Abstract
Adhesion turnover is critical for cell motility and invasion. We previously demonstrated that the adaptor molecule Breast Cancer Antiestrogen Resistance 3 (BCAR3) promotes adhesion disassembly and breast tumor cell invasion. One of two established binding partners of BCAR3 is the adaptor molecule, p130Cas. In this study, we sought to determine whether signaling through the BCAR3/Cas complex was responsible for the cellular functions of BCAR3. We show that the entire pool of BCAR3 is in complex with Cas in invasive breast tumor cells and that these proteins co-localize in dynamic cellular adhesions. While accumulation of BCAR3 in adhesions did not require Cas binding, a direct interaction between BCAR3 and Cas was necessary for efficient dissociation of BCAR3 from adhesions. The dissociation rates of Cas and two other adhesion molecules, α-actinin and talin, were also significantly slower in the presence of a Cas-binding mutant of BCAR3, suggesting that turnover of the entire adhesion complex was delayed under these conditions. As was the case for adhesion turnover, BCAR3-Cas interactions were found to be important for BCAR3-mediated breast tumor cell chemotaxis toward serum and invasion in Matrigel. Previous work demonstrated that BCAR3 is a potent activator of Rac1, which in turn is an important regulator of adhesion dynamics and invasion. However, in contrast to wildtype BCAR3, ectopic expression of the Cas-binding mutant of BCAR3 failed to induce Rac1 activity in breast cancer cells. Together, these data show that the ability of BCAR3 to promote adhesion disassembly, tumor cell migration and invasion, and Rac1 activity is dependent on its ability to bind to Cas. The activity of BCAR3-Cas complexes as a functional unit in breast cancer is further supported by the co-expression of these molecules in multiple subtypes of human breast tumors.
Keywords: Adhesion turnover, focal adhesion, breast cancer cell motility
INTRODUCTION
Cell motility is an essential feature of processes involved in development and tissue repair as well as in pathological states such as inflammation and cancer. A signaling node comprised of the adaptor molecules Breast Cancer Antiestrogen Resistance 3 (BCAR3) and p130Cas (Cas; also known as BCAR1) has been established as a regulator of several aspects of motility, including cell protrusion, adhesion, migration and invasion.1–5 BCAR3 is a member of the novel SH2 domain-containing protein (NSP) family of adaptor molecules, and contains an N-terminal SH2 domain and a C-terminal guanine nucleotide exchange factor (GEF)-like domain with sequence homology to the Cdc25-family of Ras GEFs.6–8 These domains promote the interaction between BCAR3 and its two established binding partners, protein tyrosine phosphatase α (PTPα) and Cas, respectively.3,9 Cas contains multiple protein-interaction domains that contribute to its localization to focal adhesions and its activity as a regulator of cell motility.10
BCAR3 and Cas bind directly to one another at their C-termini. The C-terminal domain of BCAR3 adopts a “closed” conformation, which is not only necessary for its binding to Cas but also prevents the C terminus of BCAR3 from functioning as a GEF.9 BCAR3 association with Cas has been shown to stabilize each protein and enhance Cas/c-Src (Src) interactions, Src kinase activity, and Src-mediated Cas tyrosine phosphorylation.3,5,11
Previous work from our group and others showed that BCAR3 promotes migration and invasion in breast cancer cell lines.2,4 One of the first steps in cell migration is the formation of nascent adhesions at the leading edge of a migrating cell.12 These nascent adhesions can either undergo disassembly (turnover) or they mature into focal complexes and focal adhesions. Adhesion turnover is initiated when there is a lack of tension to reinforce the adhesion. This is mediated through adaptor molecules and kinases that function in adhesions to locally activate Rac1 and inhibit RhoA GTPase signaling, thereby reducing tension and promoting adhesion disassembly.13,14 In order for the cell to move forward, adhesions in the rear of the cell must also undergo disassembly. We have previously demonstrated that the adaptor molecule BCAR3 promotes Rac1 activity and adhesion disassembly in invasive breast cancer cells.4 However, the mechanism(s) through which BCAR3 contributes to these activities remained to be elucidated.
In this study, we sought to determine the role of the BCAR3/Cas complex in BCAR3-mediated adhesion dynamics, migration, and invasion of breast cancer cells. We found that all of the BCAR3 in invasive breast cancer cells is present in a complex with Cas and that both proteins co-localize in focal adhesions. BCAR3 entry into adhesions did not require a direct interaction with Cas or an intact SH2 domain. However, the kinetics of BCAR3 dissociation from adhesions was impaired in the absence of Cas binding. This paralleled a similar delay in the dissociation of other adhesion proteins, indicating that BCAR3/Cas interactions play an important role in adhesion complex disassembly. The BCAR3/Cas complex was also found to be important for BCAR3-dependent Rac1 activation, migration, and invasion in 3D matrices. Finally, BCAR3 and Cas were found to be co-expressed in multiple subtypes of human breast tumors. Collectively, these data highlight the importance of a functional BCAR3/Cas complex in invasive breast cancer cells.
RESULTS
The entire cellular pool of BCAR3 is in complex with Cas in invasive breast cancer cells
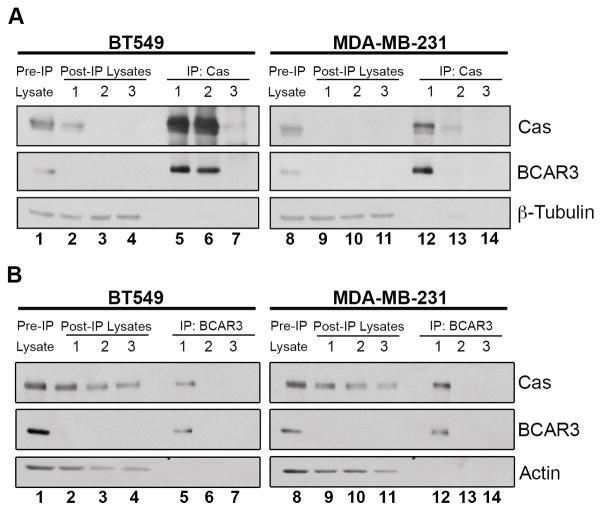
Given the evidence of a strong functional relationship between BCAR3 and Cas, we measured the steady-state levels of BCAR3/Cas complexes in invasive breast cancer cells. Lysates from BT549 and MDA-MB-231 cells were subjected to serial immunoprecipitations with either Cas or BCAR3 antibodies (Figure 1). BCAR3 was present in Cas immune complexes (Figure 1A, lanes 5–16 and 12–13) and coincidentally lost from the lysates following immune depletion of Cas (lanes 2–4 and 9–11), indicating that the majority of BCAR3 present in BT549 and MDA-MB-231 cells is in complex with Cas. In contrast, although Cas was also present in BCAR3 immune complexes (Figure 1B, lanes 5 and 12), significant amounts of Cas remained in the lysates following immune depletion of BCAR3 (lanes 2–4 and 9–11). Together, these data show that, while a substantial pool of Cas is free of BCAR3, the majority of BCAR3 in invasive breast cancer cells is in complex with Cas. Based on these dynamics, it is likely that the interaction between these molecules is critical for the biological functions of BCAR3.
Figure 1. The entire cellular pool of BCAR3 is in complex with Cas.
BT549 and MDA-MB-231 cell lysates were subjected to three serial Cas (A) or BCAR3 (B) immunoprecipitations (IP). Pre-IP lysates were separated on 8% SDS-PAGE (lanes 1 and 8) together with the proteins present in the IPs (lanes 5–7, 12–14) and post-IP lysates (2–4, 9–11). Proteins were immunoblotted with antibodies recognizing the designated proteins. The pre-IP lysate is 10% of the amount of protein used for the initial IP.
Localization of BCAR3 to adhesions does not require a functional SH2 domain or direct interaction with Cas
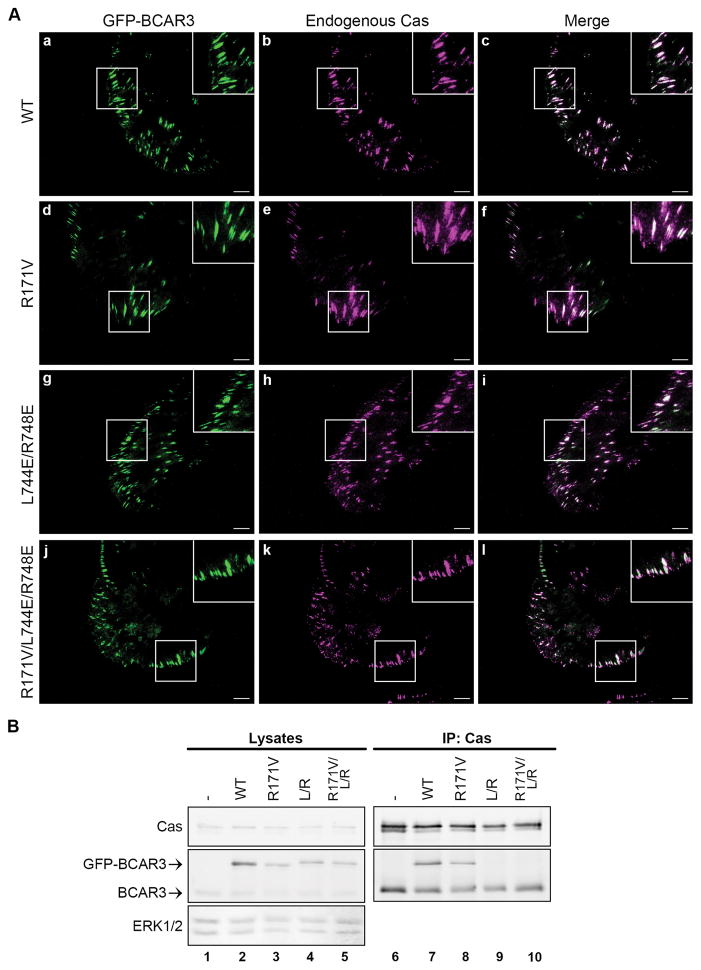
As discussed above, BCAR3 and Cas play substantial roles in motility and invasion. BCAR3 has been reported to localize to vinculin-containing adhesions in mouse embryo fibroblasts (MEFs).3 To determine whether BCAR3 also localizes to adhesions in human breast cancer cells, GFP-BCAR3 was expressed in BT549 invasive breast cancer cells and adhesions were visualized by total internal reflection fluorescence (TIRF) microscopy. Similar to MEFs, GFP-BCAR3 was present in adhesions in BT549 cells (Figure 2A, panel a). Additionally, GFP-BCAR3 co-localized with endogenous Cas in these adhesions (panels a–c).
Figure 2. BCAR3 localization in adhesions does not require a functional SH2 domain or interaction with Cas.
(A) BT549 cells were transfected with plasmids encoding WT GFP-BCAR3, R171V GFP-BCAR3, L744E/R748E GFP-BCAR3 or R171V/L744E/R748E GFP-BCAR3. Cells were incubated for 24 hours prior to plating on 10μg/ml fibronectin-coated coverslips for 4 hours. Cells were fixed, stained with polyclonal Cas antibodies (panels b, e, h, k), and subjected to TIRF microscopy to visualize adhesions. Merged images are shown in the right panels and insets show higher magnifications of the designated areas. (B) BT549 cells were transfected with plasmids encoding GFP, WT GFP-BCAR3, R171V GFP-BCAR3, L744E/R748E GFP-BCAR3 or R171V/L744E/R748E GFP-BCAR3 and lysed in a non-denaturing buffer 24 hours post-transfection. Total cell protein and Cas immune complexes (generated from 50X more protein than the lysates) were immunoblotted with antibodies to detect the indicated proteins. Left and right panels are identical exposures from the same film.
To determine which domains of BCAR3 are required for localization to adhesions, we generated functional domain mutants and expressed them in BT549 cells (Figure 2B). Since the SH2 domain was previously demonstrated to be critical for BCAR3 localization to adhesions in MEFs,3 we first investigated whether a mutant of this domain (R171V GFP-BCAR3) could localize to adhesions in breast cancer cells. This molecule was found to be present in adhesions and, like WT BCAR3, it co-localized with endogenous Cas (Figure 2A. panels d–f). This shows that, the SH2 domain of BCAR3 is not the sole determinant of adhesion targeting in breast cancer cells. Since a direct interaction between BCAR3 and Cas was reported to be important for their reciprocal stability,5 and all of the BCAR3 in these cells is bound to Cas (Figure 1), we next asked whether localization of BCAR3 to adhesions requires association with Cas. This was addressed using a BCAR3 molecule containing two point mutations, L744E and R748E, which were recently shown to prevent the interaction between BCAR3 and Cas.5 To verify that these point mutations abrogated Cas binding, Cas immune complexes were isolated from BT549 cells expressing WT GFP-BCAR3 or L744E/R748E GFP-BCAR3 (Figure 2B). As expected, endogenous BCAR3 (lower bands in lower panel, lanes 6–10) and WT GFP-BCAR3 (upper band, lane 7) were present in Cas immune complexes. However, L744E/R748E GFP-BCAR3 (L/R) failed to interact with Cas (lane 9). Despite the fact that this mutant was unable to bind to Cas, it was present in adhesions and co-localized with endogenous Cas (Figure 2A, panels g–i). This demonstrates that BCAR3 localization to adhesions does not require direct association with Cas.
While neither the SH2 domain nor the Cas-binding domain were found to be solely responsible for BCAR3 localization to adhesions, these data do not discount the possibility that both domains could contain adhesion-targeting activity. To test this, a triple BCAR3 mutant (R171V/L744E/R748E GFP-BCAR3) that lacks both a functional SH2 domain and the Cas-binding site was expressed in BT549 cells. This molecule failed to associate with Cas (Figure 2B, lane 10); however, as was the case for the individual mutants, the triple mutant was present in adhesions and co-localized with Cas (Figure 2A, panels j–l). Together, these data show that, even though PTPα (through the SH2 domain) and/or Cas (through the C-terminus) may facilitate BCAR3 localization to adhesions, other mechanisms must be available in the absence of these interactions to recruit BCAR3 to adhesion sites in breast cancer cells.
Direct interaction between BCAR3 and Cas is required for efficient adhesion disassembly in BT549 breast cancer cells
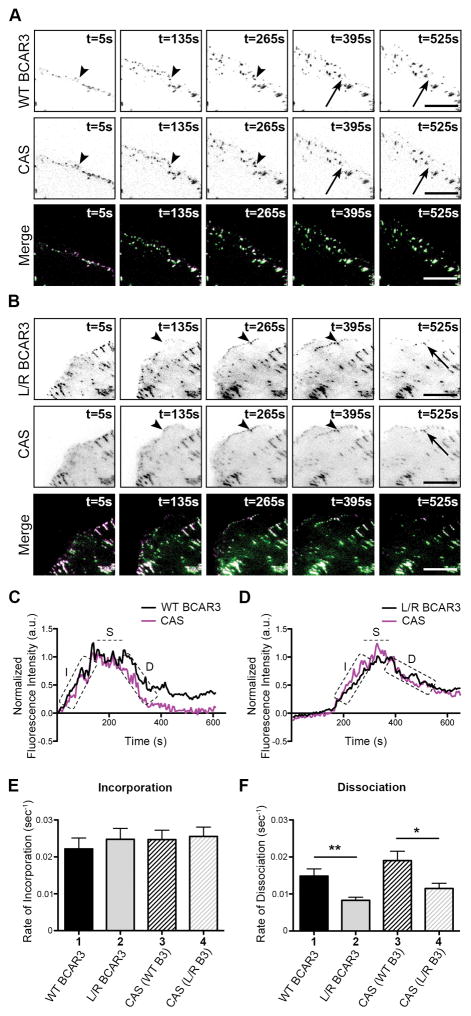
While BCAR3 localization to adhesions does not require a direct association with Cas, BCAR3 function may be dependent on this interaction. In a previous study, we demonstrated that BCAR3 promotes adhesion disassembly in invasive breast cancer cells.4 To test whether this function is dependent on BCAR3/Cas interactions, live TIRF imaging was performed on BT549 cells that were co-transfected with plasmids encoding mCherry-tagged Cas and either WT or L744E/R748E GFP-BCAR3. Under these conditions, both WT and L744E/R748E GFP-BCAR3 co-localized with Cas in dynamic adhesions (Figure 3). To quantify adhesion turnover, adhesions at peripheral, protruding edges of a cell were selected for analysis. Time-lapse images show incorporation (arrowheads) and dissociation (arrows) of BCAR3 and Cas into and from representative adhesions co-expressing Cas and either WT (Figure 3A) or L744E/R748E GFP-BCAR3 (Figure 3B). By measuring fluorescence intensity over time (Figures 3C and 3D), BCAR3 and Cas were found to incorporate into adhesions at similar rates (Figure 3E, compare bars 1 and 3). This was independent of the ability of BCAR3 to bind to Cas, as L744E/R748E GFP-BCAR3 entered adhesions at a rate similar to that of WT BCAR3 (compare bars 1 and 2). Moreover, when Cas was co-expressed with mutant BCAR3, it entered adhesions at a similar rate to when it was co-expressed with WT GFP-BCAR3 (compare bars 3 and 4). Together, these data demonstrate that BCAR3 can efficiently incorporate into adhesions without being directly bound to Cas.
Figure 3. Direct interaction between BCAR3 and Cas is required for efficient dissociation of BCAR3 from adhesions.
BT549 breast cancer cells were co-transfected with plasmids encoding WT or L744E/R748E (L/R) GFP-BCAR3 and mCherry-Cas, incubated for 24 hours, and then plated on 2μg/ml fibronectin-coated glass-bottomed TIRF dishes for 30–40 minutes prior to visualizing adhesion dynamics via live-imaging TIRF microscopy. (A, B) Representative time-lapse images show incorporation into adhesions (arrowheads) and dissociation (arrows) of the indicated proteins over the specified time course. Scale bars = 100μm. (C, D) Representative fluorescence intensity time tracings of BCAR3 (black) and Cas (magenta) present in adhesions from cells expressing WT (C) or L744ER748E (D) GFP-BCAR3. Dashed boxes/line indicate the incorporation (I), stability (S), and dissociation (D) phases of adhesion dynamics. (E, F) Quantitative analysis of the incorporation (E) and dissociation (F) rates of WT GFP-BCAR3 (bar 1), L744E/R748E (L/R) GFP-BCAR3 (bar 2), Cas co-expressed with WT GFP-BCAR3 (bar 3), and Cas co-expressed with L744E/R748E (L/R) GFP-BCAR3 (bar 4). Data presented are the mean ± SEM of 35 adhesions from 3 WT and L744E/R748E GFP-BCAR3 expressing cells from 3 independent experiments. *, p<0.05, **, p<0.01.
Using a similar approach to measure adhesion disassembly, we found that BCAR3 and Cas dissociate from adhesions at similar rates (Figure 3F, compare bars 1 and 3). However, the rate of L744E/R748E GFP-BCAR3 dissociation was significantly reduced compared to WT GFP-BCAR3 (compare bars 1 and 2), and dissociation of Cas from these adhesions was similarly impaired (compare bars 3 and 4). This suggests that direct binding between BCAR3 and Cas is required for efficient dissociation of BCAR3 and Cas from adhesions.
The reduced dissociation rate of Cas and L744E/R748E GFP-BCAR3 from adhesions could be the result of a specific delay in the dissociation of Cas and mutant BCAR3 from the adhesions, a more generalized stabilization of adhesion proteins in the adhesion complexes, or a reduction in the turnover rate of mutant BCAR3 and Cas. The latter possibility seems unlikely, as ectopic WT and L744E/R748E BCAR3 were found to have similar half-lives (Supplemental Figure S1). Moreover, these half-lives, as well as the half-life of Cas (data not shown), were found to be over 20 hours, which is far greater than the 10–12 minute timespan of the videos used to quantify adhesion disassembly.
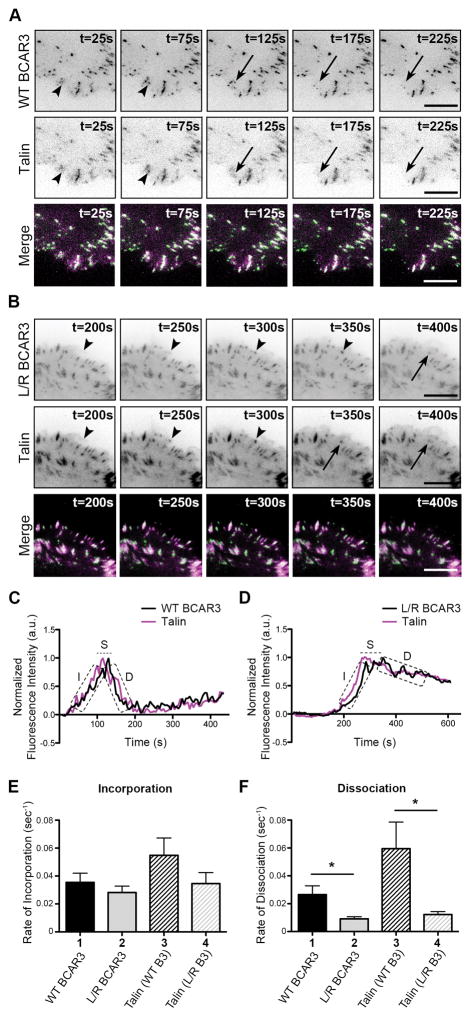
To distinguish between the first two possibilities, we examined the adhesion dynamics of another well-established adhesion protein, talin, in the presence of WT or L744E/R748E GFP-BCAR3. Unlike Cas, talin does not associate with WT BCAR3 (Supplemental Figure S2). Live TIRF imaging was performed to visualize adhesion dynamics in cells expressing mCherry-talin and either WT or L744E/R748E GFP-BCAR3, and adhesion turnover was quantified as described above (Figure 4). As before, the incorporation of BCAR3 into adhesions was not dependent on its ability to bind to Cas (Figure 4E, compare bars 1 and 2), but its rate of dissociation from adhesions was significantly reduced in the absence of Cas binding (Figure 4F, compare bars 1 and 2). The rates at which BCAR3 and talin entered and left adhesions were not significantly different (Figures 4E and 4F, bars 1 and 3). However, as was the case for Cas, the rate at which talin dissociated from adhesions was significantly reduced in the presence of L744E/R748E GFP-BCAR3 (Figure 4F, compare bars 3 and 4). This was also the case for a third adhesion protein, α-actinin (Supplemental Figure S3), which similarly does not interact with BCAR3 (Supplemental Figure S2).
Figure 4. Direct interaction between BCAR3 and Cas is required for efficient dissociation of talin from adhesions.
BT549 invasive breast cancer cells were co-transfected with plasmids encoding WT or L744E/R748E (L/R) GFP-BCAR3 and mCherry-talin, incubated for 24 hours, and then plated on 2μg/ml fibronectin-coated glass-bottomed TIRF dishes for 30–40 minutes prior to visualizing adhesion dynamics via live-imaging TIRF. (A, B) Representative time-lapse images show incorporation into adhesions (arrowheads) and dissociation (arrows) of the indicated proteins over the specified time course. Scale bars = 100μm. (C, D) Representative fluorescence intensity time tracings of BCAR3 (black) and talin (magenta) present in adhesions from cells expressing WT (C) or L744E/R748E (L/R) GFP-BCAR3 (D). Dashed boxes/line indicate the incorporation (I), stability (S), and dissociation (D) phases of adhesion dynamics. (E, F) Quantitative analysis of the incorporation (E) and dissociation (F) rates of WT GFP-BCAR3 (bar 1), L744E/R748E (L/R) GFP-BCAR3 (bar 2), Talin co-expressed with WT GFP-BCAR3 (bar 3), and Talin co-expressed with L744E/R748E (L/R) GFP-BCAR3 (bar 4). Data presented are the mean ± SEM of ≥14 adhesions from 5 separate WT BCAR3/talin or 3 separate L744E/R748E BCAR3/talin movies generated from 3 independent experiments. *, p<0.05
It is important to note that, for all of the adhesion proteins analyzed, the reduced rate at which they dissociated from adhesions in the presence of L744E/R748E BCAR3 was similar to the rate at which the mutant BCAR3 molecule left adhesions (Figures 3F, 4F, and S3F, compare bars 2 and 4). This is consistent with a stabilization of the entire adhesion complex under these conditions, suggesting that a direct interaction between BCAR3 and Cas is required for efficient adhesion complex disassembly and turnover.
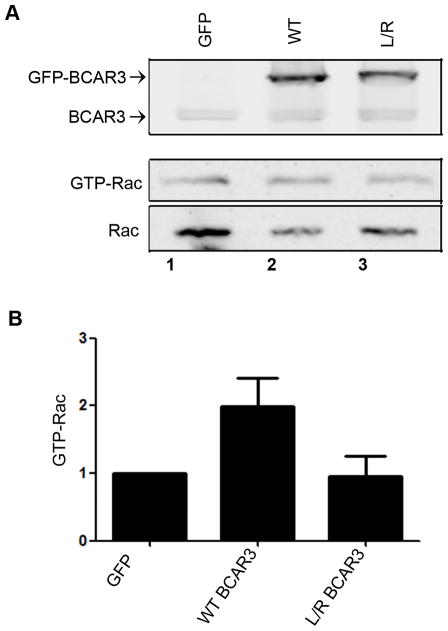
Our previous work showed that loss of BCAR3 in breast cancer cells resulted in a reduction of Rac1 activity coincident with an increase in RhoA activity, stress fiber stabilization and slower adhesion turnover.4 Because proper control of adhesion dynamics by BCAR3 required an intact Cas binding site, we hypothesized that the ability of BCAR3 to promote Rac1 activity may be dependent on its association with Cas. To test this hypothesis, active GTP-bound Rac1 was measured in extracts from BT549 cells expressing WT or L744E/R748E GFP-BCAR3 (Figure 5). Overexpression of WT BCAR3, but not the Cas binding mutant, was found to increase Rac1 activity in the cell. Together, these data show that the BCAR3/Cas complex promotes increased Rac1 activation and adhesion disassembly/turnover in breast cancer cell lines.
Figure 5. BCAR3/Cas interactions are required for BCAR3 dependent Rac activity.
BT549 cells were transfected with plasmids encoding GFP, GFP-WT BCAR3, or GFP-L744E/R748E BCAR3 and incubated for 24 hours. Cells were held in suspension for 90 minutes, then plated on 10μg/ml fibronectin for 1 hour. (A) GTP-bound Rac1 was isolated from whole cell lysates by incubation with PAK-1-binding domain agarose. Bound proteins (middle panel) and total Rac1 (bottom panel) were detected by immunoblotting with a Rac1 antibody, and BCAR3 expression was confirmed with a BCAR3-specific antibody (top panel). (B) Quantification of the relative GTP-Rac1 level is shown. Data presented are the mean ± SEM of 3 independent experiments.
Direct interaction between BCAR3 and Cas promotes breast tumor cell invasion in 3D and chemotaxis toward serum
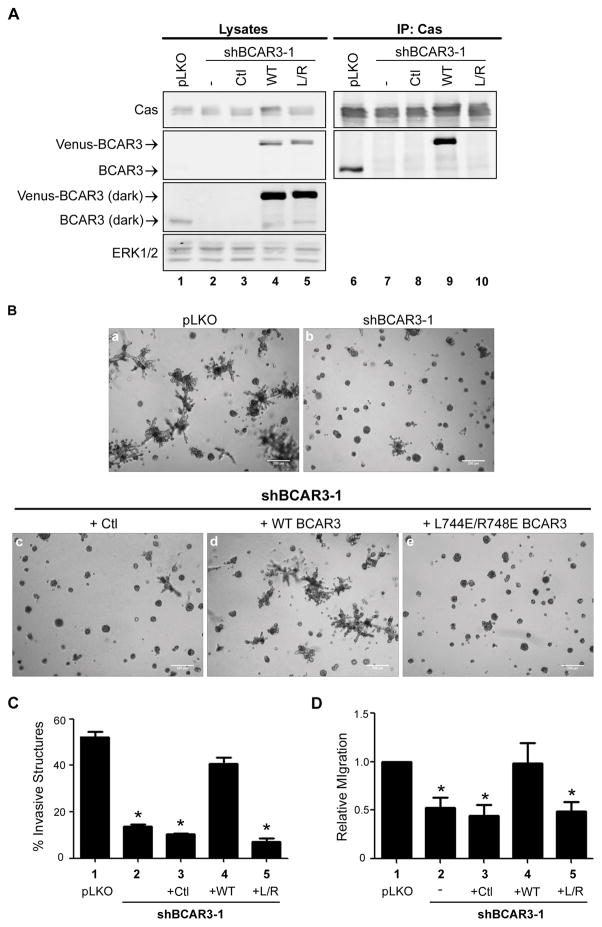
Previous studies have shown that BCAR3 promotes breast tumor cell motility and invasion.2,4 Considering that adhesion turnover is a critical facet of motility/invasion, and that BCAR3 promotes adhesion disassembly through its interaction with Cas (see above), we hypothesized that BCAR3-mediated breast tumor cell invasion and migration would similarly be dependent upon the ability of BCAR3 to bind to Cas. To test this hypothesis, stable MDA-MB-231 cells were generated that express either empty vector (pLKO) or a BCAR3-targeted short hairpin RNA (shBCAR3) (Figure 6A, lanes 1–2). The stable shBCAR3 cell lines were then infected with the lentiviral vector pLV-Venus (Figure 6A, lane 3) or shRNA-resistant wobble versions of pLV-Venus WT BCAR3 (lane 4) or the Cas binding mutant of BCAR3 (L744E/R748E, L/R) (lane 5). The expected Cas-binding capabilities of these molecules were confirmed through analysis of Cas immune complexes (lanes 7–10). Each cell line was seeded in 3D Matrigel cultures to assess whether BCAR3/Cas interactions were necessary for BCAR3-mediated invasion. As has been reported previously for parental MDA-MB-231 cells,15 control cells formed highly invasive structures when grown in 3D Matrigel culture (Figure 6B, panel a). In contrast, knockdown of BCAR3 resulted in a significant reduction in the percentage of invasive structures observed at day 8 in culture (Figure 6B, panel b, Figure 6C, compare bars 1 and 2). Cells infected with a second shRNA construct that resulted in a less robust knockdown of BCAR3 (Supplemental Figure S4A) exhibited an intermediate invasive phenotype (Figure S4B and C). The reduced invasive phenotype exhibited by cells expressing shBCAR3 was rescued by expression of WT BCAR3 protein but not the empty vector or Cas-binding mutant (Figure 6B, panels c–e, Figure 6C, bars 3–5). This requirement for direct BCAR3/Cas binding in BCAR3-mediated invasion was confirmed with a second cell line, HS-578T (Supplemental Figure S5). To determine the importance of direct binding between BCAR3 and Cas in promoting BCAR3 mediated migration, the MDA-MB-231 cells described above were plated in a modified Boyden chamber and allowed to migrate toward serum for 6 hours. Knockdown of BCAR3 resulted in a loss of migration as previously described2,(Figure 6D, bars 1, 2). The reduced migration observed in cells expressing shBCAR3 was similarly observed in cells re-expressing the empty vector and the Cas binding mutant of BCAR3 (Figure 6D, bars 3 and 5) but not in cells re-expressing WT BCAR3 (bar 4). Collectively, these data show that BCAR3 promotes both chemotaxis toward serum and invasion through its interactions with Cas.
Figure 6. Direct interaction between BCAR3 and Cas is required for invasion of MDA-MB-231 cells in 3D Matrigel culture and chemotaxis toward serum.
(A) MDA-MB-231 cells stably expressing empty vector (pLKO) or shBCAR3-1 lentiviral constructs were infected with lentiviruses encoding 3rd-base wobble variants of WT Venus-BCAR3 or L744E/R748E (L/R) Venus-BCAR3 or empty vector (pLV-Venus; Ctl). Total cell protein and Cas immune complexes were immunoblotted with antibodies to detect the indicated proteins. Left and right panels are identical exposures from the same film. (B, C) The cells described in panel A were grown in 3D Matrigel culture for 8 days. Representative phase images (B) and quantification of invasive structures (C) are shown. Data presented are the mean ± SEM of 3 independent experiments, performed in quadruplicate. Scale bars = 200μm. (D) The cells described in panel A were serum-starved overnight and plated (2.5 × 104) in the top of a Boyden chamber (6.5 mm, 8.0-μm Transwell Costar membrane; Corning Incorporated). Cells were allowed to migrate toward 10% serum for 6 hours and the cells that migrated to the lower chamber were counted. Data presented are the mean ± SEM of 7 independent experiments. *, p<0.05 relative to pLKO.
BCAR3 is co-expressed with Cas in multiple subtypes of human breast tumors
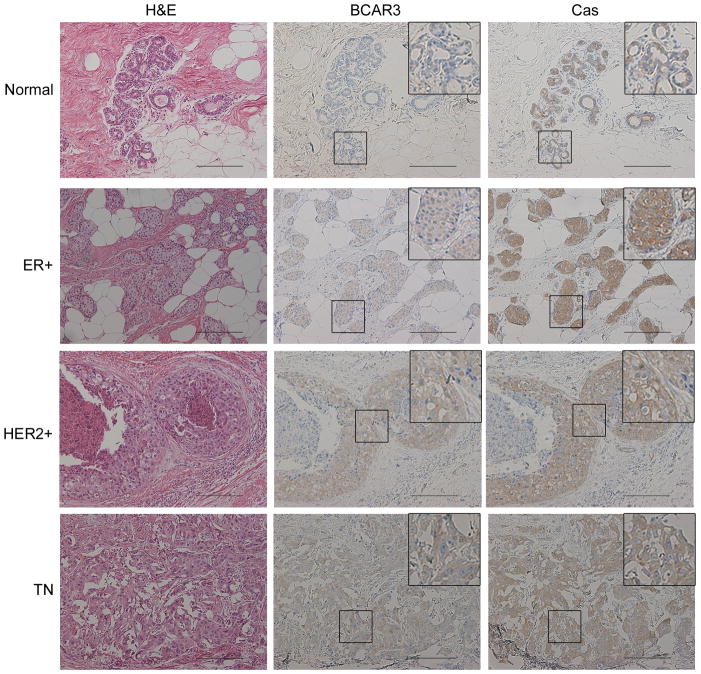
Considering the strong functional relationship between BCAR3 and Cas in breast cancer cell lines in vitro, we next sought to determine whether there was evidence for a similar functional association in human breast tumors. Sequential sections of tumor tissue were stained with hematoxylin and eosin (H&E) or antibodies recognizing BCAR3 or Cas. BCAR3 expression was found to be low to non-detectable in normal breast tissue (Figure 7, top panels) but upregulated in multiple breast tumor subtypes (bottom 3 panels). Moreover, BCAR3 was found to be co-expressed with Cas in localized regions of tumor tissue (see insets), suggesting that these two molecules may indeed function as a unit in breast cancers.
Figure 7. BCAR3 is co-expressed with Cas in multiple subtypes of human breast tumors.
Sequential sections of human tissue were stained with hematoxylin and eosin (H&E) (left panels) or immunostained with BCAR3 (middle panels) or Cas (right panels) antibodies. Insets show higher magnifications of the designated areas. Scale bars=50μM.
DISCUSSION
BCAR3 expression is upregulated in invasive breast cancer cell lines and has been shown to promote migration and invasion in these cells.2,4,16 Work from the Pasquale group demonstrated that direct binding between BCAR3 and Cas is required for enhanced Src activity and Cas phosphorylation.5 In the current study, we sought to further elucidate the importance of BCAR3/Cas complexes in BCAR3-dependent functions, particularly those associated with cell motility and invasion. The functional nature of this protein complex is underscored by our finding that all of the BCAR3 is in complex with Cas in invasive breast cancer cells.
BCAR3 targeting to adhesions is multi-factorial
Since all of the BCAR3 in BT549 and MDA-MB-231 breast cancer cells is present in BCAR3/Cas complexes, it is formally possible that, in the absence of any perturbation, endogenous BCAR3 enters adhesions together with Cas. However, there must also be Cas-independent mechanisms for adhesion targeting of BCAR3 since ectopically expressed L744E/R748E GFP-BCAR3 readily localized to adhesions despite its inability to associate with Cas (Figure 8A). The SH2 domain has been reported to mediate BCAR3 targeting in MEFs through its interaction with PTPα;3 however, the SH2 domain was dispensable for adhesion targeting in our system. Moreover, the dual SH2/Cas binding mutant (R171V/L744E/R748E GFP-BCAR3) also localized to adhesions, indicating that there are other focal adhesion targeting mechanisms that contribute to BCAR3 localization-to these sites, at least in the absence of Cas and PTPα interactions. It is unlikely that this targeting activity is a direct consequence of talin and α-actinin, as neither protein was present in WT or L744E/R748E GFP-BCAR3 immune complexes (Supplementary Figure S2). Whether other adhesion proteins are responsible for adhesion targeting of ectopic BCAR3 molecules in these circumstances remains to be determined.
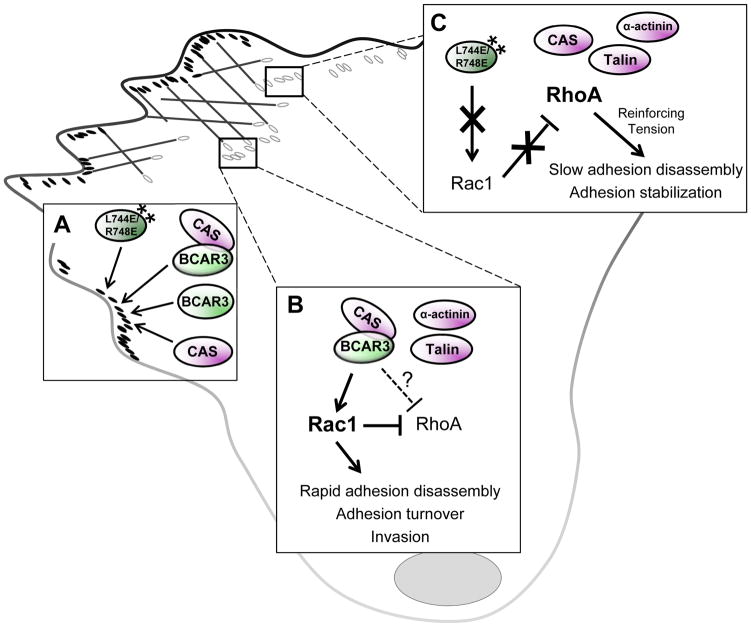
Figure 8. BCAR3/Cas interactions promote efficient adhesion complex disassembly and invasion.
(A) BCAR3 can efficiently incorporate into adhesions in the absence of a functional Cas binding and/or SH2 domain. (B) Under conditions where BCAR3/Cas interactions are enabled (i.e. WT BCAR3), rapid disassembly of multiple adhesion proteins is observed. We propose BCAR3/Cas complexes promote localized activation of Rac1 and/or suppression of RhoA under these conditions, therefore initiating rapid adhesion turnover and invasion. (C) When BCAR3/Cas interactions are prevented (i.e. L744E/R748E BCAR3), local Rac1 activation is diminished, leading to a possible rise in localized RhoA-mediated tension, which provides the reinforcement necessary to stabilize adhesions and slow the rate of disassembly.
BCAR3/Cas interactions are required for efficient BCAR3-mediated adhesion disassembly, migration, invasion, and Rac1 activity
The data presented in the current report provide the first mechanistic insight into how BCAR3 promotes adhesion disassembly and invasion of breast cancer cells. Under conditions in which BCAR3/Cas complexes were able to form (i.e. WT BCAR3), we observed rapid dissociation of multiple proteins from adhesions. However, when BCAR3/Cas interactions were blocked (i.e. L744E/R748E BCAR3), the rate of adhesion disassembly was significantly reduced. This suggests that the BCAR3/Cas complex contributes to adhesion disassembly. Recent studies have shown that the ability of BCAR3 to induce membrane ruffling/lamellipodia in 2D also requires Cas binding.5 Data presented in this report expand on these findings by showing that interactions between BCAR3 and Cas are required for the invasive phenotype of breast cancer cells in 3D as well as chemotaxis toward serum. Finally, BCAR3 expression in cells grown on plastic promotes Src-mediated Cas phosphorylation in breast cancer cells, leading to Cas/Crk coupling and Rac1 activation.2–4,10,11,17,18 We show here that BCAR3-dependent Rac1 activation also requires interaction with Cas. On 3D matrices, Rac1 activity promotes a mesenchymal phenotype, while elevated RhoA signaling promotes more rounded cell morphology.19 It is therefore interesting to speculate that BCAR3/Cas-dependent Rac1 activity may be critical for its ability to promote an invasive phenotype in 3D culture. Whether the adhesion turnover functions of BCAR3/Cas observed in 2D contribute to the BCAR3-dependent invasive phenotype in 3D remains to be determined, particularly since adhesions that form in 2D and 3D may differ significantly in protein composition, dynamics, and regulation.20,21
The co-localization of BCAR3 and Cas in adhesions suggests that BCAR3/Cas-mediated Rac1 activation is likely to occur at these sites. This activity, coincident with the possible suppression of RhoA in adhesions, could account for the faster rate of adhesion disassembly and turnover observed when WT GFP-BCAR3 and Cas are expressed in the cells (Figure 8B). Although the Cas-binding mutant of BCAR3 was also seen to efficiently localize to adhesions, it failed to promote Rac1 activity. In the absence of BCAR3-Cas interactions (or upon depletion of BCAR3 as was the case in our previous study), we speculate that the inability to locally activate Rac1, together with a possible rise in RhoA-mediated tension, provides the reinforcement necessary to stabilize adhesions and reduce the rate of disassembly (Figure 8C). This model is supported by work from the Lerner group, who showed that deletion of the C-terminus of BCAR3 abrogated both Cas binding and Rac1 activation.22 They also showed that a mutant of BCAR3 containing a single point mutation in the Cas-binding domain was still able to promote Rac1 activity despite its apparent inability to bind to Cas. It has since been shown, however, that this point mutation may not completely abrogate Cas binding in the cell.5
In conclusion, we favor a model wherein BCAR3 promotes Rac1 activation, adhesion disassembly, and an invasive phenotype through its binding to Cas, and that interfering with the interaction between these proteins short-circuits signaling network(s) responsible for these activities (Figure 8). An alternative explanation for the data presented in the current study is that L744E/748E BCAR3 may function independently of Cas to inactivate other molecules/pathways whose functions are critical for these outcomes. We consider this to be unlikely, however, largely because expression of the Cas-binding mutant of BCAR3 phenocopies the effects of BCAR3 knockdown that were reported in our previous study4 with respect to the adhesion turnover defect and diminished Rac1 activation.
BCAR3/Cas functions as an oncogenic protein complex in invasive breast tumor cells
Despite strong evidence for BCAR3 as a potent regulator of cell adhesion and invasion in breast cancer cells, the fact that a global knockout of BCAR3 fails to cause any major developmental or phenotypic abnormalities at birth23 indicates that its expression is largely dispensable for morphogenesis. BCAR3 expression is upregulated in invasive breast cancer cell lines,2,16 and it is in this context that BCAR3 appears to play a critical role in adhesion turnover and invasion. Our finding that the majority of BCAR3 in BT549 and MDA-MB-231 cells is associated with Cas suggests that the function of BCAR3 in these cells is dependent on the BCAR3/Cas complex. This is further supported by the data presented above showing that BCAR3 is co-expressed with Cas in multiple breast tumor subtypes. Together, these data suggest that BCAR3/Cas and/or its downstream effectors may prove to be effective therapeutic targets for tumors that co-express these molecules, particularly because BCAR3 is non-essential for development.
MATERIALS AND METHODS
Antibodies and reagents
Monoclonal antibodies recognizing β-Actin (A3854), β-tubulin (T4026), α-actinin (A5044), and talin (T3287) were purchased from Sigma-Aldrich. Polyclonal antibodies were obtained from the following sources: BCAR3 (Bethyl Laboratories, Inc., A301-671A); BCAR3 (for IHC) (Sigma Aldrich, HPA014858); GFP (Abcam, AB6673), ERK (Cell Signaling Technology, Inc., 9102); Texas red-conjugated goat anti-rabbit (Jackson ImmunoResearch Laboratories, Inc., 111-075-144); CasB24. Additional reagents included fibronectin (Sigma-Aldrich, F1141), EGF (Peprotech, AF-100-15), and Matrigel (Corning, 354230).
Expression vectors
BCAR3 cDNA was cloned into the EcoRI and XbaI sites of pEGFP-C1 (Clontech Laboratories, Inc.) to generate pEGFP-BCAR3 (WT GFP-BCAR3). Cas cDNA was cloned into the Xba1 and BamH1 sites of pm-Cherry-C1 to generate pm-Cherry-Cas.
Mutant R171V, L744E/R748E, and R171V/L744E/R748E GFP-BCAR3 proteins were created using the QuickChange II Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). The following primers were used (changed nucleotides are underlined, and all constructs were confirmed by sequencing):
R171V Forward: 5′-CGAGATGGTGACTTCCTAGTTGTCGACTCTCTGTCCAGCCCTGGG-3′
R171V Reverse: 5′-CCCAGGGCTGGACAGAGAGTCGACAACTAGGAAGTCACCATCTCG-3′
L744E/R748E Forward: 5′-CATGCTGAACCATGAGGCAACAGCGGAATTCATGGCCGAGGCTGC-3′
L744E/R748E Reverse: 5′-GCAGCCTCGGCCATGAATTCCGCTGTTGCCTCATGGTTCAGCATG-3′
Wobble mutants of BCAR3 were generated in the pLV-Venus vector. WT and L744E/R748E BCAR3 cDNA were cloned into the NotI and SpeI sites of the pLV-Venus vector. Site directed mutagenesis was performed using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies) to eliminate targeting by shBCAR3-1 without altering the amino acid sequence of the resultant BCAR3 protein. The following primers were used:
shB3wobble1 Forward: 5′-CCAGATTTTAACTGCGCTGTCCCGAAAATTGGAACCTCCTCCTG-3′,
shB3wobble1 Reverse: 5′-CAGGAGGAGGTTCCAATTTTCGGGACAGCGCAGTTAAAATCTGG-3′
shRNA oligonucleotides targeting BCAR3 and cloned into the TRC2-pLKO-puro vector were purchased from Sigma Aldrich. Hairpin sequences were as follows:
shBCAR3-1 shRNA ID: TRCN0000364816, sequence: 5′-CCGGTAACTGCCCTCTCGCGTAAATCTCGAGATTTACGCGAGAGGGCAGTTATTTTTG-3′
shBCAR3-2 shRNA ID: TRC0000376503, sequence: 5′-CCGGTCGGCATTGCAGTGGACATTCCTCGAGGAATGTCCACTGCAATGCCGATTTTTG-3′
Cell culture, invasion, migration, and Rac assays
BT549 and MDA-MB-231 cells (American Type Tissue Culture) were cultured as previously described2,11. HS-578T cells, generously provided by Dr. Kevin Janes (UVA), were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% FBS, 0.01mg/ml bovine insulin, and 1% Penicillin/Streptomycin. Cells lines were confirmed to be free of mycoplasma. For 3D culture of MDA-MB-231 and HS-578T cells, Matrigel (50μl) was spread evenly on the bottom of 8-well chamber slides. Cells grown in 2D monolayer culture were trypsinized and plated in the chamber slides with DMEM containing 2% (MDA-MB-231) or 10% (HS-578T) serum, 2% Matrigel, 5ng/ml EGF, and 0.5μg/ml (MDA-MB-231) or 1μg/ml (HS-578T) puromycin. Cells were grown for 6–8 days with media changes every 4 days. Phase images of representative fields were captured using an Olympus CKX41 or Zeiss Axiovert 40 CFL inverted scope. Transwell migration assays were performed as previously described2, and the cells were stained using Protocol HEMA 3 stain set (Fisher Scientific, 122-911). Rac1 assays were performed on BT549 cells transfected with plasmids encoding GFP, WT GFP-BCAR3, or L744E/R748E GFP-BCAR3 as previously described 4.
Plasmid transfection, lentivirus production and infection
Transfections were performed using Lipofectamine 2000 (Invitrogen, 11668019) following manufacturer’s specifications.
Lentiviral particles were produced by calcium phosphate transfection of HEK293T cells with a mixture of the transfer vector (pLKO-shBCAR3 or pLV-VenusBCAR3), packaging vector (psPAX2), and envelope vector (pMD2.G). Medium containing lentivirus was collected 48 hours post-transfection, filtered through 0.45μm filter, and used immediately or frozen at −80°C. Cells were infected with lentivirus in the presence of 8μg/ml polybrene.
Immunoprecipitation, immunoblotting and immunofluorescence
Cells grown in 2D were lysed in ice-cold radioimmune precipitation assay (RIPA) buffer supplemented with protease inhibitors and protein concentrations determined as previously described 2 or in a non-denaturing lysis buffer as described by Wallez et al.5 Immunoprecipitations, immunoblotting and immunofluorescence were performed as previously described 2.
Live-cell imaging and adhesion turnover analysis
BT549 cells were plated on acid-washed 2μg/ml fibronectin-coated glass bottom TIRF dishes (MatTek Corporation, Ashland, MA) and incubated for 30–40 minutes at 37°C, pH 7.4 in CCM1 media (Hyclone). Images were captured using an inverted TIRF microscope (1X70; Olympus) with a 60X objective (±1.5X magnification), a cool charged-couple device camera (Retiga Exi; Qimaging), and heated objective/stage (Bioptechs). Images were captured every 5 seconds for 10–12 minutes using MetaMorph software. To quantify adhesion turnover, adhesions at peripheral, protruding edges were manually selected for analysis. Complete fluorescence intensity time tracings for individual adhesions were (1) normalized, (2) corrected for background intensity by subtracting an average intensity value corresponding to a background region away from the cell, and (3) plotted. Both the increase (incorporation/assembly) and decrease (dissociation/disassembly) in fluorescence intensity were linear as a function of time on semi-logarithmic plots, and rate constants were determined from the slopes of these graphs. Rate constant measurements were obtained for a minimum of 13 individual adhesions on 2–5 cells.
Human breast tumor staining
Sequential sections of breast tissue were received from the University of Virginia Biorepository and Tissue Research Facility (BTRF; IRB#HSR17196). Sections were stained with hematoxylin and eosin (H&E) or immunostained with BCAR3 or Cas antibodies by the BTRF.
Statistical analysis
Statistical analyses were conducting using GraphPad Prism and the sample size was shown to have adequate power. For the adhesion turnover and invasion analysis, a Kruskal-Wallis one-way ANOVA and a Dunn’s Multiple Comparison post-test were used to compare multiple experimental groups.. For migration studies, a one-way ANOVA and the Dunnett post-test were used to compare groups, as the data followed Gaussian distribution and passed a Bartlett’s test for equal variance.
Supplementary Material
Acknowledgments
The authors would like to thank Jessica Zareno for technical assistance with TIRF microscopy and Dr. Kristen Atkins for identification and pathological assessment of human breast tumor samples. This work was supported by grants from the NIH [5 T32 CA009109 (ALW, AMB), 1 R01 CA096846 (AHB), 1 F31 CA165703 (ALW), 1 F31 CA130168 (MSG), and GM023244 (ARH)]; the American Cancer Society (PF-12-136-01-CSM to KEK); and the Women’s Oncology Research Fund and NCI Cancer Center Support Grant P30 CA44579 from the UVA Cancer Center). Breast tumor samples were obtained from the UVA tumor bank and stained by the Biorepository and Tissue Research Facility (BTRF).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Supplementary information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- 1.Riggins RB, Quilliam LA, Bouton AH. Synergistic promotion of c-Src activation and cell migration by Cas and AND-34/BCAR3. J Biol Chem. 2003;278:28264–28273. doi: 10.1074/jbc.M303535200. [DOI] [PubMed] [Google Scholar]
- 2.Schrecengost RS, Riggins RB, Thomas KS, Guerrero MS, Bouton AH. Breast cancer antiestrogen resistance-3 expression regulates breast cancer cell migration through promotion of p130Cas membrane localization and membrane ruffling. Cancer Res. 2007;67:6174–6182. doi: 10.1158/0008-5472.CAN-06-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun G, Cheng SY, Chen M, Lim CJ, Pallen CJ. Protein tyrosine phosphatase alpha phosphotyrosyl-789 binds BCAR3 to position Cas for activation at integrin-mediated focal adhesions. Mol Cell Biol. 2012;32:3776–3789. doi: 10.1128/MCB.00214-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson AL, Schrecengost RS, Guerrero MS, Thomas KS, Bouton AH. Breast cancer antiestrogen resistance 3 (BCAR3) promotes cell motility by regulating actin cytoskeletal and adhesion remodeling in invasive breast cancer cells. PloS One. 2013;8:e65678. doi: 10.1371/journal.pone.0065678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallez Y, Riedl SJ, Pasquale EB. Association of the breast cancer antiestrogen resistance protein 1 (BCAR1) and BCAR3 scaffolding proteins in cell signaling and antiestrogen resistance. J Biol Chem. 2014;289:10431–10444. doi: 10.1074/jbc.M113.541839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Brush J, Stewart TA. NSP1 defines a novel family of adaptor proteins linking integrin and tyrosine kinase receptors to the c-Jun N-terminal kinase/stress-activated protein kinase signaling pathway. J Biol Chem. 1999;274:10047–10052. doi: 10.1074/jbc.274.15.10047. [DOI] [PubMed] [Google Scholar]
- 7.Cai D, Clayton LK, Smolyar A, Lerner A. AND-34, a novel p130Cas-binding thymic stromal cell protein regulated by adhesion and inflammatory cytokines. J Immunol. 1999;163:2104–2112. [PubMed] [Google Scholar]
- 8.Vervoort VS, Roselli S, Oshima RG, Pasquale EB. Splice variants and expression patterns of SHEP1, BCAR3 and NSP1, a gene family involved in integrin and receptor tyrosine kinase signaling. Gene. 2007;391:161–170. doi: 10.1016/j.gene.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mace PD, Wallez Y, Dobaczewska MK, Lee JJ, Robinson H, Pasquale EB, et al. NSP-Cas protein structures reveal a promiscuous interaction module in cell signaling. Nat Struct Mol Biol. 2011;18:1381–1387. doi: 10.1038/nsmb.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10:858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 11.Schuh NR, Guerrero MS, Schrecengost RS, Bouton AH. BCAR3 regulates Src/p130Cas association, Src kinase activity, and breast cancer adhesion signaling. J Biol Chem. 2010;285:2309–2317. doi: 10.1074/jbc.M109.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Bio. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 14.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Near RI, Zhang Y, Makkinje A, Vanden Borre P, Lerner A. AND-34/BCAR3 differs from other NSP homologs in induction of anti-estrogen resistance, cyclin D1 promoter activation and altered breast cancer cell morphology. J Cell Physiol. 2007;212:655–665. doi: 10.1002/jcp.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akakura S, Kar B, Singh S, Cho L, Tibrewal N, Sanokawa-Akakura R, et al. C-terminal SH3 domain of CrkII regulates the assembly and function of the DOCK180/ELMO Rac-GEF. J Cell Physiol. 2005;204:344–351. doi: 10.1002/jcp.20288. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki D, Kurisu S, Takenawa T. Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene. 2009;28:1570–1583. doi: 10.1038/onc.2009.2. [DOI] [PubMed] [Google Scholar]
- 20.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–368. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrie RJ, Yamada KM. At the leading edge of three-dimensional cell migration. J Cell Sci. 2012;125:5917–5926. doi: 10.1242/jcs.093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanden Borre P, Near RI, Makkinje A, Mostoslavsky G, Lerner A. BCAR3/AND-34 can signal independent of complex formation with CAS family members or the presence of p130Cas. Cell Signal. 2011;23:1030–1040. doi: 10.1016/j.cellsig.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Near RI, Smith RS, Toselli PA, Freddo TF, Bloom AB, Vanden Borre P, et al. Loss of AND-34/BCAR3 expression in mice results in rupture of the adult lens. Mol Vis. 2009;15:685–699. [PMC free article] [PubMed] [Google Scholar]
- 24.Bouton AH, Burnham MR. Detection of distinct pools of the adapter protein p130CAS using a panel of monoclonal antibodies. Hybridoma. 1997;16:403–411. doi: 10.1089/hyb.1997.16.403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.