Abstract
Objective
Since longitudinal studies determined that anxiety is a strong risk factor for hot flashes, we hypothesized that an anxiogenic stimulus that signals air hunger (hypercapnic, normoxic gas) would trigger an exacerbated hot flash-associated increase in tail skin temperature (TST) in a rat ovariectomy (OVEX) model of surgical menopause and hot flashes in symptomatic menopausal women. We also assessed TST responses in OVEX serotonin transporter (SERT)+/− rats that models a common polymorphism that is associated with increased climacteric symptoms in menopausal women and increases in anxiety traits.
Methods
OVEX and sham-OVEX rats (initial experiment) and wildtype and SERT+/− OVEX rats (subsequent experiment) were exposed to a 5 min infusion of 20%CO2 normoxic gas while measuring TST. Menopausal women were given brief 20% and 35%CO2 challenges, and hot flashes were self-reported and objectively verified.
Results
Compared to controls, OVEX rats had exacerbated increases in TST, and SERT+/− OVEX rats had prolonged TST increases following CO2. Most women reported mild/moderate hot flashes after CO2 challenges, and the hot flash severity to CO2 was positively correlated with daily hot flash frequency.
Conclusions
The studies demonstrate that this anxiogenic stimulus is capable of inducing cutaneous vasomotor responses in OVEX rats, and eliciting hot flashes in menopausal women. In rats, the severity of the response was mediated by loss of ovarian function and increased anxiety traits (SERT+/−), and, in women, by daily hot flash frequency. These findings may provide insights into anxiety related triggers and genetic risk factors for hot flashes in thermoneutral environments.
Keywords: hypercapnia, stress, anxiety, serotonin, thermoregulation, acidosis
Introduction
Hot flashes are intense heat sensations that occur primarily in the chest and face and affect the vast majority of women at midlife or following estrogen inhibition therapies or oophorectomy (1, 2). In the laboratory setting, the subjective hot flash sensation (as indicated with button press or self-report) correlates well with objectively measured, sympathetically-mediated increases in cutaneous vasodilation which pools 37°C blood to the skin. This in turn interacts with peripheral thermal sensors in skin to signal heat. Sweating sometimes also occurs during a hot flash, but this induces an evaporative cooling sensation. Under normal circumstances, both cutaneous vasodilation and sweating are effective means of dissipating heat to maintain core body temperature (Tc) in warmer environments. While environmental temperature is often thought to affect hot flash parameters (i.e., frequency, severity, duration), many studies have found weak or inconsequential relationships to ambient temperature (3-5), and in a self-reported survey of hot flash precipitants, temperature was identified less frequently than other stimuli (6). While exact figures are unavailable, these data suggest that many women may experience hot flashes in thermoneutral environments with no clear identifiable trigger. In these circumstances, the collective heat dissipation mechanisms that occur during a hot flash could lead to decreases in Tc which does occur and could explain chills that also occur in menopausal women [(7) and see review (8)].
While there are many variables that influence hot flashes, recent findings from large longitudinal studies have identified the presence of anxiety, early life stress, and lower socioeconomic status as key risk factors for more severe and problematic hot flashes (9, 10). Notably, anxiety has been identified as the best predictor of hot flashes, even when controlling for typical confounds, and women with greater anxiety levels experience more severe hot flashes (10, 11). Additionally, many women report that stressful stimuli in everyday life precipitate hot flashes (6), and stressful stimuli in a laboratory setting have been shown to increase objectively measured hot flashes (12). Taken together, there is evidence that stressful events may provoke hot flashes, and that pre-existing anxiety states or traits may contribute to increased symptoms. However, the mechanisms by which anxiety and stress-related factors contribute to hot flashes remain poorly understood.
One contributing factor to the poor understanding of the mechanisms mediating hot flashes is a scarcity of appropriate, validated animal modeling (13). Previous models such as the morphine withdrawal model (14) elicit robust cutaneous vasomotor responses in the tail, but lack an obvious relevance to causes and triggers of hot flashes (15, 16). Consequently, the use of models for mechanistic investigation into the central and peripheral neural circuitry and associated pathways mediating hot flashes has suffered. Recently, we found profound decreases in Tc in rats following an acute 20% carbon dioxide (CO2) exposure, which produces anxiety-associated behavior (social and open field avoidance, fear-associated freezing, and avoidance of a 15-20%CO2 filled chamber in a two chamber place preference test) and sympathetic associated cardioexcitation (17-21). In human studies, 20%CO2 inhalation induces anxiety symptoms (22, 23), and in another study the participants reported anxiety that was also associated with strong heat sensations following a single inhalation of 35%CO2 (24). However, it was unknown if this would have relevance to menopause-associated symptoms.
Therefore, we designed a series of studies to determine if CO2 exposure represented a novel method of provoking hot flashes. Thus, in a rodent model, we first hypothesized that 20% CO2-induced decreases in Tc were due to cutaneous vasomotor responses in the tail which would increase the tail skin temperature (TST) to dissipate heat in a thermoneutral environment. We further posited that the TST response would be exacerbated in ovariectomized (OVEX) rats, modeling surgical oophorectomy-induced menopause, which is linked to more severe hot flashes (2, 25). Second, in a small, proof of concept study, we hypothesized that 20% CO2 and 35% CO2 inhalation tests would elicit a subjective hot flash in symptomatic menopausal women. Finally, recent work has begun to elucidate some of the genetic contributions to increased or problematic hot flashes. The short (‘s’) version of the serotonin transporter (SERT) gene, whose protein product mediates the termination of serotonin signaling, leads to reduced transcriptional efficiency and less protein, and is linked to anxiety-associated traits in humans (26-29) as well as increased climacteric symptoms in menopausal women (30). Therefore, our last hypothesis was that OVEX rats with a heterozygous null mutation of the SERT gene (SERT+/−) which leads to similar reduced transcriptional efficiency, would have exacerbated and/or prolonged hot flashes (increased TST) in response to CO2 compared to wildtype (WT) OVEX controls.
Materials and Methods
Animal Experiments
Animals and Housing Conditions
Adult female (225-250 g) Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN USA). Rats with heterozygous expression of the serotonin transporter (SERT) were purchased from Charles River Laboratories (Wilmington, MA USA) and bred in-house on a Wistar background from Wistar rats purchased from Harlan Laboratories (Indianapolis, IN). These rats possess a null mutation of the SERT generated by N-methyl-N-nitrosurea-mutation which results in an approximately 50% reduction in expression of SERT mRNA within the brain as described previously (31). New Wistars from Harlan (Indianapolis) were bred into the colony every three generations to prevent genetic drift. Female Wistar rats (225-250 g) were purchased (Harlan, Indianapolis) as wildtype controls and were housed in the same conditions as the other experimental rats (maintained at 22°C on a 12:12 light/dark cycle with lights on at 0700h) for at least 5 days prior to experimental procedures and had ad libitum access to food and water. We have also confirmed that, compared to Wistar male wildtype controls, the male SERT−/+ rats have an approximately 50% reduction in SERT mRNA within a brain region densely populated with serotonergic neurons (i.e., 300uM coronal brain punches of dorsal and median raphe nuclei (unpublished observations). All procedures were approved by the Institutional Animal Care and Use Committee at Indiana University-Purdue University, Indianapolis, and in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (NIH publication no. 80-23) revised 1996.
Ovariectomy
Female rats were anesthetized under isoflurane (2-3% by volume in atmospheric air; MGX Research Machine, Vetamac, Rossville, IN USA; Praxair Inc., Indianapolis, IN USA) delivered through a nosecone. The skin was shaved and sterilized prior to incision; the ovaries were visualized and excised bilaterally. For sham-ovariectomized rats, the ovaries were visualized but not excised. Rats were sutured, given pain medication, and allowed 12 days of recovery prior to experimental manipulations.
CO2 provocation of hot flash
Flow cages (12 in. width × 12 in. height × 24 in. length) were custom-built using Plexiglas®. When the lid of the cage was latched, gases could only enter the cage through an inlet connector (for the gas infusion) and could only exit the cage through an outlet connector. The gas flow into the cages was controlled using a 2-stage regulator (Praxair, Inc., Danbury, CT USA) at a pressure of 0.6 Bar. We previously validated the consistency of the rate of CO2 delivery using state-of-the-art infrared CO2 (ProCO2) and electrochemical O2 (ProO2) sensors (32). Specifically, concentrations of O2 remain at 21% throughout the gas infusion in the control and experimental cages [see (Johnson et al., 2005)]. The CO2 concentration remains constant at < 1 % in the control cage during exposure of rats to atmospheric air (< 1% CO2 / 21% O2/ 79% N2). Infusion of the premixed normoxic, hypercapnic gas (20% CO2 / 21% O2/ 59% N2) results in a rapid increase in CO2 concentration from < 1% CO2 up to 20% CO2 at the 5 min time point. After terminating gas infusion and opening the cages, the concentration of CO2 rapidly decreases from 20% CO2 to < 2.5% CO2 during the following 5 min. Using a portable iSTAT gas analyzer (HESKA, Des Moines, IA USA) we have also determined that this hypercapnic, normoxic challenge leads to arterial pCO2/pH levels of ~ 130mmHg/7.01 during the challenge that are back to normal physiological range (~50mmHg/7.37) within 2 min post challenge (unpublished data). Prior to placement in the apparatus, a thermistor (Omega Precision Fine Wire Thermocouples, Part #5SRTC-TT-K-30-36; Omega Engineering, Stamford, CT USA) was secured to the ventral side of the tail approximately 1 cm from the base using 3M Durapore® tape to measure tail skin temperature. The thermistor was attached to a T-type pod and connected to a Powerlab system running LabChart software (ADInstruments, Colorado Springs, CO USA) for continuous monitoring of temperature. After obtaining a stable baseline temperature in atmospheric air, rats were exposed to hypercapnic gas for 5 min, and then atmospheric air was infused again for 10 min following the challenge.
Atmospheric Air Control Experiment
To determine if the experimental environment alone caused a change in tail skin temperature, the above experiment was performed, but only atmospheric air was infused for the duration of the experiment. After obtaining a stable baseline, the air was turned off and immediately turned back on to mimic the gas change during a CO2 infusion. Rats recovered in atmospheric air for 10 min following the challenge.
Infrared Thermography
As an additional verification of temperature change, thermal images were acquired following hot flash provocations using a FLIR T440 thermal imaging camera (FLIR Systems, Boston, MA USA) with standard settings taken at a height of 1 m. Analysis of images was performed using FLIR Tools to standardize scaling between thermal images.
Human procedures
Sample
Six peri- or post-menopausal English-speaking women with at least 4 hot flashes per 24 hour day were recruited. Each participant gave written informed consent after one of the investigators explained the nature, purpose, and risks of the study. All participants were screened for medical and psychiatric history. To be eligible, all participants reported daily hot flashes (verified on 7-day diary), were afebrile, had a resting heart rate < 110 beats per minute, a respiratory rate of 8-18 breaths per minute, oxygen saturation above 94%, and were normotensive (BP < 160/100). Women were excluded if they had respiratory or cardiac disease (COPD, asthma, emphysema, hyperventilation syndrome, heart disease), hypertension, insulin-dependent diabetes, cancer, Axis I diagnosis (depression, panic disorder, generalized anxiety disorder), personal/familial history of cerebral aneurysm, were taking any medications for hot flashes, were substance abusers (including excessive smoking, i.e. >10 per day), were trained athletes, or were pregnant or breastfeeding. Participants were compensated $50 at the end of the study.
Measures
Demographic information was elicited by the investigator using a standard questionnaire. Participants completed a checklist type form to provide age, race, ethnicity, education, menopausal status, and other information.
Hot flashes were measured as follows. Using a hot flash diary, women were asked to record the number and severity of their hot flashes for seven consecutive days. This information was used to verify eligibility. To evaluate hot flash severity during the study, participants completed a numeric rating scale (NRS). During the study visit, participants rated the severity of any reported hot flashes from 0 (not at all) to 10 (extremely) during the study visit, and all self-reported hot flashes were verified with objective sternal skin conductance monitoring (Biolog, UFI Serving Science, Morro Bay, CA USA).
Anxiety was rated using two self-report scales. Using a numeric rating scale (NRS) of 0 (not at all) to 10 (extremely), participants rated how anxious they felt at baseline, after the control room air inhalation, after the CO2 challenge conditions, and at discharge. Higher scores on the NRS indicated greater anxiety. In addition, participants also completed the State version of the short form of the State-Trait Anxiety Inventory, a 10-item inventory, to assess anxiety “in the moment”. All items on the scale are positively worded (e.g., I feel calm) and each item is rated from 1 (not at all) to 4 (very much so) so that higher total scores indicate less state anxiety. The STAI was completed at baseline and immediately prior to discharge.
Procedures
Procedures were HIPAA compliant and approved by the Indiana University-Purdue University, Indianapolis Institutional Review Board prior to study implementation. Women were recruited through self-referral and emails to potentially eligible research registry members. For registry participants, once emails had been sent by the registry, the research team attempted to contact each woman by telephone. Self-referrals phoned or emailed the research team on their own. Once contact was made with a potential participant, trained personnel used a script to screen them for eligibility. If eligible, women were emailed the hot flash diary and instructed on how to complete it. Once diary responses were sent via phone, email, mail or secure fax, eligibility was confirmed (i.e., daily hot flashes met threshold) and study visits were scheduled at a mutually convenient time. Email or phone call reminders were done prior to study visits.
Each participant arrived at the university Clinical Research Center at a pre-determined time. Written informed consent and authorization to use health information was obtained. Trained research staff assessed baseline vital signs (heart rate, respiratory rate, blood pressure, and temperature), peripheral oxygen saturation levels, and height and weight, and women were connected to the hot flash monitor. Next, demographic information and anxiety scales (STAI, NRS) were completed. A non-rebreather mask was fitted for each participant. Through this mask, the participant inhaled normal room air for 10 minutes to become acclimated to the mask. The anxiety NRS was completed by the participant, followed by a 3-minute rest period. Next, the participant inhaled 20% CO2 for 40 seconds via the mask. The hot flash NRS and the anxiety NRS were completed, followed by a 15-minute rest (the rest period was extended by 5 minutes if the participant had a hot flash), and then a vital-capacity, double-breath inhalation of 35% CO2. A final anxiety NRS and STAI anxiety were completed by the participant; see Fig. 2a for a schematic of study procedures. As mentioned in the introduction, 20% and 35% CO2 inhalation procedures have been utilized previously with healthy humans to induce and study anxiety symptoms (23) and reliably produce stable autonomic responses, including increases in skin conductance and heart rate, across multiple sessions (33). Here we utilized the NRS to measure anxiety symptoms at baseline, following mask and control air and immediately post CO2 inhalation, but the NRS and STAI were also utilized to verify that there were no lingering anxiety effects 15-20 min post CO2 inhalation. Previous work has demonstrated that there are no increases in severe anxiety at 6 and 12 months following 20% CO2 challenges [compared to participants exposed to control conditions; (34)]. Vital signs were taken and the participant was discharged to home.
Figure 2. Small clinical study of hot flash provocation with CO2 challenges in highly symptomatic women.
a) Timeline for the study procedures; a room air acclimation (through a mask) was given for 10 min prior to a rest period before a 40s, 20% CO2 (normoxic) challenge. Participants rested for 15-20 min prior to the 35% CO2 administration. b) Table illustrates participants assigned number, age, total hot flashes during the day and night for a 7 day period prior to the study, average hot flashes per day, baseline STAI and NRS anxiety, NRS anxiety post control air, NRS hot flash and anxiety (respectively) severity post 20% and 35% CO2 inhalation, followed by the NRS Anxiety and STAI anxiety at rest. Participants are ranked in order of increasing daily hot flashes. 3 of the 4 participants, and 2 of the 3 participants with confirmed inhalations of 20% CO2 and 35%CO2 reported hot flashes within 5 min, respectively; hot flashes were rated from mild to moderate. Numbers below NRS hot flashes or anxiety (columns 9 and 10, respectively) post CO2 respectively represent 20% CO2 and 35% CO2 with the parenthetical number representing the mean of the confirmed inhalations (“-” indicates no confirmed inhalation for respective challenge). Correlation analyses revealed that the frequency of daily hot flashes (total per 24h day) was positively correlated with the c) mean severity of the hot flash post confirmed CO2 inhalations and d) mean severity of the anxiety response post confirmed CO2 inhalations. Correlation analysis also demonstrated that the e) mean hot flash severity following verified CO2 inhalation was positively correlated with the mean anxiety rating following verified CO2 inhalation.
Statistical Analyses
For animal experiments, dependent variables for analyses of tail skin temperature (TST) were analyzed using a one-way ANOVA with repeated measures, using gas infusion as the between-subjects factor and time as a within-subjects factor. In the presence of significant main effects or main effect × time interactions, Fisher’s Least Significant Difference (LSD) test was used for post hoc testing between-subjects comparisons. For the clinical component, an ANOVA over time was used to determine the effect of 20% CO2 on NRS anxiety scores; Dunnett’s post hoc test was used to determine specific time point differences. Pearson’s correlation was used to determine relationships between hot flash frequency (daytime, nighttime, and average daily) and NRS anxiety scores or hot flash intensity following 20% CO2 or 35%CO2 challenges. The alpha level was set at 0.05 in all cases. All statistical analyses were carried out using SPSS 21.0 (SPSS Inc., Chicago, IL, USA), and all graphs were generated using SigmaPlot 12.0 (SPSS Inc.) and an illustration program (CorelDraw X5 for Windows, Viglen Ltd., Alperton, UK).
Results
Experiments 1-2: Ovariectomized rats exhibit exacerbated hot flash-associated increases in tail skin temperature in response to hypercapnic gas infusion while atmospheric air infusion does not change tail skin temperature
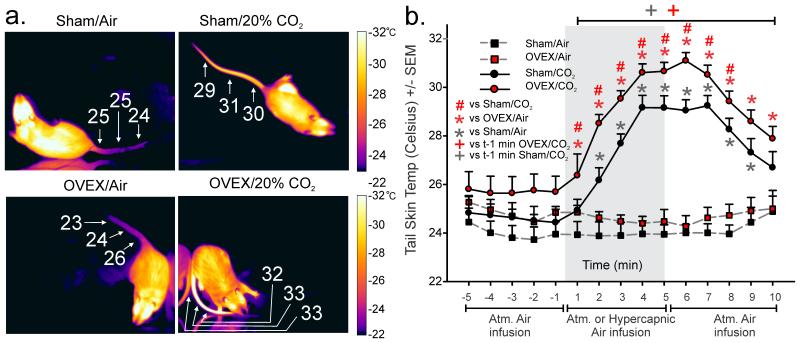
An ANOVA with ovex surgery and hypercapnic gas/CO2 as main factors and time as a repeated measure revealed that hypercapnic gas infusion produced an exacerbated increase in tail skin temperature in ovex rats while atmospheric air caused no changes in temperature [Fig 1a-Representative thermal image; Fig. 1b- CO2 tail skin temperature (n=15/group) overall CO2 effect F(1,38)=45.9, p<0.0001, overall ovex effect F(1,38)=5.3, p=0.027, and overall CO2 by time interaction F(114,532)=20.1, p<0.0001 but no ovex by time interaction F(14,532)=0.23, p=0.999 or ovex by CO2 by time interaction F(14,532)=0.34, p=0.989]. Fisher’s Least Significant Difference Test was used for post hoc testing to determine specific time point differences between groups, and Dunnett’s test was used to determine significance of specific time points against t-1.
Figure 1. Rats modeling surgical menopause exhibit exacerbated hot flash-associated tail skin temperature responses.
Effects of ovariectomy or sham surgery on baseline tail skin temperature in response to hypercapnic or atmospheric air infusion. a) Representative thermal images with scale (to the right) of a sham-OVEX (top) or OVEX rat (bottom) after 5 min exposure to atmospheric air (left) or 20% CO2 (right). b) Line graph with error bars (SEM) represents mean tail skin temperature prior to and following atmospheric air or hypercapnic gas challenge in OVEX or sham-OVEX groups assessed with a tail thermistor at the base of the tail (n=15/group for CO2 challenge and n=6/group for atmospheric air challenge). *denotes significance of surgical treatment at specific time points in (b) with Fisher’s Least Significant Difference test protected by an ANOVA, and + denotes significant differences over time from t-1 as measured by Dunnett’s test.
Experiment 3: CO2 challenges elicit hot flashes in symptomatic women
A total of 113 women were contacted (109 research registry and 4 self-referrals). Of these 113, 56 were successfully reached and completely screened for eligibility. Of the 56, 37 were not eligible and 7 were not interested, leaving 12 eligible and interested women. These 12 women were sent diaries but 5 were not eligible on diary return and 1 did not return her diary. Of the remaining 6 women, all completed the study visit.
Sample demographics were as follows for the five participants that successfully completed at least one CO2 inhalation. The participants had a mean age of 49.8 (SD=2.77) and 17.00 years of education (SD=3.32). Four of five participants were were non-Hispanic White and one was non-Hispanic Black, 4/5 were married (1 was other), 4/5 reported no difficulty paying for basics (1 reported some difficulty), and all worked full-time outside the home. Comorbidities included hypothyroidism (n=1), chronic interstitial cystitis (n=1), and non-insulin dependent diabetes (n=1). Total number of prescription medications taken per participant was 1.00 (SD=1.0, range 0 to 3).
The screening week hot flash diaries showed an average of 7.00 hot flashes during the daytime (SD=4.15, range 3.42 to 13.57), and 3.29 hot flashes during the nighttime (SD=0.96, range 2.29 to 4.86), for an average of 10.29 total hot flashes per 24-hours per participant (SD=4.24, range 5.86 to 16.43).
Five of six participants reported hot flashes after CO2 challenge (see Fig. 2b). One participant had no confirmed inhalations during either CO2 challenge and was removed from all analyses. Three of the four participants with confirmed inhalations of 20% CO2 reported hot flashes within 5 min of the challenge that ranged from mild to moderate, and two of the three participants with confirmed inhalations of 35% CO2 reported a hot flash within 5 min of the challenge. Numbers below “NRS hot flashes post CO2” respectively represent hot flash severity rating following 20%CO2 (Fig. 2b column 9), and 35%CO2 with the parenthetical number representing the mean NRS of confirmed inhalations; a “-” indicates no confirmed inhalation which was evidenced by removal of the mask and coughing, or confirmation that inhalations were not completed. Numbers below “NRS anxiety post CO2” respectively represent 20%CO2 and 35%CO2 (Fig. 2b, column 10, a “-” indicates no confirmed inhalation). Average severity of CO2-induced hot flashes was a 4 (range 3-5) after 20% CO2, and 2.5 (range 1-4) after 35% CO2. NRS ratings of anxiety approached significance following inhalation of 20%CO2, when compared to NRS anxiety ratings post inhalation of air (p=0.062 with paired Wilcoxon post hoc test). However, there was a positive correlation between average total number of hot flashes per day and the mean hot flash severity following confirmed CO2 inhalations, r=0.921, p=0.026, n=5 (Fig. 2c). There was a positive correlation between average total number of hot flashes per day and NRS scores for anxiety following verified CO2 inhalation, r=0.942, p=0.016, n=5. (Fig. 2d). There was a positive correlation between NRS hot flash severity following confirmed CO2 inhalation and NRS anxiety following confirmed inhalation, r=0.877, p=0.049, n=5 (Fig. 2e). Vital signs were in the normal range at the end of the study visit for all participants, and there were no lingering effects on anxiety or vital signs. STAI scores of 39 to 40 out of 40 possible (no anxiety) were measured in all participants at both baseline and end of the study visit.
Experiment 4: Ovariectomized SERT+/− rats exhibit prolonged hot flash-associated increases in tail skin temperature in response to hypercapnic gas infusion
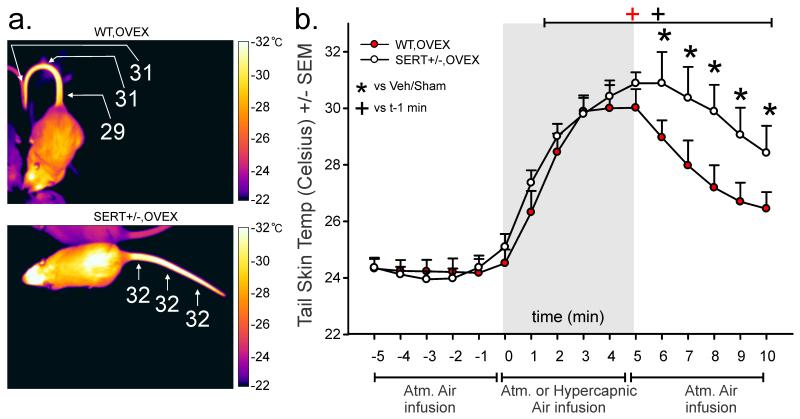
An ANOVA with genotype as the main factor and time as a repeated measure revealed that hypercapnic gas infusion produced a prolonged increase in tail skin temperature in ovex rats [Fig 3a- Representative thermal image; Fig. 3b- CO2 tail skin temperature (n=7,8) overall time effect F(13,392)=47.8, p<0.0001 and overall genotype × time interaction F(15,180)=2.1, p=0.013]. Fisher’s Least Significant Difference Test was used for post hoc testing to determine specific time point differences, and Dunnett’s test was used to determine significance of specific time points against t-1.
Figure 3. Rats with a heterozygous null mutation of the serotonin transporter (SERT+/−) have prolonged hot flash-associated tail responses to hypercapnic gas infusion.
Effects of ovariectomy in SERT+/− and WT rats tail skin temperature in response to hypercapnic gas infusion. a) Representative thermal image with scale (to the right) of a WT-ovex (top) or SERT+/− rat (bottom) after 5 min exposure to 20% CO2. b) Line graph with error bars (SEM) represents mean tail skin temperature prior to and following hypercapnic gas challenge in ovex SERT+/− or WT rats assessed with a tail thermistor at the base of the tail (n=8,7). *denotes significant effect of estrogen treatment with a two-way ANOVA p=0.001 in (a). *denotes significance of genotype at specific time points in (c) with Fisher’s Least Significant Difference test protected by an ANOVA, and + denotes significant differences over time from t-1 as measured by Dunnett’s test.
Discussion
These studies have demonstrated that an anxiogenic stimulus can precipitate an exacerbated hot flash-associated increase in TST responses in OVEX rats compared to sham-OVEX controls, which, to the best of our knowledge, is the first demonstration of threshold differences between OVEX and intact rats in response to CO2. Following similar CO2 inhalations, symptomatic menopausal women reported mild to moderate objectively verified hot flashes which approached significance for an increase in anxiety ratings following 20% CO2 inhalation. The mean number of daily hot flashes was positively correlated with the severity of hot flashes and anxiety ratings post confirmed inhalations of CO2. Our studies provide potential insights into triggers of hot flashes in thermoneutral environments and evidence that anxiety or anxiety-generating stimuli may have a causal relationship to hot flashes, not merely an associative one. Earlier clinical provocations used the drug yohimbine to facilitate hot flashes (35), which, at high doses, elicits strong anxiety (36, 37), and also produces robust TST responses in OVEX rats (38), and thus yohimbine may represent a pharmacological model preclinically and clinically. A weakness of the present rodent studies was that only CO2 was used as a stressor. However, we recently demonstrated that the GABAergic compound FG-7142 (a partial inverse benzodiazepine receptor agonist) which causes anxiety and flushing clinically (39) elicited rapid hot flash-associated increases in TST in OVEX rats but not sham control rats (40). Other studies have previously documented that stress-related stimuli, including mental arithmetic or emotionally salient films, can increase the rate of objective and subjective hot flashes (12) and self-reports implicate stressful stimuli (e.g. interpersonal family conflict or social stress) precipitate hot flashes in breast cancer patients (6). These data complement the emerging literature relating to the role of anxiety and stress-related factors in hot flashes and may help to start addressing a critical gap in knowledge of hot flash triggers.
In our preclinical rodent experiments, the results exhibit validity in three domains: 1) face validity, as the increase in TST is a rapid heat loss mechanism (cutaneous vasodilation) analogous to and what would be expected during a hot flash; 2) construct validity, as the TST change is exaggerated in ovariectomized rats, which is a model of surgical/oophorectomy-induced menopause in women (a condition with more severe hot flashes (2, 25)); and 3) predictive validity, as the same stimulus causes subjective hot flashes in menopausal women, and is exacerbated in ovariectomized SERT+/− rats, which mimic a common human polymorphism that was recently linked to increased hot flash pathology (30).
This modeling approach is unique in that the provocation does not use pharmacological manipulations; rather, it triggers an endogenous challenge to homeostasis by provoking air hunger through CO2 exposure. It is important to note that increasing concentrations of CO2 in the blood combine with water to form carbonic acid to induce acidosis, and CO2 also easily crosses the blood-brain barrier to interact with central chemoreceptive systems (41, 42). The 20%CO2 exposure used here produces a brief drop in blood pH to ~7.1-7.2 (19), and also alters pH within anxiety brain circuits (21). Importantly, these studies suggest that disease states or conditions associated with respiratory or metabolic acidosis may be risk factors for hot flashes and may reduce the threshold for a hot flash to occur. Such conditions include chronic obstructive pulmonary disease [COPD; pH has been reported to be decreased to 7.35 during sleep (43), diabetic ketoacidosis [pH 7.2 with a range of 6.78-7.39 (44), and other factors that make breathing (and expiring adequate CO2) difficult, such as in smokers, asthmatic persons, or obese persons. Indeed, obesity and smoking are well-established risk factors for hot flashes (45, 46). Additionally, stress induced hypoventilation or environments with poor ventilation and higher than normal levels of CO2 could contribute to lowering the hot flash threshold by inducing dyspnea. Finally, the anxiety response to acidosis or other stressors may also be relevant triggers and not just a response to the hot flash. Changes in pH are revealing in light of other work that has demonstrated a key role for pH in regard to thermoregulation and anxiety. The amygdala, a key brain structure for fear processing and anxiety, and 20%CO2 exposure used here produces acidosis in the amygdala, and local induction of acidosis induces robust fear associated freezing in mice (21). In persons with limbic encephalitis, which reversibly lesions the amygdala, emotional sweating is absent, yet thermoregulatory sweating was intact (47). Thus, emotional stimuli and emotional circuits also appear to be relevant to hot flashes, and it is possible that under conditions of reduced pH or elevated CO2 (which causes some peripheral vasodilation), anxiety is elicited and emotional sweating occurs. This anxiety, in combination with environmental conditions (i.e., slightly increased ambient temperature) may further exacerbate the heat dissipation response and trigger a hot flash.
Currently, the role of blood pH in hot flashes is largely unknown. Paced respiration (which could moderate blood pH) such as controlled breathing of 6-10 breaths per minute, is often recommended as a non-pharmacological treatment for reducing hot flashes, yet clinical trials have not demonstrated significantly greater efficacy than usual treatment or active comparators (48-50).
Here our challenges utilized systemic administration through either a mask or environmental exposure (the latter necessitating inhalation leading to systemic exposure). Dissection of the central and peripheral components of the CO2-induced hot flashes and TST responses in rats is beyond the scope of this investigation, but the paradigms we used most likely triggered central mechanisms, as previous applications were used to elicit and study anxiety in humans and in rodents that are accompanied by sympathetic associated cardiovascular responses. However, CO2 has also been shown to have local cutaneous vasodilatory action in both humans and rodents (51, 52). Thus, there are likely central and peripheral contributions to hot flashes (53).
In this case, evidence for central mechanisms comes from ex vivo assessments of cellular responses in rodents where 20%CO2 exposure increases activity in anxiety and thermoregulatory associated brain circuitry such as the hypothalamus and brainstem (18, 32). Within the hypothalamus, there is substantial overlap between nuclei that respond to 20% CO2 and expression patterns of estrogen receptors α and β (ERα, ERβ). Specifically, the paraventricular nucleus responds strongly to hypercapnic gas, and also has very dense expression of both ERβ (54) and an excitatory transmembrane estrogen receptor (e.g., GRP30) (55), whereas the more posterior hypothalamic regions are similarly responsive to CO2 yet expresses ERα (54) and GRP30 (55). Noradrenergic and serotonergic neurons in the midbrain pons and medulla also exhibit hypercapnic responsivity (28). Hypothalamic and brainstem sites including serotonergic nuclei (dorsal raphe and raphe pallidus) and noradrenergic nuclei (locus ceruleus) were found to significantly increase in response to FG-7142 hot flash provocation as well (40). These latter sites may be especially relevant to hot flashes in women because both the noradrenergic and serotonergic systems are treatments for hot flashes with selective serotonin and/or norepinephrine reuptake inhibitors (56, 57). Taken together, these results provide additional validity that the neural mechanisms mediating cutaneous vasomotor responses induced by CO2 may be relevant to spontaneous hot flashes.
Our rodent studies also contribute to an emerging literature concerning the 5-HTTLPR on climacteric symptoms and anxiety trait vulnerability. Short form ‘s’ carriers (s/s or s/l genotype) of the 5-HTTLPR experience higher rates of psychiatric disorders later in life, including anxiety and depression (27-29, 58), which are two conditions that have been linked to increased hot flashes (10, 59). Analyses from the multiethnic Study of Women’s Heath Across the Nation Mental Health Study found that early life stress (childhood abuse or neglect) also predicted later vasomotor symptoms (9). Menopausal women with the short form (‘s’ carrier) of the 5-HTTLPR also experience increased climacteric symptoms (30), which could be related to increased anxiety traits associated with this polymorphism. This animal model could contribute mechanistic insight into the interactions between early life events, genetic contributions, and later anxiety and physiological phenotypes, including hot flash-associated responses, which may ultimately lead to a better understanding of the role of serotonin and its complement of proteins to menopausal symptoms.
Indeed, this is an important research area in women’s health at midlife, as drugs that target the serotonin transporter are among the most frequently used pharmacotherapies for vasomotor symptom relief and currently represent the only FDA-approved non-hormonal treatment for hot flashes (60, 61). Despite this indication, SSRIs do not fully alleviate hot flashes and treatment switching is common due to tolerability issues (62). Some of this variability in response and side effects may be due to differences in SERT genotype, as persons with the s/s genotype have been shown to have a poor response to SSRIs (for treating anxiety disorders) (63). Specifically, s/s carriers are at greater risk for treatment-emergent side effects from these medications (64). Investigations into 5-HTTLPR genotype by hot flash treatment efficacy could prove useful in advancing personalized medicine by informing treatment strategies, though to our knowledge, no such studies have been performed. This is likely to be a substantial challenge, as there is evidence that the effect of the polymorphism can be complex and modulated by ethnic background; for example the ‘l’ allele, as opposed to the ‘s’ allele, confers increased risk for anxiety in a Chinese population (65).
Limitations and Future Directions
The sample size for our clinical component was small, but reflects a pilot investigation design. A follow-up study with a larger and more clinically diverse sample, including s/s or s/l carriers, women taking estrogen or other medications for hot flashes and those with additional risk factors such as asthma, is warranted to further validate this method and preclinical results. We would like to further delineate the prevalence and intensity of hot flashes following CO2 inhalation at different concentrations and accompanying physiological events (i.e. skin conductance and temperature, core body temperature, cardiovascular profile, and blood chemistry, including pH throughout the laboratory session) and time course of evoked hot flashes. These are important considerations, because current procedures to elicit hot flashes in a clinical study typically use warming methods, such as water-perfused suits and/or heating pads(66) which are relevant to hot flashes occurring in warm environments but have questionable validity for hot flashes occurring in thermoneutral environments. In addition, these methods can require up to 20 minutes for a hot flash to occur. Importantly, while the ratings of severity of the hot flashes in this preliminary investigation were mild to moderate, we did note that the subjective hot flash intensity appeared to be worse in menopausal women with more frequent hot flashes, though with the small sample size, these correlations should be considered cautiously. Future studies will also need to determine how pre-existing anxiety or stress contributes to the presentation of objective and subjective hot flashes post stress-induced hot flashes (e.g., mental arithmetic, emotionally salient films, or hypercapnic gas inhalation).
Potential Clinical Value
These studies demonstrated a novel method of provoking a hot flash using CO2 inhalation and suggests that decreased breathing during stress, or conditions associated with acidosis [e.g. respiratory (e.g., COPD, bronchitis, or asthma) or metabolic (e.g., diabetes or lactic acidosis)] may increase the risk for hot flashes by reducing the threshold for hot flashes to occur, especially in combination with anxiety or thermal associated stimuli. Further study is warranted to fully validate this method, as our sample size was small and limited to otherwise healthy symptomatic women. A fine-grained delineation of the response to CO2 will allow reverse translational studies (in highly controlled environments) that have the potential to help elucidate mechanisms underlying hot flashes.
Acknowledgments
Sources of financial support: Philip L. Johnson received financial support from: National Institute of Aging (K01AG044466); Young Investigator’s KL-2 Scholars Award (RR025760); Indiana University Simon Cancer Center Basic Science Pilot Funding Grant (23-87597); Indiana CTSI Project Development Team Pilot Grant (RR025761). Janet S. Carpenter received financial support from the Indiana CTSI Clinical Research Center (CRC) grant TR000006. Anantha Shekhar received financial support from the National Institute of Health R01 MH65702 and MH52619.
Footnotes
Conflict of interest: PLJ received funding from Janssen Research and Development, LLC within the last 36 months to complete preclinical studies unrelated to the present paper.
References
- 1.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123-33. [DOI] [PubMed] [Google Scholar]
- 2.Gallicchio L, Whiteman MK, Tomic D, Miller KP, Langenberg P, Flaws JA. Type of menopause, patterns of hormone therapy use, and hot flashes. Fertil Steril. 2006;85(5):1432–40. doi: 10.1016/j.fertnstert.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Stefanopoulou E, Gupta P, Mostafa RM, Nosair N, Mirghani Z, Moustafa K, et al. IMS study of climate, altitude, temperature and vasomotor symptoms in the United Arab Emirates. Climacteric. 2014;17(4):425–32. doi: 10.3109/13697137.2014.898266. [DOI] [PubMed] [Google Scholar]
- 4.Stefanopoulou E, Shah D, Shah R, Gupta P, Sturdee DW, Hunter MS. An International Menopause Society study of climate, altitude, temperature (IMS-CAT) and vasomotor symptoms in urban Indian regions. Climacteric. 2014;17(4):417–24. doi: 10.3109/13697137.2013.852169. [DOI] [PubMed] [Google Scholar]
- 5.Voda AM. Climacteric hot flash. Maturitas. 1981;3(1):73–90. doi: 10.1016/0378-5122(81)90022-0. [DOI] [PubMed] [Google Scholar]
- 6.Stubbs ML, Cohen SM, Carr F. Menopausal women's perceived causes of hot flash. Health Care Women Int. 2008;29(7):755–65. doi: 10.1080/07399330802179205. [DOI] [PubMed] [Google Scholar]
- 7.Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008;15(2):290–5. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman RR. Pathophysiology and Treatment of Menopausal Hot Flashes. Seminars in Reproductive Medicine. 2005;23(2) doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- 9.Thurston RC, Bromberger J, Chang Y, Goldbacher E, Brown C, Cyranowski JM, et al. Childhood abuse or neglect is associated with increased vasomotor symptom reporting among midlife women. Menopause. 2008;15(1):16–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of Menopausal Vasomotor Symptoms Over the Menopause Transition. JAMA internal medicine. 2015 doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12(3):258–66. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 12.Swartzman LC, Edelberg R, Kemmann E. Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychol. 1990;9(5):529–45. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]
- 13.Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79(6):777–81. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- 14.Simpkins JW, Katovich MJ, Song IC. Similarities between morphine withdrawal in the rat and the menopausal hot flush. Life Sci. 1983;32(17):1957–66. doi: 10.1016/0024-3205(83)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.DeFazio J, Verheugen C, Chetkowski R, Nass T, Judd HL, Meldrum DR. The effects of naloxone on hot flashes and gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab. 1984;58(3):578–81. doi: 10.1210/jcem-58-3-578. [DOI] [PubMed] [Google Scholar]
- 16.Lightman SL, Jacobs HS, Maguire AK, McGarrick G, Jeffcoate SL. Climacteric flushing: clinical and endocrine response to infusion of naloxone. British journal of obstetrics and gynaecology. 1981;88(9):919–24. doi: 10.1111/j.1471-0528.1981.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PL, Federici LM, Fitz SD, Renger JJ, Shireman B, Winrow CJ, et al. Orexin 1 and 2 Receptor Involvement in Co2 -Induced Panic-Associated Behavior and Autonomic Responses. Depress Anxiety. 2015;32(9):671–83. doi: 10.1002/da.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, et al. Induction of c-Fos in 'panic/defence'-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2011;25(1):26–36. doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012;37(8):1911–22. doi: 10.1038/npp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niel L, Weary DM. Rats avoid exposure to carbon dioxide and argon. Appl Anim Behav Sci. 2007;107(1-2):100–9. [Google Scholar]
- 21.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139(5):1012–21. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsyth JP, Eifert GH. Response intensity in content-specific fear conditioning comparing 20% versus 13% CO2-enriched air as unconditioned stimuli. J Abnorm Psychol. 1998;107(2):291–304. doi: 10.1037//0021-843x.107.2.291. [DOI] [PubMed] [Google Scholar]
- 23.Forsyth JP, Eifert GH, Canna MA. Evoking analogue subtypes of panic attacks in a nonclinical population using carbon dioxide-enriched air. Behav Res Ther. 2000;38(6):559–72. doi: 10.1016/s0005-7967(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 24.Kaye J, Buchanan F, Kendrick A, Johnson P, Lowry C, Bailey J, et al. Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. Journal of Neuroendocrinology. 2004;16:1–9. doi: 10.1111/j.0953-8194.2004.01158.x. [DOI] [PubMed] [Google Scholar]
- 25.Benshushan A, Rojansky N, Chaviv M, Arbel-Alon S, Benmeir A, Imbar T, et al. Climacteric symptoms in women undergoing risk-reducing bilateral salpingo-oophorectomy. Climacteric. 2009;12(5):404–9. doi: 10.1080/13697130902780846. [DOI] [PubMed] [Google Scholar]
- 26.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 27.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 28.Gonda X, Fountoulakis KN, Juhasz G, Rihmer Z, Lazary J, Laszik A, et al. Association of the s allele of the 5-HTTLPR with neuroticism-related traits and temperaments in a psychiatrically healthy population. European archives of psychiatry and clinical neuroscience. 2009;259(2):106–13. doi: 10.1007/s00406-008-0842-7. [DOI] [PubMed] [Google Scholar]
- 29.Gonda X, Rihmer Z, Juhasz G, Zsombok T, Bagdy G. High anxiety and migraine are associated with the s allele of the 5HTTLPR gene polymorphism. Psychiatry research. 2007;149(1-3):261–6. doi: 10.1016/j.psychres.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Grochans E, Grzywacz A, Jurczak A, Samochowiec A, Karakiewicz B, Brodowska A, et al. The 5HTT and MAO-A polymorphisms associate with depressive mood and climacteric symptoms in postmenopausal women. Progress in neuro-psychopharmacology & biological psychiatry. 2013;45:125–30. doi: 10.1016/j.pnpbp.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, et al. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146(4):1662–76. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol. 2005;19(4):327–41. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- 33.Forsyth JP, Lejuez CW, Finlay C. Anxiogenic effects of repeated administrations of 20% CO2-enriched air: stability within sessions and habituation across time. J Behav Ther Exp Psychiatry. 2000;31(2):103–21. doi: 10.1016/s0005-7916(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 34.Prenoveau JM, Forsyth JP, Kelly MM, Barrios V. Repeated exposure to 20% CO2 challenge and risk for developing panic attacks: a controlled 6- and 12-month follow-up in a nonclinical sample. J Anxiety Disord. 2006;20(8):1158–67. doi: 10.1016/j.janxdis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Freedman RR, Woodward S, Sabharwal SC. Alpha 2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol. 1990;76(4):573–8. [PubMed] [Google Scholar]
- 36.Charney DS, Heninger GR. Alpha 2-adrenergic and opiate receptor blockade. Synergistic effects on anxiety in healthy subjects. Arch Gen Psychiatry. 1986;43(11):1037–41. doi: 10.1001/archpsyc.1986.01800110023004. [DOI] [PubMed] [Google Scholar]
- 37.Charney DS, Heninger GR, Breier A. Noradrenergic function in panic anxiety. Effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Archives of general psychiatry. 1984;41(8):751–63. doi: 10.1001/archpsyc.1984.01790190025003. [DOI] [PubMed] [Google Scholar]
- 38.Morimoto Y, Aozuka Y, Shibata Y. [Effects of estrogen and keishibukuryogan on hot flash-like symptoms induced by yohimbine in ovariectomized rats] Yakugaku Zasshi. 2011;131(8):1241–50. doi: 10.1248/yakushi.131.1241. [DOI] [PubMed] [Google Scholar]
- 39.Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2(8341):98–9. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- 40.Federici LM, Caliman IF, Molosh A, Fitz SD, Truitt W, Bonaventure P, et al. Hypothalamic orexin’s role in exacerbated cutaneous vasodilation responses to an anxiogenic stimulus in a surgical menopause model. Psychoneuroendocrinology. 2016;65:127–37. doi: 10.1016/j.psyneuen.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forster HV, Smith CA. Contributions of Central and Peripheral Chemoreceptors to the Ventilatory Response to Co2/H+ J Appl Physiol. 2010 doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda Y, Sato A, Suzuki A, Trzebski A. Autonomic nerve and cardiovascular responses to changing blood oxygen and carbon dioxide levels in the rat. JAutonNervSyst. 1989;28(1):61–74. doi: 10.1016/0165-1838(89)90008-8. [DOI] [PubMed] [Google Scholar]
- 43.Koo KW, Sax DS, Snider GL. Arterial blood gases and pH during sleep in chronic obstructive pulmonary disease. Am J Med. 1975;58(5):663–70. doi: 10.1016/0002-9343(75)90502-1. [DOI] [PubMed] [Google Scholar]
- 44.Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med. 1998;31(4):459–65. doi: 10.1016/s0196-0644(98)70254-9. [DOI] [PubMed] [Google Scholar]
- 45.Thurston RC, Sowers MR, Chang Y, Sternfeld B, Gold EB, Johnston JM, et al. Adiposity and reporting of vasomotor symptoms among midlife women: the study of women's health across the nation. Am J Epidemiol. 2008;167(1):78–85. doi: 10.1093/aje/kwm244. [DOI] [PubMed] [Google Scholar]
- 46.Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–72. doi: 10.1016/s0029-7844(02)02593-0. [DOI] [PubMed] [Google Scholar]
- 47.Asahina M, Fujinuma Y, Yamanaka Y, Fukushima T, Katagiri A, Ito S, et al. Diminished emotional sweating in patients with limbic encephalitis. J Neurol Sci. 2011;306(1-2):16–9. doi: 10.1016/j.jns.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Sood R, Sood A, Wolf SL, Linquist BM, Liu H, Sloan JA, et al. Paced breathing compared with usual breathing for hot flashes. Menopause. 2013;20(2):179–84. doi: 10.1097/gme.0b013e31826934b6. [DOI] [PubMed] [Google Scholar]
- 49.Carpenter JS, Burns DS, Wu J, Otte JL, Schneider B, Ryker K, et al. Paced respiration for vasomotor and other menopausal symptoms: a randomized, controlled trial. Journal of general internal medicine. 2013;28(2):193–200. doi: 10.1007/s11606-012-2202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang AJ, Phillips S, Schembri M, Vittinghoff E, Grady D. Device-guided slow-paced respiration for menopausal hot flushes: a randomized controlled trial. Obstetrics and gynecology. 2015;125(5):1130–8. doi: 10.1097/AOG.0000000000000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diji A. Local vasodilator action of carbon dioxide on blood vessels of the hand. Journal of applied physiology. 1959;14(3):414–6. doi: 10.1152/jappl.1959.14.3.414. [DOI] [PubMed] [Google Scholar]
- 52.Ito T, Moore JI, Koss MC. Topical application of CO2 increases skin blood flow. The Journal of investigative dermatology. 1989;93(2):259–62. doi: 10.1111/1523-1747.ep12277584. [DOI] [PubMed] [Google Scholar]
- 53.Shuto H, Tominaga K, Yamauchi A, Ikeda M, Kusaba K, Mitsunaga D, et al. The statins fluvastatin and pravastatin exert anti-flushing effects by improving vasomotor dysfunction through nitric oxide-mediated mechanisms in ovariectomized animals. European journal of pharmacology. 2011;651(1-3):234–9. doi: 10.1016/j.ejphar.2010.10.084. [DOI] [PubMed] [Google Scholar]
- 54.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36(3):357–78. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 55.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O'Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202(2):223–36. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joffe H, Guthrie KA, LaCroix AZ, Reed SD, Ensrud KE, Manson JE, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA internal medicine. 2014;174(7):1058–66. doi: 10.1001/jamainternmed.2014.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289(21):2827–34. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 58.Hauser J, Leszczynska A, Samochowiec J, Czerski PM, Ostapowicz A, Chlopocka M, et al. Association analysis of the insertion/deletion polymorphism in serotonin transporter gene in patients with affective disorder. European psychiatry : the journal of the Association of European Psychiatrists. 2003;18(3):129–32. doi: 10.1016/s0924-9338(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 59.Gallicchio L, Miller SR, Kiefer J, Greene T, Zacur HA, Flaws JA. Risk factors for hot flashes among women undergoing the menopausal transition: baseline results from the Midlife Women's Health Study. Menopause. 2015 doi: 10.1097/GME.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon JA, Portman DJ, Kaunitz AM, Mekonnen H, Kazempour K, Bhaskar S, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–35. doi: 10.1097/GME.0b013e3182a66aa7. [DOI] [PubMed] [Google Scholar]
- 61.Orleans RJ, Li L, Kim MJ, Guo J, Sobhan M, Soule L, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med. 2014;370(19):1777–9. doi: 10.1056/NEJMp1402080. [DOI] [PubMed] [Google Scholar]
- 62.Handley AP, Williams M. The efficacy and tolerability of SSRI/SNRIs in the treatment of vasomotor symptoms in menopausal women: A systematic review. Journal of the American Association of Nurse Practitioners. 2015;27(1):54–61. doi: 10.1002/2327-6924.12137. [DOI] [PubMed] [Google Scholar]
- 63.Stein MB, Seedat S, Gelernter J. Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology. 2006;187(1):68–72. doi: 10.1007/s00213-006-0349-8. [DOI] [PubMed] [Google Scholar]
- 64.Perlis RH, Mischoulon D, Smoller JW, Wan YJ, Lamon-Fava S, Lin KM, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biological psychiatry. 2003;54(9):879–83. doi: 10.1016/s0006-3223(03)00424-4. [DOI] [PubMed] [Google Scholar]
- 65.Long H, Liu B, Hou B, Wang C, Li J, Qin W, et al. The long rather than the short allele of 5-HTTLPR predisposes Han Chinese to anxiety and reduced connectivity between prefrontal cortex and amygdala. Neuroscience bulletin. 2013;29(1):4–15. doi: 10.1007/s12264-013-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Germaine LM, Freedman RR. Behavioral treatment of menopausal hot flashes: evaluation by objective methods. Journal of consulting and clinical psychology. 1984;52(6):1072–9. doi: 10.1037//0022-006x.52.6.1072. [DOI] [PubMed] [Google Scholar]