Abstract
Inhalation of nitric oxide (NO) produces selective pulmonary vasodilation without dilating the systemic circulation. However, the current NO/N2 cylinder delivery system is cumbersome and expensive. We developed a lightweight, portable, and economical device to generate NO from air by pulsed electrical discharge. The objective of this study was to investigate and optimize the purity and safety of NO generated by this device. By using low temperature streamer discharges in the plasma generator, we produced therapeutic levels of NO with very low levels of nitrogen dioxide (NO2) and ozone. Despite the low temperature, spark generation eroded the surface of the electrodes, contaminating the gas stream with metal particles. During prolonged NO generation there was gradual loss of the iridium high-voltage tip (−90 µg/day) and the platinum-nickel ground electrode (−55 µg/day). Metal particles released from the electrodes were trapped by a high-efficiency particulate air (HEPA) filter. Quadrupole mass spectroscopy measurements of effluent gas during plasma NO generation showed that a single HEPA filter removed all of the metal particles. Mice were exposed to breathing 50 parts per million of electrically generated NO in air for 28 days with only a scavenger and no HEPA filter; the mice did not develop pulmonary inflammation or structural changes and iridium and platinum particles were not detected in the lungs of these mice. In conclusion, an electric plasma generator produced therapeutic levels of NO from air; scavenging and filtration effectively eliminated metallic impurities from the effluent gas.
Keywords: nitric oxide, pulsed electrical discharge, metal particles, iridium electrode
Introduction
Nitric oxide (NO) is a selective pulmonary vasodilator that was approved by the U.S. Food and Drug Administration in 1999 for the treatment of hypoxic term or near-term newborns with persistent pulmonary hypertension (PPHN) [1]. Since approval, inhaled NO has predominantly been used in major medical centers of the developed world to treat pulmonary hypertension in newborns, and children and adults with pulmonary hypertension [2; 3; 4; 5]. By 2015, an estimated half million American children and adults with pulmonary hypertension of diverse etiology had been treated with inhaled NO [6; 7]. Wider use of NO therapy is significantly limited, however, because the current delivery systems are cumbersome and expensive, requiring compressed gas cylinders, a cylinder distribution network, a complex delivery device, gas monitoring and calibration devices, and trained respiratory therapy staff. For many hospitals, inhaled NO is one of the most expensive drugs used in neonatal medicine [8]; the average cost of providing NO therapy for 5 days to a newborn patient with PPHN at Massachusetts General Hospital (MGH) is approximately $14,000. The high cost and difficulties associated with delivery have limited the use of NO in the ambulatory setting and the potential benefits of chronic NO inhalation in non-hospitalized patients have not been thoroughly investigated.
Both chemical and electric plasma discharge methods have been used to produce NO for biomedical purposes [9; 10; 11]. However, these methods produce large amounts of toxic by products, such as nitrogen dioxide (NO2) and ozone (O3), requiring complex purification systems [12; 13]. Recently, we designed, developed, and tested in lambs a lightweight, economic, and portable NO generation system using pulsed electrical discharge [14]. The prototype NO generation system produced a therapeutic range of NO (5–80 parts per million (ppm)) in air or an oxygen-enriched nitrogen containing gas [14]. Iridium or other noble metal electrodes produced the lowest fractional amount of NO2/NO compared to nickel, carbon or tungsten electrodes [14]. Iridium is a rare and inert metal. Very little is known about the toxicity of inhaled iridium aerosols, and data on the toxicity of inhaled iridium in human studies are not available. Platinum is also a noble metal and is non-reactive. There are no data on toxicity of inhalation of platinum aerosols in humans. However, inhalation of nickel aerosols can cause toxic effects in the respiratory tract and immune system [15; 16; 17]. To minimize the production of potentially harmful gases (NO2) and the release of metal particles from the surface of the electrodes, we focused on using low temperature sparks with low current flows in the plasma.
The objective of this study was to detect, and if necessary remove, potentially harmful particles produced by the electric plasma NO generator. First, we sought to determine whether electrodes underwent erosion after prolonged NO generation. Because electrode erosion was detected by scanning electron microscopy (SEM), we determined the chemical composition of particles released from the electrode using energy-dispersive X-ray spectroscopy (EDX). Second, we characterized the temperature of the plasma during NO generation to better characterize the erosion process. Third, we tested whether a 0.22 µm high-efficiency particulate air (HEPA) filter, placed in series with the NO generator, would capture metal particles in the effluent gas stream. Finally, we investigated whether any pathologic effects or metal particles were detected in murine airways and lung tissues after mice breathed electrically generated NO (50 ppm) in air for 28 days with only a Ca(OH)2 scavenger (to remove NO2) but no particulate air filter.
Materials and Methods
1.1 Determination of metallic components of electrodes
The electrodes are composed of a high voltage tip and a ground electrode. We used scanning electron microscopy (SEM) to detect morphological changes in the electrodes and energy-dispersive X-ray spectroscopy (EDX) to determine the composition of the surface metallic components of the tip and ground electrodes.
1.2 Measurement of electrode erosion
SEM was used to image the high voltage tip and the ground electrode (ACDelco 41–101, AutoZone) before (new) and after 7, 10, or 28 days of continuous plasma NO production. Four pulse pattern control variables were selected to precisely control the levels of NO production. These variables included the number of spark groups per second (B), the number of spark discharges per group (N), the time in microseconds (µsec) between two spark discharges (P), and the pulse time in µsec (H) (14). Specifically, to synthesize 50 ppm NO at 5 L/min airflow, B was set at 20, N at 30, P at 240, and H at 70. The electrode gap was set at 2 mm (see schematic of bench testing apparatus in online supplement Fig. 1). Prior to imaging, electrodes were ultrasonically cleaned with a mixture of 70% ethyl alcohol and 30% deionized water. All images of the electrodes were obtained using secondary-electron emissions in a high vacuum. Photographs of the electrode surfaces were taken either topographically or in cross section. The SEM accelerating voltage varied between 5 and 20 kV. To calculate mass (M) changes of the electrodes before (new) and after NO generation, we used the formula: M=ρπd2h/4 (ρ density, d diameter, h height).
1.3 Optical diagnostics and determination of discharge temperatures
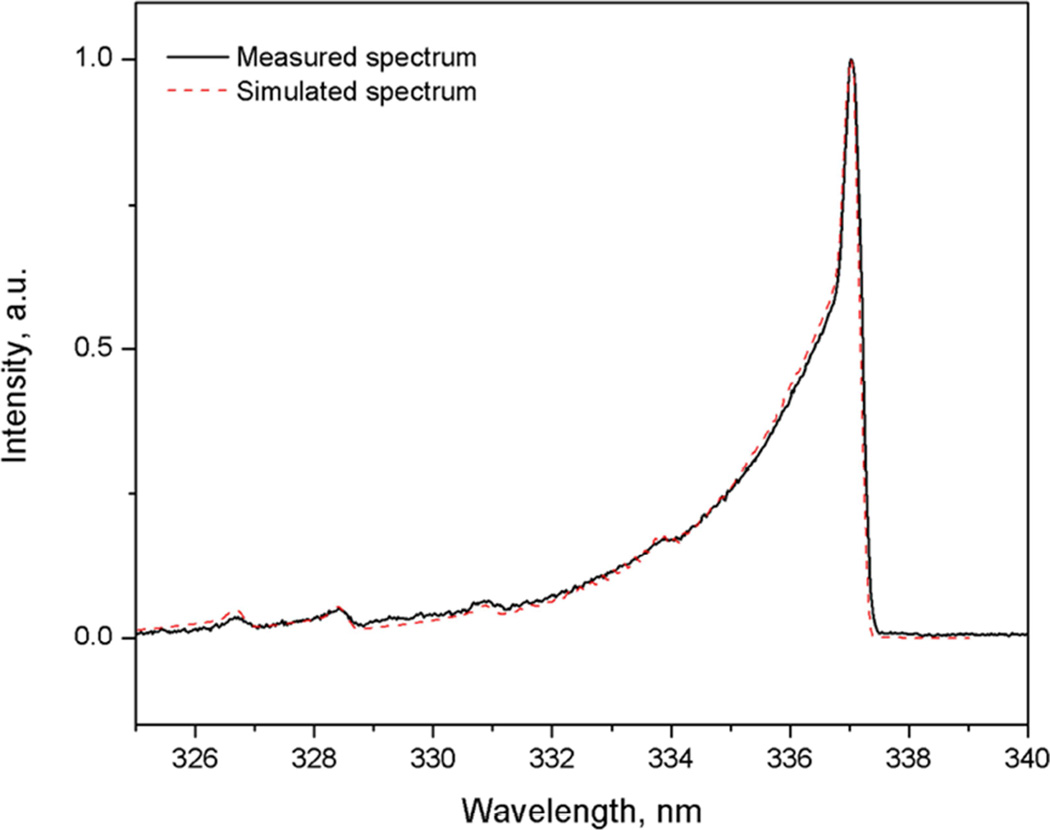
Light emitted from the electrical discharge was collected using a fiberoptic bundle (Princeton Instruments-Acton, 10 fibers −200 µm core) connected to a spectrometer (Princeton Instruments – Acton Research, TriVista TR555 spectrometer system with PIMAX digital ICCD camera, Trenton, NJ). The spectrum was integrated over a 5 sec interval. Because the discharge was non-equilibrium, the gas temperature was estimated by determining the rotational temperature from the spectrum of nitrogen due to fast collisional relaxation at atmospheric pressure [18]. One of the most reliable ways of measuring heavy particles (gas) temperature in non-thermal plasma is based on analysis of the nitrogen second positive system. Equating the rotational temperature Tr (C) of the C state and Tr (X) of the ground state of nitrogen is valid in the case of a non-equilibrium plasma. Due to the long residence time, the nitrogen ground state is in rotational–translational equilibrium with the host gas. Because the change in rotational angular momentum is small during electron impact excitation, the ground state rotational distribution is transferred to the nitrogen (C) state. The rotational temperature of nitrogen is determined by fitting a synthetic spectrum to the experimental spectrum of the (0–2) transition emission bands of the second positive system of nitrogen at 337 nm with the program SPECAIR 3.0 [19].
1.4 Detecting metal particles deposited on a HEPA filter
A 0.22 µm HEPA filter (5708, HEPA, Vital Signs Inc., Totowa, NJ) located downstream of the NO generator was used to capture metal particles during NO generation. SEM imaging and EDX were used to detect and identify metal particles on the upstream and downstream surfaces of the HEPA filter. A JSM-6010LA SEM (JEOL Ltd., Tokyo, Japan) was used for SEM imaging and EDX analysis. Back-Scattered Electron (BSE) imaging of filters was performed in a low vacuum mode. The inflow and outflow surfaces of the filter were imaged and an image of the filter in cross section was obtained after transecting the filter with a blade.
1.5 Detection and measurement of metal particles emitted by the plasma NO generation device
Quadrupole inductively-coupled plasma mass spectrometry (ICP-MS, iCAP Qc, Thermo Fisher, Bremen, Germany) was used to determine the nature and quantity of metal particles released by the plasma discharge from the electrodes while the device generated NO at an airflow of 0.05 L/min (medical grade air, Airgas, Cambridge, MA). Argon gas (0.95 L/min) was mixed with air (0.05 L/min), and then injected into the ICP-MS (producing 5% medical grade air mixed with 95% argon, see online supplement Fig. 2). The level of NO production by the metal electrodes was controlled by four pulse pattern variables: B=20, N=30, P=240, H=70, and the electrode gap was 2 mm. Metal particle generation in the outflow gas during NO generation (approximately 250 ppm NO produced at 1 L/min total gas flow) was measured under 3 conditions (1) without processing gas through a HEPA filter (0.22 µm) or scavenger, (2) with a 12 g Ca(OH)2 scavenger (to remove NO2) but no HEPA filter, and (3) with a 12 g Ca(OH)2 scavenger and a HEPA filter (the usual method of clinical application for effluent gas purification [14]).
1.6 High-resolution magnetic sector field ICP-mass spectrometric analysis of metal particles in murine lungs
High-resolution magnetic sector field ICP-MS (Element XR with Jet Interface, Thermo Fisher, Bremen, Germany) and a prepFAST Auto-Dilution/Calibration autosampler (ESI, Omaha, NE, USA) were employed to measure the quantities of iridium and platinum in the lungs of mice breathing either air or electric plasma-generated 50 ppm NO in air for 28 days. The empirically-determined detection limit for iridium and platinum using this method is <10 ng/g tissue.
1.7 Exposure of mice to electrically generated NO and histological analysis of murine lung tissues
Animal studies were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital (Boston, MA). Eight- to 10-week-old male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). We studied two groups of C57BL/6J mice (n=8/group). One group (control) breathed air for 28 days, and a second group breathed 50 ppm of electric plasma generated NO in air for 28 days. The settings for NO production were: B=25, N=30, P=240, H=70, and the gap of the iridium electrode was 3 mm. Mice were placed in two environmentally-controlled 60 L plexiglass chambers with free access to food and water (online supplement Fig. 3). Either fresh air or a flow of fresh air containing 50 ppm NO was delivered to each chamber. The flow was measured and controlled with rotameters and valves at 5 L/min. Both air and electrically generated NO in air were passed through an in-line Ca(OH)2 scavenger (75 g soda lime, Sodasorb, Smiths Medical, Dublin, OH) without HEPA filtration before delivery into the breathing chamber. In addition, approximately 250 g of Ca(OH)2 was placed on the floor of each chamber to scavenge carbon dioxide (CO2, produced by the mice) and any accumulated NO2. The Ca(OH)2 scavenger was replaced every 3 days. NO2 levels were measured daily and maintained below 1 ppm. Oxygen concentrations were measured each day at the outlet of the chambers using an oxygen analyzer (MiniOx I Oxygen Analyzer, Ohio Medical Corporation). The CO2 concentration in each chamber was measured continuously using a dedicated infrared CO2 analyzer (Extech CO2 Monitor, Extech Instruments). Humidity (40–50%) and temperature (23°C) inside the breathing chambers were maintained during the entire experiments. The levels of NO and NO2 were measured using chemiluminescence as previously described [14]. The chambers were briefly opened twice a week to weigh the mice, clean the cages, and add water and food.
At the end of the study (day 28), all mice were sacrificed with an intraperitoneal injection of pentobarbital (200 mg/kg). Murine lungs and tracheobronchial trees (main bronchus, trachea, and larynx) were harvested to detect structural changes or inflammatory cells. Tissues were perfused with 3% paraformaldehyde and 0.1% glutaraldehyde in PBS and fixed in the same solution for 24 hrs. Paraffin-embedded sections of 3–4 µm thickness were stained with hematoxylin and eosin [20; 21]. Digital images of the lung tissue were acquired via bright field microscopy (Elipse 80i, Nikon Instruments Inc., Melville, NY).
1.8 Statistical analysis
All variables were found to be normally distributed by the Shapiro-Wilk test and are expressed as mean±SD. A two-way analysis of variance with repeated measures was performed to determine the effect of breathing electrically-generated NO on the body weight of mice (GraphPad Prism; GraphPad Software Inc.). Two-tailed analyses were performed, and a P-value <0.05 was considered significant.
Results and Discussion
1.1 Determination of electrode metal components
We identified the metallic components of the electrodes by SEM and EDX. The high voltage tip is plated with pure iridium, and the platinum pad of the ground electrode is composed of 90% platinum and 10% nickel alloy (online supplement Fig. 4). In the following studies, we searched for these three metal particles during NO generation and on filters that were placed in the effluent gas outflow tract.
1.2 Measurement of electrode erosion
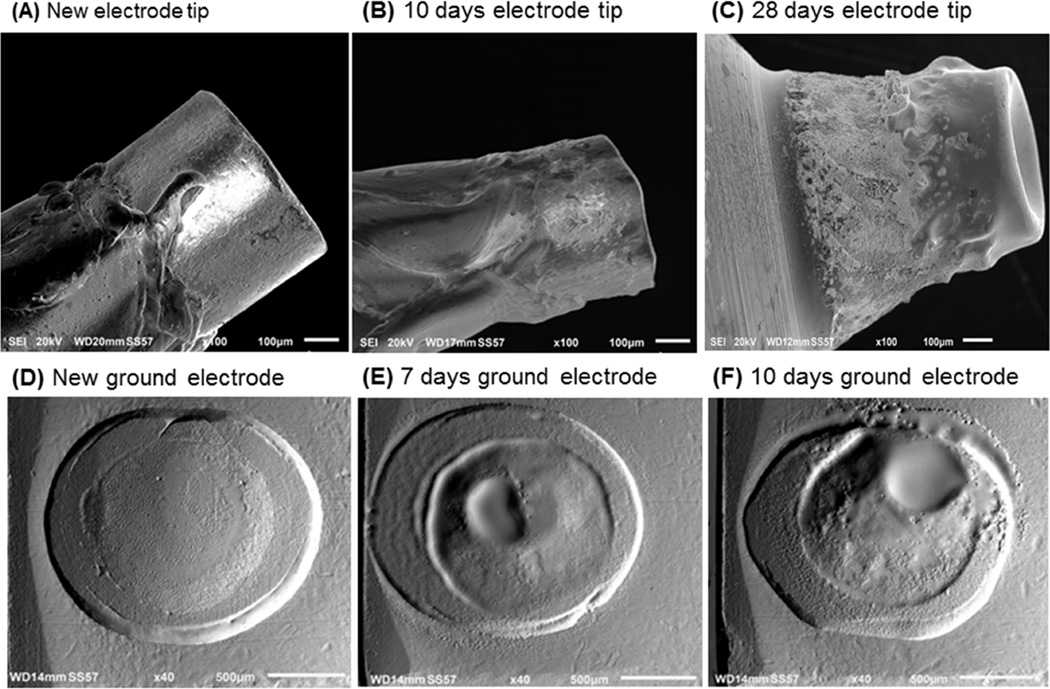
Although iridium, platinum and nickel are metals with high boiling temperatures (boiling points (bp) 4427°C, 3825°C, 2913°C, respectively), it has been reported that electrodes prepared from these metals can erode during spark generation [22; 23]. SEM was used to determine the rate of electrode erosion after prolonged NO generation in air. Because the process of obtaining images with SEM required destructive sectioning of the electrodes, the same iridium electrode could not be studied both before and after prolonged NO generation. However, the variance in the diameter and length of new iridium electrodes (n=5), was minimal (0.60±0.001 mm and 0.45±0.008 mm (mean±SD), respectively). Compared to a new, unused electrode, there was 100 µm reduction in the length of the iridium tip after use in the NO generator for 10 days. After 10 or 28 days continuous NO generation in air, the high temperatures produced by pulsed electrical discharge altered the shape and surface of the high voltage tip (Fig. 1A–C). The mass reduction rate for the high voltage tip was approximately 90 µg/day (Table 1). The surface of the ground electrode was also altered after 7 or 10 days of continuous NO generation (Table 1, Fig. 1D–F). The mass reduction rate for the ground electrode was approximately 55 µg/day. The results of these studies show that both the high voltage iridium tip and the platinum ground electrode slowly erode during continuous NO generation in air.
Figure 1.
Scanning electron microscopy (SEM) images of high voltage tips before use (A), and after 10 days (B), or 28 days (C) of NO generation. SEM images of the platinum pad of the ground electrode before use (D), and after use for 7 days (E), or 10 days (F) of NO generation.
Table 1.
Comparison of mass loss by high voltage tip and ground electrodes: new iridium spark plug, iridium spark plugs generating NO for 7, 10, or 28 days
| High voltage tip | Mass (mg) |
|---|---|
| New | 2.93 |
| Used 10 days | 2.09 (−84 µg/day) |
| Used 28 days | 0.25 (−96 µg/day) |
| Ground electrode | |
| New | 6.33 |
| Used 7 days | 5.95 (−54 µg/day) |
| Used 10 days | 5.78 (−55 µg/day) |
Inhalation of metallic fumes or compounds, such as complex platinum salts or nickel oxides can cause acute or chronic lung diseases [15; 16]. The Occupational Safety and Health Administration (OSHA) sets a limit of 1.0 mg nickel/m3 for metallic nickel and nickel compounds in workroom air to protect workers during an 8-hour shift over a 40-hour work week [24]. Based on the loss of 5.5 µg/day of nickel in the ground electrode during continuous NO generation with an air flow rate of 5 L/min, (See detailed calculations in online supplement Results and discussion) we calculated that the loss of nickel per day was 0.76×10−3 mg/m3 of unfiltered electric plasma NO generator gas, which is three orders of magnitude lower than OSHA’s nickel exposure limit (1.0 mg nickel/m3) [24]. National Institute for Occupational Safety and Health (NIOSH) recommends a platinum exposure limit of 1 mg/m3 for an 8-hour time-weighted average [25]. In the NO generator, the loss of platinum per day was 6.9×10−3 mg/m3, which is much lower than the NIOSH standard for platinum exposure. During NO generation without filtration or scavenging, the level of nickel and platinum particles released from the electrodes due to erosion is much lower than the OSHA and NIOSH exposure standards. In contrast to breathing unfiltered plasma generated NO, filtration with a single HEPA filter removed all of the metal particles produced by the plasma discharge NO generator and no particles would be inhaled.
1.3 Measurement of plasma temperature during NO generation
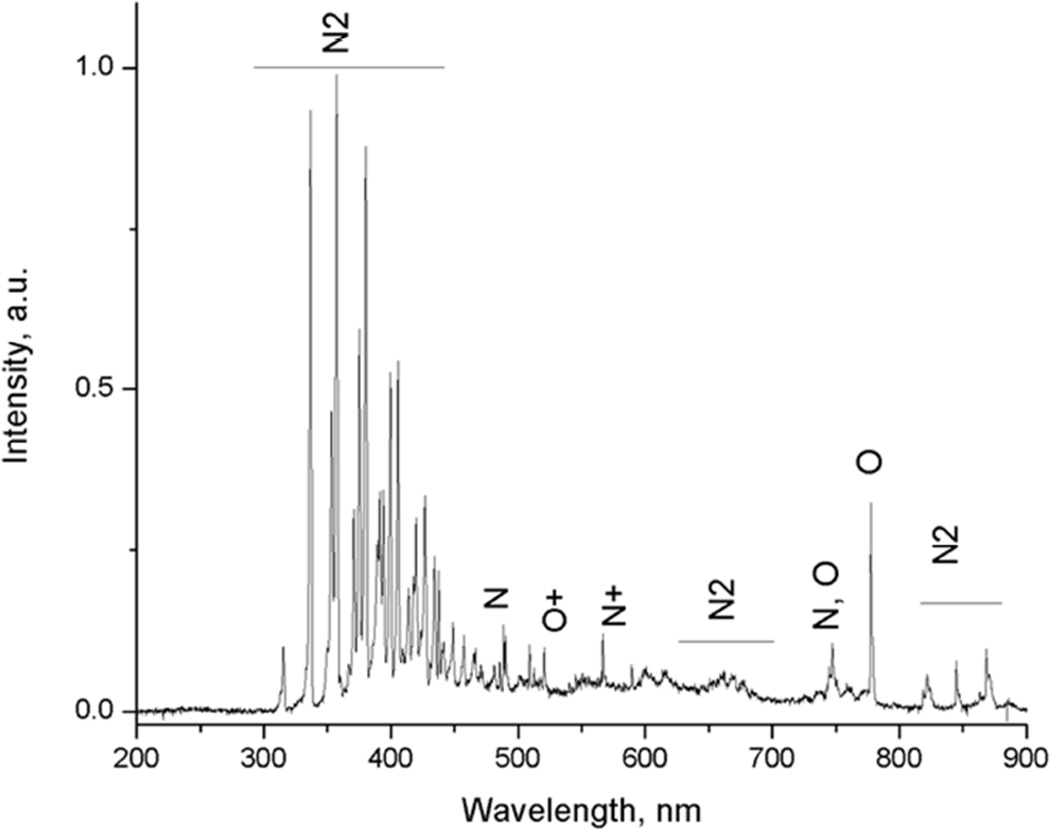
Optical emission spectrometry was used to measure the plasma gas temperature in the NO generator. The optical emission spectrum with atmospheric air in non-equilibrium plasma showed emission lines from molecular nitrogen and atomic oxygen (see Fig. 2A). The best theoretical fit (dotted line) for the nitrogen emission line is shown in Fig. 2B. The estimated gas temperature (Tg) was approximately 1470 K (1200°C). These data indicated that the NO generator produced a lower plasma temperature in gas (1200°C) than the melting points (in the range of 1500–2500°C) of the noble metals in the NO generator electrodes.
Figure 2.
Full spectrum (A) and nitrogen spectrum (B) of intensity during NO generation. Settings: iridium tip with electrode gap=2 mm, B=20, N=20, P=240, H=70, airflow 5 L/min. Tg: gas temperature=1470 K (1200°C). The simulated spectrum, as a reference, is to help calculate and estimate the real plasma temperature. The simulated spectrum is determined by fitting a synthetic spectrum to the experimental spectrum of the (0–2) transition emission bands of the second positive system of nitrogen in the range of 360–381 nm with the program SPECAIR 3.0.
Electrode erosion is likely to be the result of destruction of the metal oxide layer, which is formed on the electrode surfaces in an oxygen-rich atmosphere. The oxides of these metals boil at 1100°C for IrO2, 600°C for NiO2, 450°C for PtO2, 325°C for PtO, and 300°C for Pt3O4 (see online supplement, Table 1). The low plasma discharge temperature suggests that electrode erosion is primarily due to vaporization of the metal oxide layer, and this is consistent with the slow electrode erosion rates measured during prolonged NO generation.
In our studies, producing 50 ppm NO from air at 5 L/min we used the same electrodes continuously for 28 days. The NO production rate was constant during the 28 days despite the observed electrode erosion. The images and mass changes correlate with the slow erosion rate of the electrodes. A low plasma temperature (1200°C) was measured, suggesting the slow electrode erosion is primarily due to vaporization of the metal oxide layer, since this temperature is insufficient to vaporize these pure noble metals. However, this is a speculation since we have not measured metal oxide levels. Higher oxygen levels cause faster electrode erosion (presumably due to increased oxide formation), and producing larger quantities of NO will also augment electrode oxidation rates. However, these erosion rates are not yet studied. Clearly electrodes will have a variable length of useful duration based on O2 levels and NO generation rates.
1.4 Detecting metal particles deposited in a HEPA filter
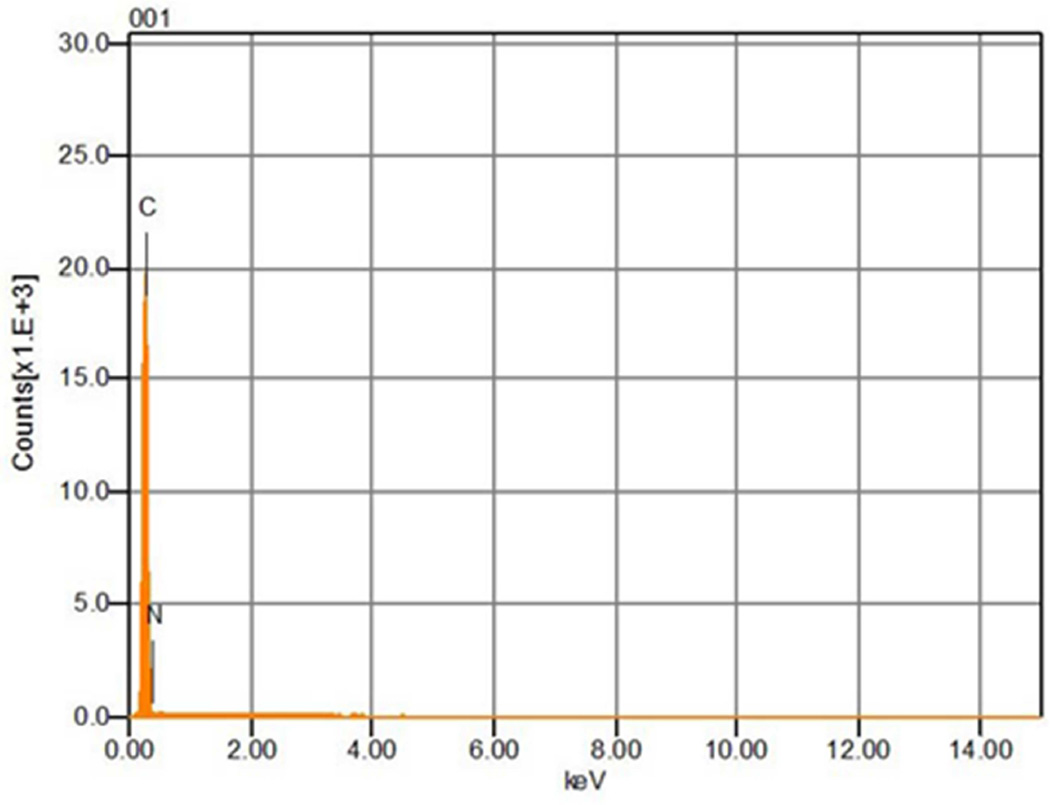
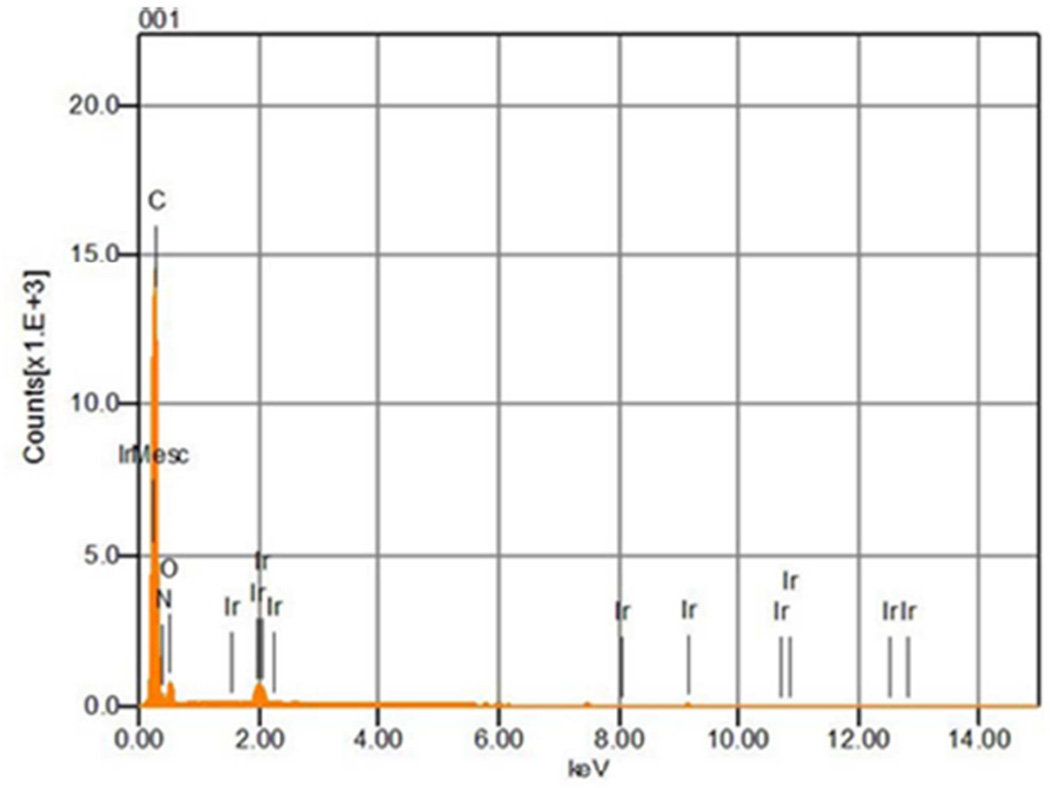
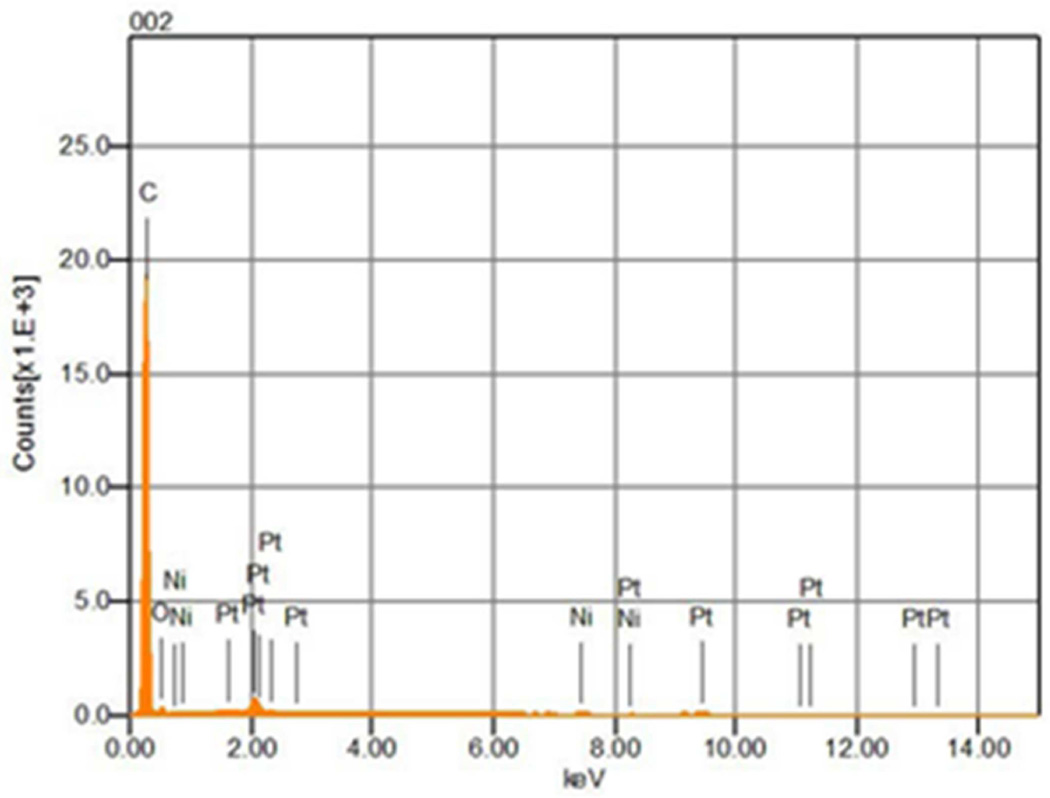
The previous SEM studies provided evidence that the electrode underwent erosion during NO generation (Fig. 1). Therefore we examined whether a 0.22 µm HEPA filter placed downstream of the NO generation device would capture metal particles by analyzing the filter with SEM and EDX. The SEM images showed there were no pre-existing metal particles on an unused HEPA filter (Fig. 3A), while metal particles were measured on a HEPA filter used for filtering effluent NO generator gas for 10 days (Fig. 3C). EDX analysis revealed that only carbon and nitrogen producing peaks (possibly from polymer materials comprising the filter) were detected on the unused filter (Fig. 3B). In contrast, iridium, nickel, and platinum peaks (in addition to carbon and nitrogen peaks) were detected on the HEPA filter after 10 days of NO production (Fig. 3D,E). The cross section SEM image showed that the proximal surface of the filter placed downstream of the NO generator was coated with metal (likely due to trapping of metal particles, online supplement Fig. 5). In contrast, EDX did not detect peaks resulting from iridium, nickel or platinum on the distal surface of the filter.
Figure 3.
Comparison of a new HEPA filter (A), and a filter used for 10 days of NO generation (C) as imaged using SEM. No metal particles were detected on the new HEPA filter (B), but iridium (D), nickel and platinum (E) particles were measured on 10-day used HEPA filter analyzed using EDX.
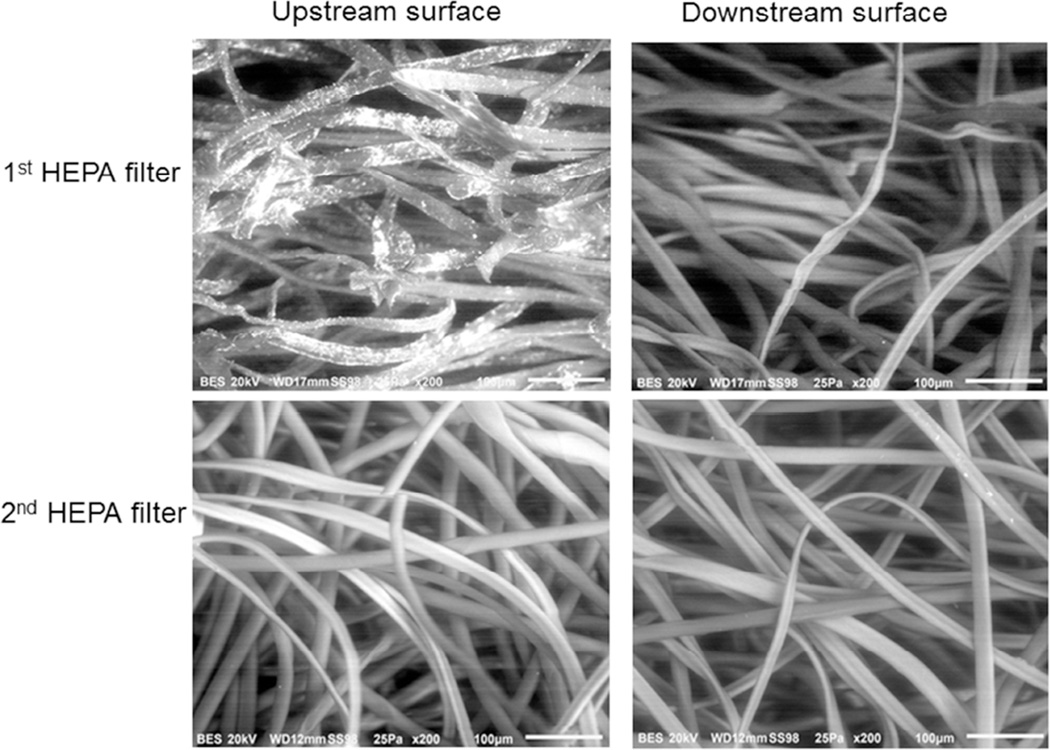
To confirm that no metal particles were able to pass through the HEPA filter, a second filter was added in-series downstream of the NO generator. SEM and EDX were used to determine whether any metal particles escaped the first filter and were trapped on the second filter (Fig. 4). Neither the upstream nor the downstream surfaces of the second filter contained metal particles. These results suggest that a single HEPA filter is sufficient to remove particles from the gas stream produced by the NO generator.
Figure 4.
Two HEPA filters were placed in series downstream of the NO generator for 10 days. SEM images of the 1st filter (Upper panels: Left, cross section upstream; right, cross section downstream), and the 2nd filter (Lower panels: left, upstream surface; right, downstream surface). Metal particles were detected on the upstream surface of the 1st HEPA filter, but no metal particles were observed on the downstream surface of the 1st filter or on either the upstream or downstream surface of the 2nd filter.
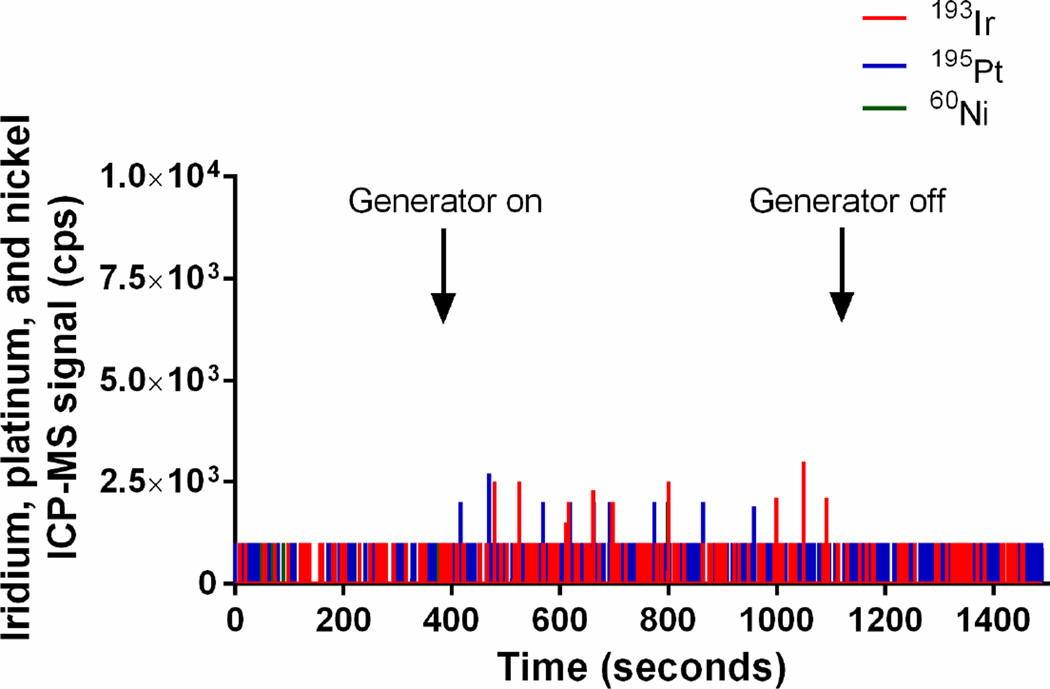
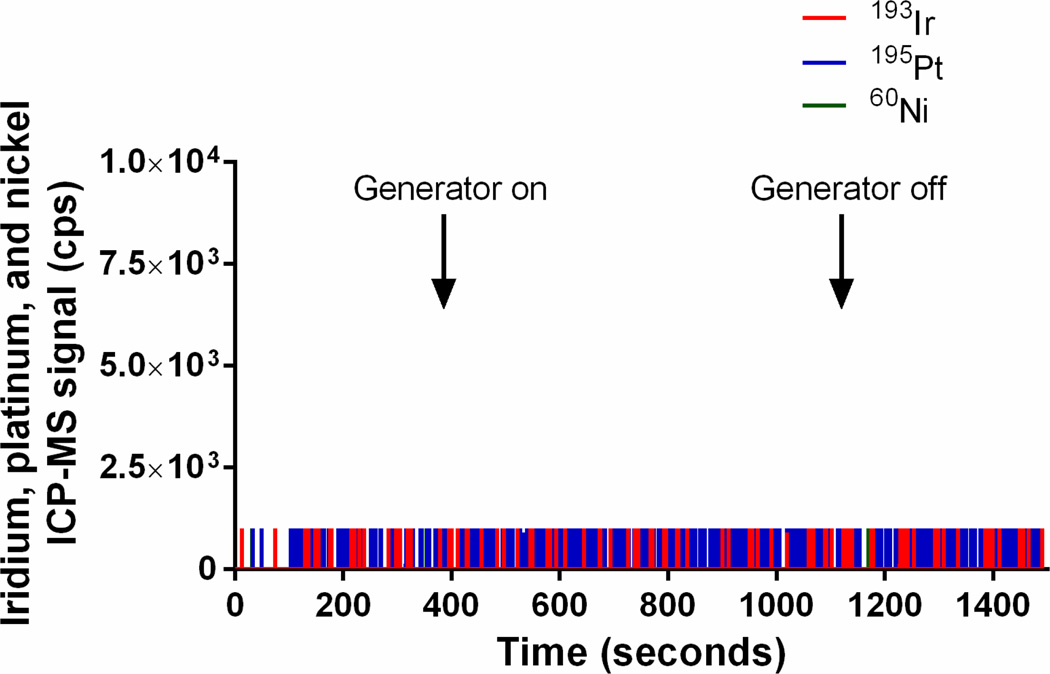
1.5 Measurement of iridium, platinum, and nickel in gas downstream of an electric NO generator
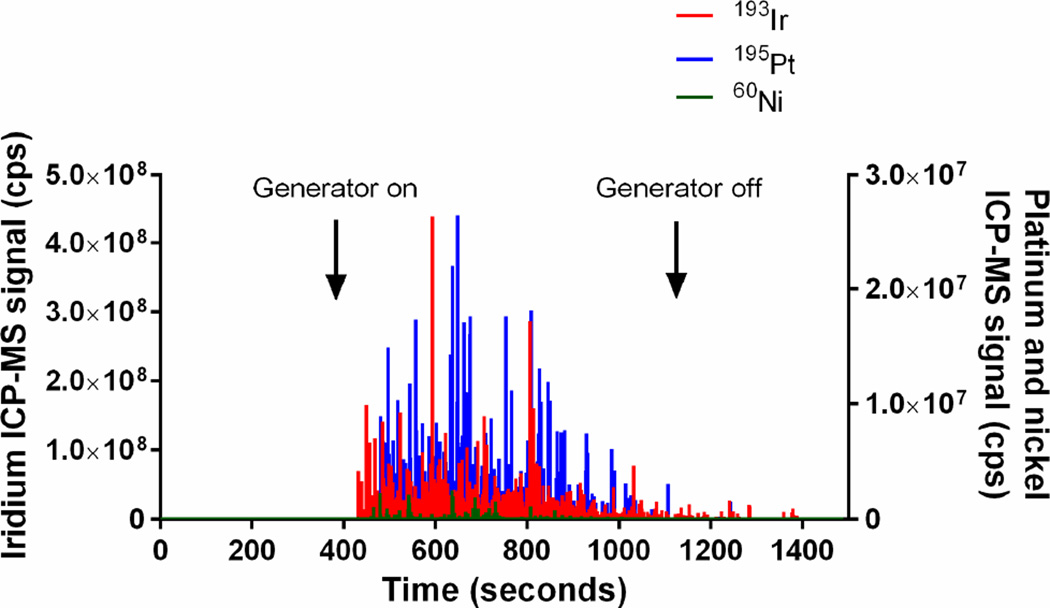
Because iridium, platinum and nickel were slowly released from the high voltage tip and the ground electrodes when generating NO, we examined the effluent gas stream for particles of metal using ICP-MS (Fig. 5). Without a HEPA filter, we measured iridium (up to 4.4×108 counts per second), platinum (2.6×107 counts per second), and nickel (2.2×106 counts per second) particles produced by the plasma during NO generation (Fig. 5A). With an in-line Ca(OH)2 (12 g) scavenger, but without a HEPA filter, there were very small amounts of iridium, platinum, and nickel particles (below 3×103 counts per second) in the outflow gas from the NO generator (Fig. 5B). With the NO generator followed by a Ca(OH)2 scavenger and a HEPA filter, no particles were detected (Fig. 5C). These data confirm that during NO generation the iridium high voltage electrode and the ground electrode release metal particles that can be effectively blocked by a scavenger and a single in-line HEPA filter.
Figure 5.
Plasma iridium, platinum, and nickel signals (counts per second, cps) in the outflow gas of the NO generator, as determined by quadrupole ICP-MS: (A) in the absence of a HEPA filter and Ca(OH)2 scavenger, (B) after a 12 g Ca(OH)2 scavenger, and (C) after both a single HEPA filter (0.22 µm) and a 12 g of Ca(OH)2 scavenger.
1.6 Measuring the levels of iridium and platinum in the lungs of mice breathing electrically generated NO for 28 days
To investigate the effects of breathing electrically generated NO on lung inflammation and potential metal deposition, we studied mice after breathing 50 ppm NO in air for 28 days. Effluent gas was treated with an in-line 75 g Ca(OH)2 scavenger to remove NO2, but without a HEPA filter. We hypothesized that the production of NO gas without a HEPA filter would provide a positive control group for the deposition of metals and subsequent inflammation in the lungs of mice. If noble metal particles were found in murine lung after breathing plasma generated NO using only the NO2 scavenger, we would then study mice breathing 50 ppm electric generated NO with both a scavenger and a HEPA filter. Mice in both the control group breathing air and the NO breathing group gained similar amounts of weight during the 28 days of study (mice breathing air gained 4±2 g versus breathing NO gained 3±2 g, P=0.2, online supplement Fig. 6). To assess for inflammation caused by breathing electrically generated NO, the murine airway (including the larynx, trachea, main bronchus) and lung tissues were histologically evaluated. All of the airway tissues, including the tracheobronchial tissue and the tissue of the distal lung, from both control and NO-breathing mice were normal. Photomicrographs of the cardiac and diaphragmatic lobes of a representative murine right lung are shown in the online supplement Fig. 7.
To detect if there were any metal particles deposited in the lung tissues of the mice, high-resolution magnetic sector field ICP-MS was applied, which is capable of definitive, interference-free elemental and isotopic identification (nominal mass resolution ~0.001 amu) and ultra-trace analytical sensitivity to metal levels as low as one part in 10−15 (one part per quadrillion, ppq). No difference was found in the levels of iridium or platinum in murine lungs between the air and NO breathing groups (online supplement, Table 2). These data suggest that it was safe for mice to breathe electrically generated 50 ppm NO for 28 days using only a Ca(OH)2 scavenger, without causing any adverse pulmonary histologic effects or iridium or platinum deposition in the lung.
Because the electrically generated NO was scavenged with Ca(OH)2 and delivered via plastic tubing into the breathing chamber, we reason that any metal particles were trapped by the scavenger or precipitated on the walls of the delivery tubing before reaching the murine lung. The system used to deliver NO to mice in this study differs from the methods that would be used to deliver NO to patients, which include nasal prongs [26] or a ventilator and endotracheal tube [27]. To mimic the NO delivery system used in patients, future studies will examine the lungs of larger animals that have been treated with plasma-generated NO delivered by nasal cannulae or a ventilator.
In conclusion, we report that electrodes used to electrically generate NO for up to 28 days underwent slow erosion. The plasma gas temperature during NO generation was approximately 1200°C. The high voltage tip is plated with pure iridium, and the platinum pad of the ground electrode is composed of 90% platinum and 10% nickel alloy. Data from SEM, EDX, and ICP-MS measurements showed that a single HEPA filter was sufficient to block all of the metal particles released during NO generation. In vivo studies showed that neither metal particles nor inflammation was found in the lungs of mice breathing electrically generated 50 ppm NO in air that was scavenged with Ca(OH)2.
Supplementary Material
Iridium electrodes erode slowly during prolonged plasma NO generation in air.
A single HEPA filter removed metallic contaminants from the effluent NO gas.
Breathing electrically generated NO for 4 weeks did not produce pulmonary injury.
Acknowledgments
This study was partially supported by grant from NHLBI B-BIC/NCAI (#U54HL119145) to W.M.Z. The portions of the study related to optical emission spectroscopy and plasma characterization were performed by a Drexel University team (D.D. and A. M.), and supported by funding from Massachusetts General Hospital. The ICP-MS study was supported by grants to L.E.G. for development of MIMS platform technology, NSF (0821304), and NIH (S10RR021131). Also, this study was supported by funding from laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.C.o.F.a. Newborn, Use of Inhaled Nitric Oxide. Pediatris. 2000;106:344–345. [PubMed] [Google Scholar]
- 2.Abman SH. Inhaled nitric oxide for the treatment of pulmonary arterial hypertension. Handb. Exp. Pharmacol. 2013;218:257–276. doi: 10.1007/978-3-642-38664-0_11. [DOI] [PubMed] [Google Scholar]
- 3.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N. Engl. J. Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 4.Breatnach CR, Flanagan F, James A, Corcoran JD, Franklin O, El-Khuffash A. The Use of Inhaled Nitric Oxide in a Tertiary Neonatal Intensive Care Unit. Ir Med J. 2015;108:275–278. [PubMed] [Google Scholar]
- 5.Sharma A, Obiagwu C, Mezue K, Garg A, Mukherjee D, Haythe J, Shetty V, Einstein AJ. Role of Vasodilator Testing in Pulmonary Hypertension. Prog Cardiovasc Dis. 2016;58:425–433. doi: 10.1016/j.pcad.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 6.INOmax. INOMAX has a well-established safety profile. 2016 [Google Scholar]
- 7.P.H.S. Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology. Drug Use Review. 2012 Oct 31;:1–9. [Google Scholar]
- 8.Subhedar N, Dewhurst C. Is nitric oxide effective in preterm infants? Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F337–F341. doi: 10.1136/adc.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namihira T, Tsukamoto S, Wang D, Katsuki S, Hackam R, Okamoto K, Akiyama H. Production of nitric monoxide using pulsed discharges for a medical application. IEEE Trans. Plasma Sci. 2000;28:109–114. [Google Scholar]
- 10.Sakai S, Matsuda M, Wang D, Kiyan T, Namihira T, Akiyama H, Okamoto K, Toda K. A compact nitric oxide supply for medical application. IEEE. 2007;1:752–755. [Google Scholar]
- 11.Lovich MA, Fine DH, Denton RJ, Wakim MG, Wei AE, Maslov MY, Gamero LG, Vasquez GB, Johnson BJ, Roscigno RF, Gilbert RJ. Generation of purified nitric oxide from liquid N2O4 for the treatment of pulmonary hypertension in hypoxemic swine. Nitric Oxide. 2014;37:66–72. doi: 10.1016/j.niox.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Hu H, Liang H, Li J, Zhao Q, He J. Study on production of inhaled nitric oxide for medical applications by pulsed discharge. IEEE Trans. Plasma Sci. 2007;35:619–625. [Google Scholar]
- 13.Samaranayake WJM, Miyahara Y, Namihira T, Katsuki S, Hackam R, Akiyama H. Ozone production by pulsed power in dry air. IEEE. 1999;2:1326–1329. [Google Scholar]
- 14.Yu B, Muenster S, Blaesi AH, Bloch DB, Zapol WM. Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy. Sci Transl Med. 2015;7:294ra107. doi: 10.1126/scitranslmed.aaa3097. [DOI] [PubMed] [Google Scholar]
- 15.Nemery B. Metal toxicity and the respiratory tract. Eur Respir J. 1990;3:202–219. [PubMed] [Google Scholar]
- 16.Kusaka Y, Sato K, Suganuma N, Hosoda Y. Metal-induced lung disease: lessons from Japan's experience. Journal of Occupational Health. 2001;43:1–23. [Google Scholar]
- 17.Rendall RE, Phillips JI, Renton KA. Death following exposure to fine particulate nickel from a metal arc process. Ann Occup Hyg. 1994;38:921–930. doi: 10.1093/annhyg/38.6.921. [DOI] [PubMed] [Google Scholar]
- 18.Machala Z, Janda M, Hensel K, Jedlovsky I, Lestinska L, Foltin V, Martisovits V, Morvova M. Emission spectroscopy of atmospheric pressure plasmas for bio-medical and environmental applications. Journal of Molecular Spectroscopy. 2007;243:194–201. [Google Scholar]
- 19.Laux CO, Spence TG, Kruger CH, Zare RN. Optical diagnostics of atmospheric pressure air plasmas. Plasma Source Science and Technology. 2003;12:125–138. [Google Scholar]
- 20.Crnkovic S, Hrzenjak A, Marsh LM, Olschewski A, Kwapiszewska G. Origin of neomuscularized vessels in mice exposed to chronic hypoxia. Respir Physiol Neurobiol. 2011;179:342–345. doi: 10.1016/j.resp.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Beloiartsev A, Rodrigues-Machado MD, Zhou GL, Tan TC, Zazzeron L, Tainsh RE, Leyton P, Jones RC, Scherrer-Crosbie M, Zapol WM. Pulmonary Hypertension After Prolonged Hypoxic Exposure In Mice With a Congenital Deficiency of Cyp2j. Am J Respir Cell Mol Biol. 2015:563–570. doi: 10.1165/rcmb.2013-0482OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osamura H, Abe N. Development of new iridium alloy for spark plug electrodes. SAE Trans. 1999;108:1063–1074. [Google Scholar]
- 23.Jehn H, Volker R, Ismail MI. Iridium losses during oxidation. Platinum Metals Review. 1978;22:92–97. [Google Scholar]
- 24.A.f.T.S.a.D. Registry. Public Health Statement for Nickel. 2005 Aug [Google Scholar]
- 25.U.S.D.o.L.a.O.S.a.H. Administration. Platinum Exposure Limits [Google Scholar]
- 26.Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, Hagar RW, Howard T, Nuss R, Okam MM, Tremonti CK, Berman B, Villella A, Krishnamurti L, Lanzkron S, Castro O, Gordeuk VR, Coles WA, Peters-Lawrence M, Nichols J, Hall MK, Hildesheim M, Blackwelder WC, Baldassarre J, Casella JF. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305:893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wessel DL, Adatia I, Thompson JE, Hickey PR. Delivery and monitoring of inhaled nitric oxide in patients with pulmonary hypertension. Crit Care Med. 1994;22:930–938. doi: 10.1097/00003246-199406000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.