Abstract
Objectives
To validate the pediatric Patient Reported Outcomes Measurement Information System short forms (PROMIS®-SFs) in childhood-onset systemic lupus erythematosus (cSLE) in a clinical setting.
Methods
At three study visits, cSLE patients completed the PROMIS-SFs (Anger, Anxiety, Depressive Symptoms, Fatigue, Physical Function-Mobility, Physical Function-Upper Extremity, Pain Interference, Peer Relationships) using the PROMIS Assessment Center, and health-related quality of life (HRQoL) legacy measures (Pediatric Quality of Life Inventory™, Childhood Health Assessment Questionnaire, Simple Measure of Impact of Lupus Erythematosus in Youngsters [SMILEY], visual analog scales [VAS] of pain and well-being). Physicians rated cSLE activity on a VAS, and completed the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). Physicians rated change of cSLE activity (GRC-MD1: better/same/worse) and change of patient overall health (GRC-MD2: better/same/worse) between study visits with a global rating scale of change. Questionnaire scores were compared in support of validity and responsiveness to change (external standards: GRC-MD1, GRC-MD2).
Results
In this population-based cohort (n=100) with a mean age of 15.8 (range: 10–20) years, the PROMIS-SFs were completed in less than five minutes in a clinical setting. The PROMIS-SF scores correlated at least moderately (Pearson’s r ≥0.5) with those of legacy HRQoL, except for the SMILEY. Measures of cSLE activity did not correlate with the PROMIS-SFs. Responsiveness to change of the PROMIS-SFs was supported by path, mixed model, and correlation analyses.
Conclusions
To assess HRQoL in cSLE, the PROMIS-SFs demonstrated feasibility, internal consistency, construct validity, and responsiveness to change in a clinical setting.
INTRODUCTION
Childhood-onset systemic lupus erythematosus (cSLE) is a chronic autoimmune disease that often negatively impacts health-related quality of life (HRQoL), especially when permanent disease damage, increased disease activity, and fatigue are present (1–4). Traditional disease measures or physician assessment of disease activity have proven insufficient to accurately assess the impact of cSLE disease on patient HRQoL (5). Hence, various patient-reported outcomes (PROs) have been developed and validated in cSLE to provide complementary information in support of optimal patient management and heightened satisfaction with care (6, 7).
Recently, the Patient Reported Outcomes Measurement Information System (PROMIS®, http://nihpromis.org), a publicly available system supported by the National Institute of Health, has become available. PROMIS offers effective PRO measurement in various HRQoL domains, flexibility in administration of measures, and electronic data collection for both adult and pediatric populations. PROMIS aims at decreasing respondent burden, and offering a comparison of PROs across disease groups, while improving the delineation of clinically relevant changes in HRQoL (8). To make full use of PROMIS to measure PROs in cSLE, the pediatric PROMIS short forms (PROMIS-SFs) require validation to determine their measurement properties. The objectives of this study were to investigate feasibility, internal consistency, construct validity, and responsiveness to change of the PROMIS-SFs when used in cSLE in a clinical setting.
METHODS AND MATERIALS
Study design and setting
This longitudinal study enrolled eligible cSLE patients at two tertiary care centers (Cincinnati Children’s Hospital Medical Center, Duke University Medical Center). Patients were consecutively recruited during routine clinic visits, between March 2012 and August 2014, and evaluated in three-month intervals for up to three visits. At each visit, patients were asked to complete legacy HRQoL questionnaires in addition to the PROMIS-SFs; the treating physician rated disease activity, damage and change of cSLE severity and patient overall health between visits. Demographic data were obtained along with information collected as part of standard clinical care of cSLE (medications, disease activity, duration and damage) at each study visit.
Approval from local research ethics boards was obtained at each site. Prior to participation, the study was explained to each eligible patient and legal guardian, and written informed consent was obtained. Written assent was also obtained from participants 11 years of age or younger. The study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki.
Study patients
Patients, 8 to 20 years of age, with a diagnosis of SLE prior to their 18th birthday (9), followed at a participating site, were approached to participate. Excluded were patients with a history of a comorbid chronic disease that might impact HRQoL besides cSLE.
Pediatric PROMIS Short forms
Eight distinct HRQoL domains probed by the PROMIS-SFs were included in this study: Anger (PROMISAnger), Anxiety (PROMISAnxiety), Depressive Symptoms (PROMISDepression), Fatigue (PROMISFatigue), Peer Relationships (PROMISPeerRel), Physical Function-Mobility (PROMISPF-Mobility), Physical Function-Upper Extremity (PROMISPF-UExt), and Pain Interference (PROMISPain). Besides the PROMISFatigue (10 items) and PROMISAnger (6 items), all other included PROMIS-SFs consist of eight items each for a total of 64 items across eight domains.
Each item included in the PROMIS-SFs has 5 ordinal response options, which considers the preceding seven days. Response options for PROMISAnger, PROMISAnxiety, PROMISDepression, PROMISFatigue, PROMISPain, PROMISPeerRel are as follows: never, almost never, sometimes, often, almost always, and for PROMISPF-Mobility and PROMISPF-UExt: no trouble, with little trouble, with some trouble, with a lot of trouble, not able to do. Additional details about PROMIS, definitions of domain framework, and domain profiles are provided elsewhere (10).
For each PROMIS-SFs, a score can be calculated using either Item response theory (IRT)-based response pattern scoring (preferred by PROMIS) or look-up tables that approximate the response pattern-based scoring. We used item response theory (IRT) scoring (11, 12), with scores reported as T-scores with normative mean values of 50 and standard deviations (SD) of 10. Additional information is provided in the PROMIS scoring manuals (12).
The PROMIS-SF scores reflect the presence of the construct measured. Hence, lower scores correspond to better HRQoL for PROMISAnger, PROMISAnxiety, PROMISDepression, PROMISFatigue, and PROMISPain. Conversely, lower scores indicate lower HRQoL for PROMISPeerRel, PROMISPF-Mobility, and PROMISPF-UExt. The internal consistency of each PROMIS-SFs when used in other pediatric populations achieved a Cronbach alpha of 0.85 or higher (13–16).
Patient and Parent completed HRQoL legacy measures
The Pediatric Quality of Life Generic Core Scale 4.0 (PedsQL-GC) is a self-report tool, comprised of 23-items divided among four domains which include: physical, emotional, social and school function. Internal reliability was α = 0.89. The Pediatric Quality of Life Rheumatology Module 3.0 (PedsQL-RM) is similar to the PedsQL-GC, but is relevant for children with rheumatic diseases, and has 22-items across five domains which include: pain and hurt, daily activities, treatment, worry and communication. Internal validity for the PedsQL-RM ranged from 0.75 to 0.86. For both the PedsQL-GC and PedsQL-RM a child (ages 8–13 years) and teen (ages 14–18 years) form was used for the age appropriate patients; items are rated on a 5-point scale (0 = never to 4 = almost always), and from the raw scores a summary score of 0 to 100 can be calculated with higher scores representative of better HRQoL.
The functional disability inventory (FDI) is a self-report measure that evaluates difficulty in physical and psychological function due to physical health. The instrument has 15 items that evaluate perception of activity limitations; total score is summed with higher scores indicative of greater disability. FDI scores less than 12 reflect no or minimal disability, score of 13 to 29 moderate disability, and greater than 30 severe disability, respectively (17). In previous studies the Cronbach alpha ranges between 0.86 and 0.91 (18).
The Simple Measure of Impact of Lupus Erythematosus in Youngsters (SMILEY) is a 26-item, cSLE specific, HRQoL questionnaire that features four domains: Effect on self, Limitations, Social and Burden of SLE. Responses are reported with a 5-faces scale. Each score ranges from 1 to 5 and the total score is transformed to a 1 to 100 scale with higher values representative of better HRQoL, and an internal reliability of 0.9 in other cSLE populations (19).
The Childhood Health Assessment Questionnaire (CHAQ) is an adaptation of the Stanford Health Assessment Questionnaire (HAQ) for pediatric use, consists of 30 items and measures physical function in eight domains: dressing/grooming, arising, eating, walking, hygiene, reach, grip, and activities. Within each domain, degree of difficulty (0 = no difficulty, 1 = some difficulty, 2 = much difficulty, 3 =unable to do), use of aids/devices, and requirement for personal assistance with tasks is assessed. The item with the highest score for any given area is used as the score for that domain. The domain scores are averaged without weighting to yield a single Disability Index score (0 – 3) (20). Internal reliability as measured by Cronbach alpha was 0.94 (21).
The Child Health Questionnaire (CHQ-PF50) is parent proxy-report of generic health status. The CHQ-PF50 is a profile measure of 50 questions that measures 10 mental and physical domains, or subscales: physical functioning (PF), bodily pain (BP), general health perceptions (GH), role/social limitations-physical (RP), role/social limitations-emotional/behavioral (REB), parent impact-time (PT) and parent impact-emotions (PE), self-esteem (SE), mental health (MH), and general behavior (BE). Each subscale score ranges from 0 to 100, with higher scores representing better health status. Two scores, physical summary (CHQ-PhS) and psychosocial summary (CHQ-PsS), are calculated by aggregation of the subscales. Scores are standardized to a mean of 50 and SD of 10, where higher scores reflect better health status (22). The internal reliability, for domains and subscales, ranges from 0.65 to 0.96 (23).
All questionnaires were administered on a laptop computer after clinic check-in, and study visits were conducted by a trained clinical research coordinator (CRC). At the end of the clinic visit, the CRC would check the questionnaires for completion and perform debriefing with the study participant. All questionnaires used in this study are child self-report except the CHQ-PF50 which is parent proxy-report.
Traditional cSLE measures
Disease damage was evaluated with Systemic Lupus International Collaborating Clinics/ACR Damage Index (SDI, range 0 to 47; 0 = absence of damage) (24, 25). Disease activity was measured using a physician global disease assessment (MD-global; 10-point Likert visual analog scale [VAS], 0 = inactive disease), the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI, range 0 to 105; 0 = inactive disease) (26), and the British Isles Lupus Activity Group index (BILAG) with alphabetical converted to numerical domain scores (A = 12, B = 8, C = 1, D = 0, E = 0; 0 = inactive disease) (27). While the SLEDAI considers only objectively measurable findings with cSLE, the BILAG score also includes subjective symptoms such as arthralgias and myalgias (28).
Measures of cSLE change
During the second and third visits, change in cSLE severity (GRC-MD1) and overall health status (GRC-MD2) were rated by the treating physician using 5-point Likert scales (much worse, somewhat worse, unchanged, somewhat better, much better). GRC-MD1 and GRC-MD2 used the sentence stem “Has there been any change in your patient’s lupus since his/her last study visit?” and “Has there been any change in your patient’s overall health since his/her last study visit?”, respectively.
Statistical analysis
Numerical variables were summarized by mean and standard deviation (SD) and binary and categorical variables were summarized by frequency and percentage. Feasibility was assessed by determining the proportion of patients who successfully completed the PROMIS-SFs, (90 percent successful completion was considered feasible) along with measuring respondent burden as time (in minutes) needed for completion along with short, informal debriefing interview after completion to assess administration and understanding of PROMIS-SFs. We also assessed internal consistency of each of the PROMIS-SFs using Cronbach’s alpha. We considered less than 0.5 “unacceptable,” 0.5 to 0.59 “poor,” 0.6 to 0.69 “questionable,” 0.7 to 0.79 “acceptable,” 0.8 to 0.89 “good,” and greater than 0.9 “excellent.”(29)
In support of construct validity (30, 31) relationships between PROMIS-SF scores, HRQoL legacy measures and traditional cSLE measures (SLEDAI, BILAG, MD-global, SDI) were assessed with Pearson’s correlation coefficient (r). Pooled or overall correlation coefficients (rpool) using data from all visits and all patients were estimated through a variance covariance matrix using a mixed effect model (32) which adjusted for dependency of observations. A correlation coefficient (r or rpool) is considered “low”; “fair”; “moderate”; “high” and “very high” if its estimated value is below 0.3; 0.30 to 0.49; 0.50 to 0.69; 0.70 to 0.89, and greater than 0.9, respectively (33).
In measuring responsiveness to change, we employed three strategies: (a) correlation of change between visits; (b) association of raw T-score change between visits; and (c) path analysis models of change across visits, with two strategies (b, c) using physician reported change in cSLE (GRC-MD1) and overall health status (GRC-MD2) as an external standard. For these analyses we condensed the GRC-MD1 and GRC-MD2 response options from five (much worse, somewhat worse, unchanged, somewhat better, much better) to three (better, same, worse).
For (a) correlation to change, we correlated changes of the legacy HRQoL measure scores with changes in PROMIS-SF scores between visits and over time, using a mixed effect model that adjusted for differences in patient demographics (age, gender and race) and within-person correlation using a random effect model. This provides information about the relationship of the responsiveness of legacy PROs to that of PROMIS-SFs.
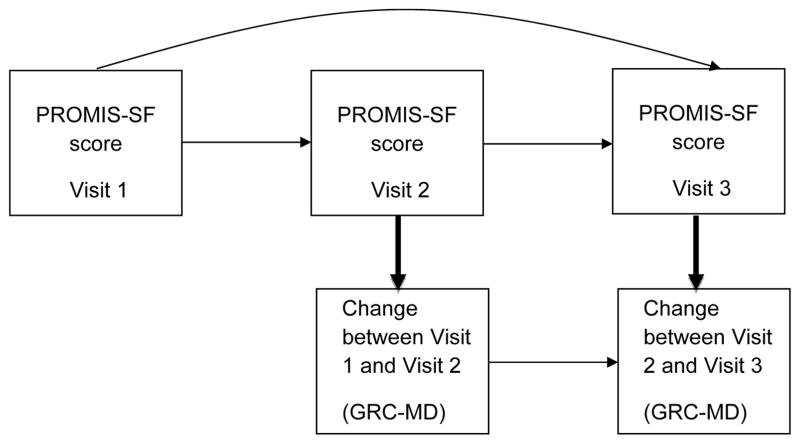
For (b) association of raw T-score change, we estimated changes in PROMIS-SF scores (T-score) over time for patients who considered better, same or worse (GRC-MD1, GRC-MD2), using a similar mixed model as detailed above. Lastly, for (c) path analysis models of change [(11, 12); see Figure 1], we evaluated the extent to which a given PROMIS-SF score at Visit 1, 2, or 3 predicted a change (better or worse) in GCR-MD1 and GCR-MD2 ratings at Visit 2 and 3. Path analysis is a special case of structural equation modeling (SEM), in which a single variable is used to measure each construct in the model (as opposed to multiple indicators per construct). Path analysis is a statistical technique that simultaneously solves a set of regression equations evaluating the hypothesized relationships among a set of variables. Figure 1 depicts a path model that examines whether a PROMIS-SF score at a given visit (e.g. Visit 2) predicts change in cSLE (GRC-MD1) or overall health (GRC-MD2) status from the previous visit to the given visit (change from Visit 1 to Visit 2), and the extent to which the Visit 1 PROMIS score predicts the Visit 2 and 3 PROMIS scores. Using Mplus 7.1 (34), we fit a path model for each PROMIS-SF across two different physician reported change variables (GCR-MD1, GCR-MD2). The path models produced a standardized coefficient (β) of how many SD the dependent variable (y; GCR-MD1, GCR-MD2 at Visit 2 and 3) changes given a SD increase in predictor variable (x; PROMIS-SFs at Visit 1, 2, and 3). The β can be interpreted similarly to a correlation coefficient (r).
Figure 1. Path Analysis Model.
Bolded arrows are paths of interest and examine whether a pediatric PROMIS Short Form score at a given visit predicts change in cSLE (GRC-MD1) and overall health (GRC-MD2) status from the previous visit to the given visit.
All statistical analyses were completed with Stata 13 and Mplus 7.1 software.
RESULTS
Demographics and PROs
A total of 100 patients were enrolled (demographics and disease features at baseline summarized in Table 1), representing all patients within the target age stratum fulfilling eligibility criteria at each site. Six patients declined study participation. Of the 100 patients that completed Visit 1, 96 completed Visit 2 and 84 completed Visit 3. Patients not completing all study visits did not significantly differ in demographics or disease features from patients who remained in the study. The same held true for the six patients declining study participation.
Table 1.
Demographics, cSLE features, and PROs at baseline (Visit 1; n = 100)
| Measure | Frequency, n (%) | Mean (SD) |
|---|---|---|
| Age, years | 15.8 (2.2) | |
| Female | 80 (80%) | |
| Race | ||
| African American | 48 (48%) | |
| Caucasian | 33 (33%) | |
| Other§ | 19 (19%) | |
| Hispanic ethnicity | 8 (8%) | |
| SLEDAI Score* | 6.0 (5.9) | |
| BILAG Score** | 6.4 (7.6) | |
| SDI Score† | 0.4 (0.7) | |
| MD-global‡ | 2.1 (1.7) | |
| Pediatric PROMIS short form domains | ||
| Anger | 51.1 (12.1) | |
| Anxiety | 48.0 (11.0) | |
| Depressive symptoms | 47.8 (11.9) | |
| Fatigue | 50.7 (14.1) | |
| Mobility | 46.9 (10.1) | |
| Upper extremity function | 46.4 (7.90) | |
| Pain | 50.3 (11.7) | |
| Peer relationships | 49.7 (13.1) | |
| Pediatric Quality of Life Inventory – Generic Score Scale 4.0 | 70.6 (17.8) | |
| Physical function | 68.9 (22.1) | |
| Emotional function | 72.1 (20.9) | |
| Social function | 79.9 (19.8) | |
| School function | 62.9 (20.6) | |
| Pediatric Quality of Life Inventory – Rheumatology Module 3.0 | 74.4 (16.2) | |
| Pain & hurt | 65.1 (28.0) | |
| Daily activity | 86.7 (17.6) | |
| Treatment | 76.9 (17.3) | |
| Worry | 65.2 (25.3) | |
| Communication | 69.6 (27.6) | |
| Simple Measure of Impact of Lupus Erythematosus in Youngsters | 62.3 (13.9) | |
| Effect on self | 56.2 (19.7) | |
| Limitations | 68.3 (15.6) | |
| Social | 64.1 (17.6) | |
| Burden of cSLE | 58.9 (18.3) | |
| Functional Disability Inventory | 8.4 (9.2) | |
| Childhood Health Assessment Questionnaire | 0.47 (0.6) | |
| Dressing/grooming | 0.36 (0.7) | |
| Arising | 0.46 (0.7) | |
| Eating | 0.33 (0.7) | |
| Walking | 0.29 (0.6) | |
| Hygiene | 0.32 (0.7) | |
| Reach | 0.64 (0.8) | |
| Play | 0.82 (0.9) | |
| Grip | 0.57 (0.8) | |
| Childhood Health Questionnaire P-50 | ||
| Psychosocial summary score | 50.4 (10.6) | |
| Physical summary score | 40.9 (12.5) | |
| Physical functioning | 50.4 (25.5) | |
| Bodily pain | 67.1 (25.8) | |
| General health perception | 53.5 (17.0) | |
| Role/social-physical | 82.8 (25.6) | |
| Role/social-emotional/behavioral | 80.4 (28.6) | |
| Parent impact-time | 76.1 (29.1) | |
| Parent impact-emotional | 63.4 (29.8) | |
| Self-esteem | 79.2 (19.8) | |
| Mental health | 78.5 (17.3) | |
| Behavior | 80.0 (17.7) | |
Systemic Lupus Erythematosus Disease Activity Index 2000
British Isles Lupus Activity Group index (A = 12, B = 8, C = 1, D = 0, E = 0)
Systemic Lupus international Collaborating Clinics/ACR Damage Index
MD global; 10 point Likert scale, 0 = inactive disease
Other represents another option for completion of the questionnaires if the provided choices did not apply or there was an overlap of the choices.
All questionnaires are child self-report except Child Health Questionnaire P50 which is parent proxy-report
As summarized in Table 1, the cohort was 80% (80/100) female with a mean age of 15.8 years (SD 2.2). 33% (33/100) self-identified as Caucasian, 48% (48/100) African American and 11% (11/100) other with 8% (8/100) reporting Hispanic ethnicity. At Visit 1, there were 16% (16/100) and 14% (14/100) of patients with SLEDAI and BILAG scores of 0 and 73% (73/100) had no damage (SDI = 0). PRO responses for all HRQoL measures at baseline (Visit 1) are also shown in Table 1.
Feasibility
For all completed visits, 100 percent of the patients successfully completed the PROMIS-SFs. Electronic completion through the assessment center of all eight PROMIS-SFs averaged less than five minutes with an individual item average of five seconds. Time to complete the individual PROMIS-SF domains averaged 37 seconds (range 28 to 48 seconds). PROMISFatigue completion took the longest (48 seconds), and the PROMISPF-UExt the least amount of time (28 seconds) to complete. None of the patients reported difficulty understanding the items per informal debriefing after completion of the PROMIS-SFs.
Reliability
Cronbach alpha (α) for all PROMIS-SFs ranged between 0.88 and 0.96 for Visit 1 and exceeded 0.91 for Visit 2 and 3. Cronbach alpha for all PROMIS-SFs pooled data across all visits (αpool) ranged between 0.91 and 0.97. Details are provided online in the Supplemental Table 1.
Construct validity
As summarized in Table 2, the PROMIS-SFs scores were moderately-to-highly correlated (rpool > 0.5) with the summary scores of the HRQoL legacy measures (PedsQL-GC, PedsQL-RM, CHQ-PSS, CHQ-PHS, CHAQ, FDI). The highest correlations were observed for the PedsQL-GC and PedsQL-RM scores and the lowest with the SMILEY. The psychosocial PROMIS-SFs (PROMISAnger, PROMISAnxiety, PROMISDepression, PROMISPeerRel) correlated highly with the CHQ-PSS and FDI, while the physical function PROMIS-SF domains (PROMISPF-Mobility, PROMISPF-Uext, PROMISFatigue, PROMISPain) correlated highly with CHQ-PHS, CHAQ, and FDI. Further summarized in Table 2, legacy subscale correlations are similar to legacy summary score correlations with the PROMIS-SFs.
Table 2.
Bivariate correlation (rPool) between pediatric PROMIS short forms and legacy measure subscales
| PROMIS Short Forms/Subscales | Anger | Anxiety | Depressive Symptoms | Fatigue | Mobility | Upper Extremity | Pain | Peer Relationships | |
|---|---|---|---|---|---|---|---|---|---|
| SLEDAI** | 0.12 | 0.06 | 0.03 | 0.11 | −0.23 | −0.12 | 0.16 | 0.11 | |
| BILAG† | 0.01 | 0.07 | −0.03 | 0.08 | −0.26 | −0.13 | 0.21 | 0.11 | |
| MD global‡ | 0.10 | 0.09 | 0.07 | 0.08 | −0.16 | −0.02 | 0.12 | 0.08 | |
| SDI§ | −0.07 | −0.06 | −0.07 | −0.01 | −0.11 | −0.14 | −0.00 | −0.05 | |
| Functional Disability Inventory | 0.37* | 0.48* | 0.42* | 0.62* | −0.70* | −0.55* | 0.62* | −0.19 | |
| PedsQL-GC | Summary Score | −0.57* | −0.68* | −0.64* | −0.78* | 0.69* | 0.49* | −0.74* | 0.30* |
| Physical function | −0.44* | −0.53* | −0.48* | −0.70* | 0.72* | 0.50* | −0.68* | 0.16 | |
| Emotional function | −0.63* | −0.73* | −0.72* | −0.67* | 0.51* | 0.38* | −0.61* | 0.25 | |
| Social function | −0.46* | −0.50* | −0.49* | −0.54* | 0.49* | 0.38* | −0.54* | 0.44* | |
| School function | −0.45* | −0.58* | −0.53* | −0.71* | 0.52* | 0.33* | −0.66* | 0.26 | |
| PedsQL-RM | Summary Score | −0.52* | −0.66* | −0.62* | −0.74* | 0.62* | 0.51* | −0.72* | 0.25 |
| Pain & hurt | −0.44* | −0.52* | −0.47* | −0.72* | 0.68* | 0.44* | −0.72* | 0.12 | |
| Daily activity | −0.34* | −0.43* | −0.41* | −0.53* | 0.56* | 0.61* | −0.54* | 0.19 | |
| Treatment | −0.47* | −0.54* | −0.56* | −0.51* | 0.43* | 0.39* | −0.51* | 0.24 | |
| Worry | −0.35* | −0.52* | −0.43* | −0.53* | 0.36* | 0.25 | −0.52* | 0.09 | |
| Communication | −0.35* | −0.50* | −0.45* | −0.49* | 0.32* | 0.24 | −0.44* | 0.30 | |
| SMILEY | Summary Score | 0.26 | 0.26 | 0.25 | 0.28 | −0.22 | −0.14 | 0.29 | 0.02 |
| Effect on self | 0.33* | 0.34* | 0.34* | 0.39* | −0.30 | −0.18 | 0.39* | −0.09 | |
| Limitations | 0.10 | 0.07 | 0.06 | 0.03 | −0.05 | −0.03 | 0.03 | 0.12 | |
| Social | −0.46* | −0.50* | −0.49* | −0.54* | 0.49* | 0.38* | −0.54* | 0.44* | |
| Burden of cSLE | 0.30 | 0.33* | 0.28 | 0.36* | −0.31* | −0.20 | 0.38* | −0.08 | |
| CHAQ | Summary Score | 0.24 | 0.28 | 0.28 | 0.42* | −0.62* | −0.74* | 0.45* | −0.11 |
| Dressing/grooming | 0.23 | 0.31* | 0.28 | 0.42* | −0.58* | −0.66* | 0.41* | −0.10 | |
| Arising | 0.24 | 0.28 | 0.27 | 0.46* | −0.68* | −0.54* | 0.51* | −0.16 | |
| Eating | 0.18 | 0.25 | 0.22 | 0.38* | −0.55* | −0.74* | 0.40* | −0.11 | |
| Walking | 0.23 | 0.29 | 0.24 | 0.36* | −0.61* | −0.55* | 0.42* | −0.09 | |
| Hygiene | 0.27 | 0.26 | 0.31* | 0.43* | −0.61* | −0.67* | 0.45* | −0.12 | |
| Reach | 0.26 | 0.28 | 0.29 | 0.46* | −0.58* | −0.65* | 0.50* | −0.10 | |
| Play | 0.32* | 0.37* | 0.34* | 0.53* | −0.68* | −0.60* | 0.59* | −0.14 | |
| Grip | 0.28 | 0.33* | 0.31* | 0.44* | −0.54* | −0.69* | 0.49* | −0.18 | |
| CHQ P-50 | Psychosocial Summary Score | −0.37* | −0.32* | −0.34* | −0.36* | 0.40* | 0.21 | −0.36* | 0.32* |
| Physical Summary Score | −0.16 | −0.26 | −0.21 | −0.37* | 0.52* | 0.37* | −0.41* | 0.03 | |
| Physical functioning | −0.17 | −0.26 | −0.22 | −0.35* | 0.57* | 0.33* | −0.35* | 0.06 | |
| Bodily pain | −0.21 | −0.29 | −0.25 | −0.41* | 0.52* | 0.33* | −0.43* | 0.08 | |
| General health perception | −0.11 | −0.27 | −0.18 | −0.25 | 0.28 | 0.21 | −0.30 | 0.02 | |
| Role/social-physical | −0.24 | −0.20 | −0.27 | −0.28 | 0.34* | 0.32* | −0.33* | 0.18 | |
| Role/social- emotional/behavioral | −0.16 | −0.25 | −0.25 | −0.35* | 0.49* | 0.35* | −0.37* | 0.17 | |
| Self-esteem | −0.32* | −0.32* | −0.35* | −0.35* | 0.48* | 0.19 | −0.28 | 0.32* | |
| Mental health | −0.45* | −0.37* | −0.40* | −0.37* | 0.37* | 0.20 | −0.38* | 0.21 | |
| Behavior | −0.34* | −0.19 | −0.23 | −0.25 | 0.14 | 0.06 | −0.23 | 0.31* | |
| Mental health | −0.45* | −0.37* | −0.40* | −0.37* | 0.37* | 0.20 | −0.38* | 0.21 | |
Values are pooled correlation coefficients (r pool) across visits (n = 280 patient visits)
Represent p-value < 0.001 and r > 0.30
Systemic Lupus Erythematosus Disease Activity Index 2000
British Isles Lupus Activity Group index (A = 12, B = 8, C = 1, D = 0, E = 0)
MD global; 10 point Likert scale, 0 = inactive disease
Systemic Lupus International Collaborating Clinics/ACR Damage Index (range 0 to 47; 0 = absence of damage)
The SMILEY summary score correlated only weakly at best with the PROMIS-SFs, however, a few of the SMILEY subscales had mild-to-moderate correlation with the PROMIS-SFs. In addition, PROMIS-SF scores were no more than weakly correlated (rpool < 0.26) with measures of disease activity (SLEDAI, BILAG, or MD global) or damage (SDI).
Responsiveness to Change
Based on GCR-MD1 and GRC-MD2, 33 (34%) and 25 (26%) patients remained stable (unchanged) between Visit 1 and 2, and 38 (45%) and 34 (40%) patients between Visit 2 and 3, respectively. Between Visit 1 and 2, 52 (54%) and 56 (58%) patients were considered improved (somewhat or much better), and between Visit 2 and 3, 32 (38%) and 36 (43%) patients, respectively. Few patients worsened between study visits (GRC-MD1/GRC-MD2 rated as somewhat worse or much worse; between Visit 1 and 2: 11 and 15 patients, and between Visit 2 and 3: 14 and 14 patients).
For the first strategy (a), correlation analysis of PROs change scores for patients who improved or worsened between visits showed mostly low-to-fair correlation (rpool ≤ 0.42; p-value < 0.001) of change of the legacy measure summary scores (FDI, CHAQ, CHQ-PsS, CHQ-PhS, PedsQL-GC, PedsQL-RM, SMILEY) with the change of the scores of the PROMIS-SFs (See Supplemental Table 2).
As summarized in Table 3, the second strategy (b) that evaluated changes of the PROMIS-SF scores with changes of cSLE and overall health as rated by the physician (GRC-MD1, GRC-MD2), using mixed repeated-measure models showed appropriate parallel changes in scores of PROMIS-SFs with physician rated change. Significant improvement (p-value < 0.05) in PROMISAnger, PROMISAnxiety, PROMISPF-Mobility, and PROMISPain were observed with improvement in cSLE and overall health, while worsening cSLE was associated with significant (p-value < 0.05) decrease in PROMISPF-Mobility score. Also, PROMIS-SF scores remained stable in the setting of stable (unchanged/same) cSLE and overall health status. Further, cSLE disease activity improvement was accompanied by significant (p-value < 0.05) improvement in scores for PROMISAnger (SLEDAI, MD-global), PROMISPF-Mobility (SLEDAI, BILAG), PROMISPF-UExt and PROMISPain (BILAG only). Further, MD-global worsening was associated with statistically significant (p-value < 0.05) increase in PROMISDepression score.
Table 3.
Pediatric PROMIS short form domain score† change across visits and relationship to change in cSLE, health status and disease activity
| Variable/Category | Anger | Anxiety | Depressive Symptoms | Fatigue | Mobility | Upper Extremity Function | Pain Interefence | Peer Relationships | |
|---|---|---|---|---|---|---|---|---|---|
| Change in cSLE (GRC-MD1) | Better | −3.2(1.4)* | −2.7 (1.3)* | −1.3 (1.4) | −2.6 (1.6) | 2.9 (1.2)* | 2.8 (1.5) | −3.2 (1.3)* | −0.0 (1.5) |
| Same | −0.5 (1.3) | 1.0 (1.1) | 1.7 (1.2) | 0.3 (1.4) | 0.5 (1.1) | 0.1 (1.3) | −0.1 (1.2) | −0.3 (1.4) | |
| Worse | 0.4 (2.0) | 1.1 (1.8) | 2.0 (1.9) | −0.2 (2.1) | −4.0 (1.7)* | −1.4 (2.0) | 1.3 (1.8) | −0.9 (2.1) | |
|
| |||||||||
| Change in Health (GRC-MD2) | Better | −3.3 (1.4)* | −3.1 (1.3)* | −1.4 (1.4) | −2.5 (1.5) | 3.1 (1.2)* | 2.4 (1.5) | −3.3 (1.3)* | −0.6 (1.5) |
| Same | −0.3 (1.3) | 1.3 (1.2) | 2.1 (1.2) | 0.6 (1.4) | −0.5 (1.1) | −0.4 (1.3) | 0.2 (1.2) | 0.1 (1.4) | |
| Worse | 0.4 (1.9) | 1.2 (1.7) | 1.6 (1.8) | −0.9 (2.0) | −1.7 (1.6) | 0.1 (1.9) | 0.9 (1.7) | −0.9 (2.0) | |
|
| |||||||||
| Change in MD-global (MD-VAS) | Better | −5.2 (1.9)* | −1.7 (1.7) | −1.6 (1.7) | −1.2 (2.1) | 2.4 (1.6) | 2.2 (1.5) | −2.5 (1.9) | −1.9 (2.1) |
| Same | −0.5 (1.0) | 0.6 (0.9) | 0.8 (0.9) | −0.4 (1.1) | −0.1 (0.9) | −0.4 (0.8) | 0.4 (1.0) | −0.5 (1.1) | |
| Worse | −0.9 (2.5) | 1.9 (2.2) | 5.0 (2.3)* | −1.4 (2.7) | −0.8 (2.1) | 2.9 (1.8) | −1.2 (2.4) | 3.1 (2.6) | |
|
| |||||||||
| Change in SLEDAI | Better | −4.1 (1.8)* | −0.7 (1.6) | −0.8 (1.7) | −1.7 (2.0) | 4.2 (1.5)* | 1.8 (1.4) | −2.2 (1.8) | −2.0 (2.0) |
| Same | −0.6 (1.1) | 0.9 (0.9) | 1.7 (1.0) | −0.2 (1.2) | −0.8 (0.9) | −0.2 (0.8) | 0.6 (1.0) | −0.3 (1.1) | |
| Worse | −1.6 (2.1) | −1.1 (1.8) | −0.3 (1.9) | −0.3 (2.3) | −1.7 (1.8) | 0.8 (1.6) | −1.1 (2.1) | 0.2 (2.3) | |
|
| |||||||||
| Change in BILAGE | Better | −2.2 (1.5) | −1.0 (1.3) | 1.3 (1.4) | 0.7 (1.7) | 3.1 (1.3)* | 3.1 (1.1)* | −3.3 (1.5)* | −2.2 (1.6) |
| Same | −1.2 (1.2) | 1.2 (1.1) | 1.0 (1.1) | 0.0 (1.3) | −1.0 (1.0) | −1.0 (0.9) | 1.0 (1.2) | −0.6 (1.3) | |
| Worse | −1.2 (1.8) | −0.2 (1.6) | −0.1 (1.6) | −3.2 (1.9) | −1.7 (1.5) | −0.4 (1.3) | 1.4 (1.7) | 1.7 (1.9) | |
Values are T-score means (Standard Error) across visits – pooled data
Represents p-value < 0.05
GRC-MD1: Visit 2: Better 52 (54%); Same 33 (34%); Worse 11(11%); Visit 3: Better 32 (38%); Same 38 (45%); Worse 14 (17%)
GRC-MD2: Visit 2: Better 56 (58%); Same 25 (26%); Worse 15 (16%); Visit 3: Better 36 (43%); Same 34 (40%); Worse 14 (17%)
Summarized in Table 4(c) the results of the path analysis models of the PROMIS-SFs predicting change in cSLE and overall health (GRC-MD1; GRC-MD2), demonstrated appropriate prediction change in cSLE and overall health. Statistically significant (p-value < 0.05) change was predicted in the setting of cSLE improvement when PROMISAnger, PROMISFatigue, PROMISPain, and PROMISPF-UExt improved and predicted cSLE worsening when PROMISPF-Mobility worsened. Similar results were observed for change in overall health. We did not observe statistically significant relationships for PROMISAnxiety, PROMISDepression or PROMISPeerRel but expected patterns were present.
Table 4.
Path analysis of the pediatric PROMIS short forms and prediction of change in cSLE and health over time
| Variable Category | Anger | Anxiety | Depressive Symptoms | Fatigue | Mobility | Upper Extremity | Pain Interference | Peer Relationships | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Change in cSLE (GRC-MD1) | Between Visit 1 and 2 | Better | −0.7 (0.3)* | −0.3 (0.2) | −0.4 (0.3) | −0.7 (0.3)* | 0.4 (0.2) | 0.7 (0.3)* | −0.6 (0.3)* | −0.2 (0.3) |
| Worse | −0.2 (0.4) | −0.2 (0.4) | 0.0 (0.3) | −0.3 (0.5) | 0.3 (0.3) | 0.6 (0.3) | −0.3 (0.3) | 0.4 (0.4) | ||
|
| ||||||||||
| Between Visit 2 and 3 | Better | 0.1 (0.2) | −0.1 (0.1) | 0.0 (0.2) | 0.0 (0.2) | 0.3 (0.1) | 0.1 (0.1) | −0.1 (0.1) | 0.1 (0.2) | |
| Worse | 0.2 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.0 (0.2) | −0.4 (0.1)* | −0.2 (0.1) | 0.3 (0.2) | −0.1 (0.2) | ||
|
| ||||||||||
| Change in Health (GRC-MD2) | Between Visit 1 and 2 | Better | −0.6 (0.3)* | −0.3 (0.2) | −0.4 (0.3) | −0.7 (0.3)* | 0.3 (0.2) | 0.6 (0.3)* | −0.6 (0.3)* | −0.1 (0.3) |
| Worse | −0.0 (0.3) | −0.2 (0.3) | 0.1 (0.3) | −0.0 (0.4) | −0.1 (0.3) | 0.2 (0.3) | −0.2 (0.3) | 0.2 (0.4) | ||
|
| ||||||||||
| Between Visit 2 and 3 | Better | 0.0 (0.1) | −0.0 (0.1) | 0.0 (0.2) | 0.0 (0.1) | 0.2 (0.1) | 0.2 (0.1) | −0.1 (0.1) | 0.1 (0.2) | |
| Worse | 0.1 (0.2) | 0.1 (0.1) | 0.1 (0.2) | 0.1 (0.1) | −0.6 (0.1)* | −0.2 (0.1) | 0.3 (0.2) | 0.1 (0.2) | ||
Values are standardized coefficients (Standard Error)
Represents p-value < 0.05
DISCUSSION
The objectives of this study were to investigate the feasibility, internal consistency, construct validity and responsiveness to change of the PROMIS-SFs in a pediatric population with cSLE in a clinical setting. Our study focused on eight distinct PROMIS-SFs: Anger, Anxiety, Depressive Symptoms, Fatigue, Physical Function-Mobility, Physical Function-Upper Extremity, Pain Interference and Peer Relationships, i.e. health domains that seemed most relevant to cSLE, among the PROMIS-SFs available for pediatric populations at the time of the study. Our findings that PROMIS-SFs offer a valid option to efficiently collect PROs in cSLE in a clinical setting, given outstanding feasibility, and minimal time burden.
Construct validity of the PROMIS-SFs was demonstrated with high correlation with similar constructs (e.g. PROMISPF-Mobility and CHAQ, FDI, and Fatigue and FDI) and low correlation with dissimilar constructs (e.g. PROMISPeerRel and SLEDAI, Pain and SDI). This held true when PROMIS-SFs were compared with the legacy subscales too (e.g. high correlation of PROMISPF-Mobility and CHAQ: arising, PROMISPain and PedsQL-RM: pain & hurt; and low correlation of PROMISPeerRel and PedsQL-GC: physical function and PROMISAnxiety and CHAQ: eating). As expected, the physical domains moved together and the psychosocial domains to trended together.
Responsiveness of the PROMIS-SFs was demonstrated by three complementary strategies, all supporting sensitivity to change. The different approaches provide strong support for the usefulness of the PROMIS-SFs to capture clinically relevant changes in HRQoL in patients with cSLE. As there was relatively low disease activity at baseline and only few patients experienced a significant cSLE flare during the study as rated by the GRC-MD1 scale, statistical significance was not consistently reached when the PROMIS-SF scores were used as predictors of change in cSLE (GRC-MD1).
In all of the analyses that used the GRC-MD scales for assessing responsiveness of the PROMIS-SFs, physician ratings rather than patient ratings served as external standards. Based on the known weakness of physicians to gauge patient HRQoL, lower estimates of responsiveness might have been expected. Arguably, the strongest support for the responsiveness of the PROMIS-SFs is provided in our analyses (strategy b) showing moderate associations with the score changes of patient-completed legacy HRQoL measures.
On the other hand, physicians’ rating of improvement in cSLE and overall health tracked well with improved disease control (SLEDAI, BILAG, MD-global) and was associated with important changes in physical function, anger, anxiety, depression and pain as measured by the PROMIS-SFs. There was also high correlation of fatigue with FDI scores and, as expected, the PROMISFatigue score underlines the relevance of addressing patients’ complaints of fatigue in the medical management of cSLE. Together, this stresses the profound impact of cSLE on many aspects of physical health and HRQoL.
Another objective of PROMIS is “to develop meaningful, precise instruments while reducing respondent burden” (35). In this study the entire battery of PROMIS-SFs was completed in approximately five minutes which equates to less than one minute per domain. Our experience is in line with observations made in previous studies (36, 37). This supports a decreased respondent burden when compared with legacy measures that require 5 to 15 minutes each for completion (38–40).
PROMIS offers flexibility and customization as PROMIS-SFs can be selected, collected and maintained electronically through assessment center. While the electronic features of the assessment center can improve data management and improve feasibility, we encountered difficulties with assessmentcenter.net, resulting in loss of data or replicated data. It is anticipated that such shortcomings can be addressed quite easily by minimal programming changes or instructions for the use of the software.
Offering comparison of PROs across disease groups is another aim of PROMIS (35), and as the PROMIS-SFs are validated for different disease groups, comparison across the disease groups can be achieved. In this study we validated the PROMIS-SF for use in cSLE, giving providers another tool to use to measure HRQoL in cSLE, but also allowing for comparison of HRQoL between cSLE and other chronic diseases to enhance understanding of HRQoL in different chronic diseases.
There are several limitations to our study. During the study period, only English versions of the PROMIS-SFs were available, and as a result only English-speaking participants were enrolled in this study. The PROMIS-SFs are now available in Spanish and Dutch, with PROMISAnger, PROMISFatigue, PROMISPain, PROMISPeerRel, PROMISPF-Mobility PROMISPF-UExt translation efforts underway for simplified Chinese, Portuguese, German, and Swedish. Also, as expected for cSLE, the majority of patients were teenagers. Thus findings and experiences with the electronic data capture may be different in younger children with cSLE. Nonetheless, our study included children as young as 10 years of age. As in many studies in pediatric rheumatology, sample size was limited and likely contributed to difficulty demonstrating responsiveness to change for all of the PROMIS-SFs included in this study. This is especially true as it pertains to worsening of cSLE and overall health. However, use of three complementary strategies to support sensitivity to change are reported, all suggesting that PROMIS-SFs are suitable to capture clinically relevant change in HRQoL in cSLE. As this study took place at two centers, a center bias cannot be excluded, and next steps would include expanding this study to other centers. There was an excellent retention rate throughout the study with few missing data values for the cohort, and patients studied were well phenotyped and representative of the cSLE populations followed at the two tertiary pediatric centers, adding to the validity of analyses presented.
In conclusion, this study shows preliminary evidence of validation of the PROMIS-SFs for use as HRQoL measurement in cSLE. The PROMIS-SFs are a reliable, responsive and efficient choice for PRO measurement in cSLE, taking advantage of easy interpretation of scores and change in scores, thereby, reducing respondent burden and making HRQoL assessment feasible in research and clinical care settings as well as across disease groups.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
The pediatric Patient Reported Outcomes Measurement Information System short forms (PROMIS®-SFs) demonstrated construct validity, internal consistency, and responsiveness to change in cSLE.
The PROMIS-SFs are easily completed in a clinical setting, thus decreasing the burden of HRQoL measurement compared to currently used HRQoL legacy measures.
Different from some HRQoL measures, the PROMIS-SFs did not correlate with overall cSLE disease activity or damage.
Acknowledgments
Financial Support
Lupus Foundation of America Research Award, Michael Jon Barlin research program, National Institutes of Health AR-5U01AR067166 and the Center for Clinical & Translational Science and Training Award.
The authors would like to acknowledge Shannon Nelson, Kasha Wiley, Alexa Greenler and Courtney Paffett of Cincinnati Children’s Hospital Medical Center for help with data collection, and Alexa Greenler for additional help with data management.
Footnotes
Conflicts of interest
All authors declare they have no conflicts of interest.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Jones had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Brunner, Morgan DeWitt, Schanberg
Acquisition of data. Jones, Wootton, Liberio, Lee
Analysis and interpretation of data. Jones, Ying, Carle, Morgan DeWitt, Brunner
References
- 1.Brunner HI, Higgins GC, Wiers K, Lapidus SK, Olson JC, Onel K, et al. Health-related quality of life and its relationship to patient disease course in childhood-onset systemic lupus erythematosus. J Rheumatol. 2009;36(7):1536–45. doi: 10.3899/jrheum.081164. [DOI] [PubMed] [Google Scholar]
- 2.Ruperto N, Buratti S, Duarte-Salazar C, Pistorio A, Reiff A, Bernstein B, et al. Health-related quality of life in juvenile-onset systemic lupus erythematosus and its relationship to disease activity and damage. Arthritis Rheum. 2004;51(3):458–64. doi: 10.1002/art.20412. [DOI] [PubMed] [Google Scholar]
- 3.Thumboo J, Strand V. Health-related quality of life in patients with systemic lupus erythematosus: an update. Ann Acad Med Singapore. 2007;36(2):115–22. [PubMed] [Google Scholar]
- 4.Jones JT, Cunningham N, Kashikar-Zuck S, Brunner HI. Pain, Fatigue and Psychological Impact on Health-related Quality of Life in Childhood-onset Lupus. Arthritis care & research. 2015 doi: 10.1002/acr.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmeding A, Schneider M. Fatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2013;27(3):363–75. doi: 10.1016/j.berh.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Eiser C, Jenney M. Measuring quality of life. Arch Dis Child. 2007;92(4):348–50. doi: 10.1136/adc.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware JE., Jr The Patient-Reported Outcomes Measurement Information System (PROMIS) seeks to improve and standardize measures of five generic health-related QOL domains. 2007 [Google Scholar]
- 9.Silva CA, Avcin T, Brunner HI. Taxonomy for systemic lupus erythematosus with onset before adulthood. Arthritis Care Res (Hoboken) 2012;64(12):1787–93. doi: 10.1002/acr.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domain Frameworks PROMIS Adult Self-Reported Health. www.nihpromis.org2015. Available from: http://www.nihpromis.org/measures/domainframework1.
- 11.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45(5 Suppl 1):S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 12.PROMIS Scoring Manuals. www.assessmentcenter.net/Manuals.aspx.2015.
- 13.DeWitt EM, Stucky BD, Thissen D, Irwin DE, Langer M, Varni JW, et al. Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: built using item response theory. J Clin Epidemiol. 2011;64(7):794–804. doi: 10.1016/j.jclinepi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varni JW, Stucky BD, Thissen D, Dewitt EM, Irwin DE, Lai JS, et al. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11(11):1109–19. doi: 10.1016/j.jpain.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19(4):595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin DE, Stucky BD, Langer MM, Thissen D, DeWitt EM, Lai JS, et al. PROMIS Pediatric Anger Scale: an item response theory analysis. Qual Life Res. 2012;21(4):697–706. doi: 10.1007/s11136-011-9969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, et al. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain. 2011;152(7):1600–7. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121(1–2):77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorthy LN, Peterson MG, Baratelli M, Harrison MJ, Onel KB, Chalom EC, et al. Multicenter validation of a new quality of life measure in pediatric lupus. Arthritis Rheum. 2007;57(7):1165–73. doi: 10.1002/art.22988. [DOI] [PubMed] [Google Scholar]
- 20.Meiorin S, Pistorio A, Ravelli A, Iusan SM, Filocamo G, Trail L, et al. Validation of the Childhood Health Assessment Questionnaire in active juvenile systemic lupus erythematosus. Arthritis Rheum. 2008;59(8):1112–9. doi: 10.1002/art.23912. [DOI] [PubMed] [Google Scholar]
- 21.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37(12):1761–9. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 22.Landgraf JM, Abetz LN, Ware JE. The Childhood Health Questionsnnaire user’s manual. 2. Boston (MA): HealthAct; 1999. [Google Scholar]
- 23.Asmussen L, Olson LM, Grant EN, Landgraf JM, Fagan J, Weiss KB. Use of the child health questionnaire in a sample of moderate and low-income inner-city children with asthma. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1215–21. doi: 10.1164/ajrccm.162.4.2001067. [DOI] [PubMed] [Google Scholar]
- 24.Gladman DD, Goldsmith CH, Urowitz MB, Bacon P, Fortin P, Ginzler E, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000;27(2):373–6. [PubMed] [Google Scholar]
- 25.Brunner HI, Silverman ED, To T, Bombardier C, Feldman BM. Risk factors for damage in childhood-onset systemic lupus erythematosus: cumulative disease activity and medication use predict disease damage. Arthritis Rheum. 2002;46(2):436–44. doi: 10.1002/art.10072. [DOI] [PubMed] [Google Scholar]
- 26.Brunner HI, Feldman BM, Bombardier C, Silverman ED. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42(7):1354–60. doi: 10.1002/1529-0131(199907)42:7<1354::AID-ANR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Yee CS, Cresswell L, Farewell V, Rahman A, Teh LS, Griffiths B, et al. Numerical scoring for the BILAG-2004 index. Rheumatology (Oxford) 2010;49(9):1665–9. doi: 10.1093/rheumatology/keq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44(7):902–6. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 29.George DM, Mallery Paul. SPSS for Windows step by step: A simple guide and reference. 4. Boston: Allyn & Bacon; 2003. p. 400. 11.0 Update. [Google Scholar]
- 30.Westen D, Rosenthal R. Quantifying construct validity: two simple measures. J Pers Soc Psychol. 2003;84(3):608–18. doi: 10.1037//0022-3514.84.3.608. [DOI] [PubMed] [Google Scholar]
- 31.Singh JA, Solomon DH, Dougados M, Felson D, Hawker G, Katz P, et al. Development of classification and response criteria for rheumatic diseases. Arthritis Rheum. 2006;55(3):348–52. doi: 10.1002/art.22003. [DOI] [PubMed] [Google Scholar]
- 32.Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biom J. 2006;48(2):286–301. doi: 10.1002/bimj.200510192. [DOI] [PubMed] [Google Scholar]
- 33.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 34.Muthen LK, Muthen BO. Mplus User’s Guide. 7. Lost Angeles, CA: 1998–2012. [Google Scholar]
- 35.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khanna D, Maranian P, Rothrock N, Cella D, Gershon R, Khanna PP, et al. Feasibility and construct validity of PROMIS and “legacy” instruments in an academic scleroderma clinic. Value Health. 2012;15(1):128–34. doi: 10.1016/j.jval.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Senders A, Hanes D, Bourdette D, Whitham R, Shinto L. Reducing survey burden: feasibility and validity of PROMIS measures in multiple sclerosis. Mult Scler. 2014;20(8):1102–11. doi: 10.1177/1352458513517279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varni JW, Seid M, Smith Knight T, Burwinkle T, Brown J, Szer IS. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory Generic Core Scales and Rheumatology Module. Arthritis Rheum. 2002;46(3):714–25. doi: 10.1002/art.10095. [DOI] [PubMed] [Google Scholar]
- 39.Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML) Arthritis care & research. 2011;63(Suppl 11):S420–30. doi: 10.1002/acr.20637. [DOI] [PubMed] [Google Scholar]
- 40.Hersh A. Measures of health-related quality of life in pediatric systemic lupus erythematosus: Childhood Health Assessment Questionnaire (C-HAQ), Child Health Questionnaire (CHQ), Pediatric Quality of Life Inventory Generic Core Module (PedsQL-GC), Pediatric Quality of Life Inventory Rheumatology Module (PedsQL-RM), and Simple Measure of Impact of Lupus Erythematosus in Youngsters (SMILEY) Arthritis care & research. 2011;63(Suppl 11):S446–53. doi: 10.1002/acr.20559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.