Abstract
The use of vaporized nicotine products (VNPs), especially e-cigarettes and to a lesser extent pressurized aerosol nicotine products and heat-not-burn tobacco products, are increasingly being adopted as an alternative to smoking combusted products, primarily cigarettes. Considerable controversy has accompanied their marketing and use. We propose a framework that describes and incorporates patterns of VNP and combustible cigarette use in determining the total amount of toxic exposure effects on population health. We begin by considering toxicity and the outcomes relevant to population health. We then present the framework and define different measures of VNP use, namely, trial and long-term use for exclusive cigarette smokers, exclusive VNP and dual (cigarette and VNP) use. Using a systems thinking framework and decision theory we considered potential pathways for current, former and never users of VNPs. We then consider the evidence to-date and the likely impacts of VNP use on public health, the potential effects of different policy approaches, and the possible influence of the tobacco industry on VNP and cigarette use.
INTRODUCTION
In the United States, smoking rates have fallen by 50% since their peak in the 1960’s as a result of tobacco control policies (1), but smoking still contributes to high rates of premature mortality. The 2014 Surgeon General’s Report stated, “the burden of death and disease from tobacco in the U.S. is overwhelmingly caused by cigarettes and other combusted tobacco products; rapid elimination of their use will dramatically reduce this burden.”
While all are agreed that efforts to discourage combustible tobacco products, especially cigarettes should continue, there is more controversy about the marketing of new vaporized nicotine products (VNPs), especially e-cigarettes, because of disagreements about whether they will complement or undermine successful tobacco control efforts (2, 3). VNP use has increased markedly in many high income countries (4–7) as a result of increased marketing (8, 9), the use of VNPs for smoking cessation (10), and policies that have made cigarettes less affordable (11). In the U.S., increasing e-cigarette use (5, 6) has been accompanied by an unusually large reduction in adult (12) and youth (6, 13) smoking prevalence.
Although the types of available VNPs vary and are rapidly evolving (14, 15), these products expose users to substantially lower levels of toxicants than combustible cigarettes (16–18). Consequently, VNPs could reduce harm to never smokers who would have otherwise initiated long-term cigarette use, and reduce harm to current smokers by helping them to quit, to switch to exclusive VNP use, or to substantially reduce their smoking. If, however, VNP use encourages the long-term use of cigarettes, or VNPs are used by those who would not have otherwise smoked, the net societal benefit would be diminished and VNPs could incur population level harm.
Despite growing evidence of the possible benefits of VNPs, 55 of 123 countries surveyed (19) have bans or laws that prohibit or restrict the sale of VNPs and 71 have laws that regulate the minimum purchase age, marketing, or taxation of VNPs. In April 2014, the U.S. FDA’s Center for Tobacco Products proposed deeming regulations that would assert their jurisdiction over e-cigarettes (20). Before imposing these regulations, the FDA must consider scientific evidence on the likely benefit and harm to individuals and the population as a whole.
This paper proposes a systems level model (21) of the possible harm-increasing and harm-reducing effects that is used to estimate the potential net effects of VNPs on population health. This framework employs decision theory to consider potential pathways of cigarette and VNP product use by current, former, and never smokers. We begin by considering the toxicity of VPN. We then present the framework and consider different measures of use, distinguishing trial from different forms of long-term use. Finally, we consider the available evidence and likely impacts on public health, the potential effects of different policies, and the possible influence of the tobacco industry on VNP and cigarette use. We focus on the US, where VNPs are now largely unregulated.
MORTALITY RISKS OF EXCLUSIVE AND DUAL VNP USE
A multi-criteria decision analysis (22) estimated that exclusive VNP use is associated with 5% of the mortality risks of smoking. This is comparable to the estimated risks of low-nitrosamine smokeless tobacco (23). In the absence of long term experience, the precise percentage of reduced harm may be difficult to quantify but studies using major biomarkers of cancer and other chemicals in e-cigarettes indicate substantially lower (e.g. 9–450 times) levels compared to cigarette smoke (16–18).
For dual users, VNP use may translate to a lower quantity and duration of cigarettes smoked. Both may decrease lung cancer and COPD risk (24, 25), with the amount depending on the proportion of total harm exposure obtained from each source. Studies find considerable variation in VNP use and quantity of cigarettes smoked (26), including ≥ 50% reduction in consumption. The potential to reduce risk is likely to depend on the age of initial dual use. Although much use now begins at later ages, VNP use is likely to occur at earlier ages in more recent cohorts of smokers, and thereby provide a greater reduction in cigarette use and toxic exposures over longer periods of use. In addition, initiating VNP use before cigarette smoking may delay or prevent smoking initiation and thereby reduce smoking risks.
FRAMEWORK AND MEASURES OF USE
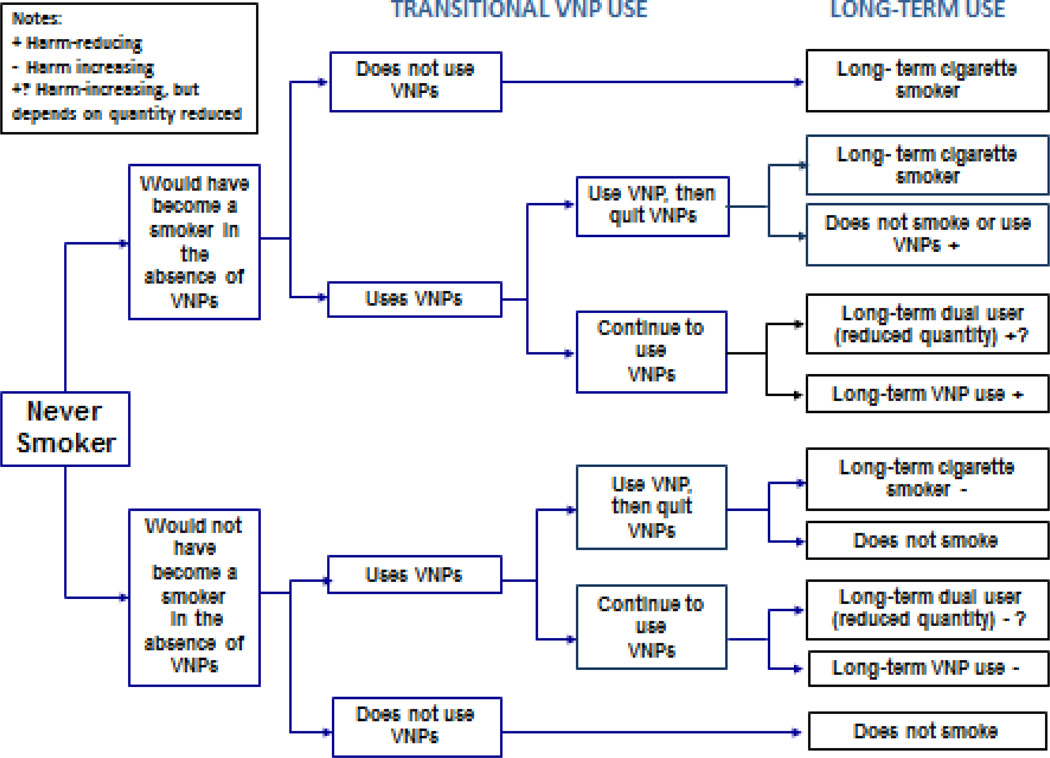
The use of tobacco products over a prolonged period is necessary to detect reductions in life expectancy (25, 27). This is also likely to apply to the use VNPs. We consider short-term (“trial”) use, which may determine transitions to long-term (“prolonged”) use and may help gauge the immediate effects of public policies. Possible transitions are shown for never, current and former smokers in Figures 1–3. Harm-reducing effects are indicated by a “+” and harm-increasing effects by a “−.” A “?” indicates that the amount of change depends on the pattern of use. In each case, the impact on population health will depend on how VNP use influences the long-term prevalence of: exclusive cigarette smoking, exclusive VNP use, dual use, and abstinence compared to the counterfactual scenario in the absence of VNPs.
Figure 1.
The public health impact of VNP use among never smokers
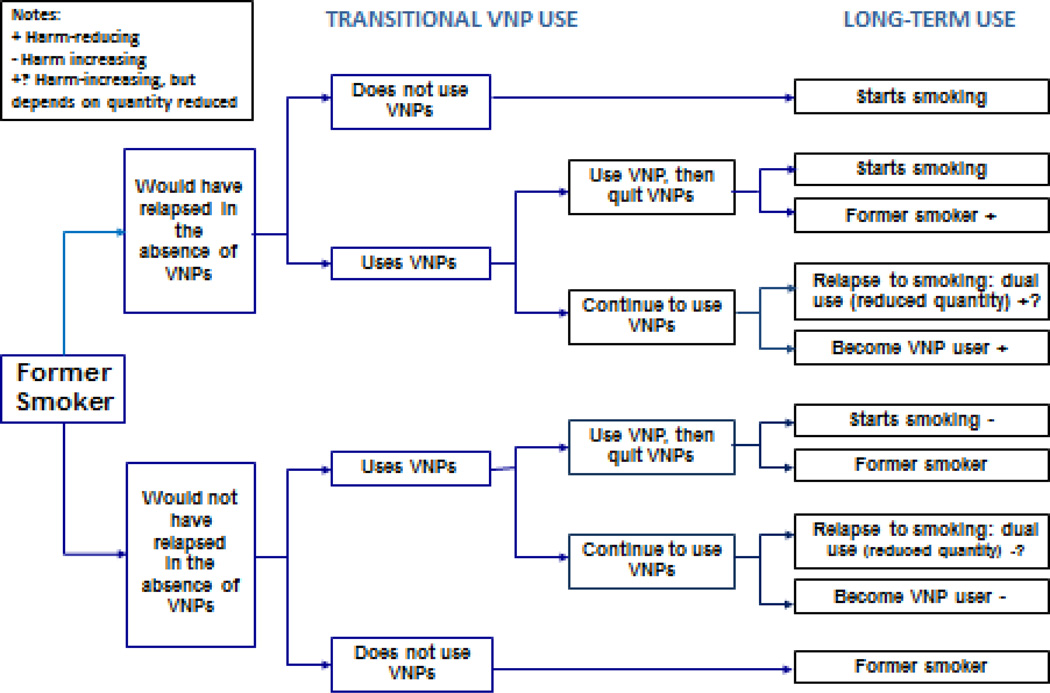
Figure 3.
The public health impact of VNP use among former smokers
Studies on e-cigarettes to date have mostly measured “ever-use” or “past 30-day use”(28), with the ratio of current to ever use averaging 30% across 27 European countries (29) in 2012 and 30% among US college students (30) and adults (31). While current use is often described by past-30 day use, evidence suggests that much reported use is infrequent (31) and so unlikely to lead to substantial harm to health. Harm is determined by how many users transition to more frequent use of cigarettes or VNPs. More established use can be assessed by inquiring about the number of days used in the last month (29, 32), daily use (33) and number of times used (34–36).
Accurate measures of long-term exclusive and dual use require sufficient time to transition from smoking, possibly through dual use, to final use states (e.g., abstinence from either cigarettes or from VPNs or both) (37). For example, recent former cigarette smokers (quit ≤ 1 year) were twice as likely as longer-term former smokers (quit 2–3 years) and four times as likely as current cigarette smokers (31) to be daily VNP users. In addition, transitions may differ by cohort depending on perceived risk, ability to satisfy cravings or withdrawal symptoms, differences between early and late adopters, socio-economic status and current tobacco control policies (38, 39).
TRANSITIONS FROM NEVER SMOKER
As shown in Figure 1, a never smoker may transition from trying VNP to exclusive VNP use, exclusive cigarette use, dual use, or quit using cigarettes and VNPs. The population health impact depends critically on whether the never smoker who tries VNPs would have smoked cigarettes in the absence of VNPs. Health impacts are harm-increasing when VNPs lead to someone who would otherwise never smoke to initiate cigarette smoking. VNP and dual use and are harm-reducing when those who would otherwise smoke cigarettes transition to no use, substantially reduce their cigarette use, or exclusively use VNPs.
Studies indicate that adolescents and young adult VNP users are far more likely to have already smoked cigarettes than to have never smoked (40). A 2014 Great Britain survey (ages 11–18) found past month use at 0.2% among never smokers and 13.5% among smokers. Only 8.2% of those who ever used a VNP smoked a cigarette for the first time after using VNPs compared to 69.8% who smoked a cigarette before trying a VNP (41). Studies of youth and young adults use from the US (42–44) and other countries (45–48) using different use measures have found current smokers at least 15 times more likely to use VNPs than never smokers.
Only a few studies have considered more established VNP use (49, 50). Of 13.4% of high school students reporting any past 30-day VNP use, 74% had tried VNPs on 1 to 9 days, while ≥20 days use was reported by only 15.5% of users who comprised 2% of the population (49). Among college students, cigarette smokers were more likely to continue VNP use (8.0%) than non-smokers (0.4%), and more non-smokers who tried VNPs were non users at follow-up (96.8%) than smokers (68.1%) (50).
Adolescents and young adult who use VNPs are most likely to be those at higher risk of initiating cigarette smoking (51, 52). Young VNP experimenters are more likely to engage in other risky behaviors (30, 53, 54) and have executive function deficits (55, 56) like those found in cigarette smokers (56, 57). These findings suggest a common liability model is more plausible than a gateway from VNP use to cigarette smoking (58, 59). In testing the role of common liability and gateway effects of VNP use, statistical techniques are required to adequately control for the factors that determine initial VNP use and the transition from experimental to regular use, i.e., those that correct for confounding and selection bias (60, 61).
TRANSITIONS FROM CURRENT CIGARETTE SMOKING
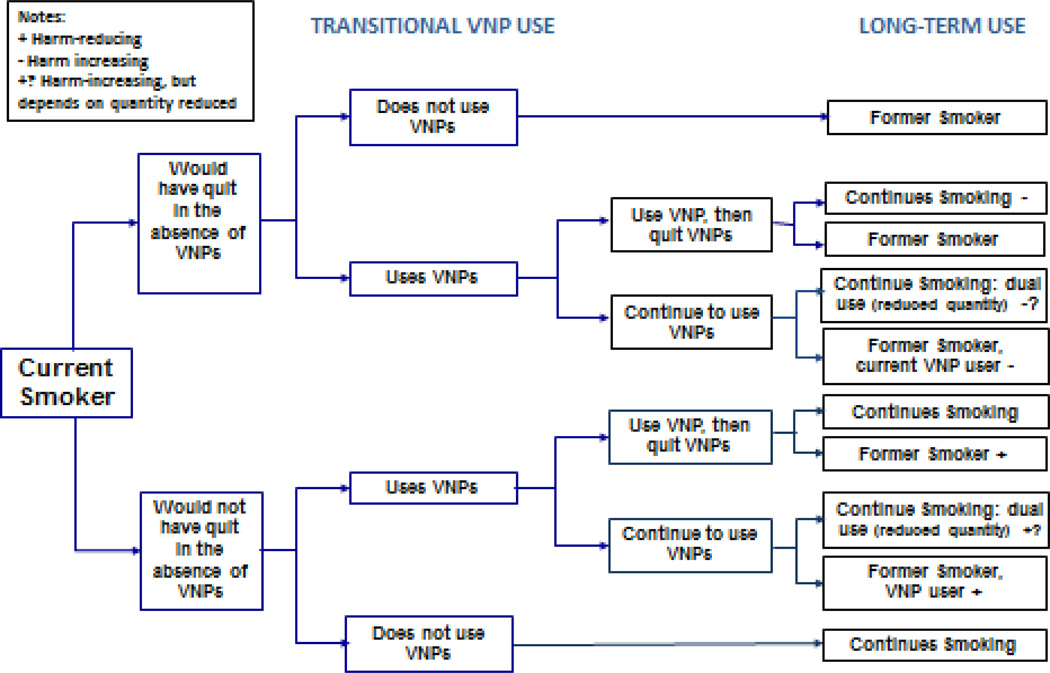
Figure 2 shows that the public health impact on VNP use on cigarette smokers will depend on how VNP use affects the likelihood of quit success, i.e., how many smokers would quit in their absence. The effect of VNPs on cessation is likely to depend on their desirability and the ability to deliver nicotine at a sufficient dose to reduce craving or withdrawal symptoms from cigarettes (4, 62). Both may vary with product type and preparedness of smokers to use them for prolonged periods. Several studies have reported higher smoking cessation rates among users of VNP tank systems (62). Other studies indicate that more regular use (e.g., daily) of VNPs is correlated with being an ex-smoker (31, 33, 34), increased numbers of quit attempts and greater reductions in number of cigarettes smoked (63).
Figure 2.
The public health impact of VNP use among smokers
Two randomized controlled trials have found that VNPs can help some smokers to quit or reduce their cigarette consumption (64, 65). Rates of smoking cessation in the VNP groups were similar to those seen in clinical trials of nicotine replacement therapy (NRT) (66). In uncontrolled prospective studies, one with carbon monoxide (CO) testing found a similar success rate (67) while four others found higher rates of smoking abstinence (68–71). One review (72) that reported lower cessation rates among VNP users included studies that were not prospective, defined ever-use or past 30 day use as sufficient exposure to VNPs to impact abstinence, and suffered from other methodological weaknesses (e.g., selection bias). A more recent review (73) concluded, “Smokers who have tried other methods of quitting without success could be encouraged to try e-cigarettes to stop smoking…There is evidence that EC can encourage quitting or cigarette consumption reduction.”
Because VNPs are more widely available and often more appealing to smokers than conventional NRT (10), they have the potential for having a larger impact on the rate of smoking cessation in the population (2, 74). However, evidence suggests that VNPs are not especially attractive to longer-term ex-smokers; only 0.8% of long-term former smokers who had quit for more than four years used VNPs compared to 13% of recent quitters (31).
Ultimately, the ability to identify the public health impact of VNP use will depend on measurement of factors that predict willingness to try VNPs and transitions to long-term VNP use by different groups (i.e., current smokers, ex-smokers, never smokers). For example, quit success may depend on intent (e.g., whether it is used to quit) and on whether smokers who use VNPs are more addicted or have a history of unsuccessful use of other cessation techniques (10, 62, 75, 76). Studies (29, 76, 77) find that current VNP use is associated with past quit attempts. One study found that the relationship between VNP use and cigarette smoking cessation depended upon the ability to statistically control for factors related to success of past quit attempts and intention to quit (75).
TRANSITIONS FROM FORMER SMOKER
Figure 3 shows that VNP use may increase harm for former smokers who would not have otherwise relapsed if after trying VNPs they relapse to exclusive or dual cigarette use. It will reduce harm in former smokers who use VNPs to prevent a relapse to cigarette smoking. Beneficial effects of VNP use are suggested by a longitudinal observational study (78) that found 6% of former smokers who used VNP daily at baseline relapsed to cigarette smoking at one month and 6% at one year. Eight percent of recent quitters relapsed to occasional smoking at one month and 5% at one year but none relapsed to daily smoking. These rates compare favorably to typical relapse rates for smoking after cessation using other methods (79). However, we do not yet have enough evidence on the effects of VNP use on relapse, because of their limited use by former smokers who did not use VNPs before quitting (80, 81).
THE ROLE OF POLICY: INTENDED AND UNINTENDED EFFECTS
Any assessment of the effect of policies towards VNPs depends on understanding that cigarettes and VNPs are potentially substitutable goods (82–84). Liberal regulation of VNPs may mean that transitions to VNPs result in more long term VNP use rather than their short term use as cessation aids. On the other hand, restrictive policies towards VNPs may mean that cigarette smokers are less likely to switch to VNPs. A recent study (84), for example, found that states with minimum VNP purchase age laws had lower rates of VNP uptake and more cigarette uptake than states without such restrictions.
Stronger cigarette control policies (e.g., bans on menthol and other flavors to reduce their appeal, toxicity or addictiveness) may encourage cessation by those smokers who are more likely to quit. As many as 40% of smokers make a quit attempt each year in most high income nations but only 3%–5% remain abstinent for 6-months or longer (85, 86), indicating that many smokers who try to quit soon relapse to smoking. Studies (32, 76) indicate that most smokers use VNPs with the intention of quitting smoking cigarettes. While stronger cigarette policies may initially lead to dual use, they may also lead to complete cessation of cigarettes if the policies are sufficiently strong.
The effect of policies towards VNPs will depend on how they affect dual versus exclusive use. Product regulations that limit toxicity may increase VNP use as a substitute for smoking, especially if that information is publicized, and thereby substantially reduce risk per unit exposure. However, if regulations discourage VNP innovations that make VNPs more attractive to smokers, they reduce cessation among smokers who would use better VNPs. Outright bans on VNP sales may be more likely to discourage cessation than reduce VNP use, as indicated by their use in countries where VNP sales are prohibited (87, 88). Bans may not stop some young people from taking up vaping, as experience with cannabis use shows.
Concerns have been raised that cigarette smoking will be re-normalized by VNP use (89, 90). This issue can be addressed by the media and public health campaigns that encourage norms that are hostile to cigarette smoking and at the same time distinguishing clearly between VNP and cigarette risks, discourage dual use and encourage exclusive VNP use. Indeed, the availability of VNPs may provide a justification for stronger policies to discourage cigarette smoking because smokers, particularly those of lower socio-economic status and with mental health issues, are given a less risky and potentially less costly alternative way to service their need for nicotine.
THE ROLE OF THE TRADITIONAL TOBACCO AND VAPING INDUSTRIES
In coordinating tobacco and VNP control strategies, we need to gauge how they will influence the 4-Ps of tobacco marketing: Product, Price, Promotion and Place (91, 92).
The VNP industry is made up of many different manufacturers, most of whom are not affiliated with cigarette companies. By contrast the cigarette, cigar, and smokeless tobacco industries are largely consolidated and controlled by a few large multinational cigarette companies. With the rapid growth of the VNP market (93), major cigarette makers such as such as Phillip Morris (MarkTen, IQOS, Marlboro Heat Stick), Imperial (Blu), Reynolds American (Vuse, Revo), and BAT (Vype) have introduced VNP products. However, cigarette companies do not control VNPs like they do the rest of the tobacco business; many manufacturers of e-cigarettes like NJOY do not sell cigarettes and there are thousands of vape shops that are independent of the cigarette industry. The diversity of the VNP business influences the distribution channels and the cost differential between VNP and conventional tobacco products.
Cigarette companies that have entered the smokeless tobacco market (94, 95) have encouraged dual rather than exclusive use and are likely to do the same with VNPs. By contrast VNP companies that are unaffiliated with cigarette manufacturers want smokers to switch completely from cigarettes to VNPs. Product content regulations that create regulatory hurdles that only large firms can get over are likely to favor the cigarette industry and discourage innovation by firms outside the cigarette industry. For example, a regulation restricting VNP tank devices will favor firms selling the “cigalike” VNPs sold by cigarette companies (71) that are less attractive to smokers (63).
Increasing VNP prices by taxing them the same way as cigarettes will discourage youth VNP use, but also discourage use by smokers of lower socioeconomic status who are trying to switch or quit. However, if VNP taxes are accompanied by even higher cigarette taxes, youth VNP use may be reduced and initiation into smoking discouraged, while switching and cessation among current smokers would be encouraged (96). In the case of marketing restrictions, retailer point-of-sale restrictions, which limit subsidies by cigarette manufacturers to provide shelf space and price promotions, can reduce price discounting and discourage advertisement displays (97). This could provide greater shelf space for VNP products to be sold by independent firms.
FINAL COMMENTS
From a public health perspective, VNP policies should aim to discourage experimental and regular use of VNPs by never smokers who would not have otherwise smoked while encouraging innovations in VNP products that promote smoking cessation. The evidence suggests a strong potential for VNP use to improve population health by reducing or displacing cigarette use in countries where cigarette prevalence is high and smokers are interested in quitting. Rising VNP use is a global phenomenon in low- and middle-income countries as well as in high-income countries (87). Yet evidence is lacking on their impact in countries where cigarette smoking prevalence is low (e.g., Sub-Saharan African countries) or where interest in quitting among smokers may be low (e.g., China).
The primary aim of tobacco control policy should therefore be to discourage cigarette use while providing the means for smokers to more easily quit smoking, even if that means switching for some time to VNPs rather than quitting all nicotine use. Countries whose policies discourage VNP use run the risk of neutralizing a potentially useful addition to methods of reducing tobacco use. We must collect better information on VNP use and its consequences to better assess this potential. Although large cross-sectional surveys can be used to estimate transition probabilities (98), we need longitudinal data, such as the large-scale longitudinal US Population Assessment of Tobacco and Health (PATH) survey and the International Tobacco Control surveys (87), to more directly track transitions to and from VNP use. As we gain better knowledge of the effects of cigarette-oriented and VNP-oriented policies, a long-term view that reduces the use of the most toxic combusted tobacco nicotine delivery products will become a more realistic goal.
Our framework identifies the critical information required, but this information will need to be continually updated. VNPs will change over time, and the extent of product innovation will depend on industry structure and how tobacco control policies are applied to cigarettes and VNPs. As the product and population of users change, the characteristics of experimenters and long-term VNP users, their transitions to exclusive and dual cigarette and VNP use and associated health risks may change. While there is more uncertainty about the health risks of exclusive and dual VNP use than of cigarette use, the substantially lower levels of toxins than cigarettes make VNPs far less harmful, although by exactly how much is unclear. If the harms of VNP use are substantially more than indicated by current evidence, then policies will be needed to discourage long term VNPs use.
Clearly, we need better measures of longer-term and longitudinal patterns of VNP use, product toxicity and addictive potential, and appropriate methods to study critical transitions in patterns of VNP and cigarette use. With multiple potential interactions between VNP and cigarette use and the differential effects of policies on these use rates, modeling provides a “virtual population laboratory” to synthesize existing evidence, to project the likely impacts without regulatory action, and to compare the impact of different possible interventions (99–102). However, until better data are available, our ability to understand the impact of VNP use will be need to be based on careful and prudent extrapolations of their likely benefits and harms from shorter term evidence.
Acknowledgments
Funding was received from the Food and Drug Administration through the National Institute on Drug Abuse under grant 1R01DA036497-01. All authors contributed to the writing of this manuscript
Footnotes
Declaration of Interests. The authors declare no conflicts of interest.
Contributor Information
David T. Levy, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
K. Michael Cummings, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA.
Andrea C. Villanti, The Schroeder Institute for Tobacco Research and Policy Studies at Truth Initiative, Washington, DC, USA.
Ray Niaura, The Schroeder Institute for Tobacco Research and Policy Studies at Truth Initiative, Washington, DC, USA.
David B. Abrams, The Schroeder Institute for Tobacco Research and Policy Studies at Truth Initiative, Washington, DC, USA; Dept. of Health, Behavior and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
Geoffrey T. Fong, Department of Psychology, University of Waterloo, Waterloo, Ontario, Canada; School of Public Health and Health Systems, University of Waterloo, Waterloo, Ontario, Canada; Ontario Institute for Cancer Research, Toronto, Ontario, Canada.
Ron Borland, Nigel Gray Distinguished Fellow in Cancer Prevention, VicHealth Centre for Tobacco Control, The Cancer Council Victoria, Melbourne, Victoria, Australia; Cancer Council Victoria, Australia.
References
- 1.Holford TR, Meza R, Warner KE, Meernik C, Jeon J, Moolgavkar SH, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311:164–171. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311:135–136. doi: 10.1001/jama.2013.285347. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. In: U.S. Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, editor. Atlanta, GA: 2014. [Google Scholar]
- 4.Dawkins L, Turner J, Roberts A, Soar K. 'Vaping' profiles and preferences: an online survey of electronic cigarette users. Addiction (Abingdon, England) 2013;108:1115–1125. doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- 5.King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17:219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, et al. Tobacco use among middle and high school students - United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2015;64:381–385. [PMC free article] [PubMed] [Google Scholar]
- 7.McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob Res. 2015;17:1195–1202. doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- 8.Kim AE, Lee YO, Shafer P, Nonnemaker J, Makarenko O. Adult smokers' receptivity to a television advert for electronic nicotine delivery systems. Tob Control. 2015;24:132–135. doi: 10.1136/tobaccocontrol-2013-051130. [DOI] [PubMed] [Google Scholar]
- 9.Kornfield R, Alexander GC, Qato DM, Kim Y, Hirsch JD, Emery SL. Trends in exposure to televised prescription drug advertising, 2003–2011. Am J Prev Med. 2015;48:575–579. doi: 10.1016/j.amepre.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J, Beard E, Kotz D, Michie S, West R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction. 2014;109:1531–1540. doi: 10.1111/add.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campaign for Tobacco Free Kids. Cigarette Tax Increases by State per Year 2001–2015. 2015 [Google Scholar]
- 12.Centers for Disease Control and Prevention. National Health Interview Survey. Early Release. 2014 [Google Scholar]
- 13.University of Michigan. Monitoring the Future Survey. Ann Arbor: 2015. [Google Scholar]
- 14.Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, Foulds J. Factors Associated With Electronic Cigarette Users' Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine Tob Res. 2015;17:1242–1246. doi: 10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(Suppl 3):iii3–iii9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 18.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17:704–709. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute for Global Tobacco Control. Country Laws Regulating E-cigarettes: A Policy Scan. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; 2015. May, [Google Scholar]
- 20.Federal Register. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. 2014 [PubMed] [Google Scholar]
- 21.Mabry PL, Olster DH, Morgan GD, Abrams DB. Interdisciplinarity and systems science to improve population health: a view from the NIH Office of Behavioral and Social Sciences Research. Am J Prev Med. 2008;35:S211–S224. doi: 10.1016/j.amepre.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nutt DJ, Phillips LD, Balfour D, Curran HV, Dockrell M, Foulds J, et al. Estimating the harms of nicotine-containing products using the MCDA approach. European addiction research. 2014;20:218–225. doi: 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 23.Levy DT, Mumford EA, Cummings KM, Gilpin EA, Giovino G, Hyland A, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: estimates of a panel of experts. Cancer Epidemiol Biomarkers Prev. 2004;13:2035–2042. [PubMed] [Google Scholar]
- 24.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res. 2003;63:6556–6562. [PubMed] [Google Scholar]
- 25.Burns D, Garfinkel L, Samet J. Smoking and Tobacco Control Monograph 8. Bethesda, MD: National Institutes of Health, National Cancer Institute; 1997. Changes in Cigarette-Related Disease Risks and Their Implication for Prevention and Control. [Google Scholar]
- 26.Rahman MA, Hann N, Wilson A, Mnatzaganian G, Worrall-Carter L. E-cigarettes and smoking cessation: evidence from a systematic review and meta-analysis. PLoS One. 2015;10:e0122544. doi: 10.1371/journal.pone.0122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirie K, Peto R, Reeves GK, Green J, Beral V Million Women Study C. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepper JK, Brewer NT. Electronic nicotine delivery system (electronic cigarette) awareness, use, reactions and beliefs: a systematic review. Tob Control. 2014;23:375–384. doi: 10.1136/tobaccocontrol-2013-051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vardavas CI, Filippidis FT, Agaku IT. Determinants and prevalence of e-cigarette use throughout the European Union: a secondary analysis of 26 566 youth and adults from 27 Countries. Tob Control. 2015;24:442–448. doi: 10.1136/tobaccocontrol-2013-051394. [DOI] [PubMed] [Google Scholar]
- 30.Saddleson ML, Kozlowski LT, Giovino GA, Hawk LW, Murphy JM, MacLean MG, et al. Risky behaviors, e-cigarette use and susceptibility of use among college students. Drug Alcohol Depend. 2015;149:25–30. doi: 10.1016/j.drugalcdep.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Delnevo CD, Giovenco DP, Steinberg MB, Villanti AC, Pearson JL, Niaura RS, et al. Patterns of Electronic Cigarette Use Among Adults in the United States. Nicotine Tob Res. 2015 Nov 2; doi: 10.1093/ntr/ntv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amato MS, Boyle RG, Levy D. How to define e-cigarette prevalence? Finding clues in the use frequency distribution. Tob Control. 2015 doi: 10.1136/tobaccocontrol-2015-052236. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17:127–133. doi: 10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giovenco DP, Lewis MJ, Delnevo CD. Factors Associated with E-cigarette Use: A National Population Survey of Current and Former Smokers. Am J Prev Med. 2014;47:476–480. doi: 10.1016/j.amepre.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozlowski L, Giovino G. Softening of monthly cigarette use in youth and the need to harden 1243 measures in surveillance. Prev Med Rep. 2014;1:53–55. doi: 10.1016/j.pmedr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner K. The remarkable decrease in cigarette smoking by American youth: further evidence. Prev Med Rep. 2015;2:259–261. doi: 10.1016/j.pmedr.2015.04.001. 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klesges RC, Ebbert JO, Morgan GD, Sherrill-Mittleman D, Asfar T, Talcott WG, et al. Impact of differing definitions of dual tobacco use: implications for studying dual use and a call for operational definitions. Nicotine Tob Res. 2011;13:523–531. doi: 10.1093/ntr/ntr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cobb NK, Abrams DB. The FDA, e-cigarettes, and the demise of combusted tobacco. N Engl J Med. 2014;371:1469–1471. doi: 10.1056/NEJMp1408448. [DOI] [PubMed] [Google Scholar]
- 39.Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction (Abingdon, England) 2013;108:1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- 40.Bauld L, MacKintosh AM, Ford A, McNeill A. E-Cigarette Uptake Amongst UK Youth: Experimentation, but Little or No Regular Use in Nonsmokers. Nicotine Tob Res. 2016;18:102–103. doi: 10.1093/ntr/ntv132. [DOI] [PubMed] [Google Scholar]
- 41.Eastwood B, Dockrell MJ, Arnott D, Britton J, Cheeseman H, Jarvis MJ, et al. Electronic cigarette use in young people in Great Britain 2013–2014. Public Health. 2015;129:1150–1156. doi: 10.1016/j.puhe.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Dutra LM, Glantz SA. Electronic Cigarettes and Conventional Cigarette Use Among US Adolescents: A Cross-sectional Study. JAMA Pediatr. 2014;168:610–617. doi: 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biener L, Song E, Sutfin EL, Spangler J, Wolfson M. Electronic Cigarette Trial and Use among Young Adults: Reasons for Trial and Cessation of Vaping. Int J Environ Res Public Health. 2015;12:16019–16026. doi: 10.3390/ijerph121215039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saddleson ML, Kozlowski LT, Giovino GA, et al. Risky behaviors, e-cigarette use and susceptibility of use among college students. Drug Alcohol Depend. 2015;149:25–30. doi: 10.1016/j.drugalcdep.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54:684–690. doi: 10.1016/j.jadohealth.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babineau K, Taylor K, Clancy L. Electronic Cigarette Use among Irish Youth: A Cross Sectional Study of Prevalence and Associated Factors. PLoS one. 2015;10:e0126419. doi: 10.1371/journal.pone.0126419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goniewicz ML, Zielinska-Danch W. Electronic cigarette use among teenagers and young adults in Poland. Pediatrics. 2012;130:e879–e885. doi: 10.1542/peds.2011-3448. [DOI] [PubMed] [Google Scholar]
- 48.Stenger N, Chailleux E. Survey on the use of electronic cigarettes and tobacco among children in middle and high school. Rev Mal Respir. 2016;33:56–62. doi: 10.1016/j.rmr.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Neff LJ, Arrazola RA, Caraballo RS, Corey CG, Cox S, King BA, et al. Frequency of Tobacco Use Among Middle and High School Students--United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:1061–1065. doi: 10.15585/mmwr.mm6438a1. [DOI] [PubMed] [Google Scholar]
- 50.Loukas A, Batanova M, Fernandez A, Agarwal D. Changes in use of cigarettes and non-cigarette alternative products among college students. Addict Behav. 2015;49:46–51. doi: 10.1016/j.addbeh.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, et al. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob Res. 2015;17:228–235. doi: 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coleman BN, Apelberg BJ, Ambrose BK, Green KM, Choiniere CJ, Bunnell R, et al. Association between electronic cigarette use and openness to cigarette smoking among US young adults. Nicotine Tob Res. 2015;17:212–218. doi: 10.1093/ntr/ntu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wills TA, Knight R, Williams RJ, Pagano I, Sargent JD. Risk factors for exclusive e-cigarette use and dual e-cigarette use and tobacco use in adolescents. Pediatrics. 2015;135:e43–e51. doi: 10.1542/peds.2014-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes K, Bellis MA, Hardcastle KA, McHale P, Bennett A, Ireland R, et al. Associations between e-cigarette access and smoking and drinking behaviours in teenagers. BMC Public Health. 2015;15:244. doi: 10.1186/s12889-015-1618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pentz MA, Shin H, Riggs N, Unger JB, Collison KL, Chou CP. Parent, peer, and executive function relationships to early adolescent e-cigarette use: a substance use pathway? Addictive behaviors. 2015;42:73–78. doi: 10.1016/j.addbeh.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummins SE, Zhu SH, Tedeschi GJ, Gamst AC, Myers MG. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2014;23(Suppl 3):iii48–iii53. doi: 10.1136/tobaccocontrol-2013-051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belendiuk KA, Molina BS, Donovan JE. Concordance of adolescent reports of friend alcohol use, smoking, and deviant behavior as predicted by quality of relationship and demographic variables. J Stud Alcohol Drugs. 2010;71:253–257. doi: 10.15288/jsad.2010.71.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell K, Keane H. All gates lead to smoking: The 'gateway theory', e-cigarettes and the remaking of nicotine. Soc Sci Med. 2014;119C:45–52. doi: 10.1016/j.socscimed.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, et al. Common liability to addiction and "gateway hypothesis": theoretical, empirical and evolutionary perspective. Drug Alcohol Depend. 2012;123(Suppl 1):S3–S17. doi: 10.1016/j.drugalcdep.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heckman J. Sample Selection Bias as a Specification Error Econometrica. 1979;47:153–166. [Google Scholar]
- 61.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations Between E-Cigarette Type, Frequency of Use, and Quitting Smoking: Findings From a Longitudinal Online Panel Survey in Great Britain. Nicotine Tob Res. 2015;17:1187–1194. doi: 10.1093/ntr/ntv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160–1168. doi: 10.1111/add.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 65.Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PloS one. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tobacco Use and Dependence Guideline Panel. Rockville (MD): US Department of Health and Human Services; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 67.Manzoli L, Flacco ME, Fiore M, La Vecchia C, Marzuillo C, Gualano MR, et al. Electronic Cigarettes Efficacy and Safety at 12 Months: Cohort Study. PloS one. 2015;10:e0129443. doi: 10.1371/journal.pone.0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the Electronic Cigarette: An Eight-Week Flemish Study with Six-Month Follow-up on Smoking Reduction, Craving and Experienced Benefits and Complaints. Int J Environ Res Public Health. 2014;11:11220–11248. doi: 10.3390/ijerph111111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health. 2014;14:1159. doi: 10.1186/1471-2458-14-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Brien B, Knight-West O, Walker N, Parag V, Bullen C. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis. 2015;13:5. doi: 10.1186/s12971-015-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Polosa R, Caponnetto P, Cibella F, Le-Houezec J. Quit and smoking reduction rates in vape shop consumers: a prospective 12-month survey. International journal of environmental research and public health. 2015;12:3428–3438. doi: 10.3390/ijerph120403428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNeill A, Brose L, Calder R, Hitchman S, Hajek P, McRobbie H. E-cigarettes: an evidence update. London, England: Public Health; 2015. [Google Scholar]
- 74.Fiore MC, Schroeder SA, Baker TB. Smoke, the chief killer--strategies for targeting combustible tobacco use. N Engl J Med. 2014;370:297–299. doi: 10.1056/NEJMp1314942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearson JL, Stanton CA, Cha S, Niaura RS, Luta G, Graham AL. E-Cigarettes and Smoking Cessation: Insights and Cautions From a Secondary Analysis of Data From a Study of Online Treatment-Seeking Smokers. Nicotine Tob Res. 2015;17:1219–1227. doi: 10.1093/ntr/ntu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutten LJ, Blake KD, Agunwamba AA, Grana RA, Wilson PM, Ebbert JO, et al. Use of e-Cigarettes among Current Smokers: Associations among Reasons for Use, Quit Intentions, and Current Tobacco Use. Nicotine Tob Res. 2015;17:1228–1234. doi: 10.1093/ntr/ntv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pulvers K, Hayes RB, Scheuermann TS, Romero DR, Emami AS, Resnicow K, et al. Tobacco Use, Quitting Behavior, and Health Characteristics among Current Electronic Cigarette Users in a National Tri-Ethnic Adult Stable Smoker Sample. Nicotine Tob Res. 2015;17:1085–1095. doi: 10.1093/ntr/ntu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39:491–494. doi: 10.1016/j.addbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 79.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33:1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–1766. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu SH, Gamst A, Lee M, Cummins S, Yin L, Zoref L. The Use and Perception of Electronic Cigarettes and Snus among the U.S. Population. PloS One. 2013;8:e79332. doi: 10.1371/journal.pone.0079332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang J, Tauras J, Chaloupka FJ. The impact of price and tobacco control policies on the demand for electronic nicotine delivery systems. Tob Control. 2014;23(Suppl 3):iii41–iii47. doi: 10.1136/tobaccocontrol-2013-051515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quisenberry AJ, Koffarnus MN, Hatz LE, Epstein LH, Bickel WK. The Experimental Tobacco Marketplace I: Substitutability as a Function of the Price of Conventional Cigarettes. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv230. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Friedman AS. How does electronic cigarette access affect adolescent smoking? J Health Econ. 2015;44:300–308. doi: 10.1016/j.jhealeco.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Burns DM, Anderson C, Major J, Vaughn J, Shanks T. Population Based Smoking Cessation Monograph No 12. Washington, DC: National Institutes of Health. National Cancer Institute; 2000. Cessation and cessation measures among daily adult smokers: National- and State-specific data. NIH Publication No. 00-4804, 2000.39. [Google Scholar]
- 86.Hyland A, Li Q, Bauer JE, Giovino GA, Steger C, Cummings KM. Predictors of cessation in a cohort of current and former smokers followed over 13 years. Nicotine Tob Res. 2004;6(Suppl 3):S363–S369. doi: 10.1080/14622200412331320761. [DOI] [PubMed] [Google Scholar]
- 87.Gravely S, Fong GT, Cummings KM, Yan M, Quah AC, Borland R, et al. Awareness, trial, and current use of electronic cigarettes in 10 countries: Findings from the ITC project. Int J Environ Res Public Health. 2014;11:11691–11704. doi: 10.3390/ijerph111111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yong HH, Borland R, Balmford J, McNeill A, Hitchman S, Driezen P, et al. Trends in E-Cigarette Awareness, Trial, and Use Under the Different Regulatory Environments of Australia and the United Kingdom. Nicotine Tob Res. 2015;17:1203–1211. doi: 10.1093/ntr/ntu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKee M, Chapman S, Daube M, Glantz S. The debate on electronic cigarettes. Lancet. 2014;384:2107. doi: 10.1016/S0140-6736(14)62366-7. [DOI] [PubMed] [Google Scholar]
- 90.Fairchild AL, Bayer R, Colgrove J. The Renormalization of Smoking? E-Cigarettes and the Tobacco "Endgame". The New England journal of medicine. 2013 doi: 10.1056/NEJMp1313940. [DOI] [PubMed] [Google Scholar]
- 91.Henriksen L. Comprehensive tobacco marketing restrictions: promotion, packaging, price and place. Tob Control. 2012;21:147–153. doi: 10.1136/tobaccocontrol-2011-050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cummings KM, Morley CP, Horan JK, Steger C, Leavell NR. Marketing to America's youth: evidence from corporate documents. Tob Control. 2002;11(Suppl 1):I5–17. doi: 10.1136/tc.11.suppl_1.i5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rose SW, Barker DC, D'Angelo H, Khan T, Huang J, Chaloupka FJ, et al. The availability of electronic cigarettes in U.S. retail outlets, 2012: results of two national studies. Tob Control. 2014;23(Suppl 3):iii10–iii16. doi: 10.1136/tobaccocontrol-2013-051461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carpenter CM, Connolly GN, Ayo-Yusuf OA, Wayne GF. Developing smokeless tobacco products for smokers: an examination of tobacco industry documents. Tob Control. 2009;18:54–59. doi: 10.1136/tc.2008.026583. [DOI] [PubMed] [Google Scholar]
- 95.PEETERS S, GILMORE AB. Transnational tobacco company interests in smokeless tobacco in Europe: analysis of internal industry documents and contemporary industry materials. PLoS Med. 2013;10:e1001506. doi: 10.1371/journal.pmed.1001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chaloupka FJ, Sweanor D, Warner KE. Differential Taxes for Differential Risks--Toward Reduced Harm from Nicotine-Yielding Products. N Engl J Med. 2015;373:594–597. doi: 10.1056/NEJMp1505710. [DOI] [PubMed] [Google Scholar]
- 97.Levy DT, Lindblom EN, Fleischer NL, Thrasher J, Mohlman MK, Zhang Y, et al. Public Health Effects of Restricting Retail Tobacco Product Displays and Ads. Tob Regul Sci. 2015;1:61–75. doi: 10.18001/trs.1.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen X, Lin F. Estimating Transitional Probabilities with Cross-Sectional Data to Assess Smoking Behavior Progression: A Validation Analysis. J Biomet Biostat. 2012;S1:4–8. doi: 10.4172/2155-6180.S1-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cobb C, Villanti A, Pearson J, Stanton C, Levy D, Abrams D, et al. A Markov model to estimate population-level patterns of cigarette and e-cigarette use. Tob Reg Sci. 2015;1:129–141. [Google Scholar]
- 100.Levy DT, Bauer JE, Lee HR. Simulation modeling and tobacco control: creating more robust public health policies. Am J Public Health. 2006;96:494–498. doi: 10.2105/AJPH.2005.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villanti AC, Vargyas EJ, Niaura RS, Beck SE, Pearson JL, Abrams DB. Food and Drug Administration regulation of tobacco: integrating science, law, policy, and advocacy. Am J Public Health. 2011;101:1160–1162. doi: 10.2105/AJPH.2011.300229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vugrin ED, Rostron BL, Verzi SJ, Brodsky NS, Brown TJ, Choiniere CJ, et al. Modeling the potential effects of new tobacco products and policies: a dynamic population model for multiple product use and harm. PloS one. 2015;10:e0121008. doi: 10.1371/journal.pone.0121008. [DOI] [PMC free article] [PubMed] [Google Scholar]