Abstract
Background
Major depressive disorder (MDD) has been associated with changes in mean telomere length and mitochondrial DNA (mtDNA) copy number. This study investigates if clinical features of MDD differentially impact these molecular markers.
Methods
Data from a large, clinically ascertained sample of Han Chinese women with recurrent MDD were used to examine whether symptom presentation, severity, and comorbidity were related to salivary telomere length and/or mtDNA copy number (maximum N=5284 for both molecular and phenotypic data).
Results
Structural equation modeling revealed that duration of longest episode was positively associated with mtDNA copy number, while earlier age of onset of most severe episode and a history of dysthymia were associated with shorter telomeres. Other factors, such as symptom presentation, family history of depression, and other comorbid internalizing disorders, were not associated with these molecular markers.
Conclusions
Chronicity of depressive symptoms is related to more pronounced telomere shortening and increased mtDNA copy number among individuals with a history of recurrent MDD. As these molecular markers have previously been implicated in physiological aging and morbidity, individuals who experience prolonged depressive symptoms are potentially at greater risk of adverse medical outcomes.
Keywords: depression, biological markers, genetics, international, dysthymic disorder
Introduction
Major depressive disorder (MDD) is a common psychiatric disease that incurs substantial personal, social, and economic costs.[1] It is moderately genetically influenced, with a heritability estimate of 37%,[2] with the balance of risk attributable to environmental factors. Previous studies have demonstrated that MDD is associated with molecular markers beyond DNA sequence variation, including telomere length and quantity of mitochondrial DNA (mtDNA) (e.g., [3]), molecular changes that have functional consequences on cellular physiology. Clarifying the relationships between these molecular markers and MDD symptoms may improve our understanding of the pathology of MDD. Currently, there is a relative paucity of data on the relationship between clinical characteristics of MDD and telomere length or mtDNA in large well-powered samples.
Telomere length declines with age as a function of cell division [4], and telomere shortening has been associated with age-related medical conditions such as heart disease and cancer,[5] as well as with psychiatric disorders such as MDD, as described in a recent meta-analysis.[6] However, aging per se is not the only determinant of telomere length. Prior studies have reported shorter telomeres under circumstances of greater chronicity of stress,[7] higher degree of perceived stress,[8] cumulative childhood stress exposure,[9] longer exposure to depression,[10; 11] and depression severity.[8; 10; 12] Given that these associations remain after controlling for age, telomere length is perhaps more accurately conceptualized as a marker of biological rather than chronological aging.[13] Several of these studies’ statistical power was limited by small sample sizes (N<300).[7-9]
The association between mtDNA and depression is inconsistent, with some studies reporting an increase in mtDNA among MDD cases,[3; 8] another reporting the opposite,[14] and another reporting no association;[15] the latter two studies had limited sample sizes. Few studies are available on clinical characteristics of MDD and their relationship with mtDNA, though Tyrka et al. [8], using a modest sample, reported no association between mtDNA copy number and depressive symptoms or perceived stress.
A previous study of the current sample examined telomere length and mtDNA copy number with respect to MDD case-control status.[3] The authors reported shorter telomeres and more abundant mtDNA among cases of MDD, and demonstrated that these associations were independent of a history of childhood sexual abuse and other stressful life events. These findings suggests that shorter telomeres and more abundant mtDNA may in part be contingent on the depressive condition itself rather than being predominantly due to the experience of external stressors. However, stressful life events certainly still serve as significant precipitating risk factors for the onset of MDD. Further analyses, based on a mouse model, raised the possibility that changes to these molecular markers are potentially state-dependent, i.e., a molecular recovery might be observed among individuals whose MDD remits.
The current study uses a structural equation modeling (SEM) approach designed to test the hypotheses that telomere length and mtDNA copy number may differ within MDD cases as a function of the clinical presentation of the disorder. Specifically, individuals with more severe or chronic characteristics of depression may exhibit more pronounced telomeric loss and/or higher mtDNA copy number due to the detrimental impact of sustained psychological distress. We test these hypotheses by examining the association between a variety of clinical characteristics of MDD and both mean telomere length and mtDNA copy number in a sample of Chinese women with recurrent MDD.
Methods and Materials
Sample
The current study draws upon participants in the China, Oxford and Virginia Commonwealth University Experimental Research on Genetic Epidemiology (CONVERGE) sample. Cases in the CONVERGE study were collected across 58 mental health centers and psychiatric departments in 23 Chinese provinces, were at least 30 years of age, had a history of recurrent MDD, and were of Han Chinese descent (all four grandparents were born in China). Controls were recruited from patients undergoing minor surgery at general hospitals; for this study, only MDD cases were included. Participants provided saliva samples for DNA extraction, as described previously.[3; 16]
All cases were interviewed by trained personnel, using a computerized assessment system that determined MDD status based on the Composite International Diagnostic Interview (CIDI) (WHO lifetime version 2.1; Chinese version), which used DSM-IV diagnostic criteria. After quality control filters were applied, outcome data was available for 5284 individuals.
The study protocol was approved by the Ethical Review Board of Oxford University and by participating data collection sites. All participants provided written informed consent.
Potential predictors
We selected potential predictors based on their relevance to: i) symptom-based clinical presentation, ii) general severity of MDD, or iii) comorbidity. With respect to symptom-based clinical presentation, 27 MDD-related items from the interview were included in an initial exploratory factor analysis. These items correspond to disaggregated MDD symptoms, e.g., loss of interest, trouble concentrating, loss of weight, increase in appetite, etc.,[17] and were subjected to an initial exploratory factor analysis. Items were retained for the comprehensive model as appropriate. To assess general severity of MDD, we included: age at onset, age at worst episode, number of episodes, symptom count during worst episode, duration of longest episode, and number of family members with a history of depression. Finally, with respect to comorbidity, we included a history of generalized anxiety disorder (GAD), phobia (any), panic disorder, dysthymia, and postnatal depression. Diagnoses were established with the CIDI (WHO lifetime version 2.1; Chinese version) as previously described.[18] Stressful life events were assessed as has been described previously.[19] Briefly, participants were asked whether they had ever experienced any of 16 traumatic life events (see Supplementary Material), which have been previously assessed for their association with MDD in CONVERGE.[19] A summary score was assigned to participants to capture the number of events experienced.
Mitochondrial copy number and telomeric length outcomes
DNA was prepared and sequenced and mitochondrial DNA (mtDNA) copy number and telomere lengths were estimated as previously described.[3] As only cases were included in the current analyses, transformations of raw mtDNA and telomere length estimates were conducted within cases only, in contrast to Cai et al. [3] Prior to transformations, age was negatively correlated with both mean mtDNA (Pearson's r= −0.0352, p=0.01) and telomere length (r= −0.0833, p=1.32×10−9). For mtDNA copy number, the initial estimates were corrected/adjusted for batch, mean read depth on chromosome 20, sample age, and five ancestry principle components using linear regression. We then transformed the residuals using a quantile normal function in R. Mean telomere length was treated similarly, correcting for batch, sample age, and the five ancestry principle components, then transforming the residuals to normality.
Data analysis
Exploratory Factor Analysis and Item Analysis
To identify the main dimensional features of the MDD symptom criteria item set, exploratory and item level analyses were conducted. An initial exploratory factor analysis (EFA) of the 27 dichotomously coded (0=absent, 1=present) MDD symptom criteria items was carried out. Based on the model fits for solutions extracting from 1 to 6 factors, additional confirmatory item level factor analyses were conducted to identify sets of items that performed well in defining each of the MDD clinical features factors. These analyses focused on estimating and evaluating the threshold and factor loading item characteristics for each MDD related item. These psychometric considerations provide the basis for calibrating individual differences on each of the MDD clinical feature factors that will be included in the full SEM model for predicting mitochondrial copy number and telomere length.
Comprehensive Structural Model
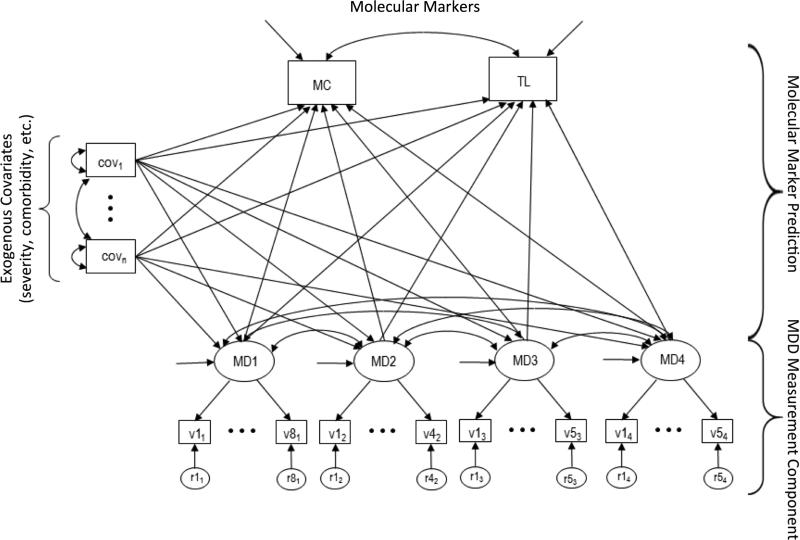
To investigate the relationships between the clinical features of MDD and the variation in telomere length and mitochondrial copy number count, a latent variable SEM was developed. The full model can be organized around 3 main components: (1) The transformed mtDNA copy number and telomere length variables were included as joint outcomes; (2) a measurement model is specified for four MDD factors and their binary indicators, and (3) a set of selected covariate predictors (described above). All modeling was performed using Mplus version 7.31.[20] Because the MDD factor item indicators were binary, the weighted least squares mean and variance (WLSMV) adjusted robust estimator available in Mplus was used. In this application, the binary indicator variables are treated categorical. For each indicator an unobserved continuous response variable is assumed on which a single threshold is estimated. Telomere length and mtDNA were regressed onto each of the four symptom-based latent factors determined from the EFA results and item analyses (see below). Both the latent MDD factors and DNA based outcomes were regressed onto the other relevant covariate predictors. These included each of the 6 measures of severity and the 5 comorbid conditions. Outcome variables (transformed mtDNA and telomere length) were allowed to correlate. Inter-factor correlations among the four MDD factors were also allowed. All correlations amongst the included set of covariate predictors were freely estimated. A schematic of the full model is provided in the Figure. Due to the manner in which Mplus handles missing data with the WLSMV estimator, the final sample size for the comprehensive model was 4078 cases.
Figure.
General structure of the comprehensive model predicting telomere length and mtDNA amount. MD1-MD4 represent the symptom-based factors described in the main text; each loads onto multiple items. Covariates related to comorbidity, severity, etc., are represented by cov1-covn. MC and TL represent the mtDNA count and telomere length outcomes, respectively. Double-headed arrows represent correlations; single-headed arrows between variables represent regression paths.
Results
Factor model of depressive symptoms
Initial EFA model fitting for the 27 MDD related symptom criteria items converged on a reasonably fitting and interpretable four-factor solution (Comparative Fit Index [CFI] = .965, Tucker-Lewis Index [TLI] = .951, root mean square error of approximation [RMSEA] = .03) (Supplementary Table 1). However, this oblique four-factor solution was complex in that significant cross-loadings for some items were present (Supplementary Table 2). Additional confirmatory factor analysis (CFA) model fitting was carried out to identify items that reasonably fit a simple structure model (i.e., each symptom criterion item being an indicator for only one factor) for each of the four MDD factors. Item characteristics were examined and used to select items for the final measurement model. Items that varied in their threshold locations (i.e., optimal point of discrimination on the latent factor) and showed good discrimination (i.e., peaked information curves indicates by higher factor loadings) were retained for the four-factor MDD measurement model. This item selection process resulted in dropping 5 of the original 27 MDD related items and some choices for items having significant cross-loadings. This more restrictive simple structure CFA measurement model resulted in some reduction in overall model fit compare to the more highly parameterized EFA fit (CFI = .901, TLI = .890, RMSEA = 0.045). Based on factor loadings for the final simple structure four-factor measurement model, the following descriptive labels were assigned to the MDD factors; a general depression factor (MDF1); a weight/appetite factor (MDF2); a Beck like factor (MDF3), after Beck's cognitive theory of depression;[21] and an agitation/anxiety factor (MDF4).
Comprehensive model predicting mtDNA copy number and telomere length
In the full structural model, depicted in the Figure, the telomere length and mtDNA copy number were regressed onto each of the four MDD factors with the chosen covariate set allowed to have direct effects on both the molecular markers and MDD factors simultaneously. The full model contained a total of 133 parameters, which included factor loadings, item thresholds (measurement model), and directed prediction effects (structural model). The molecular markers were significantly correlated with one another (r= −.277, p<0.001; shorter telomeres were associated with higher mtDNA copy number). The MDD symptom factors were significantly correlated with one another (r= −.068-.364, p= <.001-0.020), with the exception of the weight/appetite and agitation/anxiety factors (r=.050, p=.185).
None of the linear effects of the 4 symptom factors predicting either mtDNA copy number or telomere length while simultaneously taking into account the various severity covariates (e.g., age of onset, number of episodes, etc.) were significant at the p ≤ .05 level. With respect to severity-related covariates, duration of longest depressive episode significantly and positively predicted mtDNA copy number, and earlier age of onset of worst depressive episode was significantly associated with shorter telomeres (Table). Finally, dysthymia significantly (p=0.01) predicted telomere length, such that MDD cases with a history of dysthymia had, on average, shorter telomere lengths compared to those without a history of dysthymia. A complete description of path estimates is available in Supplementary Table 3. In general, the prediction effects sizes were relatively small for both mtDNA copy number and telomere length as indicated by their R2 values (~0.01 for both mtDNA copy number and telomere length).
Table.
Standardized path estimates, standard errors, and nominal p-values for predictors of normalized telomere length and mtDNA copy number.
| Telomere Lenath | mtDNA count | ||||||
|---|---|---|---|---|---|---|---|
| Predictor | N | Estimate | SEa | p-value | Estimate | SE | p-value |
| Symptom Presentation | |||||||
| General depression | 5284 | 0.038 | 0.030 | 0.211 | -0.035 | 0.030 | 0.241 |
| Weight/Appetite | 5284 | 0.021 | 0.023 | 0.363 | 0.002 | 0.024 | 0.940 |
| Beck symptoms | 5284 | 0.034 | 0.022 | 0.125 | -0.015 | 0.022 | 0.493 |
| Agitation/Anxiety | 5284 | -0.047 | 0.031 | 0.131 | -0.002 | 0.031 | 0.952 |
| Severity | |||||||
| Age at onset | 5210 | -0.004 | 0.002 | 0.127 | 0.000 | 0.002 | 0.903 |
| Age at worst episode | 5173 | 0.007 | 0.003 | 0.032 | 0.001 | 0.003 | 0.836 |
| No. episodes | 5230 | -0.003 | 0.021 | 0.899 | 0.017 | 0.021 | 0.410 |
| Symptom count | 4579 | -0.021 | 0.039 | 0.596 | 0.032 | 0.037 | 0.389 |
| Duration | 5233 | -0.010 | 0.013 | 0.437 | 0.032 | 0.013 | 0.014 |
| FH count | 5171 | 0.009 | 0.010 | 0.397 | -0.012 | 0.010 | 0.221 |
| Comorbidity | |||||||
| Panic disorder | 5207 | 0.051 | 0.065 | 0.439 | 0.045 | 0.066 | 0.492 |
| GAD | 5222 | -0.005 | 0.039 | 0.903 | 0.037 | 0.038 | 0.334 |
| Dysthymia | 5244 | -0.136 | 0.053 | 0.010 | 0.071 | 0.052 | 0.169 |
| Postnatal depression | 4748 | 0.009 | 0.045 | 0.836 | 0.027 | 0.045 | 0.546 |
| Phobia | 5194 | -0.001 | 0.034 | 0.969 | 0.057 | 0.034 | 0.095 |
SE=standard error
Post-hoc analyses
Only a subset of the available measures of chronicity was associated with the molecular markers, potentially due to associations among predictors (i.e., perhaps the observed effect of dysthymia functions as a proxy for the other measures). Therefore, we tested whether dysthymia was associated with these other measures of chronicity. We found that cases with a history of dysthymia had experienced significantly more MDD episodes (8.6 vs. 4.8, p<0.0001), a longer duration episode (77.3 vs. 43.4 weeks, p<0.0001), and earlier age of onset (30.7 vs. 35.3 years, p<0.0001).
Finally, we considered the possibility that stressful life events (SLEs) might be related to molecular markers. We therefore conducted an analysis in which SLE was included as a predictor in the SEM (note that the sample size decreased in this model from N=4078 to N=3805). Model fit was nearly identical, SLE was not associated with mtDNA copy number or telomere length, and the previously associated predictors’ effects remained consistent and significant.
Discussion
The current study examined relationships between clinical characteristics of MDD and molecular markers, namely telomere length and mtDNA copy number, in a large sample of Chinese women with severe, recurrent MDD. We report modest but significant associations between chronicity of MDD and the molecular markers: dysthymia and age at worst episode were associated with telomere length (p=0.010 and p=0.032, respectively), and duration of longest episode was associated with mtDNA amount (p=0.014). These findings suggest that the molecular changes in mtDNA and telomeres could be related to, or even consequences of, particular aspects of MDD. Previous evidence in this sample indicates that shortened telomeres and increase mtDNA copy number are consequences, rather than predictors, of MDD.[3] To the extent that these molecular markers are potentially modifiable[22] and may impact prognosis, these results provide a nascent framework for considering the physiological consequences of MDD.
Our findings are largely consistent with prior studies of the relationship between MDD (or stress exposure) and telomere length. Though exceptions have been reported [23], a preponderance of evidence indicates that more severe or longer-lasting MDD or stress is associated with shorter telomeres.[7; 8; 10; 11] The current results, which benefit from a far larger sample size than previous studies, implicate chronicity in particular: variables that are characterized by their chronic and persistent nature such as dysthymia were predictors of telomere shortening. However, some other factors that could be considered markers of chronicity – e.g., number of episodes, duration of longest episode – were also not associated with telomere length. This is possibly due to the strong associations between dysthymia and other measures of chronicity. Thus, we might generally expect indicators of chronicity to be implicated across studies, though the specific predictor may differ.
The observed relationship between duration of longest depressive episode and mtDNA copy number is of note because the current sample size is the largest (to our knowledge) to have been assessed for such a relationship. Results from previous studies have been equivocal, and often based on small samples with less severe MDD. While there is some evidence that perturbed mitochondrial functioning is correlated with MDD (or stress),[24] data are inconsistent with regards to whether MDD is associated with increased or decreased mtDNA copy number.[8; 14; 15] Furthermore, very little prior research has been conducted examining clinical characteristics of MDD and their association with mtDNA copy number.
With the current results, we speculate that the relative increase in mtDNA copy number represents a physiological response to the prolonged experience of depressive symptoms, as was the case with shortening of telomeres. As mitochondrial functioning declines with age,[25] due in part to oxidative stress,[26] mtDNA content has been found to increase.[27] Increased oxidative stress has been reported in MDD and other psychiatric and psychological conditions,[28] and it has been proposed that this physiological stressor accelerates cellular aging, the molecular manifestations of which include increased mtDNA.[29] It was also suggested that increase in mtDNA copy number could be a compensatory response to inefficiencies or dysfunction in mitochondrial machinery. For instance, mtDNA increase in autistic subjects was accompanied by a reduction in mitochondrial function.[30] One or both of these mechanisms may explain the increase in mtDNA copy number in chronic stress, though the current study is not designed to test such mechanisms.
Cai and colleagues[3] reported on the relationship among these molecular markers, MDD case-control status, and SLE in the full CONVERGE sample. A series of conditional regression analyses suggested that the predictive power of SLE on molecular markers was mediated through MDD. Nonetheless, we considered the possibility that SLE might impact the current results, and respecified the model including SLE as a predictor in the SEM. That there were no substantive changes to the results suggests that the current findings are effectively independent of SLE, raising the possibility that the association between stress and telomere length or mtDNA in previous studies may be due to another factor not included in those models. For example, it is possible that previously observed associations between SLEs/stress and molecular markers are largely indirect, and would be mediated by psychopathological measures if such measures were included in the model. Still, SLEs may be a critical component of the causal pathway to perturbation of molecular markers: indeed, they are robust predictors of MDD.
Cai et al. [3] further reported that telomere length and mtDNA copy number were perturbed in mice exposed to stressful circumstances (e.g., tail suspension, forced swim), but returned to baseline after a recovery period. These results raise the possibility that the same molecular markers in humans can recover after MDD remits. We were unable to test this hypothesis due to the absence of data on onset and remission of specific depressive episodes. However, there is prior evidence for telomere lengthening over time in humans;[22] indeed, a non-trivial proportion (~15-25%) of individuals exhibit lengthening over several years.[31-33] Furthermore, telomerase activity has been shown to increase with reduction of psychological distress,[34-37] which could mitigate telomere attrition. Many of these studies were conducted using blood cells, which are a component of saliva samples (which were used in the current study).
Few studies have explored stress-induced changes in mtDNA copy number, though there is some evidence that it is modifiable;[38] more studies are needed. Whether telomere lengthening and/or normalization of mtDNA copy number could be reliably induced via behavioral or pharmacological interventions is of utmost clinical interest: if cellular aging is the consequence of MDD and the cause of medical morbidity, then interventions along the pathways leading to cellular aging may be useful in mitigating that morbidity.
Limitations
The current study is not without limitations. First, telomere length and mtDNA copy number were assessed cross-sectionally, precluding the ability to determine whether differences existed prior to MDD and/or the clinical characteristics used as predictors. However, longitudinal studies strongly suggest that these molecular markers change as a function of risk exposure rather than as a predictor of risk exposure. Second, all cases are severely affected – for example, over 76% of cases endorsed all nine MDD criteria – thus limiting our ability to examine whether variation in severity was associated with the outcomes. More broadly, the use of a stringently ascertained sample has implications for detection of effects under circumstances of reduced variation, and for generalizability to other, less severely affected, samples. Third, we extracted DNA from saliva samples. Most previous studies have used blood samples, and comparability across the sample types is unknown, though leukocyte telomere length is correlated with telomere length in target tissues for other complex diseases,[5] and mtDNA in blood and saliva behave similarly.[3] Furthermore, we have previously provided evidence [3] that case-control differences in mtDNA are unlikely to be due to discrepancies in cellular composition, but it remains possible that such variation might impact the current findings. Fourth, the mechanism by which changes in telomere length/mtDNA in peripheral cells are relevant to MDD has not yet been determined. These changes are unlikely to be representative of telomere length in brain regions relevant to MDD, given differences between peripheral tissues and neurons. Data available in CONVERGE do not have the capacity to explore this question. Fifth, we report nominal p-values without correcting for preliminary tests that informed the final model structure. Finally, the clinical characteristics included here accounted for only a small proportion of the total variance in mtDNA copy number and telomere length; other critical factors must be considered in future analyses.
In spite of these limitations, the current study provides evidence that the chronicity of MDD impacts telomere shortening and mtDNA copy number, using one of the largest samples assessed to date. To the extent that these molecular markers in turn impact morbidity and mortality,[5] this implies that the chronicity of MDD affects medical outcomes to which it might not otherwise be related via shared genetic or environmental risk. The present findings provide context for future work exploring the consequences of changes in molecular markers on subsequent MDD (e.g., remittance time course), other psychopathology, and aging-related medical outcomes.
Supplementary Material
Acknowledgments
This work was funded by the Wellcome Trust (WT090532/Z/09/Z, WT083573/Z/07/Z, WT089269/Z/09/Z) and the National Institutes of Health (MH100549 to KSK; AA021399 to ACE; ARD and REP are supported by MH020030). All authors are part of the China, Oxford and VCU Experimental Research on Genetic Epidemiology (CONVERGE) consortium and gratefully acknowledge the support of all partners in hospitals across China. N.C. is supported by the Agency of Science, Technology and Research (A*STAR) Graduate Academy. Research was in part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London.
References
- 1.Greenberg PE, Kessler RC, Birnbaum HG, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–75. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–62. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 3.Cai N, Chang S, Li Y, et al. Molecular signatures of major depression. Curr Biol. 2015;25(9):1146–56. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579(4):859–62. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Price LH, Kao HT, Burgers DE, et al. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32(4):229–38. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- 7.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyrka AR, Parade SH, Price LH, et al. Alterations of Mitochondrial DNA Copy Number and Telomere Length with Early Adversity and Psychopathology. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalev I, Moffitt TE, Sugden K, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013;18(5):576–81. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhoeven JE, Revesz D, Epel ES, et al. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry. 2014;19(8):895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- 11.Wolkowitz OM, Mellon SH, Epel ES, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011;6(3):e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Needham BL, Mezuk B, Bareis N, et al. Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindqvist D, Epel ES, Mellon SH, et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–64. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MY, Lee JW, Kang HC, et al. Leukocyte mitochondrial DNA (mtDNA) content is associated with depression in old women. Arch Gerontol Geriatr. 2011;53(2):e218–21. doi: 10.1016/j.archger.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 15.He Y, Tang J, Li Z, et al. Leukocyte mitochondrial DNA copy number in blood is not associated with major depressive disorder in young adults. PLoS One. 2014;9(5):e96869. doi: 10.1371/journal.pone.0096869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CONVERGE Consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015 doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Aggen S, Shi S, et al. The structure of the symptoms of major depression: exploratory and confirmatory factor analysis in depressed Han Chinese women. Psychol Med. 2014;44(7):1391–401. doi: 10.1017/S003329171300192X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Aggen S, Shi S, et al. Subtypes of major depression: latent class analysis in depressed Han Chinese women. Psychol Med. 2014:1–14. doi: 10.1017/S0033291714000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao M, Li Y, Xie D, et al. Examining the relationship between lifetime stressful life events and the onset of major depression in Chinese women. J Affect Disord. 2011;135(1-3):95–9. doi: 10.1016/j.jad.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthén BO, Muthén LK. Mplus User's Guide. Seventh Edition Los Angeles, CA: 2012. [Google Scholar]
- 21.Beck AT. Cognitive therapy of depression. Guilford Press; New York: 1979. [Google Scholar]
- 22.Epel E. How “reversible” is telomeric aging? Cancer Prev Res (Phila) 2012;5(10):1163–8. doi: 10.1158/1940-6207.CAPR-12-0370. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27(12):1111–6. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- 24.Manji H, Kato T, Di Prospero NA, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13(5):293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 25.Kopsidas G, Kovalenko SA, Heffernan DR, et al. Tissue mitochondrial DNA changes. A stochastic system. Ann N Y Acad Sci. 2000;908:226–43. doi: 10.1111/j.1749-6632.2000.tb06650.x. [DOI] [PubMed] [Google Scholar]
- 26.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 27.Barrientos A, Casademont J, Cardellach F, et al. Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Brain Res Mol Brain Res. 1997;52(2):284–9. doi: 10.1016/s0169-328x(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 28.Maurya PK, Noto C, Rizzo LB, et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19(11):1156–62. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu F, Chauhan V, Kaur K, et al. Alterations in mitochondrial DNA copy number and the activities of electron transport chain complexes and pyruvate dehydrogenase in the frontal cortex from subjects with autism. Transl Psychiatry. 2013;3:e299. doi: 10.1038/tp.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W, Kimura M, Kim S, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66(3):312–9. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farzaneh-Far R, Lin J, Epel E, et al. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010;5(1):e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–57. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 35.Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917–28. doi: 10.1016/j.psyneuen.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57–65. doi: 10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664–81. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Menshikova EV, Ritov VB, Fairfull L, et al. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–40. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.