Abstract
Background
Anxiety patients exhibit deficits in cognitive tasks that require prefrontal control of attention, including those that tap working memory (WM). However, it is unclear whether these deficits reflect threat-related processes or symptoms of the disorder. Here we distinguish between these hypotheses by determining the effect of shock threat vs. safety on the neural substrates of WM performance in anxiety patients and healthy controls.
Methods
Patients, diagnosed with generalized and/or social anxiety disorder, and controls performed blocks of an N-back WM task during periods of safety and threat of shock. We recorded BOLD activity during the task, and investigated the effect of clinical anxiety (patients vs. controls) and threat on WM load-related BOLD activation.
Results
Behaviorally, patients showed an overall impairment in both accuracy and reaction time compared to controls, independent of threat. At the neural level, patients showed less WM load-related activation in the dorsolateral prefrontal cortex, a region critical for cognitive control. In addition, patients showed less WM load-related deactivation in the ventromedial prefrontal cortex and posterior cingulate cortex, which are regions of the default mode network. Most importantly, these effects were not modulated by threat.
Conclusions
This work suggests that the cognitive deficits seen in anxiety patients may represent a key component of clinical anxiety, rather than a consequence of threat.
Keywords: anxiety/anxiety disorders, functional MRI, cognition, GAD/generalized anxiety disorder, stress
Individuals with anxiety disorders frequently suffer from attentional problems, such as being easily distracted and unable to focus on ongoing tasks. In fact, one of the core symptoms of generalized anxiety disorder (GAD) is “difficulty concentrating”1. Such susceptibility to distraction has been linked to competition between task-relevant and task-irrelevant (e.g., worry) thoughts in working memory (WM)2. Although anxiety has been frequently shown to disrupt WM3–5, negative studies have also been reported6. Inconsistent results might be related to the use of different operational definitions of anxiety in these studies. Some studies examine changes in aversive state (state anxiety) usually using within-subject designs, while others define anxiety as a predisposition (trait or dispositional anxiety). In addition, anxiety also refers to a disorder (clinical anxiety), with high levels of trait and state anxiety. Therefore, it is difficult to determine the root cause of the WM deficits reported in previous work. Furthermore, because clinical anxiety includes symptoms related to both dimensions, it is difficult to determine whether the WM deficits in these individuals arise primarily from disease characteristics, or state-dependent effects like threat-related processing.
Studies on the relationship between dispositional anxiety (i.e., high trait anxiety) and WM performance suggest that impairments in WM potentially arise from two alternative mechanisms, (1) an inefficient cognitive control system, or (2) an inability to engage control mechanisms to screen out task-irrelevant (threat) processing and focus on the task. In line with the first interpretation, individuals with dispositional anxiety over-engage the dorsolateral prefrontal cortex (dlPFC) due to interference from threat-related processing/worry2,6,7. In line with the second interpretation, individuals with dispositional anxiety under-engage the dlPFC, even in the absence of distractors, leading to less dlPFC activation during task performance8–10. Using fMRI, the present study will test these alternative hypotheses.
However, these interpretations do not control for increases in state anxiety in response to an actual or perceived threat stimulus. Therefore, the purpose of this study was to determine whether WM-related deficits in clinical anxiety are chronic, or whether they arise due to threat-related processing (e.g. worry). In the latter case, one would expect that experimentally-inducing threat would exacerbate the deficits. To manipulate state anxiety we used threat of shock, which is a well-validated11,12, translational procedure13–16, previously shown to increase state anxiety3,17–19, cause worry20,21, and interfere with task performance22–26. We tested WM performance during threat of shock and safety in anxiety patients and healthy controls, while recording brain activity with functional magnetic resonance imaging (fMRI). Subjects performed a spatial N-back working memory (WM) task comprising 4 levels of difficulty (i.e., cognitive load), 0-back, 1-back, 2-back and 3-back conditions. We chose to study spatial working memory because it has been found to activate the dlPFC proportionally to the load level27,28, and, unlike verbal WM4, has been previously shown to be influenced by anxiety at both low and high loads4,29,23.
Using this paradigm, we tested two competing hypotheses: 1) Exaggerated dlPFC activation in clinical anxiety arises transiently in anxiogenic (threat) situations, where processing of threat-related information interferes with task demands7,30; 2) Poor engagement of the prefrontal cortex (particularly dlPFC) in clinical anxiety reflects a core component of the disorder, rather than a transient effect of threat-related processing8–10. The former hypothesis would be supported by a Diagnosis x Threat (threat, safe) interaction or Diagnosis x Load x Threat interaction, where patients actually show greater dlPFC activation relative to the controls, but only in the threat condition. The latter hypothesis would be supported by a diagnosis main effect or Diagnosis x Load interaction, where patients show reduced dlPFC activation compared to healthy controls, independent of threat.
Methods
Participants
Forty-one healthy volunteers (27 female; M(SD): 28.65(7.14) yo) and 28 anxiety patients (20 female; M(SD): 30.96(9.87) yo) from the Washington DC metropolitan area were recruited into the present study. Following an initial telephone screen, participants visited the National Institutes of Health Clinical Center for a comprehensive screening by a clinician. Inclusion criteria for clinical anxiety were: (1) no other current Axis I psychiatric disorder or past psychosis as assessed by SCID-I/P31, (2) no first-degree relative with a known psychotic disorder, (3) no interfering acute or chronic medical condition, (4) no brain abnormality on MRI as assessed by a licensed radiologist, (5) negative urine drug screen, and (6) right-handedness. Of the sixty-nine participants recruited, 6 participants (5 patients) were excluded from the analysis because of issues with their fMRI data (e.g. excessive motion, imaging artifacts, etc.). All included datasets were free from excessive motion (defined below) and obvious imaging artifacts.
Anxiety patients were diagnosed with either generalized anxiety disorder (GAD, n = 7), social anxiety disorder (SAD, n = 3) or comorbid GAD/SAD (n = 13) using DSM-IV classifications1. Additional inclusion criteria for healthy volunteers were no current or past history of any Axis I psychiatric disorder as assessed by SCID-I/NP. All participants gave written informed consent approved by the National Institute of Mental Health (NIMH) Combined Neuroscience Institutional Review Board and received compensation for participating.
Psychometric data
Participants completed measures of anxiety (Beck Anxiety Inventory; BAI19, State/Trait Anxiety Scale20), depression (Beck Depression Inventory; BDI21, and intelligence (Wechsler Abbreviated Scale of Intelligence; WASI22).
A task-specific questionnaire was administered after each block to assess the participants’ emotional state during the task. This questionnaire included the following questions, which were all scored on 1 (not at all) to 9 scales (extremely): 1) During the previous (threat/safe) blocks, how much did each task distract you from anxiety related to the shock? 2) During the previous (threat/safe) blocks, how much did your anxiety related to the shock interfere with your performance on each task? 3) Please rate your level of anxiety/ fear when you were in the (threat/safe) blocks. 4) Please rate the level of difficulty of the task when you were in the (threat/safe) blocks. 5) Please rate the intensity of the electrical stimulation during the previous run.
Stimuli and apparatus
Stimuli were presented to participants using the Presentation software package (version 14.6, Neurobehavioral Systems, Berkeley, CA) via a back-projection system. We used a Digitimer constant current stimulator (DS7A; Digitimer, Letchworth Garden City, UK) to deliver 2 ms shocks to the subjects’ left wrist via 2 Ag / AgCl 6 mm electrodes. The intensity of the shock could range from 0mA to 100mA and was calibrated prior to the experiment. Responses were collected using a 4-button fiber optic response device (Current Designs, Philadelphia, PA).
Procedure
Upon arrival to the scanning suite, subjects were briefed on the experiment, assessed by a nurse, and given an opportunity to review the informed consent form. They then completed the battery of psychological tests (See Psychometric Data) and practiced the task. Next, subjects were escorted into the scanning room, and situated comfortably on the gurney. Scanning began with the collection of an MPRAGE, followed by two EPI task runs. After completion of the task, the subject was removed from the scanner and completed a post-experimental and a debriefing questionnaire.
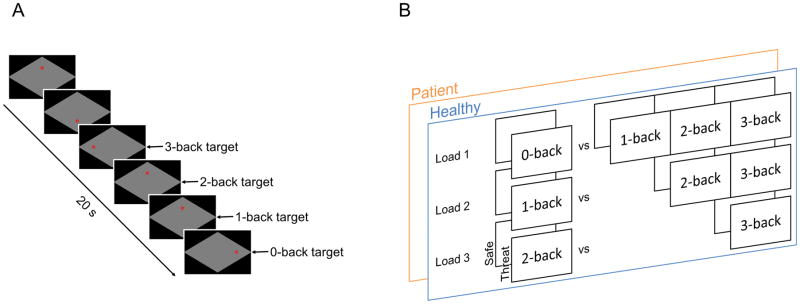
During the task runs the subject performed several blocks of the N-back task, which occurred during periods of safety and threat of shock (See Figure 1). For the duration of the experiment, subjects viewed a diamond-shaped area in the center of the screen, surrounded by a colored box. The color of the surrounding box informed the subject whether they were in a safe block (blue) or a threat block (red). The target stimulus (*) could appear in one of four quadrants of the viewing area, corresponding to the corners of the diamond. During the 1-, 2- and 3-back blocks, the subject had to indicate whether the current stimulus was in the same position as the stimulus presented 1, 2, or 3 trials previously. During the 0-back condition, the subject had to indicate whether the position of the stimulus matched the target position (uppermost quadrant). As a result, there were 8 types of blocks: Safe (0-, 1-, 2-, and 3-back) and Threat (0-, 1-, 2-, and 3-back).
Figure 1. Schematic of experimental design and statistical comparisons.
A) Subjects tracked the location of a red asterisk on the corners of a black diamond (up, down, right, left), and had to indicate whether the location of the current stimulus (*) matched the location of the target. In the 0-back condition, the subject indicated whether the stimulus was located at the top of the diamond (target-up position). B) We performed a general linear model with the following factors: 1) patients vs. controls, 2) threat vs. safe, and 3) load. We modeled the load factor using the three orthogonal planned comparisons depicted.
Blocks were 40 s long, separated by an 8 s interblock interval. Prior to each block, the block type (0-, 1-, 2-, or 3-back) was specified at the top of the screen for a period of 2 s, after which the border changed color to indicate the condition (Safe or Threat). Each block consisted of 18, 2.5 s trials. During each trial, the target stimulus was presented for 500 ms, followed by a 2 s intertrial interval. Blocks were grouped into two, 760 s runs, each with 16 blocks (2 per type). Blocks were pseudorandomly organized, such that blocks from the same condition (safe or threat) or load level (0-, 1-, 2-, or 3-back) were not presented sequentially. In addition, block order was counterbalanced across subjects, such that half of the subjects began with a safe block and the other half began with a threat block. Participants were told they would receive shocks unpredictably during the threat condition. Over the course of the experiment, participants received a total of 8 shocks. The shocks were delivered at unpredictable moments during the threat blocks, and shock delivery was varied across counterbalance orders. We included a regressor of no interest corresponding to the shock delivery. To create this regressor we modeled a gamma variate function beginning at the TR of each shock presentation.
Shock intensity calibration
Shock intensity varied across participants based on their subjective rating of stimulus discomfort. The shock work-up procedure was completed prior to the task to determine a strength which was “uncomfortable, but not painful.” Acceptable subjective stimulus rating ranged from 3 to 4.5 on a 1–5 scale of discomfort (1 = not at all, 5 = extreme).
fMRI acquisition
We collected two runs of 380 echo-planar images (EPI) using a 3T Siemens MAGNETOM Skyra (Erlangen, Germany) fMRI system, and a 32-channel head coil. Thirty-five interleaved 3 mm slices (Matrix 64 mm x 64 mm; FOV = 192 x 192) were collected parallel to the AC-PC line (TR = 2 s; TE = 30 ms; Flip angle = 70°), resulting in whole-brain coverage with 3 mm isotropic voxels. Prior to the first functional run we acquired a T1-weighted MPRAGE (TR 1900; TE 2.13; Flip angle 9). We acquired 192 0.9 mm axial slices (Matrix 256 mm x 256 mm; FOV 240 mm x 240 mm), which were later co-registered to the EPI images.
fMRI analysis
fMRI data were analyzed using the AFNI software package32. MPRAGE images were first processed with Freesurfer33 to obtain segmentation masks corresponding to the brain (skull-stripped), white matter, and ventricles. The whole-brain masks were normalized to MNI space using the ICBM 2009a Nonlinear Symmetric atlas34–36 and the AFNI program 3dQwarp, which performs a non-linear warp to a template brain. For display purposes, the skull-stripped MPRAGE images in MNI space were averaged across subjects. This average serves as the underlay for Figures 3–5. The following preprocessing steps were done to the EPI data using the afni_proc.py script: despiking the timeseries (despike), slice timing correction (tshift), co-registration with the MPRAGE (align), volume registration across the timeseries (volreg), and normalization (scale). To correct for motion, we censored images where the derivative of the motion regressors from 3dvolreg had a Euclidean norm above 0.5 mm. These preprocessed EPI timeseries were then warped to MNI space using the parameters obtained from 3dQwarp, and blurred within the whole-brain mask using a 6 mm FWHM Gaussian filter. In addition, we excluded subjects with more than 76 (10% of total) censored TRs. Six subjects were excluded based on these criteria.
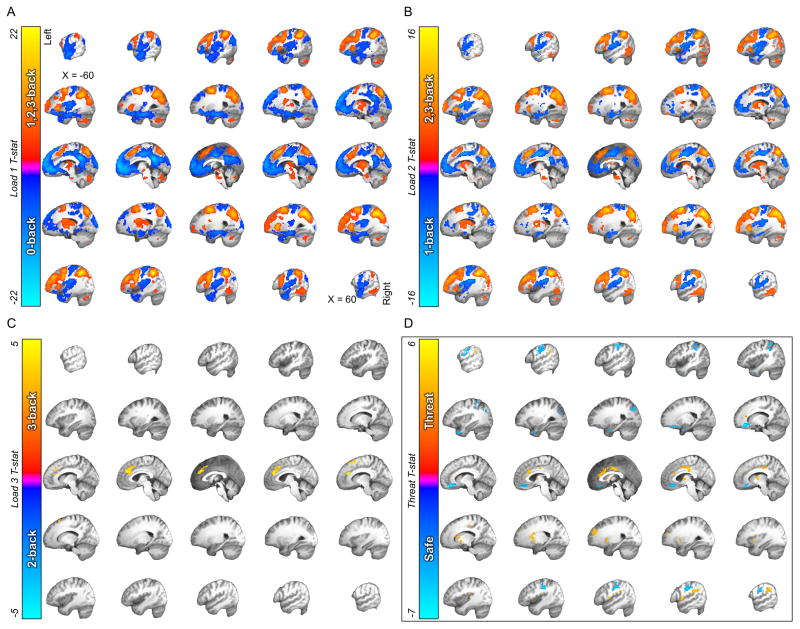
Figure 3. The effects of WM load and threat on BOLD activation.
A) Thresholded t-map for the 0- vs. 1-, 2-, 3-back comparison. B) Thresholded t-map for the 1- vs. 2-, 3-back comparison. C) Thresholded t-map for the 2- vs. 3-back comparison. (Warm colors represent WM load-related increases in BOLD activity. Cool colors represent WM load-related decreases in BOLD activity.) D) Thresholded t-map for the safe vs. threat comparison. (Warm colors represent threat related increases in BOLD activity. Cool colors represent threat related decreases in BOLD activity.)
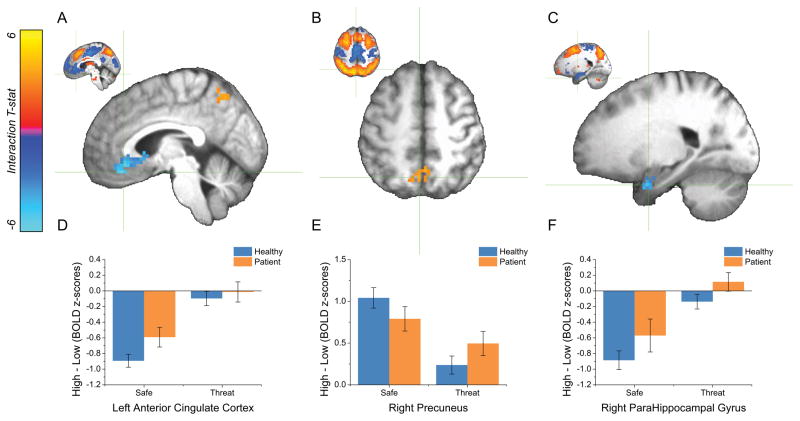
Figure 5. The effect of Threat on WM load-related activity.
A, B, C ) Thresholded t-maps for the Threat x Load 2 (1- vs. 2-, 3-back) interaction. (Warm colors represent decreases in task-positive activity as a function of threat. Cool colors represent decreases in task-negative activity as a function of threat. Crosshairs depict the location of the voxel with the peak activation for a given cluster. Coordinates are reported in Table 3. Insets depict the WM load-related effect at the corresponding location.) D, E, F ) Pattern of activity seen in the clusters depicted in the above panels. (Bars represent the mean ± SEM.)
For the first level analysis we extracted statistical parametric maps using a general linear model, as implemented in the AFNI program 3dDeconvolve. Regressors based on a fourth-order polynomial were used to model the baseline. Regressors of no interest were created from 6 motion parameters, timeseries from the white matter and ventricles, and shock onsets. The shock onsets were convolved with a gamma variate function to account for BOLD responses to the shock stimuli. Given that block designs typically result in better signal to noise ratio37, and the n-back task requires blocks of regularly spaced trials of a particular type (e.g. 1-back, 2-back, etc.), we chose a block design to model the BOLD response for the different conditions. Regressors corresponding to the 40 s blocks were convolved with a gamma variate function and entered into the GLM. The resulting partial correlation coefficients for each condition were extracted, and converted to z-scores using the following formula ([β − Mβ]/SDβ) to account for individual differences in overall BOLD responses.
To determine the effect of WM-load, diagnosis, and threat on the BOLD responses, we performed a general linear model with Load, Diagnosis, and Threat as fixed factors, and subject as a random factor to analyze the group data (blurred beta maps). Diagnosis and Threat were each dummy-coded using a single degree of freedom. Because there were unequal Ns in our Diagnostic groups, we weighted the group codes for that regressor.
Although previous studies examining WM in anxiety have treated load as a singular construct4,5,23, increasing the number of items in the n-back task incorporates multiple cognitive processes38–40. For instance, moving from a 0-back target detection task to a 1-back task requires the maintenance of the 1-back item across time, while moving from a 1-back task to a 2-back task requires the maintenance of the 1 and 2-back items, and the additional suppression of the 1-back item, which is maintained but not acted upon. Models using multiple degrees of freedom, like linear or quadratic trends, may miss neural activity uniquely engaged by these distinct processes41. Therefore, we chose to model the effects of this factor using 3 orthogonal comparisons to capture specific increases in WM load-related processing as the number of to-be-maintained items increased. Accordingly, we dummy-coded the following regressors: load comparison 1 (0-back vs. 1-, 2-, and 3-back), load comparison 2 (1-back vs. 2-, and 3-back), and load comparison 3 (2-back vs. 3-back). To code the 2-way and 3-way interactions between the fixed factors, we simply cross-multiplied each of the main effect regressors. In addition, because we saw differences in BDI scores across groups, we included BDI as a covariate. We then entered these regressors into the AFNI program 3dRegAna. In addition, we used the same general linear model to predict accuracy and reaction time.
The resulting statistical group maps were then corrected for multiple comparisons using a cluster-thresholding technique. We began by estimating the smoothness of noise by passing the subjects’ residual timeseries from 3dDeconvolve through the AFNI program 3dFWHMx, resulting in an estimated average smoothness of 7.43 mm. We then ran 10,000 monte carlo simulations using the AFNI program 3dClustSim to estimate a minimum cluster size threshold based on the estimated smoothness of our noise (s = 7.43 mm) and a voxel-wise p-threshold (p < 0.001). Based on these simulations we chose minimum cluster size threshold of 24 3 mm voxels (648 μL), which yielded a corrected alpha threshold at the cluster level of 0.01.
Results
Psychometric data
To examine differences between patients and controls on dimensional measures of anxiety, depressive symptoms, and intelligence, we administered a battery of psychological tests, and compared group means using independent samples t-tests. As expected, patients reported elevated state anxiety, both before and after the experiment. Patients also reported higher trait anxiety, and scored higher on both the BAI and BDI, but their WASI scores did not differ from that of the controls (See Table 1 for summary and statistics).
Table 1.
Psychometric data
| Questionnaire | Healthy Controls | Anxiety Patients | T-test |
|---|---|---|---|
| Trait Anxiety | 28.65 (1.10) | 49.61 (2.30) | t(61) = 9.07; p < 0.01 |
| State Anxiety | |||
| Pretest | 25.45 (1.03) | 43.09 (2.20) | t(59) = 8.02; p < 0.01 |
| Posttest | 32.23 (1.49) | 47.43 (2.76) | t(54) = 5.20; p < 0.01 |
| BDI | 1.10 (0.26) | 9.83 (1.63) | t(61) = 6.71; p < 0.01 |
| BAI | 3.03 (0.90) | 10.59 (1.71) | t(60) = 4.24; p < 0.01 |
| WASI | 120.33 (2.28) | 113.00 (7.44) | t(44) = 1.14; p = 0.26 |
Note: Values reflect mean (SD).
Subjective experience of the task
To determine the effect of diagnosis and threat on retrospective cognitive and emotional ratings, a general linear model was performed with Diagnosis and Threat as fixed factors, and Subject as a random factor for each of the scales (See Table 2).
Table 2.
Task Questionnaires
| Condition | Safe | Threat |
|---|---|---|
| Healthy Controls | ||
| Distract | 3.05 (0.34) | 4.76 (0.26) |
| Interfere | 2.03 (0.19) | 4.16 (0.26) |
| Difficulty | 2.33 (0.22) | 5.04 (0.32) |
| Anxiety | 3.90 (0.23) | 5.75 (0.29) |
| Shock rating | 5.11 (0.25) | |
| Anxiety Patients | ||
| Distract | 3.64 (0.38) | 4.85 (0.26) |
| Interfere | 3.01 (0.36) | 5.01 (0.41) |
| Difficulty | 3.51 (0.40) | 5.97 (0.42) |
| Anxiety | 5.28 (0.34) | 6.86 (0.33) |
| Shock rating* | 5.60 (0.33) | |
Diagnosis
Overall, patients reported more anxiety during the experiment (i.e. during both safe and threat; t(119) = 3.03; p < 0.01), greater anxiety-related task interference (t(119) = 3.01; p < 0.01), and more effort required to perform the task (i.e. more difficult; t(119) = 4.03; p < 0.01). Importantly, these differences were present even though groups rated the aversiveness of the shock similarly (t(54) = 1.16; p = 0.25).
Threat
Threat of shock increased anxiety ratings during the experiment, (Threat > Safe; t(119) = 7.56; p < 0.01). Subjects also reported that their shock-related anxiety interfered with performance more during the threat blocks than the safe blocks (t(119) = 6.76; p < 0.01), and that the task was more difficult during the threat blocks than the safe blocks (t(119) = 5.62; p < 0.01). Subjects also reported that the task distracted them from their anxiety more during the threat blocks than during the safe blocks (t(119) = 4.32; p < 0.01).
Diagnosis by Threat
There were no significant Diagnosis by Threat interactions.
Performance
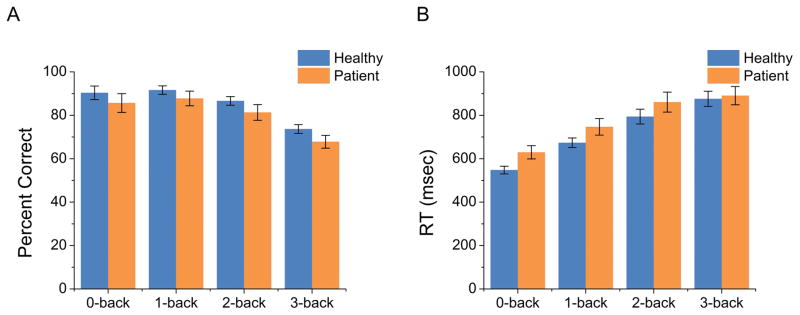
To determine the effects of load, diagnosis, and threat on performance, we performed a general linear model with Load, Diagnosis, and Threat as fixed factors, and Subject as random factor for accuracy and reaction time. Diagnosis had a significant main effect on both accuracy and reaction time (See Figure 2). Healthy controls were both faster (t(487) = 3.39; p < 0.01) and more accurate (t(487) = 3.36; p < 0.01) compared to the anxious patients. In addition, there was a significant main effect of Load for all three comparisons. In all load comparisons, subjects were faster (Load 1; t(487) = 11.62; p < 0.01, Load 2; t(487) = 7.37; p < 0.01, Load 2; t(487) = 2.68; p < 0.01) and more accurate (Load 1; t(487) = 3.94; p < 0.01, Load 2; t(487) = 6.93; p < 0.01, Load 3; t(487) = 6.53; p < 0.01) for the easier condition, suggesting a linear decrease in speed and accuracy as task difficulty increased. There was no significant Threat main effect (Accuracy: Threat; p = 0.88; RT: Threat; p = 0.49) and no significant interactions (Accuracy: Diagnosis by Threat; p = 0.7; Diagnosis by Load 1; p = 0.92; Diagnosis by Load 2; p = 0.63; Diagnosis by Load 3; p = 0.89; Threat by Load 1; p = 1; Threat by Load 2; p = 0.86; Threat by Load 3; p = 0.33; Diagnosis by Threat by Load 1; p = 0.99; Diagnosis by Threat by Load 2; p = 0.7; Diagnosis by Threat by Load 3; p = 0.92; RT: Diagnosis by Threat; p = 0.98; Diagnosis by Load 1; p = 0.45; Diagnosis by Load 2; p = 0.44; Diagnosis by Load 3; p = 0.28; Threat by Load 1; p = 0.65; Threat by Load 2; p = 0.55; Threat by Load 3; p = 0.09; Diagnosis by Threat by Load 1; p = 0.69; Diagnosis by Threat by Load 2; p = 0.93; Diagnosis by Threat by Load 3; p = 0.82).
Figure 2. Accuracy and reaction time performance for the N-back task.
A) As WM load increases, accuracy decreases (A) and reaction time increases (B). Across all levels of load patients are slower and less accurate than controls. (Bars represent the mean ± SEM)
WM load-related and Threat-related BOLD, across the whole sample
First, the effects of the task manipulations (i.e., WM Load and Threat) were examined.
WM Load
Extensive and largely overlapping activation clusters were revealed for the first two contrasts (Load 1: 0-back vs. 1-, 2-, 3-back, Load 2: 1-back vs. 2-, 3-back; See Figure 3A and 3B, respectively), which largely replicate activity patterns identified in previous studies of WM38–40. In brief, higher load was associated with greater activation of task-positive networks such as the frontoparietal attention network (dlPFC and SPL), the sensory motor network (SMA and thalamus), and the cingulo-opercular network (dmPFC and anterior insula). In contrast, we found load-related deactivation in regions of the task-negative default mode network (DMN; vmPFC, PCC, and the hippocampus). Finally, the third comparison (2-back vs. 3-back) yielded significant clusters in the left dorsal anterior cingulate cortex and right supplemental motor area.
Threat
The Threat manipulation was associated with a pattern of activation (See Figure 3D) that largely replicated findings of previous studies42–47. Threat vs. safety was associated with greater activation in regions involved with emotional expression (dorsal ACC, medial dorsal thalamus, and anterior insula), and less activation in regions involved with emotion regulation (vmPFC)48,49.
WM load-related BOLD is modulated by both Diagnosis and Threat
Next we determined whether Diagnosis and Threat influenced WM load-related BOLD activity. Because we were specifically interested in whether these factors affected task-related patterns of activity (such as frontal-parietal cognitive control regions), we limited our analysis to the areas of the brain that specifically contributed to the task, as indicated by the Load main effects (See Table 3 and Figure 5 for summary).
Table 3.
Significant interaction clusters in WM load-related regions.
| Label | # Voxels | t-value | Peak Activation
|
||
|---|---|---|---|---|---|
| RL | AP | IS | |||
| Anxiety x Load 1 (0-back vs. 1-, 2-, 3-back) | |||||
| Right Superior Frontal Gyrus | 41 | 3.85 | −29 | −11 | 55 |
| Right Middle Frontal Gyrus | 36 | 4.04 | −44 | −26 | 40 |
| Right Supplementary Motor Area | 36 | 4.12 | −5 | −17 | 58 |
| Right Cuneus | 34 | 3.93 | −11 | 89 | 22 |
| Left Posterior Cingulate Cortex | 34 | 3.62 | 11 | 50 | 25 |
| Right Middle Frontal Gyrus | 30 | 3.83 | −35 | −41 | 16 |
| Threat x Load 2 (1-back vs. 2-, 3-back) | |||||
| Left Anterior Cingulate Cortex | 220 | 4.19 | −5 | −35 | −17 |
| Right Precuneus | 32 | 3.72 | −5 | 62 | 52 |
| Right Parahippocampal Gyrus | 28 | 3.85 | −23 | −2 | −29 |
Diagnosis x Load
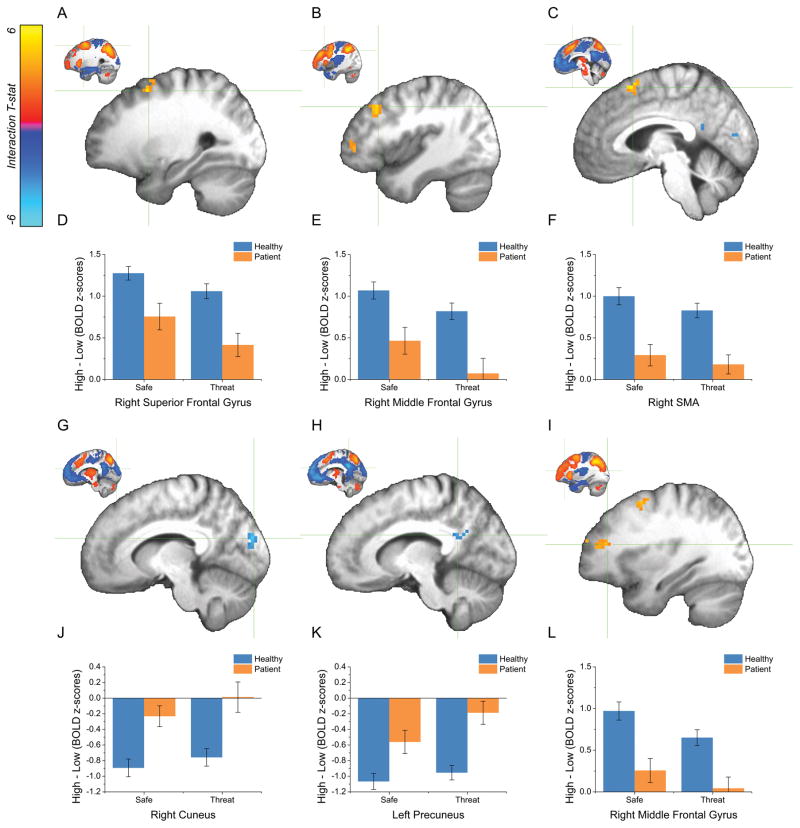
WM load-related BOLD activity was modulated by Diagnosis in 6 statistically significant clusters (See Table 3). In each of these regions, patients with anxiety disorders showed less differential activity as a function of WM load compared to healthy controls. In 4 of the 6 clusters, anxiety patients showed a reduction in task positive activity (i.e. decrease in positive load effect) for the Load 1 comparison (0-back vs. 1-, 2-, 3-back; See Figure 4 Panels D, E, F, & L). Three of these clusters (right superior frontal gyrus [Figure 4A], right middle frontal gyrus [posterior portion, Figure 4B], and right middle frontal gyrus [anterior portion, Figure 4I]), were located along the anterior to posterior axis of the dlPFC. There was an additional prefrontal cortex region showing the same pattern (right supplementary motor area [Figure 4C]). In the remaining 2 clusters, anxiety patients showed a reduction in task negative activity (i.e. a decrease in the negative load effect) for the Load 1 comparison (0-back vs. 1-, 2-, 3-back; See Figure 4 Panels J, & K). These regions were located in the right cuneus (Figure 4G) and left posterior cingulate cortex (Figure 4H).
Figure 4. The effect of Diagnosis on WM load-related activity.
A, B, C, G, H, I ) Thresholded t-maps for the Diagnosis x Load 1 (0- vs. 1-, 2-, 3-back) interaction. (Warm colors represent decreases in task-positive activity as a function of diagnosis. Cool colors represent decreases in task-negative activity as a function of diagnosis. Crosshairs depict the location of the voxel with the peak activation for a given cluster. Coordinates are reported in Table 3. Insets depict the WM load-related effect at the corresponding location.) D, E, F, J, K, L ) Pattern of activity seen in the clusters depicted in the above panels. (Bars represent the mean ± SEM.)
Threat x Load
WM load-related BOLD activity was modulated by Threat in 3 statistically significant clusters (See Table 3). As with Diagnosis, Threat reduced the magnitude of differential WM load-related activity in these regions. In 2 of the 3 clusters (left ventromedial prefrontal cortex [Figure 5A] and right parahippocampal gyrus [Figure 5c]), threat reduced task negative activity for the Load 2 comparison (1-back vs. 2-, 3-back; See Figure 5 Panels D, & F). In the remaining cluster (right precuneus [Figure 5B]), threat reduced task positive activity for the Load 2 comparison (1-back vs. 2-, 3-back; See Figure 5 Panel E).
WM load-related BOLD is not differentially modulated by Threat in patients vs. controls
Diagnosis x Threat x Load
Consistent with the performance data, WM load-related BOLD activations did not vary as a function of the combination of Diagnosis and Threat. The absence of the 3-way interaction reflected the fact that the interaction of Diagnosis by Load was not modulated by threat (i.e., state anxiety). In other words, the performance deficits and reduced prefrontal cortical activity seen in the anxiety patients cannot be attributed to greater state anxiety.
Discussion
The goal of the current experiment was to determine the effect of threat on WM performance in anxiety patients and healthy controls. We used threat of shock to increase state anxiety and subjects reported more state anxiety during the threat periods. However, results also show that patients were impaired relative to controls across all levels of the N-back task, and that this performance impairment was independent of the threat manipulation. Similarly, the task-positive BOLD activity in the dlPFC was reduced in patients relative to controls, in three distinct regions along the anterior to posterior axis. In addition, task-negative BOLD activity in the PCC was reduced in patients relative to controls, and a similar reduction of task-negative activity was observed as a function of Threat in the vmPFC and right parahippocampal gyrus. Importantly, although threat and clinical anxiety had similar effects on WM performance and WM-related neural activity, threat did not exacerbate cognitive deficits (at least with WM) or reduction in prefrontal activity in patients relative to the controls. Consistent with the second hypothesis raised in the introduction, these results suggest that poor engagement of the prefrontal cortex (particularly dlPFC) in clinical anxiety reflects a core component of the disorder rather than a transient effect of threat-related processing8–10.
Our results seem to be inconsistent with several other studies of individuals with high trait anxiety. Subjects with high trait anxiety have been shown to have greater dlPFC activity compared to subjects with low trait anxiety during Sternberg WM trials that require manipulation of the to-be-remembered information6. One key difference between high trait anxious “healthy” individuals and patients with clinical anxiety is impairment. Anxiety patients (by definition) suffer from impairment in daily life to a greater extent than high trait anxious individuals. In addition, high trait anxious healthy individuals may be resilient due to a potentially greater ability to recruit dlPFC activity during cognitive tasks6,7, relative to their impaired counterparts. Consistent with this hypothesis, our results show a performance impairment in anxiety patients compared to controls. Likewise, Bishop et. al (2009) show slower reaction time in their high trait anxiety sample9. In contrast, Basten et al. (2012) found similar performance for high and low trait anxious healthy individuals6. Taken together, these results could suggest that dlPFC activity is reduced by anxiety only when there is a performance deficit. One possible explanation is that dispositionally anxious individuals engage the dlPFC during off-task thinking, and that impaired individuals have difficulty recruiting this region for cognitive tasks. Consistent with this potential explanation, our dlPFC ROI closely matches the ROI where Forster et al., (2015)50 found an association between dlPFC activation and intrusive thoughts. Also consistent with this hypothesis is that individuals with anxiety disorders report less control over their negative intrusive thoughts when compared to healthy controls with similar levels of worry51.
Task-negative activity and anxiety
The current results replicate the common finding that decreased DMN activity accompany increased cognitive load38–40. In the current study, this effect was reduced as a function of both threat (vmPFC and hippocampal/parahippocampal gyrus) and clinical anxiety (PCC). The vmPFC plays a key role in emotion regulation52,53, fear extinction48,54,55, and active efforts to deal with fearful stimuli56. Therefore, this anxiety-related DMN interaction effect (reduced WM-related DMN deactivation) may represent the effort to overcome anxiety regardless of task conditions. Additionally, in anxiety patients, this effect may reflect a general deficit in the patients’ ability to flexibly disengage their attention from ongoing, potentially threat-related, thoughts during tasks57–59.
Organization of the dlPFC
Anxiety patients showed reduced task-positive activity in 3 regions of the dlPFC, and these regions are organized along the anterior to posterior axis of the dlPFC. Interestingly, there is growing evidence that the lateral prefrontal cortex is organized hierarchically, with increasingly abstract information being processed in the more anterior regions60. Some have suggested that the most anterior portions of the lateral prefrontal cortex are involved in decision making based on abstract contextual principles61. Although the pattern of activity is similar across the 3 regions, the magnitude of the interaction effect seems to increase along the anterior to posterior gradient, consistent with the hypothesis that contextual information flows from anterior to posterior regions62.
Strengths & Limitations
One of the strengths of this study is that we recruited only medication free participants who met the diagnostic criteria for an anxiety disorder, which sets it apart from studies investigating trait anxiety in otherwise healthy individuals. Another strength is that threat was manipulated according to a within-subject design, using a well-validated, translational procedure (i.e. threat of shock)42–47, which increased anxiety, interfered with task performance, and made the task more difficult according to the subjective reports. However, because of the aversive nature of the test, the recruitment of patients was more difficult and the number of patients excluded because of artifacts was greater compared to studies that take place in more neutral contexts. As a result, this study has a smaller sample size of patients than controls. Although this can be seen as a limitation of the current work, it should be noted that we corrected for group size in our statistical models, and that our sample size is comparable to several other recent studies published63–68.
Another limitation is that we do not replicate the effect that elevated state anxiety reduces WM performance4,5,23. One possible reason for this null result might be that our threat of shock manipulation did not increase anxiety or increased anxiety only minimally in our subjects. However, subjects reported significantly more anxiety during threat blocks than during safe blocks, and this effect size is comparable to that of our previous studies3–5. In addition, there was significantly more activity in the anterior insula, and dACC during threat blocks than during safe blocks for both patients and controls, which has been reported previously in similar threat studies17,69. Another possible explanation is that our load manipulation was not properly titrated to support a performance effect, particularly when considering possible effects of the scanning environment, which can impact performance70. However, we see clear behavioral differences as a function of load at all levels of analysis, and these differences accurately replicate the bulk of the findings with spatial WM38–40,71,72.
Finally, although we studied healthy subjects and individuals with either GAD or SAD, it is unclear whether our effects are specific to these disorders. For instance, as expected, our patient population had higher depression (BDI) scores than the healthy subjects, and similar hypofrontality has been seen previously in individuals with depression73,74. However, our effects were not due to higher levels of depression in the anxiety patients because the results remain the same after covarying out BDI scores in the final analysis of the BOLD activity. Another question is whether the WM deficit and dlPFC reductions generalize to patients with other anxiety disorders. For instance, individuals with PTSD have also been shown to suffer from WM deficits75–78; additional research should be conducted to determine whether these deficits share similar etiologies.
Conclusions
Previous studies have shown that trait anxious individuals exhibit deficits in cognitive control mediated by the dlPFC. However, until now it was not clear whether this deficit was a core characteristic of individuals with high trait anxiety or a consequence of ongoing threat-related processing (e.g. worry). In this study, we experimentally manipulated threat and found that anxiety patients demonstrated working memory performance deficits and reduced dlPFC activity, independent of this threat manipulation. These results suggest that poor cognitive control is a stable trait in anxiety patients. Furthermore, these results generate a testable hypothesis that working memory deficits may predict future symptom severity or treatment outcome.
Acknowledgments
Financial support of this study was provided by the Intramural Research Program of the National Institute of Mental Health, ZIAMH002798 (ClinicalTrial.gov Identifier: NCT00047853: Protocol ID 02-M-0321).
Footnotes
The authors report no conflicts of interest.
Financial Disclosure: The authors report no conflicts of interest. Financial support of this study was provided by the Intramural Research Program of the National Institute of Mental Health, ZIAMH002798 (ClinicalTrial.gov Identifier: NCT00047853: Protocol ID 02-M-0321).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Dsm-5. Arlington, VA: Amer Psychiatric Pub Incorporated; 2013. [Google Scholar]
- 2.Eysenck MW, Derakshan N, Santos R, et al. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–53. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 3.Robinson OJ, Vytal KE, Cornwell BR, et al. The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum Neurosci. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vytal KE, Cornwell BR, Letkiewicz AM, et al. The complex interaction between anxiety and cognition: insight from spatial and verbal working memory. Front Hum Neurosci. 2013;7:93. doi: 10.3389/fnhum.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vytal KE, Cornwell BR, Arkin N, et al. Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–52. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basten U, Stelzel C, Fiebach CJ. Trait anxiety and the neural efficiency of manipulation in working memory. Cogn Affect Behav Neurosci. 2012;12:571–88. doi: 10.3758/s13415-012-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fales CL, Barch DM, Burgess GC, et al. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci. 2008;8:239–53. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- 8.Basten U, Stelzel C, Fiebach CJ. Trait anxiety modulates the neural efficiency of inhibitory control. J Cogn Neurosci. 2011;23:3132–45. doi: 10.1162/jocn_a_00003. [DOI] [PubMed] [Google Scholar]
- 9.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–8. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 10.Telzer EH, Mogg K, Bradley BP, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79:216–22. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grillon C, Ameli R. Effects of threat of shock, shock electrode placement and darkness on startle. Int J Psychophysiol. 1998;28:223–31. doi: 10.1016/s0167-8760(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 12.Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–95. [PubMed] [Google Scholar]
- 13.Grillon C. Greater sustained anxiety but not phasic fear in women compared to men. Emotion. 2008;8:410–3. doi: 10.1037/1528-3542.8.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grillon C, Ameli R, Goddard A, et al. Baseline and fear-potentiated startle in panic disorder patients. Biol Psychiatry. 1994;35:431–9. doi: 10.1016/0006-3223(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 15.Morgan Ca, Grillon C, Southwick SM, et al. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry. 1995;38:378–85. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- 16.Robinson OJ, Overstreet C, Allen PS, et al. Neuropsychopharmacology. Vol. 37. Nature Publishing Group; 2012. Acute tryptophan depletion increases translational indices of anxiety but not fear: serotonergic modulation of the bed nucleus of the stria terminalis? pp. 1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vytal KE, Overstreet C, Charney DR, et al. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: a mechanism for maintaining an anxious state in healthy adults. J psychiatry Neurosci. 2014:1–9. doi: 10.1503/jpn.130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunning JP, Deldonno S, Hajcak G. Biol Psychol. Vol. 94. Elsevier B.V; 2013. The effects of contextual threat and anxiety on affective startle modulation; pp. 130–5. [DOI] [PubMed] [Google Scholar]
- 19.Hansen AL, Johnsen BH, Thayer JF. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety Stress Coping. 2009;22:77–89. doi: 10.1080/10615800802272251. [DOI] [PubMed] [Google Scholar]
- 20.Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–75. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- 21.Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–37. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balderston NL, Mathur A, Adu-Brimpong J, et al. Effect of anxiety on behavioural pattern separation in humans. Cogn Emot. 2015;9931:1–11. doi: 10.1080/02699931.2015.1096235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke R, Johnstone T. Prefrontal inhibition of threat processing reduces working memory interference. Front Hum Neurosci. 2013;7:228. doi: 10.3389/fnhum.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavric A, Rippon G, Gray JR. Threat-evoked anxiety disrupts spatial working memory performance: An attentional account. Cognit Ther Res. 2003;27:489–504. [Google Scholar]
- 25.Robinson OJ, Krimsky M, Grillon C. The impact of induced anxiety on response inhibition. Front Hum Neurosci. 2013;7:69. doi: 10.3389/fnhum.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson OJ, Letkiewicz AM, Overstreet C, et al. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn Affect Behav Neurosci. 2011;11:217–27. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomasi D, Ernst T, Caparelli EC, et al. Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito F, Bertolino A, Scarabino T, et al. Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res Bull. 2006;70:263–9. doi: 10.1016/j.brainresbull.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Shackman AJ, Sarinopoulos I, Maxwell JS, et al. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- 30.Derakshan N, Ansari TL, Hansard M, et al. Anxiety, inhibition, efficiency, and effectiveness: An investigation using the Antisaccade task. Exp Psychol. 2009;56:48–55. doi: 10.1027/1618-3169.56.1.48. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Publishing; 2012. [Google Scholar]
- 32.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 33.Morey Ra, Petty CM, Xu Y, et al. Neuroimage. Vol. 45. Elsevier B.V; 2009. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes; pp. 855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonov VS, Evans AC, McKinstry RC, et al. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47(Supple):S102. [Google Scholar]
- 35.Fonov V, Evans AC, Botteron K, et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–27. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins DL, Zijdenbos AP, Baare WFC, et al. ANIMAL+INSECT: Improved cortical structure segmentation. Inf Process Med Imaging, Proc. 1999;1613:210–23. [Google Scholar]
- 37.Amaro E, Barker GJ. Study design in fMRI: Basic principles. Brain Cogn. 2006;60:220–32. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Linden DEJ. The working memory networks of the human brain. Neuroscientist. 2007;13:257–67. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- 39.Owen AM, McMillan KM, Laird AR, et al. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nee DE, Brown JW, Askren MK, et al. A meta-Analysis of executive components of working memory. Cereb Cortex. 2013;23:264–82. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdi H, Williams LJ. Contrast analysis. In: Salkind N, editor. Encycl Res Des. Thousand Oaks, CA: SAGE Publications; 2010. pp. 243–51. [Google Scholar]
- 42.Hasler G, Fromm S, Alvarez RP, et al. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–9. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez RP, Biggs A, Chen G, et al. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–9. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold AL, Morey Ra, McCarthy G. Biol Psychiatry. Elsevier; 2014. Amygdala-Prefrontal Cortex Functional Connectivity During Threat-Induced Anxiety and Goal Distraction; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiddick L. Neurosci Biobehav Rev. Vol. 35. Elsevier Ltd; 2011. There is more than the amygdala: potential threat assessment in the cingulate cortex; pp. 1007–18. [DOI] [PubMed] [Google Scholar]
- 46.Etkin A, Egner T, Kalisch R. Trends Cogn Sci. Vol. 15. Elsevier Ltd; 2011. Emotional processing in anterior cingulate and medial prefrontal cortex; pp. 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nitschke JB, Sarinopoulos I, Mackiewicz KL, et al. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 48.Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 49.Blair KS, Otero M, Teng C, et al. Neuroimage. Vol. 78. Elsevier B.V; 2013. Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias; pp. 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forster S, Nunez Elizalde AO, Castle E, et al. Unraveling the Anxious Mind: Anxiety, Worry, and Frontal Engagement in Sustained Attention Versus Off-Task Processing. Cereb Cortex. 2015 doi: 10.1093/cercor/bht248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruscio AM, Borkovec TD. Experience and appraisal of worry among high worriers with and without generalized anxiety disorder. Behav Res Ther. 2004;42:1469–82. doi: 10.1016/j.brat.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Delgado MR, Nearing KI, Ledoux JE, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diekhof EK, Geier K, Falkai P, et al. Neuroimage. Vol. 58. Elsevier Inc; 2011. Fear is only as deep as the mind allows A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect; pp. 275–85. [DOI] [PubMed] [Google Scholar]
- 54.Milad MR, Quinn BT, Pitman RK, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci. 2005;102:10706–11. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milad MR, Wright CI, Orr SP, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Nili U, Goldberg H, Weizman A, et al. Neuron. Vol. 66. Elsevier Ltd; 2010. Fear thou not: activity of frontal and temporal circuits in moments of real-life courage; pp. 949–62. [DOI] [PubMed] [Google Scholar]
- 57.Northoff G, Qin P, Nakao T. Trends Neurosci. Vol. 33. Elsevier Ltd; 2010. Rest-stimulus interaction in the brain: A review; pp. 277–84. [DOI] [PubMed] [Google Scholar]
- 58.Kucyi a, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci. 2013;110:18692–7. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–35. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Donoso M, Collins AGE, Koechlin E. Human cognition. Foundations of human reasoning in the prefrontal cortex. Science. 2014;344:1481–6. doi: 10.1126/science.1252254. [DOI] [PubMed] [Google Scholar]
- 63.Mennin DS, Fresco DM, Ritter M, et al. AN OPEN TRIAL OF EMOTION REGULATION THERAPY FOR GENERALIZED ANXIETY DISORDER AND COOCCURRING DEPRESSION. Depress Anxiety. 2015;32:614–23. doi: 10.1002/da.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strawn JR, Wehry AM, Chu W-J, et al. NEUROANATOMIC ABNORMALITIES IN ADOLESCENTS WITH GENERALIZED ANXIETY DISORDER: A VOXEL-BASED MORPHOMETRY STUDY. Depress Anxiety. 2013;30:842–8. doi: 10.1002/da.22089. [DOI] [PubMed] [Google Scholar]
- 65.Strawn JR, Bitter SM, Weber WA, et al. NEUROCIRCUITRY OF GENERALIZED ANXIETY DISORDER IN ADOLESCENTS: A PILOT FUNCTIONAL NEUROIMAGING AND FUNCTIONAL CONNECTIVITY STUDY. Depress Anxiety. 2012;29:939–47. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- 66.Hettema JM, Kettenmann B, Ahluwalia V, et al. Pilot multimodal twin imaging study of generalized anxiety disorder. Depress Anxiety. 2012;29:202–9. doi: 10.1002/da.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenberg T, Carlson JM, Cha J, et al. VENTROMEDIAL PREFRONTAL CORTEX REACTIVITY IS ALTERED IN GENERALIZED ANXIETY DISORDER DURING FEAR GENERALIZATION. Depress Anxiety. 2013;30:242–50. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- 68.Andreescu C, Gross JJ, Lenze E, et al. Depress Anxiety. Vol. 28. Wiley Subscription Services, Inc., A Wiley Company; 2011. Altered cerebral blood flow patterns associated with pathologic worry in the elderly; pp. 202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cornwell BR, Baas JMPJ, Johnson L, et al. Neuroimage. Vol. 37. Elsevier Inc; 2007. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography; pp. 282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blair KS, Vythilingam M, Crowe SL, et al. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2012;43:1–11. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barch DM, Braver TS, Nystrom LE, et al. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–80. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 72.Brookes MJ, Wood JR, Stevenson CM, et al. Neuroimage. Vol. 55. Elsevier Inc; 2011. Changes in brain network activity during working memory tasks: a magnetoencephalography study; pp. 1804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diener C, Kuehner C, Brusniak W, et al. Neuroimage. Vol. 61. Elsevier Inc; 2012. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression; pp. 677–85. [DOI] [PubMed] [Google Scholar]
- 74.Rayner G, Jackson G, Wilson S. Neurosci Biobehav Rev. Vol. 61. Elsevier Ltd; 2015. Cognition-related brain networks underpin the symptoms of unipolar depression: evidence from a systematic review; pp. 53–65. [DOI] [PubMed] [Google Scholar]
- 75.Dretsch MN, Thiel KJ, Athy JR, et al. Mood symptoms contribute to working memory decrement in active-duty soldiers being treated for posttraumatic stress disorder. Brain Behav. 2012;2:357–64. doi: 10.1002/brb3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honzel N, Justus T, Swick D. Posttraumatic stress disorder is associated with limited executive resources in a working memory task. Cogn Affect Behav Neurosci. 2014;14:792–804. doi: 10.3758/s13415-013-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J, Xiong K, Qiu M, et al. Brain Res. Vol. 1531. Elsevier; 2013. Negative emotional distraction on neural circuits for working memory in patients with posttraumatic stress disorder; pp. 94–101. [DOI] [PubMed] [Google Scholar]
- 78.Morey Ra, Dolcos F, Petty CM, et al. J Psychiatr Res. Vol. 43. Elsevier Ltd; 2009. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder; pp. 809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]