Abstract
The generation of axon collateral branches is a fundamental aspect of the development of the nervous system and the response of axons to injury. Although much has been discovered about the signaling pathways and cytoskeletal dynamics underlying branching, additional aspects of the cell biology of axon branching have received less attention. This review summarizes recent advances in our understanding of key factors involved in axon branching. Here we focus on how cytoskeletal mechanisms, intracellular organelles, such as mitochondria and the endoplasmic reticulum, and membrane remodeling (exocytosis and endocytosis) contribute to branch initiation and formation. Together this growing literature provides valuable insight as well as a platform for continued investigation into how multiple aspects of axonal cell biology are spatially and temporally orchestrated to give rise to axon branches.
INTRODUCTION
The proper function of the nervous system is dependent upon the establishment of complex patterns of stereotypical synaptic connectivity. As an example, there are an estimated 15–16 ×108 synapses per mm3 in the human neocortex (DeFelipe 1999). Although each neuron generates only a single axon, this axon establishes synaptic connections with an expansive array of postsynaptic partners, often in disparate regions of the nervous system. The generation of such complex patterns of synaptic connectivity is in large part due to the developmental branching of axons. Developing axons ramify through two main mechanisms; the bifurcation of the extending growth cone, which initiates two branches of the main axon, and the de novo formation of collateral branches from the axon shaft after the growth cone has extended toward its main target region (Gibson & Ma 2010). The vast majority of studies have focused on this latter form of branching, which is the focus of this review. The initiation and stabilization of nascent collateral branches, which begin as plasma membrane protrusions, involves the reorganization of the dynamic cytoskeleton to generate protrusive force against the plasma membrane. This dramatic change in axonal morphology also involves substantial expansion of the axonal plasma membrane, which requires addition and remodeling of membrane material. The cytoskeletal dynamics and membrane turnover underlying branching must be coordinated in space and time, as well as receive support from relevant organelles, such as the endoplasmic reticulum and mitochondria, which control ionic and metabolic aspects of the cytoplasm.
The final pattern of connectivity adopted by a given neuron is ultimately dependent on both the elaboration, maintenance and pruning of axon collateral branches (Kalil & Dent 2014). The formation of axon collateral branches also occurs in response of the nervous system to injury (Hagg 2006; Onifer et al. 2011), in which case the branching can have adaptive or maladaptive consequences (Weaver et al. 2006; Hagg 2006). Here we review the cellular processes that are involved in collateral branch generation, including cytoskeletal dynamics, organelle transport and function, and membrane addition and removal. We also discuss the current understanding of the relationship between the emergence of collateral branches and synapse formation and function.
The cytoskeletal foundation of axon collateral branches
Localized reorganization of the axonal cytoskeleton underlies the generation of axon collateral branches. The initial step in the formation of a collateral branch is the appearance of an actin filament-based protrusion from the axon shaft (Figure 1A). This protrusive phase of branching takes the form of a filopodium most commonly, or occasionally a lamellipodium (Figure 1A) (Gallo 2011; Kalil & Dent 2014). Filopodia are finger-like protrusions comprising a linear array of bundled actin filaments (Gupton & Gertler 2007), whereas lamellipodia are veil-like protrusions supported by an interconnected array of actin filaments (Mejillano et al. 2004). Relative to the growth cone at the tip of the extending axon, the axon shaft contains fewer actin filaments and exhibits little protrusive activity, due to axonal consolidation (Dent & Gertler 2003; Burnette et al. 2008), which actively suppresses protrusive activity along the axon. Although mechanisms of axonal consolidation maintain the position of the growth cone at the tip of the extending axon, they must be overcome to permit collateral branching of the axon, making collateral axon branch formation distinct from other forms of actin-based protrusions (Loudon et al. 2006; Mingorance-Le Meur & O'Connor 2008), including growth cone splitting. The axonal actin filament cytoskeleton consists of localized patches of actin filaments, sub-membranous rings of actin filaments circumferential to the main axis of the axon and a smaller population of filaments arranged longitudinally (Spillane et al. 2011; Hu et al. 2012; Xu et al. 2013; Ganguly et al. 2015). Axonal actin patches serve as the precursors to the emergence of localized protrusive activity along the axon shaft (Figure 1A). Whereas the axon generates many actin patches that are transient in nature, only a subset of such patches gives rise to protrusive activity, and the remaining patches dissipate without exhibiting protrusive activity. The formation of axonal actin patches is a major regulatory point in the mechanism used by some branch-inducing signals to increase the level of protrusive activity along the axon (e.g., nerve growth factor/NGF in dorsal root ganglion neurons) (Mingorance-Le Meur & O'Connor 2008; Ketschek & Gallo 2010). Thus, the localized regulation of the cytoskeleton is one of the earliest events in the progression of collateral branch formation.
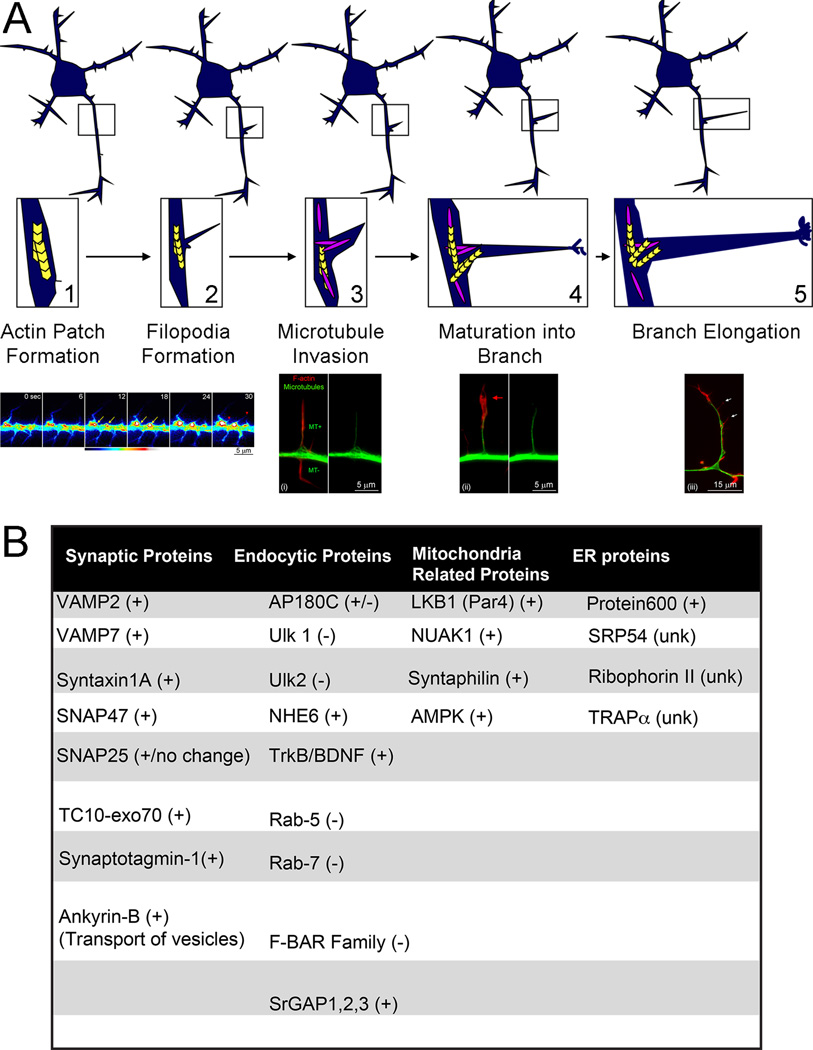
Figure 1.
Sequence of cytoskeletal events underlying the collateral branching of axons. (A) Schematic of the sequence of events leading to the formation of a collateral branch (top, A1–A5). Examples of cytoskeletal dynamics and organization during the branching of embryonic sensory axons are also provided (bottom). Axonal filopodia are the first morphogenetic step in the formation of a branch (A2). Filopodia emerge from pre-existing patches of axonal actin filaments (A1). The time-lapse sequence (A1 bottom) shows the formation of axonal actin patches and the emergence of filopodia from the patches (eYFP-β-actin imaging; time is shown in seconds in each panel). Actin patches form and elaborate (i.e., grow brighter and larger) as denoted by the yellow arrows at 12–18 sec. The red arrowheads and 30 sec denote filopodia that have emerged from the two patches labeled in prior panels. The targeting of one or more microtubules (MT+) into a filopodium is a required event for the maturation of the filopodium into a branch (A3). Filopodia that are targeted by microtubules can then mature into a branch (A4). The maturation of the filopodium is characterized by the development of a polarized distribution of actin filaments (red arrow, A4 bottom), which form a small growth cone-like structure at the tip of the nascent branch. As the branch elongates actin filaments remain mostly polarized at its tip, although the branch can also give rise to filopodia along its shaft (white arrows, A5 bottom), and in turn secondary branches. (B) Table featuring the proteins discussed in the text and denoting whether they promote (+) or impede (−) branching. (unk) denotes proteins with insufficient data regarding their role in axon branching.
As the majority of axonal filopodia do not give rise to a branch and are instead retracted, the subset that proceed to branch formation are somehow stabilized. The invasion of axonal filopodia by the polymerizing tips of axonal microtubules, or their active transport into filopodia, is a requisite step in this stabilization and thus the formation of collateral branches (Gallo & Letourneau 1999; Dent & Kalil 2001)(Figure 1A). However, entry of a microtubule into a filopodium is not sufficient for the maturation of the filopodium into a branch. This is likely due to the dynamic nature of microtubules, where their plus ends switch between phases of polymerization and depolymerization, a process termed dynamic instability (Mitchison & Kirschner 1984; Brouhard 2015). Microtubule depolymerization can result in the removal of the microtubule from the filopodium, and destabilization of the nascent axonal protrusion. Thus, the stabilization of microtubules within axonal filopodia is also considered a necessary step in the subsequent maturation of the filopodium into a branch (Gallo & Letourneau 1999; Dent & Kalil 2001; Dent et al. 2004; Gallo 2011). Also notable, the axonal microtubule array, which is normally characterized by linearly aligned microtubules, undergoes splaying at sites of axonal protrusive activity and branching (Gibney & Zheng 2002; Ketschek et al. 2015). This localized reorganization of the axonal microtubule array likely promotes interactions between actin filaments and microtubules during the early steps of branching, however this aspect of branching requires further investigation to elucidate its mechanisms.
The defining event in the maturation of a filopodium into a collateral branch is the reorganization of its actin filament array from a bundle of aligned filaments into a polarized structure. This structure is supported by microtubules and exhibits actin filaments localizing predominantly at the tip of the nascent branch in a growth cone-like structure (Figure 1A). The maturation of a branch can thus be considered a form of cell polarization at the level of a single filopodium. However, the mechanism that drives this polarization remains poorly understood. The maturation of an axonal filopodium into a branch shares similarities with the initial establishment of the main axon. In both cases, a filopodium is converted into a microtubule containing structure with a polarized actin filament cytoskeleton (Smith 1994; Dehmelt et al. 2003; Dent et al. 2007).
Beyond the cytoskeleton
Collateral branching increases the total volume and surface area of the axon, thus the delivery of cellular components, such as membrane material and organelles are critical to axon branch generation and maturation (Pfenninger & Johnson 1983; Pfenninger 2009). Collateral branches eventually mature into a functional compartment of the main axon, capable of supporting synapse formation and activity. As such, axonal transport cargoes must be delivered into the branch from the main axon, and organelles must populate the nascent branch in order to maintain normal physiology. Sites of axon branching exhibit accumulation of vesicles and also organelles (e.g., mitochondria) (Yu et al. 1994; Greif et al. 2013; Courchet et al. 2013; Tao et al. 2013; Spillane et al. 2013), indicating that membranous organelles and membrane recycling systems likely have roles in axonal branching. For the balance of this review, the role of organelles, pre-synaptic exocytic machinery, and membrane recycling machinery will be considered vis-à-vis the delineated cytoskeletal-based steps of the mechanism of axon branching described above (a summary of proteins discussed in the review is presented in Figure 1B).
Mitochondria
The lengthening and branching of axons necessitates high ATP production to power local protein synthesis and changes in neuronal morphology. Given that distal axon branches are positioned far from the soma, local energy production is a chief function of mitochondria found within the axon. Mitochondria undergo active bi-directional transport in axons through a kinesin-mediated mechanism (Saxton & Hollenbeck 2012; Sheng 2014). However, between 60–70% of axonal mitochondria remain stalled at specific locations along the axon for prolonged time periods (Miller & Sheetz 2006; Roossien et al. 2014). Mitochondrial density increases in growing axon branches as the influx of the organelles into the branch outweighs efflux from branches (Ruthel & Hollenbeck 2003). This mitochondrial influx is due to organelle specific motility and not passive axoplasmic flow, suggesting that mitochondria may play a key role in axon branch maturation. In embryonic mouse cortical neurons, deletion of the kinase Lkb1 (Par4), its downstream effector kinase Nuak1, or the microtubule and mitochondria linker protein syntaphilin, decreases the percentage of stalled mitochondria present along the axon and the number of mature axon branches (Courchet et al. 2013). Overexpression of any of these three proteins increases the number of stalled mitochondria and associated axon branches. These data suggest that LKB1, NUAK1 and syntaphilin control the stalling of mitochondria within the axon, which allows for local energy production and consequently the extension of axon branches.
In embryonic chick sensory neurons, axonal sites containing both a previously stable filopodium and a stalled mitochondrion mature into stable axon branches in response to bath application of NGF (Spillane et al. 2013), suggesting mitochondrial derived energy is necessary for this transition (Figure 1A). Inhibiting mitochondrial respiration, or locally ablating subsets of mitochondria using chromophore assisted light inactivation (CALI) on neurons expressing a mitochondrially-targeted killer-red (KR) construct blocks maturation of filopodia into branches. Similarly, reversal of nascent branches to their previously filopodial-like morphology occurs in response to targeted ablation of stalled mitochondria at their branch point bases.
The induction of axon branching by NGF requires the intra-axonal translation of mRNAs coding for actin regulatory proteins (e.g., cortactin and Arp2; (Spillane et al. 2012). Localized mRNA translation can be visualized in axons using a myrGFP mRNA that includes the 3’UTR of Arp2 or cortactin mRNAs. Mitochondria colocalize with areas of active mRNA translation, suggesting that mitochondria may fuel local translation of cytoskeletal components involved in axon branching (Spillane et al. 2012; Spillane et al. 2013). Accordingly, the formation of translational ‘hotspots’ associated with mitochondria decreases upon CALI ablation of mitochondria or pharmacological inhibition of mitochondrial respiration. Taken together these results suggest that stalled, actively respiring mitochondria are required for the formation of ‘hot spots’ of localized axonal translation, which are in turn required for localized branch formation (Spillane et al. 2012).
Mitochondrial accumulation also contributes to activity-dependent changes in axonal morphology, indicating that synaptic function can create feedback to modulate the morphology and connectivity of the axon. In rat postnatal granule cells, KCl-induced depolarization increases the length and number of axon branches (Tao et al. 2013). Both the initiation and maintenance of longer and more numerous branches are associated with accumulations of mitochondria. The induction of axon branch formation by depolarization and correlated mitochondrial presence requires AMPK activation. These data indicate that mitochondria involved in axon branching are not only regulated by extracellular cues like NGF, but also by membrane depolarization, which may play an integral role in defining which potential synaptic partners are innervated by the axon during developmental activity-dependent circuit formation.
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a network of membranous cisternae critical in the synthesis and transport of proteins and membrane lipids, many of which are destined for the axonal compartment and axonal membrane. In addition to supplying cellular materials, the ER maintains Ca2+ homeostasis, and interacts with and regulates mitochondria. The ER pervades the axon as parallel 20–40 nm wide interconnected tubules (Broadwell 1984). Whereas the ER has been implicated in the establishment of axonal polarity, axon guidance and dendritic arborization, little is known regarding its role in axon branching (Renvoisé & Blackstone 2010). However, the ER accumulates at the base of axon branches, where it colocalizes with mitochondria. As axon branches elongate, the ER reaches further into their bases, suggesting the ER may play a role in branch stabilization (Spillane et al. 2013). Current data suggest that the close interaction between the ER and the microtubule cytoskeleton is requisite in both axon branching and elongation. For instance, in primary cortical mouse neurons, silencing Protein 600, a microtubule associated protein that binds to the ER, destabilizes neuronal processes and decreases neurite extension (Shim et al. 2008). These data suggest that the scaffolding of ER to microtubules within neuronal processes is required for process maintenance, presumably including axon branches. Whereas axons have been presumed devoid of rough ER, recent work suggests that developing axons contain components functionally equivalent to rough ER for local protein synthesis (Merianda et al. 2009). Further, in cultured rat dorsal root ganglion neurons and Xenopus retinal ganglion cells, intra-axonal immunoreactivity and colocalization was observed for ER-specific proteins including SRP54, Ribophorin II and TRAPα (Merianda et al. 2009). As the literature points to a potential role for both smooth and a specialized version of rough ER in the axon (Tang & Kalil 2005), but does not examine axon branching in depth, this is a research area open to new development that warrants a functional analysis of the role of the ER in branching.
Characterization of synaptic proteins
A substantial amount of cellular material must reach the plasma membrane during axon branching. The fusion or exocytosis of secretory vesicles not only secretes soluble cargo, but also delivers membrane material and membrane proteins to the expanding plasma membrane during axonal elongation and branching (Pfenninger 2009). Mature synaptic vesicles are rich in proteins, particularly those associated with membrane trafficking, which are present in multiple copies (Takamori et al. 2006). Interestingly, presynaptic exocytic machinery is present well before the onset of synaptogenesis and some components contribute to the growth cone-mediated extension and branching of the primary axon (Greif et al. 2013; Winkle et al. 2014). Developing axons are capable of neurotransmitter release before target contact, indicating that the fundamental molecular mechanism of synaptic vesicle clustering and Ca2+ dependent exocytosis is already in place in growing axons (Kraszewski et al. 1995; Verderio et al. 1999; Ahmari et al. 2000). Activity-associated fusion events contribute to the modeling and remodeling of developing axonal arbors, suggesting that neurotransmitter release via early-expressed synaptic machinery contributes to morphology of axon terminals. Whereas neurotransmitter release at the synapse is a highly regulated form of exocytosis, it represents an elaboration of the more general process of vesicle trafficking and fusion with the plasma membrane known as the SNARE hypothesis (Söllner et al. 1993; Rothman 1994; Südhof & Rothman 2009).
The discovery of homologous proteins to SNARE components in yeast and non-neuronal tissue (Bennett & Scheller 1993; Aalto et al. 1993; Brennwald et al. 1994) and the high degree of conservation of other vesicle proteins across species suggests that shared mechanisms control synaptic and other forms of secretory vesicle fusion (Söllner et al. 1993; Bennett & Scheller 1994; Rothman 1994), including in developing neurons. Among the large group of proteins implicated in vesicular trafficking and fusion, the SNARE complex components synaptobrevins (also known as vesicle associated membrane proteins, or VAMPs), presynaptic membrane syntaxins and SNAP-25, and vesicle-associated synaptotagmins (syts) are among the most well-studied. VAMPs form a stable, SDS-resistant SNARE complex with syntaxin and SNAP-25 that promotes the close apposition of the vesicular and destination membrane. This complex has been proposed to provide the energy necessary for fusing two membranes (Hanson et al. 1997; Wiederhold & Fasshauer 2009).
Components of the SNARE complex interact with presynaptic membrane components of the syt, Munc/Sec and exocyst families (Li & Chin 2003; Südhof & Rothman 2009; Deák et al. 2009) to mediate regulated and localized vesicle fusion and neurotransmitter release. Syt1 is proposed to serve as a central regulator of Ca2+-triggered SNARE-mediated vesicle fusion at the presynaptic terminal (Rizo & Rosenmund 2008; Rizo & Südhof 2012). Syt1 also interacts with phospholipids in the plasma membrane via its C2 domains (Perin et al. 1990; Perin et al. 1991; Brose et al. 1992; Bai et al. 2003), suggesting that it may promote interactions between vesicles and the plasma membrane. Newly-developing axons contain clusters of vesicles (Matteoli et al. 1992; Kraszewski et al. 1995; Foletti et al. 2000), and syt1 is expressed during axon outgrowth of cortical neurons in vivo (Chun & Shatz 1988). In addition, Rab GTPases, synaptophysin, synapsins and several other presynaptic terminal proteins are implicated in regulated exocytosis. Synapsin I and synaptophysin, are also expressed prior to neurite outgrowth (Fletcher et al. 1991) and are observed in the branches of developing axon arbors in the retinotectal system of Xenopus embryos (Pinches & Cline 1998). Together these findings suggest that an extensive number of exocytic proteins may play roles in axon branching and development (Chen & Scheller 2001; Südhof 2004; Rizo & Rosenmund 2008; Südhof & Rothman 2009; Rizo & Südhof 2012)
Synaptic proteins and the development of axons
Recent evidence suggests that membrane vesicles may be targeted to selected sites by interactions with the cytoskeleton, and that both exocytic and endocytic events contribute to the dynamic developmental remodeling of processes (Zakharenko & Popov 1998; Sabo & McAllister 2003; Alberts et al. 2006; Meyer & Smith 2006; Tojima et al. 2007; Erez et al. 2007; Gupton & Gertler 2010; Tojima et al. 2014). Growing axons navigate through a complex extracellular environment, changing direction in response to cues (Gallo & Letourneau 2000; Dent et al. 2003; Horton & Ehlers 2003; Lowery & Van Vactor 2009; Gallo 2013). Evidence suggests that response to positive/attractant cues might involve addition of membrane by controlled exocytosis via SNAREs (Cotrufo et al. 2011; Winkle et al. 2014; Tojima et al. 2014; Ros et al. 2015), contributing to the steering of growth cones towards positive signals, while repulsive signals might be avoided in part by regulated endocytosis of membrane material (Tojima & Kamiguchi 2015; Pfenninger 2009). Selective asymmetric insertion of membrane into growth cones in response to guidance cues suggests a role for controlled exocytosis in axonal outgrowth and guidance (Sakisaka et al. 2004; Tojima et al. 2007; Tojima et al. 2014)
Synaptic proteins are implicated in the regulation of axon elongation. For example the family of synapsins is linked to regulation of axon outgrowth (Ferreira et al. 2000; Kao et al. 2002; Fornasiero et al. 2009). The TC10- exo70 exocyst complex is reported to be an essential component for axon outgrowth (Vega & Hsu 2001; Dupraz et al. 2009). The results of manipulation of SNAREs and related proteins on axon outgrowth are variable (Tojima & Kamiguchi 2015). Whereas VAMP2-mediated exocytosis is observed in growth cones (Sabo & McAllister 2003; Tojima et al. 2007), cleavage of VAMP by tetanus toxins does not alter axon outgrowth (Osen-Sand et al. 1996), although it does block intrinsic neuritogenesis (Gupton & Gertler 2010). In contrast, overexpression of VAMP2 in the neuronal-like PC12 cell line promotes neurite elongation (Kimura et al. 2003). The varied roles suggest specific vSNAREs may function at distinct developmental stages or in response to various extracellular signals, and may deliver distinct cargo to the expanding plasma membrane. Cleavage of SNAP-25 by Botulinum toxins or reductions in expression by antisense oligonucleotides inhibits axon growth (Osen-Sand et al. 1993; Osen-Sand et al. 1996). However, thalamo-cortical axon outgrowth from Snap25−/− knockout mice observed in E16-18 organotypic cultures is indistinguishable from that in Snap25+/+ cultures (Blakey et al. 2012). Knockout of Munc18 and Munc13 reduces axon outgrowth in organotypic cultures and in dissociated hippocampal neurons (Broeke et al. 2012). Blocking the C2A domain of sytI/II with antibodies reduces neurite outgrowth in PC12 cells, whereas overexpression promotes neurite outgrowth (Kabayama et al. 1999). Possible explanations for observed inconsistencies include functional redundancy among members of protein families or high levels of expression of synaptic proteins localized within growth cones. Thus, while the synaptic machinery is strongly implicated in multiple aspects of axon development and guidance, the roles of specific components remain to be elucidated.
Synaptic proteins and axon branching
As outlined above, synaptic proteins are heavily implicated in axon extension, guidance and neurotransmitter release, but their roles in axon branching are less well documented. In vivo live imaging of axon branch formation in retinal ganglion cells reveals a correlation between sites of branch formation and the accumulation of the synaptic vesicle marker synaptobrevin II (Cohen-Cory & Lom 2004). Axon branching in cortical and hippocampal neurons is stimulated by the secreted axon guidance cue netrin-1 (Dent et al. 2004; Winkle et al. 2014). Total internal reflection fluorescence microscopy (TIRFM) of murine cortical neurons expressing VAMP2-pHlourin or VAMP7-pHluorin revealed that netrin-1 treatment stimulates the fusion of vesicles positive for either v-SNARE particularly in neurites (Winkle et al. 2014). TeNT-mediated cleavage of VAMP2 or longin-mediated inhibition of VAMP7 individually fails to decrease netrin-dependent axon branching independently, however simultaneous inhibition of both vSNAREs decreases axon branching, indicating the function of either vSNARE is sufficient for plasma membrane expansion and axon branching. Acute Botulinum Neurotoxin A-mediated cleavage of SNAP25, which forms a SNARE complex with either VAMP2 or VAMP7 decreases netrin-dependent axon branching, in a similar fashion to the inhibition of both v-SNAREs, suggesting that exocytic vesicle fusion is required for plasma membrane expansion during axon branching (Winkle et al. 2014). These data suggest that VAMP2 and VAMP7 may function during netrin-stimulated axon branching (Winkle et al. 2014).
SNAP47 is a SNARE protein also present in the nervous system, which forms SNARE complexes with syntaxin-1A and VAMP2 in cultured neurons, and facilitates exocytic fusion events (Holt et al. 2006). VAMP2, SNAP25 and SNAP47 mediate the local vesicular release of the neurotrophic factor BDNF. Moreover, ablation of the function of SNAP47, BDNF or the BDNF receptor TrkB disrupts callosal axon branching both in vitro and in vivo, suggesting that SNAP47 may also function in both exocytosis and axon branching (Shimojo et al. 2015). SNAP47 interacts with VAMP7 and Trans-Golgi associated VAMP4, suggesting that SNAP47 pairs with diverse SNARE proteins to potentially fulfill multiple distinct trafficking requirements in developing neurons (Kuster et al. 2015). As such, silencing SNAP47 dramatically changes the cellular localization of VAMP4, whereas SNAP47 overexpression inhibits VAMP7-dependent exocytic activity. As the literature regarding SNAP47 is relatively sparse, further investigations of its roles in the regulation of secretory transport in the developing neuron are warranted.
The synaptotagmin syt1 is implicated in axon branching as well. Syt1 mRNA is enriched in immature axons and growth cones compared to more mature axons (Gumy et al. 2011). Syt1 immunoreactivity is present within filopodia in embryonic chicken forebrain neurons in vitro (Greif et al. 2013). Overexpression of syt1 increases filopodial initiation and stability, leading to increased numbers of axonal filopodia and subsequent primary axon branches. Conversely, knockdown of endogenous syt1 by RNAi reduces the number of axonal filopodia and branches (Greif et al. 2013). Axonal branching is also reduced in thalamo-cortical co-cultures by a dominant-negative construct of syt1 with a mutated C2B Ca2+-binding domain, which reduces vesicle cycling (Granseth et al. 2013). Transfection with the mutant construct also blocks increases in axonal branching in response to BDNF.
The numerous other synaptotagmin proteins likely play critical roles in axon outgrowth as well. For example, both process outgrowth and branching in vitro are defective in sympathetic neurons from syt7-defective mice (Arantes & Andrews 2006). Ca2+-dependent translocation of syt1 to the plasma membrane of dendrites of developing rat hypothalamic neurons also suggests that syt1 specifically and synaptotagmins in general also may contribute to dendritic morphology as well (Schwab et al. 2001). Syt1 also contributes to the morphology of neuronal-like processes in non-neuronal cells. Ectopic expression of syt1 in non-neuronal cells produces extensive filopodia-like structures (Feany & Buckley 1993; Johnsson & Karlsson 2012). The polybasic sequence on syt1 directly stimulates actin polymerization via interactions with anionic phospholipids in non-neuronal cells (Johnsson & Karlsson 2012), suggesting a potential interaction between synaptic vesicles and the cytoskeletal reorganization associated with filopodial development that represents the initial phase of axon branching (Figure 1A).
Axon branching requires membrane addition for increased surface area. Whether vesicle fusion occurs locally or globally during axon branching and how this changes over development remains to be seen. However, if the plasma membrane is as fluid as suggested in the literature this membrane may not necessarily need to be inserted locally. Since local increases in Ca2+ are associated with filopodia and branch development, local control of SNARE-mediated membrane fusion may provide an effective mechanism to couple cytoskeletal reorganization with membrane addition. Furthermore, the proteins involved in the docking and fusion of vesicles with the presynaptic membrane are general components of synapses, regardless of transmitter type (Chen & Scheller 2001; Südhof 2004; Südhof & Rothman 2009). Since early neural activity can modulate the organization, architecture and strength of synapses and this is associated with the regulation of axonal branching morphogenesis in multiple systems (O'Leary & McLaughlin 2005; Uesaka et al. 2006; Hayano & Yamamoto 2008; Yamamoto & López-Bendito 2012), future work is necessary to further define the spatial and temporal regulation of neural activity as it relates to arborization.
Endocytosis and endocytic trafficking in branching
After synaptic vesicle fusion, vesicle membrane is recycled via dynamin-mediated endocytosis and subsequently vesicles are refilled with neurotransmitter (Südhof 2004). Similarly, axon outgrowth involves a balance between addition and removal of membrane; evidence suggests that endocytosis of membrane components also play a role in axonal morphogenesis (Lasiecka & Winckler 2011). Although the regulation of endocytosis and trafficking of endosomes is crucial for the development and maintenance of axons (Yap & Winckler 2015), in comparison to exocytosis, there is relatively little published on the function of endocytosis in axon branching. Endocytosis and protrusion can be considered opposing cellular behaviors. The first results in the removal of plasma membrane and the formation of an endocytic vesicle within the cytoplasm, whereas the second results in the formation of a filopodium or lamellipodium supported by membrane expansion, which could mature into an axon collateral as outlined (Figure 1A). In the growth cone, endocytosis can modulate growth cones turning away from a repellent signal. In elegant studies, Kamiguchi and colleagues have demonstrated that asymmetric endocytosis on one side the of the growth cone is sufficient to induce turning away from repulsive signals (Tojima & Kamiguchi 2015; Tojima et al. 2010). They propose that axon guidance may be a balance between exocytosis and endocytosis, acting in an antagonistic fashion. If these findings are extrapolated to axon branching and collateralization then one might expect that endocytosis plays a role in the inhibition of branching through the endocytic retrieval of cell adhesion molecules and growth factor receptors.
Consistent with these findings, inhibition of endocytosis is thought to increase axon branching. For example, inhibition of fibroblast growth factor receptor (FGFR1) endocytosis results in increased axon branching of adult DRG neurons (Hausott et al. 2011). Another study in thalamo-cortical neurons, demonstrated that BDNF stimulation increases axon branching and the inhibition of clathrin-mediated endocytosis, via overexpression of AP180C, has a biphasic effect, decreasing branching at low BDNF concentrations and increasing branching at high BDNF concentrations (Granseth et al. 2013). Since high concentrations of AP180C result in sequestration of clathrin, it follows that inhibiting clathrin-mediated endocytosis in thalamo-cortical axons results in increased branching. Presumably, inhibition of endocytosis allows growth factor receptors to build up on the axonal plasma membrane and increases the likelihood of activation by growth factors such as FGF-2 and BDNF. Consistent with these data, knockdown of Ulk1 and/or Ulk2, two UNC-51-like kinases impaired endocytosis of NGF, resulting in increased axon branching of embryonic sensory neurons (Zhou et al. 2007). Thus, inhibiting endocytosis appears to leave more receptors on the membrane surface and results in increased receptor signaling, leading to increased branching.
Conversely, disrupting endosomal pH can decrease axon branching. Mutation of NHE6, the endosomal Na+/H+ exchanger that is mutated in Christianson syndrome, decreases axon branching in cultured hippocampal neurons (Ouyang et al. 2013). NHE6 is present on early, late and recycling endosomes and opposes endosomal luminal acidification by the V-ATPase. Knockout of the Na+/H+ exchanger abrogates axon branching and is rescued by re-expression of a functional exchanger or excess BDNF. The authors propose that the over-acidification and increased pH-sensitive proteolysis in endosomes and/or the expansion of lysosomes that occurs upon knockout of NHE6, results in decreased signaling and premature degradation of TrkB receptors. Thus, the loss of functional TrkB receptors in endosomes leads to a decrease in axon branching, presumably because subsequent exocytosis of these over-acidified vesicles results in fewer functional TrkB receptors on the membrane surface.
Two recent studies by Halloran and colleagues demonstrated that endocytic trafficking is also important for peripheral sensory neuron branching in vivo (Ponomareva et al. 2014; Ponomareva et al. 2016). Studying the Rohan-Beard neurons in living zebrafish, this group showed that constitutively active Rab5, which favors enlargement of early endosomes and disrupts the conversion to late endosomes, has no effect on axon outgrowth but decreases axon branching (Ponomareva et al. 2014). By photoactivating GFP-Rab5 and imaging its transport, they detected Rab5-labeled vesicles accumulating at axon branching points. Disruption of Rab5 transport by knockdown of the kinesin adapter protein calsyntenin-1 similarly results in decreased axon branching, suggesting that transport of Rab5-containing vesicles is important for peripheral axon branching. Moreover, constitutively active mutations in Rab7 and point mutations that give rise to Charcot-Marie Tooth syndrome, also decrease axon branching in these peripheral sensory axons (Ponomareva et al. 2016). Together, these studies indicate a properly functioning endocytic trafficking system is crucial to branch formation during development. Presumably, this occurs by targeting specific endocytic populations with their associated receptor cargo to distinct regions along the axonal arbor and subsequent insertion of these receptors results in a localized signaling cascade, cytoskeletal polymerization and formation of a new collateral branch.
F-BAR proteins in plasma membrane remodeling and branching
One large family of proteins implicated in endocytosis, cell migration and morphogenesis is the FER/CIP4 Homology Bin-Amphiphysin-RVS (F-BAR) superfamily of proteins. F-BAR proteins are present in many tissues and are involved in sensing and inducing plasma membrane curvature through their N-terminal F-BAR domain (Chitu & Stanley 2007; Aspenström 2008; Suetsugu et al. 2014). F-BAR proteins also contain other domains involved in binding active Rho GTPases (HR1 domains) or domains that act as GTPase-activating domains (GAP domains). Many F-BAR proteins also contain a C-terminal SH3 domain(s) that binds dynamin, the major vesicle scission protein, as well as a host of actin-associated proteins, such as N-WASP, WAVE and the formin DAAM1 (Suetsugu et al. 2014). F-BAR proteins exist as homo and heterodimers in cells (Coutinho-Budd et al. 2012) and act to elongate endocytic tubules by associating as end-to-end polymers that form helical coats around vesicles (Shimada et al. 2007; Frost et al. 2008). Their ability to bridge the plasma membrane to actin polymerization positions them as potentially important actors in filopodia, lamellipodia and subsequent axon branch formation (Figure 1A).
Several studies implicate F-BAR proteins as negative regulators of axon branch formation, consistent with their roles in endocytosis and tubulation. For example, knockdown of TOCA-1, a CIP4 family F-BAR protein, increases axon branching slightly in cultured hippocampal neurons (Kakimoto et al. 2006). Knockdown of syndapin I, another F-BAR family member, results in a pronounced increase in axon branching, whereas overexpression of syndapin I resulted in fewer axon branches in hippocampal neurons (Dharmalingam et al. 2009). Together, these data suggest that decreasing endocytosis increases branching, while driving endocytosis decreases branching. Although these studies did not delineate the pathways between endocytosis and branching the results are consistent with studies mentioned in the previous section.
Conversely, the inverse F-BAR (iF-BAR) protein srGAP2 promotes axon arborization (Guerrier et al. 2009). Instead of resulting in elongation of endocytic vesicles, overexpression of the iF-BAR protein srGAP2 resulted in filopodial-like structures that require actin polymerization to form, but surprisingly do not require F-actin for their maintenance (Guerrier et al. 2009; Carlson et al. 2011). Thus, srGAP2, as well as family members srGAP1 and 3 (Coutinho-Budd et al. 2012), are thought to form helical polymeric rings along the entire inner surface of the extended filopodial-like structure. Since filopodia are important for branch formation (Figure 1A), it follows that knockdown of srGAP2 decreases axon branching in cortical neurons (Guerrier et al. 2009). Although studies of F-BAR proteins in branching are limited, the few mentioned here suggest these proteins may be acting to bridge plasma membrane deformation and cytoskeletal rearrangements. Since both endocytosis and protrusion play key roles in the regulation of branching, further studies are likely to implicate other F-BAR proteins in the process of axon branching.
Concluding Remarks
In conclusion, axon branching is a complex cell biological problem involving the coordination of the cytoskeleton, the secretory pathway, and the organelles that provide the material and energy to elaborate the axon. We now have a working “parts lists” of the many cellular components that fuel this important stage of neuronal morphogenesis, although surely more are to be uncovered. Future work to determine how these different systems are coordinated and regulated is a critical area of research. Although we have touched upon many proteins that regulate both axonal outgrowth and axonal branching, further delineation of the similarities and differences between these dynamic stages of axonal morphogenesis is an important area of investigation. As noted in a prior review (Table 1 in (Gallo 2011), experimental manipulation of specific proteins can have disparate effects on axon branching and axon outgrowth. In the case of syt1, reviewed herein, along the axon syt1 was found to promote the formation of filopodia and thus branching, but had no effect on axon length. This might reflect the role of syt1 in mediating filopodia formation which is crucial to branching but dispensable, if not antagonistic, for axon extension (Argiro et al. 1984; Kleitman & Johnson 1989). Furthermore, axons can extend in the absence of actin filaments but collateral branching from the same axons is strictly dependent on actin filaments and protrusive activity (Dent & Kalil 2001). These considerations indicate that it will be important to dissect the roles of specific proteins in the morphogenetic events leading to axon branching or outgrowth, beyond the characterization of a function for the protein of interest in axon branching or outgrowth.
Axonal branching likely involves a number of scaffolding components at the plasma membrane that concentrate these different cellular machines in spatially clustered units, such as F-BAR proteins. Furthermore, there are likely a plethora of enzymes that catalyze post-translational modifications or otherwise alter the function, localization or stability of the cellular machines that promote stages of axon branching both spatially and temporally. As in non-neuronal cells, members of both the Rho and Rab families of small GTPases are integral to this function. There are also likely a number of neuronal-specific proteins necessary for this unique cellular shape change. For example, the brain enriched E3 ubiquitin ligase TRIM9 inhibits SNARE complex formation and exocytosis through binding to SNAP25 (Li et al. 2001; Winkle et al. 2014). Additionally TRIM9 ubiquitinates and inhibits the actin polymerase VASP, which reduces filopodial stability (Menon et al. 2015). Interestingly, TRIM9 also directly interacts with the netrin receptor DCC, and netrin stimulation inhibits these negative regulatory functions of TRIM9 in order to promote axon branching axon branching (Winkle et al. 2014). In addition to netrin, a number of other axon guidance cues can promote or block axon branching (Kennedy & Tessier-Lavigne 1995; Dent et al. 2004). Elucidation of the signaling pathways activated downstream of these distinct cues and how they converge upon the cellular machines necessary for branching is a key component to how these shape changes are spatially and temporally controlled in vivo.
Recently a number of non-canonical membrane delivery schemes have been identified which involve the direct coupling of the ER to the neuronal plasma membrane (Giordano et al. 2013; Petkovic et al. 2014). Although the coupling of the ER to the membrane has not been implicated in axon branching as of yet, the ER accumulates at sites of branching (Spillane et al. 2013) and since ER-based mechanisms may contribute to the delivery of plasma membrane material to the axolemma, they are well poised to also modulate axon branching. Continued investigation into the mechanism of axon branching is likely to yield novel insights into the coordination and regulation of the cytoskeleton, membranous compartments and membrane turnover.
Acknowledgments
This work was supported by awards to CCW (NIH F31-NS087837), KT (NIH T01-GM07507), EWD (NIH R01-NS080928), GG (NIH R01-NS078030), KFG (Pennsylvania Health Research Formula Fund and Bryn Mawr College) and SLG (NIH R01-GM108970).
Footnotes
No authors have a conflict of interest to report.
Referenced Literature
- Aalto MK, Ronne H, Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouvière C, Galli T. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17:1194–1203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes RME, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26:4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiro V, Bunge MB, Johnson MI. Correlation between growth form and movement and their dependence on neuronal age. J Neurosci. 1984;4:3051–3062. doi: 10.1523/JNEUROSCI.04-12-03051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenström P. International Review of Cell and Molecular Biology. Vol. 272. San Diego: 2008. Roles of F-BAR/PCH Proteins in the Regulation of Membrane Dynamics and Actin Reorganization; pp. 1–31. [DOI] [PubMed] [Google Scholar]
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2003;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Scheller RH. A Molecular Description of Synaptic Vesicle Membrane Trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- Blakey D, Wilson MC, Molnár Z. Termination and initial branch formation of SNAP-25-deficient thalamocortical fibres in heterochronic organotypic co-cultures. European Journal of Neuroscience. 2012;35:1586–1594. doi: 10.1111/j.1460-9568.2012.08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P, Kearns B, Champion K, Keränen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Broadwell RD. The neuronal endoplasmic reticulum: Its cytochemistry and contribution to the endomembrane system. II. Axons and terminals. The Journal of Comparitive Neurology. 1984;230:231–248. doi: 10.1002/cne.902300208. [DOI] [PubMed] [Google Scholar]
- Broeke JHP, Roelandse M, Luteijn MJ, Boiko T, Matus A, Toonen RF, Verhage M. Munc18 and Munc13 regulate early neurite outgrowth. Biology of the Cell. 2012;102:479–488. doi: 10.1042/BC20100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Petrenko A, Sudhof T, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Brouhard GJ. Dynamic instability 30 years later: complexities in microtubule growth and catastrophe. Molecular biology of the cell. 2015;26:1207–1210. doi: 10.1091/mbc.E13-10-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BR, Lloyd KE, Kruszewski A, Kim IH, Rodriguiz RM, Heindel C, Faytell M, Dudek SM, Wetsel WC, Soderling SH. WRP/srGAP3 Facilitates the Initiation of Spine Development by an Inverse F-BAR Domain, and Its Loss Impairs Long-Term Memory. J Neurosci. 2011;31:2447–2460. doi: 10.1523/JNEUROSCI.4433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Chitu V, Stanley ER. Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions. Trends Cell Biol. 2007;17:145–156. doi: 10.1016/j.tcb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Chun J, Shatz CJ. Redistribution of synaptic vesicle antigens is correlated with the disappearance of a transient synaptic zone in the developing cerebral cortex. Neuron. 1988;1:297–310. doi: 10.1016/0896-6273(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Lom B. Neurotrophic regulation of retinal ganglion cell synaptic connectivity: from axons and dendrites to synapses. Int J Dev Biol. 2004;48:947–956. doi: 10.1387/ijdb.041883sc. [DOI] [PubMed] [Google Scholar]
- Cotrufo T, Perez-Branguli F, Muhaisen A, Ros O, Andres R, Baeriswyl T, Fuschini G, Tarrago T, Pascual M, Urena J, et al. A Signaling Mechanism Coupling Netrin-1/Deleted in Colorectal Cancer Chemoattraction to SNARE-Mediated Exocytosis in Axonal Growth Cones. J Neurosci. 2011;31:14463–14480. doi: 10.1523/JNEUROSCI.3018-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Jr, Lee S, Courchet V, Liou D-Y, Aizawa S, Polleux F. Terminal Axon Branching Is Regulated by the LKB1-NUAK1 Kinase Pathway via Presynaptic Mitochondrial Capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Budd J, Ghukasyan V, Zylka MJ, Polleux F. The F-BAR domains from srGAP1, srGAP2 and srGAP3 regulate membrane deformation differently. J Cell Sci. 2012;125:3390–3401. doi: 10.1242/jcs.098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F, Xu Y, Chang W-P, Dulubova I, Khvotchev M, Liu X, Südhof TC, Rizo J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Estimation of the Number of Synapses in the Cerebral Cortex: Methodological Considerations. Cerebral Cortex. 1999;9:722–732. doi: 10.1093/cercor/9.7.722. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23:9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Tang F, Kalil K. Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms. Neuroscientist. 2003;9:343–353. doi: 10.1177/1073858403252683. [DOI] [PubMed] [Google Scholar]
- Dent, Kwiatkowski, Mebane, Philippar, Barzik, Rubinson, Gupton, Veen V, Furman, Zhang, et al. Filopodia are required for cortical neurite initiation. Nat Cell Biol. 2007;9:1347–1359. doi: 10.1038/ncb1654. [DOI] [PubMed] [Google Scholar]
- Dharmalingam E, Haeckel A, Pinyol R, Schwintzer L, Koch D, Kessels MM, Qualmann B. F-BAR Proteins of the Syndapin Family Shape the Plasma Membrane and Are Crucial for Neuromorphogenesis. J Neurosci. 2009;29:13315–13327. doi: 10.1523/JNEUROSCI.3973-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz S, Grassi D, Bernis ME, Sosa L, Bisbal M, Gastaldi L, Jausoro I, Caceres A, Pfenninger KH, Quiroga S. The TC10-Exo70 Complex Is Essential for Membrane Expansion and Axonal Specification in Developing Neurons. J Neurosci. 2009;29:13292–13301. doi: 10.1523/JNEUROSCI.3907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez H, Malkinson G, Prager-Khoutorsky M, De Zeeuw CI, Hoogenraad CC, Spira ME. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J Cell Biol. 2007;176:497–507. doi: 10.1083/jcb.200607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Buckley KM. The synaptic vesicle protein synaptotagmin promotes formation of filopodia in fibroblasts. Nature. 1993;364:537–540. doi: 10.1038/364537a0. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Kao HT, Feng J, Rapoport M, Greengard P. Synapsin III: developmental expression, subcellular localization, and role in axon formation. J Neurosci. 2000;20:3736–3744. doi: 10.1523/JNEUROSCI.20-10-03736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TL, Cameron P, De Camilli P, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foletti DL, Lin R, Finley MA, Scheller RH. Phosphorylated syntaxin 1 is localized to discrete domains along a subset of axons. J Neurosci. 2000;20:4535–4544. doi: 10.1523/JNEUROSCI.20-12-04535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasiero EF, Bonanomi D, Benfenati F, Valtorta F. The role of synapsins in neuronal development. Cell Mol Life Sci. 2009 doi: 10.1007/s00018-009-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J Neurosci. 1999;19:3860–3873. doi: 10.1523/JNEUROSCI.19-10-03860.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Neurotrophins and the dynamic regulation of the neuronal cytoskeleton. J Neurobiol. 2000;44:159–173. doi: 10.1002/1097-4695(200008)44:2<159::aid-neu6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Gallo G. The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol. 2011;71:201–220. doi: 10.1002/dneu.20852. [DOI] [PubMed] [Google Scholar]
- Gallo G. Mechanisms Underlying the Initiation and Dynamics of Neuronal Filopodia: From Neurite Formation to Synaptogenesis. International Review of Cell and Molecular Biology. 2013;301:95–156. doi: 10.1016/B978-0-12-407704-1.00003-8. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Tang Y, Wang L, Ladt K, Loi J. A dynamic formin-dependent deep F-actin network in axons. J Cell Biol. 2015;210:410–417. doi: 10.1083/jcb.201506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney J, Zheng JQ. Cytoskeletal dynamics underlying collateral membrane protrusions induced by neurotrophins in cultured Xenopus embryonic neurons. J Neurobiol. 2002;54:393–405. doi: 10.1002/neu.10149. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development. 2010;138:183–195. doi: 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI (4, 5) P 2-dependent and Ca 2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Fukushima Y, Barnes AM, Sugo N, Tang F, Lagnado L, Yamamoto N. Regulation of thalamocortical axon branching by BDNF and synaptic vesicle cycling. Front Neural Circuits. 2013;7:202. doi: 10.3389/fncir.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greif KF, Asabere N, Lutz GJ, Gallo G. Synaptotagmin-1 promotes the formation of axonal filopodia and branches along the developing axons of forebrain neurons. Dev Neurobiol. 2013;73:27–44. doi: 10.1002/dneu.22033. [DOI] [PubMed] [Google Scholar]
- Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin W-L, Frost A, Polleux F. The F-BAR Domain of srGAP2 Induces Membrane Protrusions Required for Neuronal Migration and Morphogenesis. Cell. 2009;138:990–1004. doi: 10.1016/j.cell.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumy LF, Yeo GSH, Tung Y-CL, Zivraj KH, Willis D, Coppola G, Lam BYH, Twiss JL, Holt CE, Fawcett JW. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 2011;17:85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T. Collateral sprouting as a target for improved function after spinal cord injury. J Neurotrauma. 2006;23:281–294. doi: 10.1089/neu.2006.23.281. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Heuser JE, Jahn R. Neurotransmitter release — four years of SNARE complexes. Current Opinion in Neurobiology. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- Hausott B, Hausott B, Rietzler A, Rietzler A, Vallant N, Vallant N, Auer M, Auer M, Haller I, Haller I, et al. Inhibition of fibroblast growth factor receptor 1 endocytosis promotes axonal branching of adult sensory neurons. Neuroscience. 2011;188:13–22. doi: 10.1016/j.neuroscience.2011.04.064. [DOI] [PubMed] [Google Scholar]
- Hayano Y, Yamamoto N. Activity-dependent thalamocortical axon branching. Neuroscientist. 2008;14:359–368. doi: 10.1177/1073858408317272. [DOI] [PubMed] [Google Scholar]
- Holt M, Varoqueaux F, Wiederhold K, Takamori S, Urlaub H, Fasshauer D, Jahn R. Identification of SNAP-47, a Novel Qbc-SNARE with Ubiquitous Expression. J Biol Chem. 2006;281:17076–17083. doi: 10.1074/jbc.M513838200. [DOI] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T, Gallo G, Spiliotis ET. Septin-Driven Coordination of Actin and Microtubule Remodeling Regulates the Collateral Branching of Axons. Current Biology. 2012;22:1–7. doi: 10.1016/j.cub.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A-K, Karlsson R. Synaptotagmin 1 causes phosphatidyl inositol lipid-dependent actin remodeling in cultured non-neuronal and neuronal cells. Exp Cell Res. 2012;318:114–126. doi: 10.1016/j.yexcr.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Kabayama H, Takei K, Fukuda M, Ibata K, Mikoshiba K. Functional involvement of synaptotagmin I/II C2A domain in neurite outgrowth of chick dorsal root ganglion neuron. Neuroscience. 1999;88:999–1003. doi: 10.1016/s0306-4522(98)00547-8. [DOI] [PubMed] [Google Scholar]
- Kakimoto T, Katoh H, Negishi M. Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination. J Biol Chem. 2006;281:29042–29053. doi: 10.1074/jbc.M604025200. [DOI] [PubMed] [Google Scholar]
- Kalil K, Dent EW. Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nature Reviews Neuroscience. 2014;15:7–18. doi: 10.1038/nrn3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H-T, Song H-J, Porton B, Ming G-L, Hoh J, Abraham M, Czernik AJ, Pieribone VA, Poo M-M, Greengard P. A protein kinase A–dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci. 2002;5:431–437. doi: 10.1038/nn840. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Tessier-Lavigne M. Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Current Opinion in Neurobiology. 1995;5:83–90. doi: 10.1016/0959-4388(95)80091-3. [DOI] [PubMed] [Google Scholar]
- Ketschek A, Gallo G. Nerve Growth Factor Induces Axonal Filopodia through Localized Microdomains of Phosphoinositide 3-Kinase Activity That Drive the Formation of Cytoskeletal Precursors to Filopodia. J Neurosci. 2010;30:12185–12197. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek A, Jones S, Spillane M, Korobova F, Svitkina T, Gallo G. Nerve growth factor promotes reorganization of the axonal microtubule array at sites of axon collateral branching. Dev Neurobiol. 2015;75:1441–1461. doi: 10.1002/dneu.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Mizoguchi A, Ide C. Regulation of Growth Cone Extension by SNARE Proteins. Journal of Histochemistry & Cytochemistry. 2003;51:429–433. doi: 10.1177/002215540305100404. [DOI] [PubMed] [Google Scholar]
- Kleitman N, Johnson MI. Rapid growth cone translocation on laminin is supported by lamellipodial not filopodial structures. Cell Motil Cytoskeleton. 1989;13:288–300. doi: 10.1002/cm.970130407. [DOI] [PubMed] [Google Scholar]
- Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster A, Nola S, Dingli F, Vacca B, Gauchy C. The Q-soluble N-Ethylmaleimide-sensitive Factor Attachment Protein Receptor (Q-SNARE) SNAP-47 Regulates Trafficking of Selected Vesicle-associated Membrane Proteins (VAMPs) J Biol Chem. 2015;290:28056–28069. doi: 10.1074/jbc.M115.666362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiecka ZM, Winckler B. Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes. Mol Cell Neurosci. 2011;48:278–287. doi: 10.1016/j.mcn.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chin LS. The molecular machinery of synaptic vesicle exocytosis. Cell Mol Life Sci. 2003;60:942–960. doi: 10.1007/s00018-003-2240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chin LS, Weigel C, Li L. Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis. J Biol Chem. 2001;276:40824–40833. doi: 10.1074/jbc.M106141200. [DOI] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66:847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Perin MS, Südhof TC, De Camilli P. Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejillano MR, Kojima S-I, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Menon S, Boyer NP, Winkle CC, McClain LM, Hanlin CC, Pandey D, Rothenfußer S, Taylor AM, Gupton SL. The E3 Ubiquitin Ligase TRIM9 Is a Filopodia Off Switch Required for Netrin-Dependent Axon Guidance. Dev Cell. 2015;35:698–712. doi: 10.1016/j.devcel.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JSY, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Direct evidence for coherent low velocity axonal transport of mitochondria. J Cell Biol. 2006;173:373–381. doi: 10.1083/jcb.200510097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingorance-Le Meur A, O'Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2008;28:248–260. doi: 10.1038/emboj.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- O'Leary DDM, McLaughlin T. Mechanisms of retinotopic map development: Ephs, ephrins, and spontaneous correlated retinal activity. Prog Brain Res. 2005;147:43–65. doi: 10.1016/S0079-6123(04)47005-8. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Smith GM, Fouad K. Plasticity After Spinal Cord Injury: Relevance to Recovery and Approaches to Facilitate It. Neurotherapeutics. 2011;8:283–293. doi: 10.1007/s13311-011-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, JONES KA, Ayala G, Knowles J, Grenningloh G, Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Staple JK, Naldi E, Schiavo G, Rossetto O, Petitpierre S, Malgaroli A, Montecucco C, Catsicas S. Common and distinct fusion proteins in axonal growth and transmitter release. J Comp Neurol. 1996;367:222–234. doi: 10.1002/(SICI)1096-9861(19960401)367:2<222::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Lizarraga SB, Schmidt M, Yang U, Gong J, Ellisor D, Kauer JA, Morrow EM. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin MS, Brose N, Jahn R, Südhof TC. Domain structure of synaptotagmin (p65) J Biol Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Petkovic M, Jemaiel A, Daste F, Specht CG, Izeddin I, Vorkel D, Verbavatz J-M, Darzacq X, Triller A, Pfenninger KH, et al. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat Cell Biol. 2014;16:434–444. doi: 10.1038/ncb2937. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH, Johnson MP. Membrane biogenesis in the sprouting neuron. I. Selective transfer of newly synthesized phospholipid into the growing neurite. J Cell Biol. 1983;97:1038–1042. doi: 10.1083/jcb.97.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nature Reviews Neuroscience. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- Pinches EM, Cline HT. Distribution of synaptic vesicle proteins within single retinotectal axons ofXenopus tadpoles. J Neurobiol. 1998;35:426–434. [PubMed] [Google Scholar]
- Ponomareva OY, Eliceiri KW, Halloran MC. Charcot-Marie-Tooth 2b associated Rab7 mutations cause axon growth and guidance defects during vertebrate sensory neuron development. Neural Development. 2016;11:2. doi: 10.1186/s13064-016-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomareva OY, Holmen IC, Sperry AJ. Calsyntenin-1 regulates axon branching and endosomal trafficking during sensory neuron development in vivo. J Neurosci. 2014;34:9235–9248. doi: 10.1523/JNEUROSCI.0561-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvoisé B, Blackstone C. Emerging themes of ER organization in the development and maintenance of axons. Current Opinion in Neurobiology. 2010;20:531–537. doi: 10.1016/j.conb.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Südhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Annu Rev Cell Dev Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- Roossien DH, Lamoureux P, Miller KE. Cytoplasmic dynein pushes the cytoskeletal meshwork forward during axonal elongation. J Cell Sci. 2014;127:3593–3602. doi: 10.1242/jcs.152611. [DOI] [PubMed] [Google Scholar]
- Ros O, Cotrufo T, Martinez-Marmol R, Soriano E. Regulation of Patterned Dynamics of Local Exocytosis in Growth Cones by Netrin-1. J Neurosci. 2015;35:5156–5170. doi: 10.1523/JNEUROSCI.0124-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo SL, McAllister AK. Mobility and cycling of synaptic protein–containing vesicles in axonal growth cone filopodia. Nat Neurosci. 2003;6:1264–1269. doi: 10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Baba T, Tanaka S, Izumi G, Yasumi M, Takai Y. Regulation of SNAREs by tomosyn and ROCK: implication in extension and retraction of neurites. J Cell Biol. 2004;166:17–25. doi: 10.1083/jcb.200405002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125:2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab Y, Mouton J, Chasserot-Golaz S, Marty I, Maulet Y, Jover E. Calcium-dependent translocation of synaptotagmin to the plasma membrane in the dendrites of developing neurones. Brain Res Mol Brain Res. 2001;96:1–13. doi: 10.1016/s0169-328x(01)00244-3. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H. Mitochondrial trafficking and anchoring in neurons: New insight and implications. J Cell Biol. 2014;204:1087–1098. doi: 10.1083/jcb.201312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SY, Wang J, Asada N, Neumayer G. Protein 600 is a microtubule/endoplasmic reticulum-associated protein in CNS neurons. J Neurosci. 2008;28:3604–3614. doi: 10.1523/JNEUROSCI.5278-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, et al. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell. 2007;129:761–772. doi: 10.1016/j.cell.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Shimojo M, Courchet J, Pieraut S, Torabi-Rander N, Sando R, III, Polleux F, Maximov A. SNAREs Controlling Vesicular Release of BDNF and Development of Callosal Axons. Cell Reports. 2015;11:1–14. doi: 10.1016/j.celrep.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL. Cytoskeletal movements and substrate interactions during initiation of neurite outgrowth by sympathetic neurons in vitro. J Neurosci. 1994;14:384–398. doi: 10.1523/JNEUROSCI.14-01-00384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. Nerve Growth Factor-Induced Formation of Axonal Filopodia and Collateral Branches Involves the Intra-Axonal Synthesis of Regulators of the Actin-Nucleating Arp2/3 Complex. J Neurosci. 2012;32:17671–17689. doi: 10.1523/JNEUROSCI.1079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol. 2011 doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Reports. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu S, Kurisu S, Takenawa T. Dynamic Shaping of Cellular Membranes by Phospholipids and Membrane-Deforming Proteins. Physiol Rev. 2014;94:1219–1248. doi: 10.1152/physrev.00040.2013. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane Fusion: Grappling with SNARE and SM Proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tang F, Kalil K. Netrin-1 induces axon branching in developing cortical neurons by frequency-dependent calcium signaling pathways. J Neurosci. 2005;25:6702–6715. doi: 10.1523/JNEUROSCI.0871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K, Matsuki N, Koyama R. AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Dev Neurobiol. 2013;74:557–573. doi: 10.1002/dneu.22149. [DOI] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- Tojima T, Itofusa R, Kamiguchi H. Asymmetric Clathrin-Mediated Endocytosis Drives Repulsive Growth Cone Guidance. Neuron. 2010;66:370–377. doi: 10.1016/j.neuron.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Tojima T, Itofusa R, Kamiguchi H. Steering Neuronal Growth Cones by Shifting the Imbalance between Exocytosis and Endocytosis. J Neurosci. 2014;34:7165–7178. doi: 10.1523/JNEUROSCI.5261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Kamiguchi H. Exocytic and endocytic membrane trafficking in axon development. In: Nakamura H, editor. Dev Growth Differ. Vol. 57. 2015. pp. 291–304. [DOI] [PubMed] [Google Scholar]
- Uesaka N, Ruthazer ES, Yamamoto N. The role of neural activity in cortical axon branching. Neuroscientist. 2006;12:102–106. doi: 10.1177/1073858405281673. [DOI] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neurosci. 2001;21:3839–3848. doi: 10.1523/JNEUROSCI.21-11-03839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Coco S, Bacci A, Rossetto O, De Camilli P, Montecucco C, Matteoli M. Tetanus toxin blocks the exocytosis of synaptic vesicles clustered at synapses but not of synaptic vesicles in isolated axons. J Neurosci. 1999;19:6723–6732. doi: 10.1523/JNEUROSCI.19-16-06723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res. 2006;152:245–263. doi: 10.1016/S0079-6123(05)52016-8. [DOI] [PubMed] [Google Scholar]
- Wiederhold K, Fasshauer D. Is Assembly of the SNARE Complex Enough to Fuel Membrane Fusion? J Biol Chem. 2009;284:13143–13152. doi: 10.1074/jbc.M900703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, McClain LM, Valtschanoff JG, Park CS, Maglione C, Gupton SL. A novel Netrin-1-sensitive mechanism promotes local SNARE-mediated exocytosis during axon branching. J Cell Biol. 2014;205:217–232. doi: 10.1083/jcb.201311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–456. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, López-Bendito G. Shaping brain connections through spontaneous neural activity. European Journal of Neuroscience. 2012;35:1595–1604. doi: 10.1111/j.1460-9568.2012.08101.x. [DOI] [PubMed] [Google Scholar]
- Yap CC, Winckler B. Adapting for endocytosis: roles for endocytic sorting adaptors in directing neural development. Front Cell Neurosci. 2015;9:1–17. doi: 10.3389/fncel.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ahmad FJ, Baas PW. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J Neurosci. 1994;14:5872–5884. doi: 10.1523/JNEUROSCI.14-10-05872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko S, Popov S. Dynamics of Axonal Microtubules Regulate the Topology of New Membrane Insertion into the Growing Neurites. J Cell Biol. 1998;143:1077–1086. doi: 10.1083/jcb.143.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Martinez SJ, Haber M, Jones EV, Bouvier D, Doucet G, Corera AT, Fon EA, Zisch AH, Murai KK. EphA4 Signaling Regulates Phospholipase C 1 Activation, Cofilin Membrane Association, and Dendritic Spine Morphology. J Neurosci. 2007;27:5127–5138. doi: 10.1523/JNEUROSCI.1170-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]