Abstract
Liver fibrosis results from chronic injury of hepatocytes and activation of Collagen Type I producing myofibroblasts that produce fibrous scar in liver fibrosis. Myofibroblasts are not present in the normal liver but rapidly appear early in experimental and clinical liver injury. The origin of the myofibroblast in liver fibrosis is still unresolved. The possibilities include activation of liver resident cells including portal fibroblasts, hepatic stellate cells, mesenchymal progenitor cells, and fibrocytes recruited from the bone marrow. It is considered that hepatic stellate cells and portal fibroblasts are the major source of hepatic myofibroblasts. In fact, the origin of myofibroblasts differs significantly for chronic liver diseases of different etiologies, such as cholestatic liver disease or hepatotoxic liver disease. Depending on etiology of hepatic injury, the fibrogenic foci might initiate within the hepatic lobule as seen in chronic hepatitis, or primarily affect the portal areas as in most biliary diseases. It has been suggested that activated portal fibroblasts/myofibroblasts work as “myofibroblasts for cholangiocytes” while hepatic stellate cells work as “myofibroblast for hepatocytes”. This review will focus on our current understanding of the activated portal fibroblasts/myofibroblasts in cholestatic liver fibrosis.
Keywords: Portal fibroblasts, myofibroblasts, bile duct ligation, cholestatic liver fibrosis
I. Introduction
Liver fibrosis and cirrhosis are the common outcomes of chronic liver diseases. Liver cirrhosis is characterized by the deposition of extracellular matrix proteins, composed mostly of Collagen Type I, formation of fibrous scar, and loss of liver function. There is no curative therapy for advanced liver cirrhosis, often liver transplantation is the only treatment available for these patients. Dependent on the etiology, liver fibrosis is caused by cholestatic liver injury (obstruction of biliary tract) such as primary sclerosing cholangitis, primary biliary cholangitis, secondary biliary cirrhosis and biliary atresia, or hepatotoxic injury (such as hepatitis B virus infection, hepatitis C virus infection, alcoholic liver disease and non-alcoholic steatohepatitis (NASH)). Despite the differences in etiology, development of liver fibrosis is associated with several early events that play an important role in the pathogenesis of liver fibrosis, including: 1) damage to hepatic epithelial (hepatocytes and cholangiocytes) and endothelial cells; 2) release of transforming growth factor-β1 (TGF-β1), the major fibrogenic cytokine; 3) increase of intestinal permeability and endogenous bacterial products; 4) recruitment of inflammatory cells; 5) induction of reactive oxygen species; and 6) generation of extracellular matrix producing myofibroblasts, which are not present in the normal liver . Hence, myofibroblasts represent a primary target for antifibrotic therapy.
Immunophenotypically, myofibroblasts are characterized by expression of abundant pericellular matrix proteins (vimentin, α-smooth muscle actin (α-SMA), non-muscle myosin, fibronectin)(Eyden, 2008). Ultrastructurally, myofibroblasts are identified by a rough endoplasmic reticulum, a Golgi apparatus producing collagen, peripheral myofilaments, fibronexus (no lamina), and gap junctions (Eyden, 2008). Studies of fibrogenesis conducted in different organs implicated myofibroblasts in wound healing and fibroproliferative disorders (Gabbiani et al., 1971; Majno et al., 1971; Schurch et al., 1998), suggesting that myofibroblasts are the primary source of extracellular matrix. Several sources of myofibroblasts have been identified in the liver: liver resident cells (Hepatic stellate cells, HSCs, and portal fibroblasts, PFs); cells originated by epithelial-to-mesenchymal transition (EMT) and bone marrow-derived cells (fibrocytes and mesenchymal cells)(Iwaisako et al., 2014; Kisseleva et al., 2006; Nitta et al., 2008; Scholten et al., 2011). Fibrocytes were shown to contribute to 3–6% of collagen Type I expressing cells in fibrotic liver, suggesting that fibrocytes are not a significant source of extracellular matrix (Kisseleva et al., 2006). Furthermore, the contribution of EMT to liver fibrosis is still controversial. Recently, Lua et al. reported that proliferating cholangiocytes in response to bile duct ligation express collagen, which means EMT of cholangiocytes (Lua et al., 2016). But several cell fate mapping of hepatic epithelial progenitors, hepatocytes, and cholangiocytes failed to detect the presence of EMT-derived myofibroblasts in the livers following cholestatic or hepatotoxic liver injury (Chu et al., 2011; Scholten et al., 2010; Taura et al., 2010).
Despite the extensive studies, there is still an ongoing discussion regarding which cell types can give rise to the hepatic myofibroblasts in response to chronic liver injury. Still “the primary suspects”, as the major source, are the resident mesenchymal cells of the liver (Iwaisako et al., 2014; Mederacke et al., 2013; Wells, 2014); Hepatic Stellate Cells (HSCs) which have been extensively studied and aPFs/myofibroblasts which are less well characterized due to the difficulties in isolation and culturing. HSCs and aPFs/myofibroblasts have been reported to comprise > 90% of the collagen expressing cells (Iwaisako et al., 2014; Kisseleva et al., 2006), suggesting that they are the major source of collagen expressing cells in fibrotic liver. HSCs are generally accepted as major contributors to liver fibrosis that give rise to hepatic myofibroblasts in response to toxic liver injury. aPFs/myofibroblasts, on the other hand, have been implicated in pathogenesis of cholestatic liver fibrosis (Iwaisako et al., 2014). While experimental data validates that both HSCs and PFs can activate into myofibroblasts, the contribution of aPFs /myofibroblasts versus aHSCs to cholestatic liver fibrosis remains controversial and requires thorough examination, including lineage tracing experiments, identification and characterization of cell specific markers, and generation of new transgenic mice to study the functional properties of identified markers.
In the normal non-fibrotic liver, hepatic myofibroblasts become locally and transiently activated in response to bacterial infection. During wound healing, hepatic myofibroblasts apoptose upon completion of repair process (Iredale et al., 1998; Kisseleva et al., 2012). Therefore, pathogenic hepatic fibrosis could be viewed as a chronic state of hepatic myofibroblast activation and finding ways to terminate that activation or induce apoptosis in those aberrantly activated myofibroblasts may be the key to arresting hepatic fibrosis.
Recent studies provide potential experimental models of fibrosis reversal upon cessation of liver injury, or successful pharmacological treatment of underlying causative liver injury (Iredale et al., 1998). Experimental reversal of liver fibrosis has been closely associated with disappearance of hepatic myofibroblasts (Iredale, 2001; Iredale et al., 1998). The mechanism of hepatic myofibroblast disappearance during regression of liver fibrosis in these experiments has been suggested for aHSC-derived myofibroblasts, but remains unknown for myofibroblasts originated from aPFs /myofibroblasts. Thus, 50% aHSC-derived myofibroblasts undergo senescence (Schrader et al., 2009) and concomitant apoptosis (Iredale, 2001, 2007; Iredale et al., 1998) during regression of liver fibrosis. The cell-fate mapping-based studies have demonstrated that 50% of aHSCs survive during regression of liver fibrosis, and obtain “inactivated” phenotype (iHSCs). iHSCs downregulate myofibroblast-specific genes, such as Collagen Type I, α-SMA. Spp1, TIMP1, and others, and upregulate some of genes associated with quiescent phenotype in qHSCs, therefore reverting to a quiescent-like state (Kisseleva and Brenner, 2008; Troeger et al., 2012). Clearly, aHSC-derived myofibroblasts are attractive primary targets for anti-fibrotic therapy (Friedman and Bansal, 2006; Ghiassi-Nejad et al., 2013). Like aHSCs, aPFs /myofibroblasts can give rise to activated myofibroblasts that drive hepatic fibrosis and therefore may offer another anti-fibrotic therapy target particularly in the case of cholestatic injury. Little is known about pathways of PF activation due to difficulties of isolating and characterizing aPFs /myofibroblasts. Also, it remains unknown if aPFs /myofibroblasts can "inactivate" during regression of cholestatic fibrosis. This review will summarize the most recent evidence supporting (or objecting) the contribution of aPFs /myofibroblasts to cholestatic liver fibrosis, and discuss their potential functional properties.
II. Hepatic myofibroblasts
Hepatic Stellate Cells (HSCs)
In the normal liver, HSCs that reside in the space of Disse which is the space between hepatocyte cluster and sinusoidal endothelial cells, account for about 5–8% of cells (Lepreux and Desmouliere, 2015). HSCs in the healthy liver display a quiescent phenotype, and express neural markers (glial fibrillar acidic protein (GFAP), synaptophysin (Bataller and Brenner, 2005), nerve growth factor (NGF) receptor p75 (Kendall et al., 2009; Sachs et al., 2007)) and desmin. qHSCs also serve as the major storage of vitamin A within the normal liver (Geerts, 2001; Iredale, 2007; Senoo et al., 2007). Due to the close association of HSCs with endothelial cells they function as pericytes for liver sinusoids. In response to injury, qHSCs decrease vitamin A lipid droplets and undergo activation to become collagen type I-producing myofibroblasts (Fallowfield et al., 2007; Kisseleva and Brenner, 2006). Following chronic injury induced by CCl4 treatment, a large number of HSC-derived myofibroblasts rapidly proliferate and accumulate around central veins (Lukita-Atmadja and Subowo, 1993; Seifert et al., 1994), giving rise to a population of centrilobular myofibroblasts comprising up to 14% of total liver cells (Kisseleva et al., 2012).
Activated portal fibroblasts/myofibroblasts
Portal fibroblasts (PFs) normally comprise a small population of the fibroblastic cells that surround the portal vein to maintain integrity of portal tract. They were first described as “mesenchymal cells not related to sinusoids”, and since then were called “periductular fibroblasts” or portal/periportal mesenchymal cells” and are now associated with the pathogenesis of cholestatic liver injury (Beaussier et al., 2007). In response to chronic injury, PFs become activated in α-SMA-expressing myofibroblasts and may proliferate, and synthesize extracellular matrix (Tuchweber et al., 1996), which here we call aPFs/myofibroblasts. PFs are characterized by the expression of ectonucleotidase 2 (NTPDase 2), but the expression is down regulated when they become myofibroblasts in biliary cirrhotic livers (Dranoff et al., 2004; Jhandier et al., 2005). Therefore, the origin of portal fibroblasts in the liver fibrogenesis is still controversial. aPFs/myofibroblasts have heterogeneous populations, which are defined as nonHSC-derived myofibroblasts not bone marrow derived myofibroblasts, and characterized by the expression of collagen1a1 and other markers described later.
Activated portal fibroblasts/myofibroblasts contribute to cholestatic fibrosis
There is a growing body of evidence supporting the concept of "etiology-driven cirrhosis" (Pinzani, 2015). Cholestatic liver diseases and hepatotoxic liver diseases show totally different symptoms and phenotypes in their early stages. The former is characterized by jaundice with dominant elevation of alkaline phosphatase and direct bilirubin in the serum, while the latter represents the symptoms of general fatigue and shows dominant elevation of ALT and AST in the serum. Histologically it is possible to identify disease specific patterns of myofibroblast activation dependent on the etiology of the underlying chronic liver injury. For example, portal-central septa are characteristics of chronic viral hepatitis and intercellular fibrosis. On the other hand, the deposition of extracellular matrix around the sinusoids is characteristic of alcoholic and NASH. Proliferation of reactive bile ductules and periductular extracellular matrix deposition are characteristics of primary biliary cirrhosis, primary sclerosing cholangitis, and biliary atresia (Pinzani, 2015). Our group (Iwaisako et al., 2014) and Beaussier et al (Beaussier et al., 2007) have reported that PFs activate at the onset of cholestatic liver injury. However, many questions remain controversial. For example, myofibroblasts, activated during development of portal fibrosis, arise either from HSCs or PFs, the contribution of HSCs and PFs to collagen type I deposition, as well as the kinetics of their activation and proliferation in the damaged liver. For instance, cell fate mapping-based experiments, designed to track activation of HSCs in the fibrotic liver reported by Mederacke et al (Mederacke et al., 2013)., suggest that HSCs are the critical source of hepatic myofibroblasts. The difficulty of the analysis comes from the fact that mouse models of cholestatic disease do not fully recapitulate the course of cholestatic injury in patients. Furthermore, the etiology of cholestatic fibrosis is not well understood, as well as the role of aPFs/myofibroblasts in activation of cholangiocytes and regulation of their proliferation (ductular reaction) and vice versa.
III. New insights into aPF biology
Experimental methods of cholestatic liver injury
The most commonly used mouse model for cholestatic fibrosis is bile duct ligation (BDL), a surgical procedure involving the double ligation of the common bile duct (Symeonidis and Trams, 1957). The pathology resulting from BDL is similar to that seen in human chronic cholestatic disease: initiation of a ductular reaction and activation of myofibroblasts surrounding biliary tracts. Development of advanced cholestatic fibrosis is usually observed in post-BDL mice at day 21. However, complications from surgical intervention and the severity of cholestatic injury in mice, especially genetically altered mice, are often associated with increased mortality and limit the long-term use of BDL in mice.
Another model of cholestatic fibrosis is caused by exposure to Methapyrilene. Methapyrilene [N,N-dimethyl-N’-pyridyl-N’’ (2-thienylmethyl- 1,2- ethanediamine] is an histamine-1 receptor antagonist that was used as a sedative and anti-histamine until its withdrawal in the late 1970s, when it was demonstrated to promote hepatocellular carcinoma in chronically-dosed rats (Lijinsky et al., 1980; Probert et al., 2014). Subsequent works demonstrated that Methapyrilene acts as a periportal hepatotoxin in rats. Methapyrilene is bio-activated in the liver by cytochromes P450, leading to the production of reactive oxygen species intermediates. The intermediates cause hepatic inflammation in periportal regions, proliferation of cholangiocytes (ductular reaction), expansion of aPFs/myofibroblasts in biliary tract area, and concomitant development of liver fibrosis. Immunohistochemical evaluation of aPFs/myofibroblasts in the Methapyrilene model needs to be performed. The histological changes that accompany Methapyrilene -mediated liver fibrosis resemble cholestatic fibrosis similar to that observed in BDL model in mice (Probert et al., 2014). Although this model has been originally designed for rats, potentially it could be adopted to mice, allowing utilization of the variety of transgenic and knockout mice to study pathogenesis of cholestatic fibrosis. Methapyrilene is administered intraperitoneally; the duration of the injury is about 21 days. Modulation of the regimen and/or dose of the Methapyrilene administration may provide a better insight in the pathogenesis of cholestatic liver fibrosis, and mimic the stages of cholestatic fibrosis in mice. In addition, Methapyrilene model might become a useful tool to study reversibility of cholestatic fibrosis, as an alternative to reversal of BDL-injury in mice (which requires a very sophisticated and technically challenging surgery to reverse obstruction of the common bile duct) and is currently performed only by limited number of laboratories.
The multidrug resistance gene 2 knockout mouse (mdr2−/−) represents an established animal model for chronic cholestatic disorders (Fickert et al., 2004), Mice deficient in the canalicular phospholipid flippase (Mdr2/Abcb4−/− mice) spontaneously develop liver injury (Fickert et al., 2004). Hepatic injury in Mdr2−/− mice resembles progressive familial intrahepatic cholestasis type 3 with which mutations of human ortholog gene multidrug resistance 3 (MDR3) are associated and may recapitulate some aspects of the pathogenesis of primary sclerosing cholangitis (Colombo et al., 2011; de Vree et al., 1998; Degiorgio et al., 2007; Delaunay et al., 2009; Deleuze et al., 1996; Trauner et al., 2010). The pathogenesis of liver injury in Mdr2−/− mice is characterized by disruption of tight junctions and basement membranes of bile ducts, bile leakage into the portal tract, and formation of periportal biliary fibrosis (Fickert et al., 2004). These mice also suffer from the absence of phosphatidylcholine from bile, which disrupts the formation of mixed micelles and subsequently leads to “toxic bile” triggering an inflammatory cascade (Miethke et al., 2016; Smit et al., 1993). These mice show myofibroblasts restricted to bile duct areas, while no myofibroblasts are observed in the hepatic lobule (Strack et al., 2011). The majority of the myofibroblasts come from aPFs/myofibroblasts (Baghdasaryan et al., 2010), but bone marrow derived fibrocytes may be involved in the pathogenesis (Strack et al., 2011). This model has been used for novel therapies for chronic cholestasis, targeting anti-cholestatic, anti-inflammatory and antifibrogenic pathways (Baghdasaryan et al., 2010; Miethke et al., 2016; Stiedl et al., 2015; Strack et al., 2011).
Methods of identification and purification of aPFs/myofibroblasts
The contribution of aPFs/myofibroblasts to liver fibrosis of different etiologies is not well understood, mainly because of the difficulties in the isolation of PFs and myofibroblasts, e.g. cell outgrowth from dissected bile segments (Uchio et al., 2002), enzymatic digestion of the biliary tree followed by size selection (Kruglov et al., 2002; Wen et al., 2012). Unfortunately, this techniques require multiple passaging and prolonged culturing (up to 2–3 weeks) which may yield outgrowth of myofibroblasts of non-aPFs/myofibroblasts origin (Lepreux and Desmouliere, 2015), activation of myofibroblasts derived by plastic-mediated EMT (Taura et al., 2010), or simply change the original phenotype of aPFs/myofibroblasts (Dranoff and Wells, 2010). A more physiological method of studying PFs is precision-cut liver slices, designed to maintain cell-cell and cell-matrix interactions and mimic the natural microenvironment of PFs, but this does not enable the study of purified populations of PFs. Only a few markers are available to identify PFs in the myofibroblast population, including Gremlin 1, Thy-1 (Dudas et al., 2007; Knittel et al., 1999a; Yovchev et al., 2009), fibulin 2 (Knittel et al., 1999a), IL-6, elastin (Goodpaster et al., 2008), ecto-AT-Pase nucleoside triphosphate diphosphohydrolase-2 (NTPD2) (Dranoff et al., 2002), collagen XV (Lemoinne et al., 2015), and coffilin 1 (Bosselut et al.). In addition, the lack of desmin, cytoglobin, GFAP, p75NGFr, and Vitamin A distinguishes PFs from HSCs (Bataller and Brenner, 2005; Dranoff and Wells, 2010; Fausther and Dranoff, 2011). Identification of additional PF markers will advance our understanding of the pathogenesis of liver fibrosis.
We have recently developed a novel method of aPFs/myofibroblasts purification using a reporter mice called Collagen-GFP mice in which GFP is expressed under the control of Collagen I(α)1 promoter/enhancer, in which all cells expressing Collagen Type I in real time also upregulate GFP. Collagen-GFP mice were generated in 1999 (Krempen et al., 1999) and now are widely used by many investigators in the US and other countries. Using GFP as marker of activated myofibroblasts in the injured liver, we can identify and separate all collagen Type I expressing myofibroblasts by flow cytometry (detected at 488 nm wave length, FL1) from hepatic nonparenchymal cell fraction (that includes HSCs, aPFs/myofibroblasts, Kupffer cells, inflammatory and endothelial cells). Moreover, hepatic stellate cells can be identified from the pool of GFP+ myofibroblasts by expression of Vitamin A (which emit autofluorescent signal upon detected at 405 nm wave length by flow cytometry). We have succeeded in separating Vitamin A+ aHSCs and vitamin A-cells from the pool of activated GFP+ myofibroblasts, extensively characterized both cellular populations and confirmed that more than 99% of the GFP+ VitA+ cells express aHSC markers and more than 90% of GFP+ VitA- cell fraction cells express markers of aPFs/myofibroblasts. Flow cytometry-based analysis of Collagen-GFP myofibroblasts allowed us to determine the composition of fibrogenic myofibroblasts dependent on etiology of underlying liver fibrosis and quantify the contribution aPFs/myofibroblasts and aHSCs. In concordance with previous studies, aPFs/myofibroblasts contribute mostly to cholestatic liver fibrosis especially in their early states of the pathogenesis, while aHSCs plays major role in the pathogenesis of hepatotoxic liver fibrosis (Iwaisako et al., 2014), and also proliferate with progression of cholestatic liver injury. More interestingly, pphenotype of HSCs activated in response to BDL has more similarities with BDL-activated PFs rather than HSCs activated in response to toxic liver injury (CCl4) (Iwaisako et al., 2014). Based on the collagen GFP and vitamin A autofluorescence, there have been more reported methods for PFs isolation using gpm6a antibody and GFP intensity to exclude the contamination of mesothelium and cholangiocytes (Lua et al., 2016).
Markers of activated Portal fibroblasts/myofibroblasts
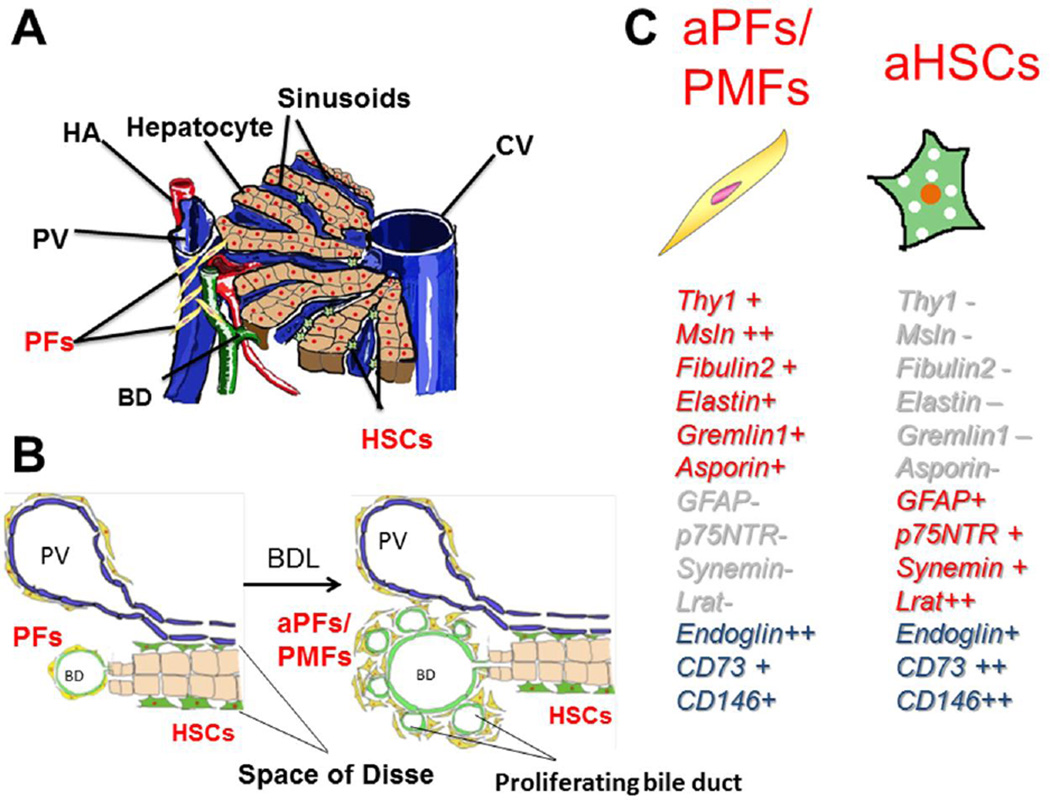
There is no single marker used to identify aPFs /myofibroblasts (Wells, 2014). Thy-1 and Gremlin1 are also expressed by mesenchymal stem cells (Kamo et al., 2007; Worthley et al., 2015), which are reportedly recruited into the liver when it is injured (Li et al., 2013). PFs share CD73 as markers with HSCs (Fausther et al., 2012). The expression of markers in myofibroblasts is summarized in Figure 1.
Figure 1. activated Portal fibroblast / myofibroblasts (aPFs/MFs) and hepatic stellate cells (HSCs).
A, PFs are located around portal triads, while HSCs are located in the space of Disse which is between sinusoidal endothelial cells and hepatocytes cluster. B, Bile ducts proliferate in response to bile duct ligation, known as “ductular reaction”. C, Representative markers are shown in the right panel. -: not expressed, +: expressed, ++: highly expressed, PV: portal vein, CV: central vein, HA: hepatic artery, BD: bile duct, BDL: bile duct ligation.
Thy-1
Thymocyte differentiation antigen-1 (Thy-1) is a glycophosphatidylinositol (GPI)-linked glycoprotein expressed on various cell types, including neurons (Leyton and Hagood, 2014), thymocytes, myofibroblasts (Dezso et al., 2007; Dudas et al., 2009; Dudas et al., 2007), endothelial cells, and mesangial cells. As a GPI-anchored protein, in neurons Thy-1 is present in the outer leaflet of lipid rafts in the cell membrane. Thy-1 has been suggested to interact with G inhibitory proteins, the Src family kinase (SFK) member c-fyn, and tubulin within lipid rafts. Thy-1 is also expressed in thymocytes and T cells, and often considered as a T cell specific marker. In activated fibroblasts, Thy1 was shown to mediate anti-fibrogenic responses via interaction with integrin avb5 (Zhou et al., 2010), induction of PPARγ (Varisco et al., 2012), and inhibition of Smad2/3 and latent TGF-β1 activation (Ramirez et al., 2011; Sanders et al., 2011). Consistently, Thy-1−/− mice have increased susceptibility to lung fibrosis. The role of Thy-1 in activation of aPFs/myofibroblasts has not been studied. Recent studies have also reported that Thy-1 is expressed on mesenchymal cells that exhibit progenitor properties, and therefore may serve as a source of hepatic myofibroblasts in fibrotic liver (Dudakovic et al., 2014).
Fibulin2
Fibulin-2 (FBLN-2) is an extracellular matrix protein of the fibulin family that binds various extracellular ligands and calcium (Pan et al., 1993). Fibulin-2 is present in the basement membrane and stroma of several tissues and may play a role in organ development, particularly during the differentiation of heart, skeletal, and neuronal structures. Knittel et al reported that FBLN2-positive myofibroblasts are detectable in the portal field, vessel walls, and hepatic parenchyma of the normal liver and their number is increased in the septal regions during liver fibrogenesis in rat models (Knittel et al., 1999a). Fibulin-2 is regulated by transforming growth factor beta-1 (TGF- β1) (Piscaglia et al., 2009).
Msln
Mesothelin is a GPI-linked glycoprotein which is upregulated in malignant mesotheliomas (Chang and Pastan, 1996) and mediates intracellular adhesion and metastatic spread (Grigoriu et al., 2009). In adult mice, Msln is expressed only in the mesothelial lining of parenchymal organs (Bera and Pastan, 2000; Rinkevich et al., 2012). Mesothelin gene encodes a 71-kDa precursor protein that is processed to yield 40-kDa mature mesothelin which is attached to the cell membrane by GPI linkage and a 31-kDa shed fragment named megakaryocyte- potentiating factor (MPF) (Chang and Pastan, 1996; Hassan et al., 2004). Msln−/− mice have been generated, and exhibit no obvious abnormalities (Bera and Pastan, 2000). Lineage tracing studies at early embryogenesis have linked expression of mesothelin to precursors of fibroblasts and smooth muscle cells (Rinkevich et al., 2012). In contrast to embryonic mesothelium, adult mesothelin-expressing cells do not proliferate until injury or stress (Rinkevich et al., 2012). However, recent studies suggested that in response to injury, aPFs/myofibroblasts originate from hepatic mesothelium (Asahina et al., 2009; Asahina et al., 2011). The role of Msln in liver fibrosis is not known and the expression is still controversial. Recently, Lua et al described that Msln is only expressed by the liver mesothelium and not a marker for the aPFs/myofibroblasts (Lua et al., 2016).
Elastin
Elastin is the main component of elastic fibers that is formed via cross-linking of soluble tropoelastin monomers into elastin polymers on a preformed microfibril scaffold (Kuang and Goldstein, 2005; Liu et al., 2004). Elastin provides the mechanical support to tissues, and plays an important role in maintaining the structural integrity (Vrhovski and Weiss, 1998). Elastin is expressed by interstitial fibroblasts and by smooth muscle cells in vascular tissues (Kuang and Goldstein, 2005).
Ecto-AT-Pase nucleoside triphosphate diphosphohydrolase-2 (NTPD2)
NTPD2 is first described as portal fibroblasts marker in 2002 by Dranoff (Dranoff et al., 2002). NTPD2 belongs to a family of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) that catalyze the hydrolysis of extracellular NTPs. In the liver, NTPD2 is expressed only in the portal fibroblast compartment and is thought to modulate extracellular ATP levels involved in cholangiocyte nucleotide signaling processes. Recently, Fausther et al have immortalized aPFs/myofibroblasts using NTPD2 (along with CD37) to select aPFs/myofibroblasts. They immortalized them with SV40 large T antigen and establish two aPFs/myofibroblast cell-lines (Fausther et al., 2015), which became a useful tool to study activation and function of aPFs/myofibroblasts.
Gremlin 1
Gremlin1, previously called as Drm, is a highly conserved 25-kDa, 184 amino acid glycoprotein. Gremlin1 is a secreted antagonist of bone morphogenetic protein (Bmp) -2, -4, and -7 and a VEGFR2 agonist (Church et al., 2015; Merino et al., 1999). Gremlin is also identified as a marker of skeletal stem cells which contribute to the embryogenesis of bone, cartilage and reticular stromal (Worthley et al., 2015). Gremlin directly, by a TGF-β independent process, activates the Smad pathway (Ma et al., 2014; Rodrigues-Diez et al., 2014). Gremlin1 has been identified as a marker of myofibroblasts in pulmonary (Koli et al., 2006), cardiac (Mueller et al., 2013), pancreatic (Staloch et al., 2015), renal (Carvajal et al., 2008; Droguett et al., 2014) and hepatic fibrosis (Zhao et al., 2014). Gremlin 1 was identified in aPFs/myofibroblasts, and its expression is localized around fibrotic septa in fibrotic livers (Ogawa et al., 2007).
CD73
CD73 also called ecto-5′-nucleotidase (ecto-5′-NT) is a GPI-linked 70-kDa cell surface enzyme found in many different cells (Yamashita et al., 1998). While Cd73 expression is found in many tissue types, Fausther (Fausther et al., 2012) et al. has shown that both hepatic stellate cells and portal fibroblasts show marked up regulation of Cd73 gene expression once activated to become myofibroblasts. Furthermore the up regulation is at the transcriptional level via SMAD and SP1 binding cites within the Cd73 promoter region (Fausther et al., 2012). The transcription factors SMAD and SP1 are known to be in the TGF-β signaling pathway during fibrosis (Ellenrieder, 2008).
Col15A1
Type 15 collagen belongs to the group of non fibrillar collagens, characterized by extensive interruptions in their collagenous sequences and a conserved noncollagenous carboxyl-terminal structure (Kivirikko et al., 1994; Muragaki et al., 1994; Myers et al., 1992). Recently Lemoinne et al have reported that col15A1 is highly upregulated in aPFs/myofibroblasts compared with hepatic stellate cells (Lemoinne et al., 2015). They described that PFs secrete vascular endothelial growth factor (VEGF) A-containing microparticles, which activated VEGF receptor 2 in ECs and largely mediated vascular remodeling.
Response of aPFs/myofibroblasts to cytokines
TGF-β1 is a potent cytokine to activate HSCs into myofibroblasts with increased expression of α-SMA, PDGF, CTGF, type I collagen, tissue inhibitor of metalloproteinase 1 (TIMP1) (Arias et al., 2002; Knittel et al., 1999b; Sysa et al., 2009) . aPFs/myofibroblasts respond rapidly to TGF-β1 by upregulation of Col-α1(I), α-SMA, TIMP1, TGF-β2, PAI-1, elastin, fibronectin, and of CD73 ecto-enzyme (Dranoff and Wells, 2010; Fausther et al., 2012; Knittel et al., 1999a; Li et al., 2007; Wells et al., 2004). FGF-2 was shown to mediate proliferation and migration of aPFs/myofibroblasts (Wells et al., 2004) via binding to its tyrosine kinase receptors FGFRs. We have recently demonstrated that unlike aHSCs, aPFs/myofibroblasts respond to stimulation with taurocholic acid and IL-25 by induction of collagen-α1(I) and IL-13, respectively (Iwaisako et al., 2014).
Osteopontin released by cholangiocytes stimulates PFs proliferation which leads to liver cirrhosis and high portal vein pressure (Pereira et al., 2015). The renin-angiotensin system is known to play a role in the activation of myofibroblasts (Osterreicher et al., 2009). RAS is locally regulated by cholangiocytes and Angiotensin II plays an important role in regulating biliary proliferation and fibrosis during extrahepatic cholestasis (Afroze et al., 2015). Several researchers reported Interleukin-6 (IL-6) as an important mediator of signaling between cholangiocytes and PFs (Yasoshima et al., 1998; Yu et al., 2008). PFs are also characterized by the expression of hyaluronic acid which causes the proliferation of cholangiocytes (He et al., 2008).
E. Functions of activated portal fibroblasts / myofibroblasts
Activated portal fibroblast/myofibroblasts serve as a significant source of extracellular matrix in cholestatic liver injury
We demonstrated that aPFs/myofibroblasts are a major source of myofibroblasts in cholestatic liver injury, contributing >70% of myofibroblasts at the onset of injury (5 days BDL) (Iwaisako et al., 2014). However, the relative contribution of aPFs/myofibroblasts decreases with progressive injury, as HSCs become activated and contribute to the myofibroblast population (14 and 20 days BDL). Since any cholangiocyte injuries are followed by hepatocyte injuries, the results suggest that the initial aPF upregulation correspond to the cholangiocytes injury and aHSC contribution in the later stages correspond to hepatocyte damages followed by the cholangiocyte damage. We have concluded that aPFs/myofibroblasts are the primary responders of cholangiocyte damages and hepatic stellate cells are the primary myofibroblasts, which respond to hepatocyte damages.
Activated portal fibroblast/myofibroblasts affect cholangiocyte physiology
Biliary obstruction causes bile acid accumulation in the liver and serum, liver toxicity, and fibrosis progressing to cirrhosis. The initial reactions of cholangiocytes in the cholestatic injuries are called as “ductular reactions”. “Ductular reactions” are characterized by cholangiocyte proliferation, expansion of transit-amplifying cells or hepatic progenitor cells (HPCs), and differentiation of the biopotential HPCs into cholangiocytes (Kim et al., 2015). These reactions are also observed in human liver diseases (Gouw et al., 2011). Reactive ductules express growth factors such as platelet-derived growth factor, connective tissue growth factor, or TGF-β2, which activate PFs and increase matrix deposition (Grappone et al., 1999; Milani et al., 1991; Sedlaczek et al., 2001). Along sides these epithelial-mesenchymal interactions, myofibroblasts produce both tenascin and type IV collagen, which are key for biliary development and activation (Lepreux and Desmouliere, 2015).
Recently, the cross talk between ductular reactions and surrounding myofibroblasts has been focused. Kim et al reported that hepatic myofibroblasts’ Jag1 expression causes cholangiocyte differentiation and proliferation and that the signal contributes to cholangiocyte injury through JAG/NOTCH signaling pathways (Kim et al., 2015). Ongoing experiments to look at growth hormones and chemokines expressed by cholangiocytes and aPFs/myofibroblasts will shed new light on the complex command and control system by which these communicate within the normal and after biliary injury.
Other than growth factors and chemokines, crosstalk between aPFs/myofibroblasts and cholangiocytes is also mediated by extracellular nucleotides. Dranoff et al reported that Nucleotides, present in the extracellular matrix, bind to the P2Y family receptors on cholangiocytes and stimulate cholangiocyte proliferation. Ectonucleotidase (NTPDase) 2 expressed by aPFs/myofibroblasts hydrolyses the nucleotides to nucleosides which cannot act as effective ligands for the P2Y receptors and blocks the signal for ductal expansion (Dranoff et al., 2002; Jhandier et al., 2005).
aPFs/myofibroblasts and liver architecture
aPFs/myofibroblasts contribute to strength, elasticity and stiffness of liver structures in response to various kind of injuries (Wells, 2014). Unlike hepatic stellate cells, PFs express elastin and fibrillin after bile duct ligation (Wells, 2014). Elastin adds flexablity to tissues (Kielty et al., 2002). Once fibrosis sets in, aPFs/myofibroblasts secrete collagen in addition to elastin. Collagen XV, which was recently identified as a aPFs/myofibroblast marker, is a structural collagen that underlies blood vessels and maintains basement membrane integrity (Lemoinne et al., 2015). PFs are located to a close proximity to cholangiocytes, therefore, they are also involved in the maintenance of the integrity of the biliary tree, and regulate cholangiocyte proliferation under physiological conditions and in response to injury, and maintain cholangiocyte polarity (He et al., 2008; Jhandier et al., 2005; Tanimizu et al., 2012).
aPFs/myofibroblasts are related to angiogenesis in the liver
The contribution of aPFs/myofibroblasts on angiogenesis is not well understood. Recently Lemoinne et al reported that aPFs/myofibroblasts promote vascular remodeling which lead to liver cirrhosis. aPFs/myofibroblasts release vascular endothelial growth factor (VEGF) A containing microparticles, which activated VEGF receptor2 in endothelial cells and mediated their proangiogenic and tubulogenesis effects (Lemoinne et al., 2015). Further studies from the viewpoint of cross talk between PFs and endothelial cells may dramatically increase our understanding of cholestatic liver fibrogenesis.
IV Conclusions
There is a growing body of evidence that aPFs/myofibroblasts play a critical role in the pathogenesis of cholestatic (versus toxic) liver fibrosis. However, functional properties of aPFs/myofibroblasts and the mechanism by which aPFs/myofibroblasts contribute to cholestatic fibrosis, e.g. ductular reaction, angiogenesis, and extracellular matrix deposition are not well understood. Therefore, generation of new tools, such as aPFs/myofibroblasts specific transgenic and knockout mice, generation of aPF-cell lines, are urgently needed to further characterize aPF biology, the mechanisms of intracellular signaling, regulation of activation and proliferation. Based on the previous studies, proliferation and activation of aPFs/myofibroblasts is specifically important at the onset of cholestatic injury, which makes aPFs/myofibroblasts an attractive target for anti-fibrotic therapy in patients with primary and secondary biliary fibrosis, and biliary atresia.
Acknowledgments
Grant support: Supported by the National Institutes of Health (DK088837, GM41804, AA15055, and DK72237), Japanese Ministry of Health, Labour and Welfare and the American Liver Foundation.
Abbreviations
- Col
collagen α1(I)
- α-SMA
α-smooth muscle actin
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- qHSCs
quiescent Hepatic Stellate Cells
- aHSCs
activated Hepatic Stellate Cells
- aPFs/myofibroblasts
activated portal fibroblasts /myofibroblasts
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TGF-β1
Transforming growth factor- β1
- GFP
green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afroze SH, Munshi MK, Martinez AK, Uddin M, Gergely M, Szynkarski C, Guerrier M, Nizamutdinov D, Dostal D, Glaser S. Activation of the renin-angiotensin system stimulates biliary hyperplasia during cholestasis induced by extrahepatic bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 2015;308:G691–G701. doi: 10.1152/ajpgi.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias M, Lahme B, Van de Leur E, Gressner AM, Weiskirchen R. Adenoviral delivery of an antisense RNA complementary to the 3' coding sequence of transforming growth factor-beta1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 2002;13:265–273. [PubMed] [Google Scholar]
- Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Jr, Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009;49:998–1011. doi: 10.1002/hep.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thuringer A, Leski K, Fickert P, Karpen SJ, Trauner M. Curcumin improves sclerosing cholangitis in Mdr2−/− mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. doi: 10.1136/gut.2009.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselut N, Housset C, Marcelo P, Rey C, Burmester T, Vinh J, Vaubourdolle M, Cadoret A, Baudin B. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics. 10:1017–1028. doi: 10.1002/pmic.200900257. [DOI] [PubMed] [Google Scholar]
- Carvajal G, Droguett A, Burgos ME, Aros C, Ardiles L, Flores C, Carpio D, Ruiz-Ortega M, Egido J, Mezzano S. Gremlin: a novel mediator of epithelial mesenchymal transition and fibrosis in chronic allograft nephropathy. Transplant Proc. 2008;40:734–739. doi: 10.1016/j.transproceed.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RH, Krishnakumar A, Urbanek A, Geschwindner S, Meneely J, Bianchi A, Basta B, Monaghan S, Elliot C, Stromstedt M, Ferguson N, Martin F, Brazil DP. Gremlin1 preferentially binds to bone morphogenetic protein-2 (BMP-2) and BMP-4 over BMP-7. Biochem J. 2015;466:55–68. doi: 10.1042/BJ20140771. [DOI] [PubMed] [Google Scholar]
- Colombo C, Vajro P, Degiorgio D, Coviello DA, Costantino L, Tornillo L, Motta V, Consonni D, Maggiore G. Clinical features and genotype-phenotype correlations in children with progressive familial intrahepatic cholestasis type 3 related to ABCB4 mutations. J Pediatr Gastroenterol Nutr. 2011;52:73–83. doi: 10.1097/MPG.0b013e3181f50363. [DOI] [PubMed] [Google Scholar]
- de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degiorgio D, Colombo C, Seia M, Porcaro L, Costantino L, Zazzeron L, Bordo D, Coviello DA. Molecular characterization and structural implications of 25 new ABCB4 mutations in progressive familial intrahepatic cholestasis type 3 (PFIC3) Eur J Hum Genet. 2007;15:1230–1238. doi: 10.1038/sj.ejhg.5201908. [DOI] [PubMed] [Google Scholar]
- Delaunay JL, Durand-Schneider AM, Delautier D, Rada A, Gautherot J, Jacquemin E, Ait-Slimane T, Maurice M. A missense mutation in ABCB4 gene involved in progressive familial intrahepatic cholestasis type 3 leads to a folding defect that can be rescued by low temperature. Hepatology. 2009;49:1218–1227. doi: 10.1002/hep.22775. [DOI] [PubMed] [Google Scholar]
- Deleuze JF, Jacquemin E, Dubuisson C, Cresteil D, Dumont M, Erlinger S, Bernard O, Hadchouel M. Defect of multidrug-resistance 3 gene expression in a subtype of progressive familial intrahepatic cholestasis. Hepatology. 1996;23:904–908. doi: 10.1002/hep.510230435. [DOI] [PubMed] [Google Scholar]
- Dezso K, Jelnes P, Laszlo V, Baghy K, Bodor C, Paku S, Tygstrup N, Bisgaard HC, Nagy P. Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver regeneration. Am J Pathol. 2007;171:1529–1537. doi: 10.2353/ajpath.2007.070273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff JA, Kruglov EA, Robson SC, Braun N, Zimmermann H, Sevigny J. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology. 2002;36:1135–1144. doi: 10.1053/jhep.2002.36823. [DOI] [PubMed] [Google Scholar]
- Dranoff JA, Kruglov EA, Toure J, Braun N, Zimmermann H, Jain D, Knowles AF, Sevigny J. Ectonucleotidase NTPDase2 is selectively down-regulated in biliary cirrhosis. J Investig Med. 2004;52:475–482. doi: 10.1136/jim-52-07-42. [DOI] [PubMed] [Google Scholar]
- Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droguett A, Krall P, Burgos ME, Valderrama G, Carpio D, Ardiles L, Rodriguez-Diez R, Kerr B, Walz K, Ruiz-Ortega M, Egido J, Mezzano S. Tubular overexpression of gremlin induces renal damage susceptibility in mice. PLoS One. 2014;9:e101879. doi: 10.1371/journal.pone.0101879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, Radel DJ, Anderson JM, Nair AA, Evans JM, Krych AJ, Smith J, Deyle DR, Stein JL, Stein GS, Im HJ, Cool SM, Westendorf JJ, Kakar S, Dietz AB, van Wijnen AJ. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem. 2014;115:1816–1828. doi: 10.1002/jcb.24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas J, Mansuroglu T, Batusic D, Ramadori G. Thy-1 is expressed in myofibroblasts but not found in hepatic stellate cells following liver injury. Histochem Cell Biol. 2009;131:115–127. doi: 10.1007/s00418-008-0503-y. [DOI] [PubMed] [Google Scholar]
- Dudas J, Mansuroglu T, Batusic D, Saile B, Ramadori G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007;329:503–514. doi: 10.1007/s00441-007-0437-z. [DOI] [PubMed] [Google Scholar]
- Ellenrieder V. TGFbeta regulated gene expression by Smads and Sp1/KLF-like transcription factors in cancer. Anticancer Res. 2008;28:1531–1539. [PubMed] [Google Scholar]
- Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;12:22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- Fausther M, Dranoff JA. New insights on the pathogenesis of biliary cirrhosis provided by studies in FXR knockout mice. J Hepatol. 2011;55:939–940. doi: 10.1016/j.jhep.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausther M, Goree JR, Lavoie EG, Graham AL, Sevigny J, Dranoff JA. Establishment and characterization of rat portal myofibroblast cell lines. PLoS One. 2015;10:e0121161. doi: 10.1371/journal.pone.0121161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausther M, Sheung N, Saiman Y, Bansal MB, Dranoff JA. Activated hepatic stellate cells upregulate transcription of ecto-5'-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am J Physiol Gastrointest Liver Physiol. 2012;303:G904–G914. doi: 10.1152/ajpgi.00015.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, Marschall HU, Denk H, Trauner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82–S88. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- Ghiassi-Nejad Z, Hernandez-Gea V, Woodrell C, Lang UE, Dumic K, Kwong A, Friedman SL. Reduced hepatic stellate cell expression of Kruppel-like factor 6 tumor suppressor isoforms amplifies fibrosis during acute and chronic rodent liver injury. Hepatology. 2013;57:786–796. doi: 10.1002/hep.26056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:347–358. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- Grappone C, Pinzani M, Parola M, Pellegrini G, Caligiuri A, DeFranco R, Marra F, Herbst H, Alpini G, Milani S. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol. 1999;31:100–109. doi: 10.1016/s0168-8278(99)80169-x. [DOI] [PubMed] [Google Scholar]
- Grigoriu BD, Grigoriu C, Chahine B, Gey T, Scherpereel A. Clinical utility of diagnostic markers for malignant pleural mesothelioma. Monaldi Arch Chest Dis. 2009;71:31–38. doi: 10.4081/monaldi.2009.374. [DOI] [PubMed] [Google Scholar]
- Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- He Y, Wu GD, Sadahiro T, Noh SI, Wang H, Talavera D, Vierling JM, Klein AS. Interaction of CD44 and hyaluronic acid enhances biliary epithelial proliferation in cholestatic livers. Am J Physiol Gastrointest Liver Physiol. 2008;295:G305–G312. doi: 10.1152/ajpgi.90229.2008. [DOI] [PubMed] [Google Scholar]
- Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, Meng F, Asagiri M, Murray LA, Hofmann AF, Iida T, Glass CK, Brenner DA, Kisseleva T. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111:E3297–E3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhandier MN, Kruglov EA, Lavoie EG, Sevigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986–22992. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- Kamo N, Yasuchika K, Fujii H, Hoppo T, Machimoto T, Ishii T, Fujita N, Tsuruo T, Yamashita JK, Kubo H, Ikai I. Two populations of Thy1-positive mesenchymal cells regulate in vitro maturation of hepatic progenitor cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G526–G534. doi: 10.1152/ajpgi.00241.2006. [DOI] [PubMed] [Google Scholar]
- Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, Iredale JP. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Kim KH, Chen CC, Alpini G, Lau LF. CCN1 induces hepatic ductular reaction through integrin alphavbeta(5)-mediated activation of NF-kappaB. J Clin Invest. 2015;125:1886–1900. doi: 10.1172/JCI79327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. Journal of gastroenterology and hepatology. 2006;21(Suppl 3):S84–S87. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc. 2008;5:338–342. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Kivirikko S, Heinamaki P, Rehn M, Honkanen N, Myers JC, Pihlajaniemi T. Primary structure of the alpha 1 chain of human type XV collagen and exon-intron organization in the 3' region of the corresponding gene. J Biol Chem. 1994;269:4773–4779. [PubMed] [Google Scholar]
- Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999a;117:1205–1221. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-alpha and TGF-beta1. J Hepatol. 1999b;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- Koli K, Myllarniemi M, Vuorinen K, Salmenkivi K, Ryynanen MJ, Kinnula VL, Keski-Oja J. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol. 2006;169:61–71. doi: 10.2353/ajpath.2006.051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempen K, Grotkopp D, Hall K, Bache A, Gillan A, Rippe RA, Brenner DA, Breindl M. Far upstream regulatory elements enhance position-independent and uterus-specific expression of the murine alpha1(I) collagen promoter in transgenic mice. Gene Expr. 1999;8:151–163. [PMC free article] [PubMed] [Google Scholar]
- Kruglov EA, Jain D, Dranoff JA. Isolation of primary rat liver fibroblasts. J Investig Med. 2002;50:179–184. doi: 10.2310/6650.2002.33431. [DOI] [PubMed] [Google Scholar]
- Kuang PP, Goldstein RH. Regulation of elastin gene transcription by proteasome dysfunction. Am J Physiol Cell Physiol. 2005;289:C766–C773. doi: 10.1152/ajpcell.00525.2004. [DOI] [PubMed] [Google Scholar]
- Lemoinne S, Cadoret A, Rautou PE, El Mourabit H, Ratziu V, Corpechot C, Rey C, Bosselut N, Barbu V, Wendum D, Feldmann G, Boulanger C, Henegar C, Housset C, Thabut D. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology. 2015;61:1041–1055. doi: 10.1002/hep.27318. [DOI] [PubMed] [Google Scholar]
- Lepreux S, Desmouliere A. Human liver myofibroblasts during development and diseases with a focus on portal (myo)fibroblasts. Front Physiol. 2015;6:173. doi: 10.3389/fphys.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton L, Hagood JS. Thy-1 modulates neurological cell-cell and cell-matrix interactions through multiple molecular interactions. Advances in neurobiology. 2014;8:3–20. doi: 10.1007/978-1-4614-8090-7_1. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, Han Y, Fan D. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013;8:e62363. doi: 10.1371/journal.pone.0062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- Lijinsky W, Reuber MD, Blackwell BN. Liver tumors induced in rats by oral administration of the antihistaminic methapyrilene hydrochloride. Science. 1980;209:817–819. doi: 10.1126/science.7403848. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Lua I, Li Y, Zagory JA, Wang KS, French SW, Sevigny J, Asahina K. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukita-Atmadja W, Subowo The stellate cells phenotypic transformation in the CCl4- injured liver fibrosis of ICR mice: their desmin immunoreactivity and vitamin A storage. Kobe J Med Sci. 1993;39:15–33. [PubMed] [Google Scholar]
- Ma B, Kang Q, Qin L, Cui L, Pei C. TGF-beta2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF. Biochem Biophys Res Commun. 2014;447:689–695. doi: 10.1016/j.bbrc.2014.04.068. [DOI] [PubMed] [Google Scholar]
- Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173:548–550. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R, Rodriguez-Leon J, Macias D, Ganan Y, Economides AN, Hurle JM. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development. 1999;126:5515–5522. doi: 10.1242/dev.126.23.5515. [DOI] [PubMed] [Google Scholar]
- Miethke AG, Zhang W, Simmons J, Taylor AE, Shi T, Shanmukhappa SK, Karns R, White S, Jegga AG, Lages CS, Nkinin S, Keller BT, Setchell KD. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–523. doi: 10.1002/hep.27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- Mueller KA, Tavlaki E, Schneider M, Jorbenadze R, Geisler T, Kandolf R, Gawaz M, Mueller II, Zuern CS. Gremlin-1 identifies fibrosis and predicts adverse outcome in patients with heart failure undergoing endomyocardial biopsy. J Card Fail. 2013;19:678–684. doi: 10.1016/j.cardfail.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Muragaki Y, Abe N, Ninomiya Y, Olsen BR, Ooshima A. The human alpha 1(XV) collagen chain contains a large amino-terminal non-triple helical domain with a tandem repeat structure and homology to alpha 1(XVIII) collagen. J Biol Chem. 1994;269:4042–4046. [PubMed] [Google Scholar]
- Myers JC, Kivirikko S, Gordon MK, Dion AS, Pihlajaniemi T. Identification of a previously unknown human collagen chain, alpha 1(XV), characterized by extensive interruptions in the triple-helical region. Proc Natl Acad Sci U S A. 1992;89:10144–10148. doi: 10.1073/pnas.89.21.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Kim JS, Mohuczy D, Behrns KE. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology. 2008;48:909–919. doi: 10.1002/hep.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Tateno C, Asahina K, Fujii H, Kawada N, Obara M, Yoshizato K. Identification of vitamin A-free cells in a stellate cell-enriched fraction of normal rat liver as myofibroblasts. Histochem Cell Biol. 2007;127:161–174. doi: 10.1007/s00418-006-0237-7. [DOI] [PubMed] [Google Scholar]
- Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM, Brenner DA. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 2009;50:929–938. doi: 10.1002/hep.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan TC, Sasaki T, Zhang RZ, Fassler R, Timpl R, Chu ML. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J Cell Biol. 1993;123:1269–1277. doi: 10.1083/jcb.123.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira TA, Syn WK, Machado MV, Vidigal PV, Resende V, Voieta I, Xie G, Otoni A, Souza MM, Santos ET, Chan IS, Trindade GV, Choi SS, Witek RP, Pereira FE, Secor WE, Andrade ZA, Lambertucci JR, Diehl AM. Schistosome-induced cholangiocyte proliferation and osteopontin secretion correlate with fibrosis and portal hypertension in human and murine schistosomiasis mansoni. Clin Sci (Lond) 2015;129:875–883. doi: 10.1042/CS20150117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M. Pathophysiology of Liver Fibrosis. Digestive diseases. 2015;33:492–497. doi: 10.1159/000374096. [DOI] [PubMed] [Google Scholar]
- Piscaglia F, Dudas J, Knittel T, Di Rocco P, Kobold D, Saile B, Zocco MA, Timpl R, Ramadori G. Expression of ECM proteins fibulin-1 and -2 in acute and chronic liver disease and in cultured rat liver cells. Cell Tissue Res. 2009;337:449–462. doi: 10.1007/s00441-009-0823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert PME, Ebrahimkhani MR, Oakley F, Mann J, Burt AD, Mann DA, Wright MC. A reversible model for periportal fibrosis and a refined alternative to bile duct ligation. Toxicol Res-Uk. 2014;3:98–109. [Google Scholar]
- Ramirez G, Hagood JS, Sanders Y, Ramirez R, Becerril C, Segura L, Barrera L, Selman M, Pardo A. Absence of Thy-1 results in TGF-beta induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab Invest. 2011;91:1206–1218. doi: 10.1038/labinvest.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR, Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14:1251–1260. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Diez R, Rodrigues-Diez RR, Lavoz C, Carvajal G, Droguett A, Garcia-Redondo AB, Rodriguez I, Ortiz A, Egido J, Mezzano S, Ruiz-Ortega M. Gremlin activates the Smad pathway linked to epithelial mesenchymal transdifferentiation in cultured tubular epithelial cells. Biomed Res Int. 2014;2014:802841. doi: 10.1155/2014/802841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, Mackenzie KF, Klussmann E, Lynch MJ, Sikorski SL, Nuriel T, Tsigelny I, Zhang J, Houslay MD, Chao MV, Akassoglou K. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders YY, Tollefsbol TO, Varisco BM, Hagood JS. Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. Am J Respir Cell Mol Biol. 2011;45:16–23. doi: 10.1165/rcmb.2010-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK, Brenner DA, Kisseleva T. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol. 2011;179:189–198. doi: 10.1016/j.ajpath.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Fallowfield J, Iredale JP. Senescence of activated stellate cells: not just early retirement. Hepatology. 2009;49:1045–1047. doi: 10.1002/hep.22832. [DOI] [PubMed] [Google Scholar]
- Schurch W, Seemayer TA, Gabbiani G. The myofibroblast: a quarter century after its discovery. Am J Surg Pathol. 1998;22:141–147. doi: 10.1097/00000478-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert WF, Roholl PJ, Blauw B, van der Ham F, van Thiel-De Ruiter CF, Seifert-Bock I, Bosma A, Knook DL, Brouwer A. Fat-storing cells and myofibroblasts are involved in the initial phase of carbon tetrachloride-induced hepatic fibrosis in BN/BiRij rats. Int J Exp Pathol. 1994;75:131–146. [PMC free article] [PubMed] [Google Scholar]
- Senoo H, Kojima N, Sato M. Vitamin a-storing cells (stellate cells) Vitam Horm. 2007;75:131–159. doi: 10.1016/S0083-6729(06)75006-3. [DOI] [PubMed] [Google Scholar]
- Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Staloch D, Gao X, Liu K, Xu M, Feng X, Aronson JF, Falzon M, Greeley GH, Rastellini C, Chao C, Hellmich MR, Cao Y, Ko TC. Gremlin is a key pro-fibrogenic factor in chronic pancreatitis. J Mol Med (Berl) 2015 doi: 10.1007/s00109-015-1308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl P, McMahon R, Blaas L, Stanek V, Svinka J, Grabner B, Zollner G, Kessler SM, Claudel T, Muller M, Mikulits W, Bilban M, Esterbauer H, Eferl R, Haybaeck J, Trauner M, Casanova E. Growth hormone resistance exacerbates cholestasis-induced murine liver fibrosis. Hepatology. 2015;61:613–626. doi: 10.1002/hep.27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack I, Schulte S, Varnholt H, Schievenbusch S, Tox U, Wendland K, Steffen HM, Drebber U, Dienes HP, Odenthal M. beta-Adrenoceptor blockade in sclerosing cholangitis of Mdr2 knockout mice: antifibrotic effects in a model of nonsinusoidal fibrosis. Lab Invest. 2011;91:252–261. doi: 10.1038/labinvest.2010.162. [DOI] [PubMed] [Google Scholar]
- Symeonidis A, Trams EG. Morphologic and functional changes in the livers of rats after ligation or excision of the common bile duct. Am J Pathol. 1957;33:13–27. [PMC free article] [PubMed] [Google Scholar]
- Sysa P, Potter JJ, Liu X, Mezey E. Transforming growth factor-beta1 up-regulation of human alpha(1)(I) collagen is mediated by Sp1 and Smad2 transacting factors. DNA Cell Biol. 2009;28:425–434. doi: 10.1089/dna.2009.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimizu N, Kikkawa Y, Mitaka T, Miyajima A. alpha1- and alpha5-containing laminins regulate the development of bile ducts via beta1 integrin signals. J Biol Chem. 2012;287:28586–28597. doi: 10.1074/jbc.M112.350488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Fickert P, Baghdasaryan A, Claudel T, Halilbasic E, Moustafa T, Wagner M, Zollner G. New insights into autoimmune cholangitis through animal models. Dig Dis. 2010;28:99–104. doi: 10.1159/000282072. [DOI] [PubMed] [Google Scholar]
- Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, Pradere JP, Friedman RA, Schwabe RF. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083. e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- Uchio K, Tuchweber B, Manabe N, Gabbiani G, Rosenbaum J, Desmouliere A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest. 2002;82:619–628. doi: 10.1038/labinvest.3780456. [DOI] [PubMed] [Google Scholar]
- Varisco BM, Ambalavanan N, Whitsett JA, Hagood JS. Thy-1 signals through PPARgamma to promote lipofibroblast differentiation in the developing lung. Am J Respir Cell Mol Biol. 2012;46:765–772. doi: 10.1165/rcmb.2011-0316OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrhovski B, Weiss AS. Biochemistry of tropoelastin. Eur J Biochem. 1998;258:1–18. doi: 10.1046/j.1432-1327.1998.2580001.x. [DOI] [PubMed] [Google Scholar]
- Wells RG. The Portal Fibroblast: Not Just a Poor Man's Stellate Cell. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- Wen JW, Olsen AL, Perepelyuk M, Wells RG. Isolation of rat portal fibroblasts by in situ liver perfusion. Journal of visualized experiments : JoVE. 2012:3669. doi: 10.3791/3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, Gross S, Renz BW, Setlik W, Martinez AN, Chen X, Nizami S, Lee HG, Kang HP, Caldwell JM, Asfaha S, Westphalen CB, Graham T, Jin G, Nagar K, Wang H, Kheirbek MA, Kolhe A, Carpenter J, Glaire M, Nair A, Renders S, Manieri N, Muthupalani S, Fox JG, Reichert M, Giraud AS, Schwabe RF, Pradere JP, Walton K, Prakash A, Gumucio D, Rustgi AK, Stappenbeck TS, Friedman RA, Gershon MD, Sims P, Grikscheit T, Lee FY, Karsenty G, Mukherjee S, Wang TC. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Hooker SW, Jiang H, Laurent AB, Resta R, Khare K, Coe A, Kincade PW, Thompson LF. CD73 expression and fyn-dependent signaling on murine lymphocytes. Eur J Immunol. 1998;28:2981–2990. doi: 10.1002/(SICI)1521-4141(199810)28:10<2981::AID-IMMU2981>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest. 1998;78:89–100. [PubMed] [Google Scholar]
- Yovchev MI, Zhang J, Neufeld DS, Grozdanov PN, Dabeva MD. Thymus cell antigen-1-expressing cells in the oval cell compartment. Hepatology. 2009;50:601–611. doi: 10.1002/hep.23012. [DOI] [PubMed] [Google Scholar]
- Yu J, Lavoie EG, Sheung N, Tremblay JJ, Sevigny J, Dranoff JA. IL-6 downregulates transcription of NTPDase2 via specific promoter elements. Am J Physiol Gastrointest Liver Physiol. 2008;294:G748–G756. doi: 10.1152/ajpgi.00208.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XK, Cheng ML, Wu RM, Yao YM, Mu M, Zhu JJ, Zhang BF, Zhou MY. Effect of Danshao Huaxian capsule on Gremlin and bone morphogenetic protein-7 expression in hepatic fibrosis in rats. World J Gastroenterol. 2014;20:14875–14883. doi: 10.3748/wjg.v20.i40.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hagood JS, Lu B, Merryman WD, Murphy-Ullrich JE. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–22393. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]