration of the study may not have been sufficient to gauge the true value of this type of personalized, web-based resource over the long-term. Additionally, we did not have information about the presence/number of current chronic health conditions (e.g., endocrine dysfunction, cardiovascular disease), which could also influence motivation to use the website.
This study must be understood in the context of other potential limitations. As this was a pilot study, we were not powered to detect small changes in self-reported knowledge or psychological constructs (i.e., health locus of control, anxiety). We were also not able to examine whether particular diagnostic groups are more vulnerable to knowledge deficits or determine when in the course of survivorship AYAs are most amenable to receiving tailored information about ongoing health-risks and ways to mitigate these risks (e.g., physical activity, dietary changes, sunscreen use). These topics warrants further exploration, as the complexity of cancer treatments and late effects risks varies markedly across cancer types, and may differentially impact knowledge within certain diagnostic groups (e.g., leukemia protocols which involve multiple combinations of chemotherapy agents).
Our measure of knowledge of past treatment, while an improvement over some prior studies that relied on self-report of patient’s understanding in the absence of medical records to check accuracy, also has limitations. Specifically, participants in this study were only asked to recall specific chemotherapy agents and not mode of delivery (e.g., intrathecal versus systemic), which is also known to contribute to risk for certain types of late effects (e.g., neurocognitive). It should also be noted that our sample consisted of survivors who were relatively well-connected to the health care system, in that they had up-to-date contact information on file and had attended an oncology appointment within the past two years, and thus may represent a more knowledgeable group than other AYA survivors who are not actively followed by an oncology provider and may have more limited access to health-related information. Finally, our sample was also predominantly white/non-Hispanic and minority survivors were under-represented. Future studies assessing cancer knowledge in more diverse samples will be important to determine whether factors associated with knowledge about cancer and late effects risks differ across racial/ethnic groups, warranting tailored intervention approaches.
Knowledge gaps exist among AYA survivors regarding potentially important aspects of their treatment histories. While providing patients with survivorship care plans confer needed information about their cancer history, late effects risks, and recommendations for ongoing surveillance, our findings suggest that this information alone (whether provided via the web or in routine clinical care) is not sufficient. Interventions targeting health beliefs and anxiety (e.g., cognitive-behavioral strategies) and incorporating motivational interviewing techniques (an approach designed to help individuals resolve ambivalence by tapping into their intrinsic motivation and values) may be additional avenues through which cancer knowledge and health behaviors could be improved. Future studies with larger sample sizes and racially/ethnically representative samples are needed to understand the efficacy of such programs and when in the course of survivorship is the best time to intervene.
Acknowledgments
This project was supported by a grant from the Lance Armstrong Foundation. Dr. Jeanne Steele was supported by a postdoctoral research traineeship in Pediatric Cancer Epidemiology [NIH T32 CA099936]. The authors wish to acknowledge the contributions of our project coordinator, Christine Jacox and website developer, Sandeep Kataria, who made substantial contributions to the study.
Appendix A: Self-Report Survey of Cancer Knowledge
| 1. | What type of cancer were you diagnosed with? Please be as specific as possible. | |||||||
| ______________________________________________________________________________ | ||||||||
| 2. | Did you receive chemotherapy? | [Circle one] | Yes | No | Don’t know | |||
| a. If yes, what chemotherapy drugs did you receive? Please be as specific as possible and list all you can recall. | ||||||||
| ______________________________________________________________________________ | ||||||||
| 3. | Did you receive radiation therapy? | [Circle one] | Yes | No | Don’t know | |||
| a. If yes, to what parts of your body did you receive radiation treatment? | ||||||||
| ______________________________________________________________________________ | ||||||||
| 4. | Did you have any surgeries as part of your treatments? | |||||||
| [Circle one] | Yes | No | Don’t know | |||||
| a. If yes, what types of surgeries? Please list below. | ||||||||
| ______________________________________________________________________________ | ||||||||
| 5. | Do you feel that previous treatments you received for cancer could cause serious future health problems? | |||||||
| [Circle one] | Yes | No | Don’t know | |||||
| ______________________________________________________________________________ | ||||||||
| 6. | I know what I can do to reduce my risk for certain kinds of complications from my cancer treatments. | |||||||
| Strongly Disagree | 1 | 2 | 3 | 4 | 5 | 6 | Strongly Agree | |
Footnotes
Statement of prior presentation: Portions presented in abstract form at the 12th International Conference on Long-Term Complications of Treatment of Children and Adolescents with Cancer, Williamsburg, VA, June 2012.
Authorship Contribution: A.K-B. performed research, analyzed data, interpreted study results, and prepared the manuscript. J.S. and A.M. designed research, interpreted study results, and reviewed and edited the manuscript; J.P.N. designed and performed research, interpreted study results, and prepared and reviewed the manuscript.
Disclosure of Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. National Academies Press; 2005. [Google Scholar]
- 2.Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, Willis A, Gansler T, Ganz PA, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center. CA: a cancer journal for clinicians. 2013;63(3):147–50. doi: 10.3322/caac.21183. [DOI] [PubMed] [Google Scholar]
- 3.Kadan-Lottick NS, Robison LL, Gurney JG, Neglia JP, Yasui Y, Hayashi R, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA : the journal of the American Medical Association. 2002;287(14):1832–9. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 4.Ford JS, Chou JF, Sklar CA. Attendance at a survivorship clinic: impact on knowledge and psychosocial adjustment. Journal of Cancer Survivorship. 2013;7(4):535–43. doi: 10.1007/s11764-013-0291-9. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Nathan PC, Kremer L. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Pediatric clinics of North America. 2008;55(1):251–73. doi: 10.1016/j.pcl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2010;102(14):1083–95. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton SE, Najita JS, Ginsburg ES, Leisenring WM, Stovall M, Weathers RE, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. The lancet oncology. 2013;14(9):873–81. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group long-term follow-up guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. Journal of Clinical Oncology. 2004;22(24):4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Henderson TO, Friedman DL, Meadows AT. Childhood cancer survivors: transition to adult-focused risk-based care. Pediatrics. 2010;126(1):129–36. doi: 10.1542/peds.2009-2802. [DOI] [PubMed] [Google Scholar]
- 10.Zebrack BJ. Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117(S10):2289–94. doi: 10.1002/cncr.26056. [DOI] [PubMed] [Google Scholar]
- 11.Kazak AE, DeRosa BW, Schwartz LA, Hobbie W, Carlson C, Ittenbach RF, et al. Psychological outcomes and health beliefs in adolescent and young adult survivors of childhood cancer and controls. Journal of Clinical Oncology. 2010;28(12):2002–7. doi: 10.1200/JCO.2009.25.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hocking MC, Hobbie WL, Deatrick JA, Lucas MS, Szabo MM, Volpe EM, et al. Neurocognitive and family functioning and quality of life among young adult survivors of childhood brain tumors. The Clinical Neuropsychologist. 2011;25(6):942–62. doi: 10.1080/13854046.2011.580284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster RH, Russell CC, Dillon R, Bitsko MJ, Godder K, Stern M. Relations among Optimism, Perceived Health Vulnerability, and Academic, Self-Regulatory, and Social Self-Efficacy in Adolescent Survivors of Childhood Cancer. Journal of psychosocial oncology. 2013 doi: 10.1080/07347332.2013.874000. just-accepted. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MH. Health Promotion in Adolescent and Young Adult Cancer Survivors Mobilizing Compliance in a Multifaceted Risk Profile. Journal of Pediatric Oncology Nursing. 2013;30(3):139–52. doi: 10.1177/1043454213486194. [DOI] [PubMed] [Google Scholar]
- 15.Tonorezos ES, Oeffinger KC. Research challenges in adolescent and young adult cancer survivor research. Cancer. 2011;117(S10):2295–300. doi: 10.1002/cncr.26058. [DOI] [PubMed] [Google Scholar]
- 16.Thompson C, Charlson M, Schenkein E, Wells M, Furman R, Elstrom R, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Annals of oncology. 2010:mdq215. doi: 10.1093/annonc/mdq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long-term (≥ 5 years) cancer survivors—a systematic review of quantitative studies. Psycho-Oncology. 2013;22(1):1–11. doi: 10.1002/pon.3022. [DOI] [PubMed] [Google Scholar]
- 18.Cox CL, Zhu L, Finnegan L, Steen BD, Hudson MM, Robison LL, et al. Survivor profiles predict health behavior intent: the Childhood Cancer Survivor Study. Psycho-Oncology. 2012;21(5):469–78. doi: 10.1002/pon.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melnyk D, Shepperd JA. Avoiding risk information about breast cancer. Annals of Behavioral Medicine. 2012;44(2):216–24. doi: 10.1007/s12160-012-9382-5. [DOI] [PubMed] [Google Scholar]
- 20.Oeffinger KC, Wallace WHB. Barriers to follow-up care of survivors in the United States and the United Kingdom. Pediatric blood & cancer. 2006;46(2):135–42. doi: 10.1002/pbc.20614. [DOI] [PubMed] [Google Scholar]
- 21.Rourke MT, Hobbie WL, Schwartz L, Kazak AE. Posttrauamatic stress disorder (PTSD) in young adult survivors of childhood cancer. Pediatric blood & cancer. 2007;49(2):177–82. doi: 10.1002/pbc.20942. [DOI] [PubMed] [Google Scholar]
- 22.Schulman D, Bickmore TW, Sidner CL, editors. An Intelligent Conversational Agent for Promoting Long-Term Health Behavior Change Using Motivational Interviewing. AAAI Spring Symposium: AI and Health Communication; 2011. [Google Scholar]
- 23.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive therapy and research. 2012;36(5):427–40. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bingen K, Kupst MJ. Evaluation of a survivorship educational program for adolescent and young adult survivors of childhood cancer. Journal of Cancer Education. 2010;25(4):530–7. doi: 10.1007/s13187-010-0077-y. [DOI] [PubMed] [Google Scholar]
- 25.Adolescent, Group YAOPR. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and the LIVESTRONG Young Adult Alliance; Bethesda, MD: 2006. (NIH Publication No. 06–6067) [Google Scholar]
- 26.Zebrack BJ, Mills J, Weitzman TS. Health and supportive care needs of young adult cancer patients and survivors. Journal of Cancer Survivorship. 2007;1(2):137–45. doi: 10.1007/s11764-007-0015-0. [DOI] [PubMed] [Google Scholar]
- 27.Bleyer A, O'leary M, Barr R, Ries L. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. 2006 [Google Scholar]
- 28.Eshelman D, Landier W, Sweeney T, Hester AL, Forte K, Darling J, et al. Facilitating care for childhood cancer survivors: integrating children’s oncology group long-term follow-up guidelines and health links in clinical practice. Journal of Pediatric Oncology Nursing. 2004;21(5):271–80. doi: 10.1177/1043454204268875. [DOI] [PubMed] [Google Scholar]
- 29.Children's Oncology Group. Long Term Follow-Up Guideliens for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 4.0. Monrovia, CA: Children's Oncology Group; 2013. [Google Scholar]
- 30.Spielberger CD. State-Trait Anxiety Inventory. Wiley Online Library; 2010. [Google Scholar]
- 31.Wallston KA. The validity of the multidimensional health locus of control scales. Journal of Health Psychology. 2005;10(5):623–31. doi: 10.1177/1359105305055304. [DOI] [PubMed] [Google Scholar]
- 32.Booth-Butterfield M, Anderson RH, Booth-Butterfield S. Adolescents' use of tobacco, health locus of control, and self-monitoring. Health communication. 2000;12(2):137–48. doi: 10.1207/S15327027HC1202_2. [DOI] [PubMed] [Google Scholar]
- 33.Regis D, Macgregor I, Balding J. Differential prediction of dental health behaviour by self-esteem and health locus of control in young adolescents. Journal of clinical periodontology. 1994;21(1):7–12. doi: 10.1111/j.1600-051x.1994.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 34.Wallston KA, Wallston BS. Health locus of control scales. Research with the locus of control construct. 1981;1:189–243. [Google Scholar]
- 35.Brinkerhoff J. Effects of a Long-Duration, Professional Development Academy on Technology Skills, Computer Self-Efficacy, and Technology Integration Beliefs and Practices. Journal of Research on Technology in Education. 2006;39(1) [Google Scholar]
- 36.Friedman AJ, Cosby R, Boyko S, Hatton-Bauer J, Turnbull G. Effective teaching strategies and methods of delivery for patient education: a systematic review and practice guideline recommendations. Journal of Cancer Education. 2011;26(1):12–21. doi: 10.1007/s13187-010-0183-x. [DOI] [PubMed] [Google Scholar]
- 37.Mohr DC, Cuijpers P, Lehman K. Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. Journal of medical Internet research. 2011;13(1) doi: 10.2196/jmir.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]