Abstract
Objective
Patients with immune-mediated necrotizing myopathy (IMNM) often have autoantibodies recognizing the signal recognition particle (SRP) or HMG-CoA reductase (HMGCR). Here, we studied a cohort of anti-SRP patients to identify factors associated with disease severity and clinical improvement; we also compared the severity of weakness in those with anti-SRP versus anti-HMGCR autoantibodies.
Methods
All anti-SRP patients in the Johns Hopkins Myositis Cohort from 2002 to 2015 were included. Longitudinal information regarding proximal muscle strength, creatine kinase (CK) levels, and immunosuppressive therapy were recorded at each visit. Univariate and multivariate multilevel regression models were used to assess prognostic factors influencing recovery. Strength in the anti-SRP patients was compared to strength in 49 previously described anti-HMGCR subjects.
Results
Data from 37 anti-SRP patients and 380 total clinic visits was analyzed. Younger age at onset was associated with more severe weakness at the first visit (p=0.02) and all subsequent visits (p=0.002). Only 50% of patients reached near-full or full strength after 4 years of treatment and most of these continued to have elevated CK levels. Rituximab appeared to be effective in 13 of 17 anti-SRP patients. Anti-SRP patients were significantly weaker than those with anti-HMGCR autoantibodies (−1.3 strength points, p=0.001).
Conclusions
Younger age at onset is associated with more severe weakness in anti-SRP myositis. Furthermore, even among anti-SRP patients whose strength improved with immunosuppression, most had ongoing disease activity as demonstrated by elevated CK levels. Finally, anti-SRP patients were significantly weaker than anti-HMGCR patients, providing evidence that these autoantibodies are associated with distinct forms of IMNM.
Keywords: myositis, autoantibody(ies), autoantigen(s), autoimmune diseases, cohort study, anti-SRP, necrotizing myositis
INTRODUCTION
The autoimmune myopathies are a heterogeneous family of diseases including polymyositis (PM), dermatomyositis (DM), and immune-mediated necrotizing myopathy (IMNM); proximal muscle weakness, elevated serum muscle enzyme levels, and abnormal muscle biopsies characterize each of these.(1) As in other systemic autoimmune diseases, autoantibodies are associated with distinct clinical phenotypes in patients with autoimmune myopathy. For example, patients with autoantibodies recognizing the signal recognition particle (SRP) or HMG-CoA reductase (HMGCR) tend to have necrotizing muscle biopsies with minimal inflammation, especially high CK levels, and relatively infrequent extramuscular involvement (2–5) which are all characteristic features of IMNM.(6)
While prior reports have emphasized that anti-SRP autoantibodies are associated with unusually severe muscle disease, not all anti-SRP-positive patients are refractory to immunosuppressive therapy. However, due to relatively small numbers of patients and lack of detailed longitudinal analysis, factors that influence the disease severity and prognosis of anti-SRP positive patients have not been well described.
Here we report the results of a detailed longitudinal cohort study of SRP patients analyzing their clinical course, prognostic factors, and treatment schemes. We also compare the strength of anti-SRP patients with the strength of anti-HMGCR patients to determine whether these autoantibodies are associated with disease severity in IMNM.
MATERIAL AND METHODS
Study populations and autoantibody testing
Between 2001 and 2015, patients with suspected myopathy were evaluated by neurologists, rheumatologists and pulmonologists at the Johns Hopkins Myositis Center and enrolled in a longitudinal study to assess the relationship between autoantibody profile and distinct clinical phenotypes. All patients who tested positive for anti-SRP autoantibodies and presented with muscle weakness in the clinical evaluation were included in the study. We also included 49 anti-HMGCR subjects that were described in a prior study (7).
Anti-SRP testing was performed by immunoprecipitation either at the Johns Hopkins Rheumatic Disease Research Core Center using previously validated methods of immunoprecipitation,(8) through the Oklahoma Medical Research Foundation, or using Quest Diagnostics myositis panels.
At the first visit, clinicians recorded the strength of neck flexors, neck extensors, arm abductors, elbow flexors, elbow extensors, wrist flexors, wrist extensors, finger flexors, finger extensors, hip flexors, hip extensors, knee flexors, knee extensors, ankle dorsiflexors, and ankle plantar flexors using the Medical Research Council (MRC) scale.(9) At each follow-up visit, the examining physician consistently evaluated the patient's arm abduction and hip flexion strength using the MRC scale. For analysis, the MRC scale was transformed to Kendall's 0-10 scale as previously described.(9) With rare exceptions, the same physician made serial strength measurements for each patient at each visit. For the purposes of regression and survival analyses, the average of right and left-side measurements for arm abduction and hip flexion strength was used for the calculations (possible range 0–10). Serum CK, aldolase, AST and ALT levels were included for the analysis if obtained within 6 weeks of the patient's visit. Additionally, the presence of cancer-associated myositis (defined as the onset of cancer 3 years before or after the onset of the inflammatory myopathy),(10) dysphagia, myositis-specific skin involvement (heliotrope rash, Gottro's sign or papules), and the predominant abnormal histological features of the muscle biopsy were recorded. Interstitial lung disease (ILD) was assessed by a multidisciplinary team that evaluated the radiologic, spirometric and clinical features on each patient. A patient was considered responsive to an immunosuppressive treatment if their strength increased 2 points or their CK levels declined by 10-fold within 6 months after its administration.
Standard protocol approvals and patient consents
This study was approved by the Johns Hopkins Institutional Review Board and written informed consent was obtained from each participant.
Statistical analysis
Qualitative variables were expressed as percentages and absolute frequencies, and quantitative features were reported as means and standard deviations (SD). Creatine kinase (CK) level, a highly positively skewed variable, was expressed as median, first, and third quartile (Q1-Q3) for descriptive purposes, and was logarithmically transformed for regression analysis.
Univariate comparisons between groups were made using Wilcoxon rank-sum or Student's t-test for continuous variables, and either a chi-square test or Fisher's exact test for categorical variables, as appropriate. Confidence intervals of percentages were performed with Wilson's method. Pearson's r was used to calculate the correlation between pairs of continuous variables except when one of the variables was the creatine kinase, where Spearman's rho was used instead. Paired t-test was used to compare the strength of different muscle groups within each patient.
The influence of non-modifiable (sex, race, and age at onset) risk factors on the patients' initial and final strength was assessed using multiple linear regression. To account for the different number of visits per patient, strength during the whole study period was analyzed using multilevel linear regression models with random intercepts. Corticosteroid dose and the administration of intravenous immunoglobulins (IVIG), rituximab, methotrexate, azathioprine, and mycophenolate were used as adjusting covariates for multilevel analysis. Treatments administered to less than 10% of the cohort were not included in the study. The effect of sex, race, and age at onset in the number of different immunosuppressant drugs was analyzed using multilevel Poisson regression models. The Kaplan-Meier method was used to study the time to reach near-full or full strength (defined as strength equal to or above 8) and Cox regression to measure the effect of different covariates over the time to reach full strength. Finally, to assess if there were differences in strength among the different subsets of patients with IMNM, we compared the strength of our sample of anti-SRP patients with that of anti-HMGCR patients (7) using an unadjusted multilevel linear regression model with random intercepts.
All statistical analyses were performed using Stata/MP 13.1 and a 2-sided p value of 0.05 or less was considered significant.
RESULTS
Clinical characteristics of patients with anti-SRP-associated myopathy
Thirty-nine of 732 (5.3%) patients tested for anti-SRP were positive. Of these, 37 (94.9%) had proximal muscle weakness and 38 (97.4%) had elevated muscle enzyme levels. The two patients who did not present with muscle weakness were excluded from further analysis. The 37 anti-SRP positive patients included in the study had a total of 380 visits, a mean of 10 visits per patient (SD:12 visits), a mean time between visits of 3.9 months (SD: 3.5 months), and a mean follow-up time of 2.9 years (SD: 2.5 years).
Of the 37 patients included in the study, 78% were women, 56% Caucasian and 42% black. Muscle biopsies revealed a necrotizing myopathy in 29 (78%), non-necrotic muscle fibers surrounded and invaded by lymphocytes in 5 (14%), and non-specific myopathic features in 3 (8%). The mean age at onset was 38.4 years (SD: 13.8) and median peak CK during the study period was 3370 IU/L (Q1-Q3: 1361-6020 IU/L). Uncommon features included cancer-associated myositis (n=1, 3%), dermatomyositis skin rash (n=1, 3%) or statin exposure before the onset of the disease (n=2, 5%). In contrast, the presence of dysphagia (n=14, 38%) or interstitial lung disease (n=8, 22%) was relatively frequent (Table 1).
Table 1.
General features of anti-SRP patients*.
| Stat (95%CI) | |
|---|---|
| n=37 | |
|
| |
| Age of onset | 38.4 (33.8,43.0) |
| Female sex | 78% (63%,89%) |
| Caucasian | 54% (38%,69%) |
| Black | 41% (26%,57%) |
| Other races | 5% (1%,18%) |
| Statin exposure | 5% (1%,18%) |
| Cancer associated myositis | 3% (0%,14%) |
| Skin involvement | 3% (0%,14%) |
| Necrotizing muscle biopsy | 78% (63%,89%) |
| Maximum CK during study period | 3370 (1813,4482) |
| Interstitial lung disease | 22% (11%,37%) |
| Dysphagia | 38% (24%,54%) |
| Corticosteroids | 95% (82%,99%) |
| Methotrexate | 65% (49%,78%) |
| Rituximab | 57% (41%,71%) |
| Azathioprine | 43% (29%,59%) |
| IVIG | 38% (24%,54%) |
| Mycophenolate | 30% (17%,46%) |
Age at onset was expressed as mean (95% confidence inteval [CIJ), creatine kinase (CK) as median (95%CI) and bivariate variables as percentage (95%CI).
At first visit, patients had weakness in a proximal > distal pattern; the most severe weakness was in hip flexors > arm abductors > hip extensors > elbow extensors > elbow flexors = neck flexors > knee flexors > knee extensors (Table 2). Univariate analysis showed that patients who were older at disease onset showed greater strength in most of these proximal muscle groups at their first visit. Black patients had higher peak CK levels than Caucasians (p=0.05).
Table 2.
Weakness pattern of anti-SRP patients at first visit across groups*.
| Sex | Race | Age of onset | Total (95%CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female | p | Male | Caucasian | p | Black | Corr. coeff | p | ||
|
| |||||||||
| Neck flexors | 7.6 | 0.4 | 8.4 | 7.5 | 0.2 | 8.8 | −0.1 | 0.5 | 7.8 (6.8,8.8) |
| Neck extensors | 9.2 | 0.3 | 10.0 | 9.0 | 0.3 | 9.8 | −0.3 | 0.2 | 9.4 (8.7,10.0) |
| Arm abductors | 6.8 | 0.3 | 5.8 | 6.5 | 1.0 | 6.6 | 0.5 | 0.003 | 6.5 (5.7,7.3) |
| Elbow flexors | 8.0 | 0.3 | 7.0 | 8.0 | 0.8 | 7.7 | 0.4 | 0.02 | 7.8 (7.0,8.6) |
| Elbow extensors | 7.7 | 0.6 | 7.1 | 7.7 | 0.8 | 7.5 | 0.4 | 0.02 | 7.6 (6.8,8.3) |
| Wrist flexors | 9.5 | 0.9 | 9.4 | 9.4 | 0.3 | 9.8 | 0.3 | 0.1 | 9.4 (9.0,9.9) |
| Wrist extensors | 9.3 | 0.6 | 9.6 | 9.2 | 0.1 | 9.9 | 0.2 | 0.3 | 9.4 (9.0,9.8) |
| Finger flexors | 9.4 | 0.6 | 9.0 | 9.2 | 0.2 | 9.9 | 0.4 | 0.06 | 9.3 (8.7,9.9) |
| Finger extensors | 9.2 | 0.3 | 10.0 | 9.2 | 0.4 | 9.8 | 0.3 | 0.2 | 9.4 (8.8,9.9) |
| Hip flexors | 5.0 | 0.4 | 3.9 | 5.0 | 0.7 | 4.7 | 0.3 | 0.08 | 4.7 (3.7,5.7) |
| Hip extensors | 7.4 | 0.6 | 6.5 | 7.8 | 0.2 | 6.0 | 0.4 | 0.08 | 7.2 (5.9,8.6) |
| Knee flexors | 8.0 | 0.8 | 7.7 | 8.1 | 0.9 | 7.9 | 0.4 | 0.04 | 8.0 (7.1,8.8) |
| Knee extensors | 8.1 | 0.9 | 8.0 | 8.2 | 0.9 | 8.1 | 0.4 | 0.04 | 8.1 (7.2,9.0) |
| Ankle flexors | 9.4 | 0.8 | 9.6 | 9.3 | 0.5 | 9.7 | 0.3 | 0.1 | 9.4 (8.8,10.0) |
| Ankle extensors | 9.4 | 0.9 | 9.5 | 9.3 | 0.4 | 9.9 | 0.3 | 0.1 | 9.5 (8.8,10.1) |
Strength values were expressed as means. Pearson's r was used to calculate the correlation coefficient.
Treatments and clinical course of anti-SRP patients
Treatment regimens varied considerably between patients. The number of different immunosuppressive medications used (not necessarily simultaneously) was 1 or 2 in 32.4%, 3 or 4 in 48.7%, and 5 or more in 18.9%. The mean number of immunosuppressive drugs per visit was 2.1 (SD: 0.9). The most commonly used drugs were corticosteroids (n=35, 94%), methotrexate (n=24, 64%) and rituximab (n=21, 56%) (Table 1). Poisson multilevel regression analysis revealed that females were treated with a slightly higher number of different drugs than males (β=0.3, p=0.03); race, age at onset, and time from onset were not associated with the number of drugs used to treat the patients.
Notably, 52% (11/21) of patients followed for at least two years remained weak, with strength scores less than 8 after 2 years of aggressive treatment. All of the persistently weak patients had sustained elevations of serum CK levels (>500 IU/L), suggesting that ongoing active disease rather than permanent muscle damage alone was responsible for the weakness. 48% (10/21) of patients recovered near-full or full strength; among these, only 40% (4/10) had CK levels below 500 IU/L, suggesting ongoing disease activity in a significant number of these patients as well.
Univariate analysis of prognostic factors associated with strength
Given that anti-SRP myositis patients have proximal greater than distal weakness and that we only consistently assessed strength of proximal muscles over time, our longitudinal analyses were restricted to an examination of the evolution of arm abduction and hip flexor strength over time. At all visits, hip flexors were about 1.8 Kendall points weaker than deltoids (all p<0.001). Univariate analysis showed that patients who were older at disease onset showed greater strength during follow-up and at their last visit. Male patients were weaker than females during follow-up (p<0.05) (Table 2 and 3).
Table 3.
Strength and creatine kinase (CK) levels of anti-SRP patients across groups at first visit, during the follow-up and at last-visit*.
| Sex | Race | Age of onset | Total (95%CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female | p | Male | Caucasian | p | Black | Corr. coeff | p | ||
|
| |||||||||
| Hip flexors strength at first visit | 5.0 | 0.4 | 3.9 | 5.0 | 0.7 | 4.7 | 0.3 | 0.08 | 4.7 (3.7,5.7) |
| Follow-up hip flexors strength | 6.5 | 0.04 | 4.2 | 6.8 | 0.07 | 5.0 | 0.3 | 0.06 | 6.0 (5.0,7.0) |
| Hip flexors strength at last visit | 6.9 | 0.02 | 3.8 | 7.3 | 0.06 | 5.0 | 0.5 | 0.004 | 6.1 (4.9,7.4) |
|
| |||||||||
| Arm abductors strength at first visit | 6.8 | 0.3 | 5.8 | 6.5 | 1.0 | 6.6 | 0.5 | 0.003 | 6.5 (5.7,7.3) |
| Follow-up arm abductors strength | 8.4 | 0.009 | 6.0 | 8.2 | 0.4 | 7.5 | 0.3 | 0.06 | 7.8 (6.9,8.6) |
| Arm abductors strength at last visit | 8.4 | 0.03 | 5.7 | 8.3 | 0.4 | 7.2 | 0.3 | 0.05 | 7.7 (6.6,8.8) |
|
| |||||||||
| CK at first visit | 2426 | 0.5 | 2638 | 2394 | 0.2 | 2900 | −0.2 | 0.3 | 2426 (1360,3506) |
| Follow-up CK | 736 | 0.4 | 1238 | 748 | 0.4 | 1270 | −0.3 | 0.2 | 854(488,1514) |
| Maximum CK | 2900 | 0.6 | 4967 | 2710 | 0.05 | 7460 | −0.1 | 0.6 | 3370 (1812,4482) |
Strength values were expressed as means and CK as medians. Bivariate comparisons were made using Student's t-testfor the strength and Wilcoxon rank-sum test for CK. Pearson's r was used to calculate the correlation coefficient for strength and Spearman's rhofor the CK. Follow-up strength was defined as the mean strength of all the visits, excluding the first one.
Comparing the initial strength of anti-SRP patients with the initial strength of 49 anti-HMGCR patients described in detail elsewhere,(7) we found that anti-SRP patients were, on average, 1.3 strength points weaker (95%CI −2.2, −0.5; p=0.001) than anti-HMGCR patients.
Multivariate analysis of prognostic factors associated with strength
To investigate these associations further we performed a regression analysis. This confirmed age at onset of disease as an independent prognostic factor for strength at all time points regardless of sex, race, or therapy (p=0.002); each 10-year addition in age at onset was associated with an increased strength of more than 0.5 strength points. Additionally, male sex was associated with weaker muscles at the last visit. Although race and sex were not associated with strength, male and black patients showed a consistent non-significant trend towards being weaker than female and Caucasian patients, respectively (Table 4).
Table 4.
Strength of anti-SRP patients across groups at first visit, all visits, and last visit.
| Strength at first visit# | Strength at all visits$ | Strength at last visit# | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate |
Univariate | Multivariate | ||
| Without treatments | With treatments | ||||||
|
|
|||||||
| Coef. [95%CI] | Coef. [95%CI] | Coef. [95%CI] | Coef. [95%CI] | Coef. [95%CI] | Coef. [95%CI] | Coef. [95%CI] | |
|
| |||||||
| Age of onset (each 10 years) | 0.73 [0.22,1.24] ** | 0.69 [0.13,1.25] * | 0.67 [0.15,1.19] * | 0.67 [0.19,1.15] ** | 0.73 [0.26,1.20] ** | 1.18 [0.37,1.99] ** | 1.26 [0.47,2.05] ** |
| Female vs Male | 1.05 [−0.79,2.88] | 0.84 [−1.02,2.70] | 1.38 [−0.33,3.09] | 1.22 [−0.26,2.70] | 1.09 [−0.34,2.52] | 2.91 [0.71,5.12] ** | 2.73 [0.77,4.70] ** |
| Black vs Caucasian | −0.16 [−1.76,1.44] | −0.09 [−1.72,1.54] | −0.76 [−2.23,0.71] | −0.57 [−1.83,0.70] | −0.63 [−1.84,0.58] | −1.68 [−3.80,0.44] | −0.49 [−2.32,1.33] |
p<0.05,
p<0.01,
p<0.001
Linear regression. Multivariate analysis adjusted by time from onset, age at onset, sex, and race.
Multilevel regression with random intercepts. Multivariate analysis adjusted by time from onset, age at onset, sex, race and immunosuppresant drugs (corticosteroid dose and treatment with intravenous immunoglobulins, rituximab, mycophenolate, methotrexate or azathioprine).
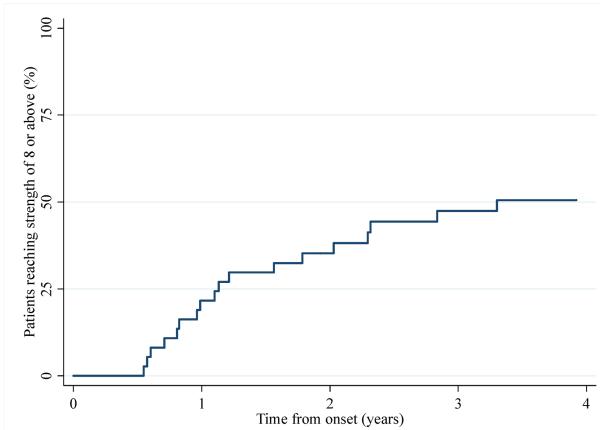
Although many patients were relatively refractory to treatment, others did recover near-full or full strength with a mean Kendall strength score of 8 or more. To determine the rate of improvement, we performed a survival analysis using Kaplan-Meier estimates. Although ~25% of anti-SRP positive patients reached near-full or full strength one year after disease onset, the rate of improvement subsequently plateaued with just 50% reaching a strength of 8 or more within 4 years of disease onset (Figure 2). Cox regression showed that the amount of time patients took to reach full strength was not significantly associated with their age at onset, sex or race (all p>0.05). Interestingly, after recovering full strength, a number of patients weakened again, often in the context of immunosuppressant treatment tapering.
Figure 2.
Rate of recovery to full or nearly full strength over time.
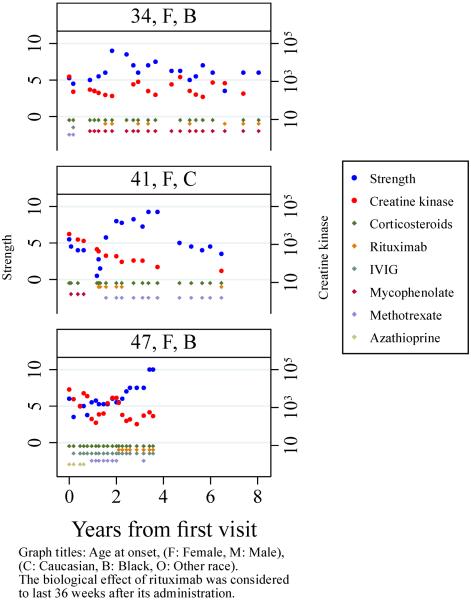
Finally, multilevel regression models showed that CK levels were significantly associated with strength (p<0.001), independently of the age at onset, time from onset, sex, race, or treatments used at each visit (β=−1.3, p<0.001). Thus, each 10-fold increase in the CK was associated with a decrease of 1.3 point of strength (95%CI: −1.9, −0.7). As exemplified in Figure 1, the CK levels tended to mirror the evolution of the strength of these patients. Aldolase, AST and ALT levels were analyzed in the same fashion as the CK but did not show a significant association with the strength levels (all p>0.05).
Figure 1.
Example of strength and creatine kinase evolution in patients treated with rituximab.
Efficacy of rituximab for anti-SRP myositis
In this cohort of patients, the treating physicians individualized therapies and so responses to particular medications could not be effectively studied. Nonetheless, in a number of patients rituximab had some evidence of efficacy, with strength gain following its administration and return of weakness after the end of its theoretical biological effect (6–9 months)(11), (Figure 1). As a rough approximation of rituximab efficacy, of the 21 patients in the current study who received rituximab, 13 were responsive to rituximab, and only 4 were not responsive (76%, 95%CI 53%–90%), 4 could not be evaluated due to lack of follow-up. The duration of the biological effect of rituximab varied, with some patients suffering a relapse less than one year after the administration of the drug and requiring frequent retreatment (patient 1, Figure 1), and others recovering strength during more than two years after the first administration (patient 2, Figure 1).
DISCUSSION
The autoimmune myopathies are a heterogeneous family of diseases, each of which has unique clinical and pathological features. Although myositis autoantibodies can identify more homogeneous subsets of autoimmune myopathy patients, even among those with the same autoantibody, clinical features and response to therapy can vary. In the current study, we studied 37 anti-SRP positive subjects who were followed longitudinally at our center. Consistent with prior reports,(5, 12, 13) we found that a majority of anti-SRP patients share similar features; most had necrotizing myopathies with very high CK levels and only a minority had rashes, lung disease, or other extramuscular manifestations. However, for the first time, we report here that younger patients were significantly weaker at the first visit than older patients. Moreover, by analyzing the evolution of strength and CK levels over time, we found that younger anti-SRP patients remained weaker at subsequent visits independent of possible confounding factors. Indeed, age at onset can be viewed as a continuous risk factor, where every 10 years of difference in patients' age is associated with a difference in strength of 0.5 points at any given time point.
Our finding that treated younger anti-SRP patients remain weaker than the treated older patients is consistent with a recent multicenter study of Japanese patients with anti-SRP myositis, which found that pediatric onset was associated with worse neurologic outcomes.(12) These observations may be somewhat surprising given that numerous prior studies have reported that younger myositis patients are more likely to go into remission than older patients.(14–18) For example, one study of 79 patients with PM and DM reported that complete remission was more frequent in younger than older patients (41.1% vs. 13.6%).(17) Similarly, a study of 77 PM and DM subjects demonstrated that patients who achieved remission were younger than those who did not.(18) While the reasons for this discrepancy are not clear, we suspect that by studying all DM and PM patients as a single group, important differences in outcomes among distinct subgroups (e.g., anti-SRP positive myositis patients) may have been overlooked. We are currently studying outcomes in other well-defined myositis disease subsets (e.g, DM patients with different DM-specific autoantibodies) to determine factors such as age that may influence prognosis in these different groups.
Although older anti-SRP positive patients were stronger at all times during follow-up compared to younger patients, this study confirmed that anti-SRP-associated myositis is a chronic and severe disease. After 1 and 4 years of treatment, respectively, only 25% and 50% of patients recovered near-full or full strength. Moreover, even among those who regained a significant degree of muscle strength, most continued to require immunosuppressive therapy. Furthermore, even among the “successfully treated” patients, almost all had persistently elevated CK levels. This suggests that treatment did not abolish the disease but only minimized it to the extent that muscle regeneration could outpace ongoing muscle damage.
The large number of patients included in this study allowed us to examine other clinical features of anti-SRP-associated myositis. For example, we found that 22% of patients in our cohort also had interstitial lung disease, which is similar to what has been previously reported.(5, 19) Interestingly, among the 8 patients with ILD in the current study, 5 had additional extramuscular features including arthritis, parotid enlargement, discoid lupus, and Raynaud phenomenon. Of note, all the patients with ILD were extensively tested for other myositis-specific and -associated autoantibodies (including the antisynthetase autoantibodies and autoantibodies associated with dermatomyositis) and none were detected, apart from one patient who was positive for anti-Ro (3 other patients without ILD were also positive for anti-Ro, the difference was not significant). We conclude that, while frequently only affecting skeletal muscle, anti-SRP may occasionally be associated with multisystem disease, similar to other forms of myositis.
It has been proposed that necrotizing myositis may be triggered by cancer(20, 21) and it is well known that the other major type of necrotizing inflammatory myopathy, anti-HMGCR disease, can be triggered by statins.(22) The rarity of these factors in our cohort suggests that anti-SRP disease is not likely to be triggered by cancer, and that statins are probably unrelated to the onset of the disease.
This study, which included a relatively large number of patients with different genders and races, allowed us to examine how these factors affect disease severity in anti-SRP myositis. Interestingly, we found that male and black patients showed a trend towards being weaker than females and Caucasians, respectively. We hypothesize that since younger, black, and male patients tend to have higher muscle mass than their respective counterparts,(23) damage to their muscles may trigger the release of greater amounts of autoantigen into the extracellular space. This could intensify an inflammatory feedback loop and lead to more aggressive disease. However, this explanation for our observation remains to be proven.
Observational studies have serious limitations in assessing the efficacy of individual treatments, especially if the number of therapies to study is large, as is true in in the current study. Nonetheless, we confirmed and expanded the results of our previously published series showing the efficacy of rituximab in 6 of 8 cases of anti-SRP myositis.(8) In the current study, we showed that rituximab administration was associated with increased strength and decreased CK levels immediately after its administration in 13 of 17 patients. Of note, this phenomenon could sometimes be observed repeatedly in the same patient, as can be seen in Figure 1. However, it is important to note that even with rituximab treatment, younger patients tended to lose strength steadily in the long term. Although conclusive evidence for making treatment recommendations is lacking, we would suggest that high intensity combination schemes containing rituximab may be considered when treating younger anti-SRP positive patients. Also, future clinical trials should consider the age at onset in their randomization strategy to ensure a balanced distribution of this variable across treatment groups.
Anti-SRP and anti-HMGCR autoantibodies are both associated with necrotizing muscle biopsies, high CK levels, and proximal muscle weakness. Indeed, maximum CK levels were no different in the anti-SRP and anti-HMGCR subjects during the course of this study (median CK: 3370 IU/L vs. 3330 IU/L, respectively) and both groups had a similar proportion of patients with necrotizing muscle biopsies (78% vs. 84%, respectively). However, we found that anti-SRP patients were significantly weaker than anti-HMGCR patients. This indicates that IMNM patients with anti-SRP autoantibodies have more severe disease than those with anti-HMGCR autoantibodies and suggests that these autoantibodies may define two unique subsets of IMNM patients. Similarly, emerging evidence suggests that DM is not a single disease and that unique clinical entities can be defined by different DM-specific autoantibodies. For example, DM patients with NXP-2 autoantibodies are at increased risk for cancer (24), frequently experience calcinosis (25), and rarely have muscle biopsies with muscle fibers surrounded and invaded by lymphocytes (i.e., primary inflammation)(26). In contrast, Mi-2 positive DM patients have a relatively low risk of cancer, rarely have calcinosis(27), and frequently have examples of primary inflammation on muscle biopsy(26). Taken together, these recent findings suggest that autoantibodies may provide a better means of categorizing myositis patients into homogeneous groups than currently accepted classification schemes recognizing only DM, PM, and IMNM as distinct disease categories.
This study has a number of limitations. First, although this is the largest single-center cohort study of anti-SRP myositis published to date, the relatively small number of patients with this exceptionally rare disease may have limited our power to detect clinically significant associations, such as the role of race and sex as prognostic factors. Second, as this study included only patients seen in an adult myositis clinic, this prevents us from extending our findings to the pediatric population. Third, some patients were included in the study more than 10 years ago and others had limited follow-up, which made the use of mixed regression models necessary to make unbiased analysis of the data and prevented us from using newer tools to define improvement (9, 11) or muscle strength (9). Fourth, as has been discussed, the study design prevented us from assessing the efficacy of many of the drugs and their combinations in an unbiased manner.
Notwithstanding these limitations, our study provides valuable information about anti-SRP myositis, including that younger patients have a worse outcome, many patients continue to have active disease even after treatment restores a significant degree of muscle strength, and that rituximab may be an effective treatment. Moreover, this study demonstrates that anti-SRP patients are weaker than anti-HMGCR patients; this suggests that these two autoantibodies may define different clinical entities. Future studies will be required to demonstrate whether earlier, more aggressive immunosuppressive regimens including rituximab, or other novel agents, might improve outcomes, especially among younger anti-SRP positive patients.
SIGNIFICANCE AND INNOVATIONS.
-
-
In anti-SRP myositis, a younger age at disease onset is associated with more severe weakness.
-
-
Even among anti-SRP myositis patients with markedly improved strength, most have active disease with persistently high CK levels.
-
-
Rituximab may be an effective treatment for anti-SRP-associated myositis.
-
-
Anti-SRP patients are weaker than anti-HMGCR patients, suggesting that IMNM includes at least two distinct forms of myositis associated with these two autoantibodies.
ACKNOWLEDGMENTS
None
FUNDING: This research was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. The Johns Hopkins Rheumatic Disease Research Core Center, where the autoantibody assays were performed, is supported by NIH grant P30-AR-053503. LC-S, SKD and the Myositis Research Database are supported by the The Huayi and Siuling Zhang Discovery Fund.
Footnotes
COMPETING INTERESTS: None
REFERENCES
- 1.Dalakas MC. Inflammatory Muscle Diseases. N Engl J Med. 2015;373:393–4. doi: 10.1056/NEJMc1506827. [DOI] [PubMed] [Google Scholar]
- 2.Reeves WH, Nigam SK, Blobel G. Human autoantibodies reactive with the signal-recognition particle. Proc Natl Acad Sci U S A. 1986;83:9507–11. doi: 10.1073/pnas.83.24.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990;33:1361–70. doi: 10.1002/art.1780330908. [DOI] [PubMed] [Google Scholar]
- 4.Miller T, Al-Lozi MT, Lopate G, Pestronk A. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry. 2002;73:420–8. doi: 10.1136/jnnp.73.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hengstman GJ, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J, et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis. 2006;65:1635–8. doi: 10.1136/ard.2006.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14:337–45. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Werner JL, Christopher-Stine L, Ghazarian SR, Pak KS, Kus JE, Daya NR, et al. Antibody levels correlate with creatine kinase levels and strength in anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Rheum. 2012;64:4087–93. doi: 10.1002/art.34673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valiyil R, Casciola-Rosen L, Hong G, Mammen A, Christopher-Stine L. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res (Hoboken) 2010;62:1328–34. doi: 10.1002/acr.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rider LG, Werth VP, Huber AM, Alexanderson H, Rao AP, Ruperto N, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S118–57. doi: 10.1002/acr.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senecal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore) 2005;84:231–49. doi: 10.1097/01.md.0000173991.74008.b0. [DOI] [PubMed] [Google Scholar]
- 11.Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314–24. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki S, Nishikawa A, Kuwana M, Nishimura H, Watanabe Y, Nakahara J, et al. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis. 2015;10:61. doi: 10.1186/s13023-015-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benveniste O, Drouot L, Jouen F, Charuel JL, Bloch-Queyrat C, Behin A, et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 2011;63:1961–71. doi: 10.1002/art.30344. [DOI] [PubMed] [Google Scholar]
- 14.Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore) 1977;56:255–86. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Koh ET, Seow A, Ong B, Ratnagopal P, Tjia H, Chng HH. Adult onset polymyositis/dermatomyositis: clinical and laboratory features and treatment response in 75 patients. Ann Rheum Dis. 1993;52:857–61. doi: 10.1136/ard.52.12.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilley H, Dennett X, Byrne E. Biopsy proven polymyositis in Victoria 1982–1987: analysis of prognostic factors. J R Soc Med. 1994;87:323–6. doi: 10.1177/014107689408700608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie I, Hatron PY, Levesque H, Hachulla E, Hellot MF, Michon-Pasturel U, et al. Influence of age on characteristics of polymyositis and dermatomyositis in adults. Medicine (Baltimore) 1999;78:139–47. doi: 10.1097/00005792-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Marie I, Hachulla E, Hatron PY, Hellot MF, Levesque H, Devulder B, et al. Polymyositis and dermatomyositis: short term and longterm outcome, and predictive factors of prognosis. J Rheumatol. 2001;28:2230–7. [PubMed] [Google Scholar]
- 19.Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum. 2004;50:209–15. doi: 10.1002/art.11484. [DOI] [PubMed] [Google Scholar]
- 20.Bronner IM, Hoogendijk JE, Wintzen AR, van der Meulen MF, Linssen WH, Wokke JH, et al. Necrotising myopathy, an unusual presentation of a steroid-responsive myopathy. J Neurol. 2003;250:480–5. doi: 10.1007/s00415-003-1027-y. [DOI] [PubMed] [Google Scholar]
- 21.Smith B. Skeletal muscle necrosis associated with cainoma. J Pathol. 1969;97:207–10. doi: 10.1002/path.1710970204. [DOI] [PubMed] [Google Scholar]
- 22.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–21. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 24.Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013;65:2954–62. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenzuela A, Chung L, Casciola-Rosen L, Fiorentino D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 2014;150:724–9. doi: 10.1001/jamadermatol.2013.10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinal-Fernandez I, Casciola-Rosen LA, Christopher-Stine L, Corse AM, Mammen AL. The Prevalence of Individual Histopathologic Features Varies according to Autoantibody Status in Muscle Biopsies from Patients with Dermatomyositis. J Rheumatol. 2015;42:1448–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Casciola-Rosen L, Mammen AL. Myositis autoantibodies. Curr Opin Rheumatol. 2012;24:602–8. doi: 10.1097/BOR.0b013e328358bd85. [DOI] [PMC free article] [PubMed] [Google Scholar]