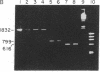
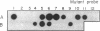Abstract
beta-Thalassemia is a hereditary disease caused by any of 90 different point mutations in the beta-globin gene. Specific populations generally carry a small number of mutations, the most common of which are those that are widely distributed regionally. The present study constitutes an extensive molecular characterization of this disease in a small, highly inbred ethnic group with a high incidence of beta-thalassemia--the Jews of Kurdistan. An unusual mutational diversity was observed. In 42 sibships 13 different mutations were identified, of which 3 are newly discovered: a C----A transversion at -88 to the cap site, a frameshift in codon 36/37, and an A----G transition in the polyadenylylation signal. Four of the mutations are unique to Kurdish Jews and have not been discovered in any other population. A fifth was found outside Kurdish Jews only in an Iranian from Khuzistan, a region bordering Kurdistan. Two-thirds of the mutant chromosomes carry the mutations unique to Kurdish Jews. We traced the origin of the mutations to specific geographic regions within Kurdistan. This information, supported by haplotype analysis, suggests that thalassemia in central Kurdistan (northern Iraq) has evolved primarily from multiple mutational events. In Turkish Kurdistan, the primary mechanism is genetic admixture with the local population. In Iranian Kurdistan, a founder effect appears to be partly responsible. We conclude that several evolutionary mechanisms contributed to the evolution of beta-thalassemia in this small ethnic isolate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abul-Hab J. Malaria vector survey in North Iraq. I. Provinces of Naynawah and Dhook. Bull Endem Dis (Baghdad) 1969 Nov;11(1):117–133. [PubMed] [Google Scholar]
- Bonné-Tamir B., Ashbel S., Bar-Shani S. Ethnic communities in Israel: the genetic blood markers of the Babylonian Jews. Am J Phys Anthropol. 1978 Nov;49(4):457–464. doi: 10.1002/ajpa.1330490405. [DOI] [PubMed] [Google Scholar]
- Cohen T. Genetic markers in migrants to Israel. Isr J Med Sci. 1971 Dec;7(12):1509–1514. [PubMed] [Google Scholar]
- GOLDSCHMIDT E., COHEN T. INTER-ETHNIC MIXTURE AMONG THE COMMUNITIES OF ISRAEL. Cold Spring Harb Symp Quant Biol. 1964;29:115–120. doi: 10.1101/sqb.1964.029.01.016. [DOI] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Horowitz A., Cohen T., Goldschmidt E., Levene C. Thalassaemia types among Kurdish Jews in Israel. Br J Haematol. 1966 Sep;12(5):555–568. doi: 10.1111/j.1365-2141.1966.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Boehm C. D. Molecular basis and prenatal diagnosis of beta-thalassemia. Blood. 1988 Oct;72(4):1107–1116. [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Dowling C. E., Waber P. G., Huang S., Lo W. H. The spectrum of beta-thalassemia genes in China and Southeast Asia. Blood. 1986 Oct;68(4):964–966. [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Markham A. F., Chapman C. R., Youssoufian H., Waber P. G. Quantification of the close association between DNA haplotypes and specific beta-thalassaemia mutations in Mediterraneans. Nature. 1984 Jul 12;310(5973):152–154. doi: 10.1038/310152a0. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr The thalassemia syndromes: molecular basis and prenatal diagnosis in 1990. Semin Hematol. 1990 Jul;27(3):209–228. [PubMed] [Google Scholar]
- Kinniburgh A. J., Maquat L. E., Schedl T., Rachmilewitz E., Ross J. mRNA-deficient beta o-thalassemia results from a single nucleotide deletion. Nucleic Acids Res. 1982 Sep 25;10(18):5421–5427. doi: 10.1093/nar/10.18.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L. Genetics of red cells and susceptibility to malaria. Blood. 1979 Nov;54(5):961–976. [PubMed] [Google Scholar]
- Niazi A. D. Malaria situation in Zakho Qadha--Mosul Liwa. Bull Endem Dis (Baghdad) 1968 Nov;10(1):185–194. [PubMed] [Google Scholar]
- Oppenheim A., Cohen S., Goldfarb A., Katzhendler J., Deutsch J., Rachmilewitz E. A. Detection of specific beta-globin mutations in Kurdish Jews with beta-thalassemia. Hemoglobin. 1988;12(1):31–38. doi: 10.3109/03630268808996880. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Antonarakis S. E., Kazazian H. H., Jr Base substitution at position -88 in a beta-thalassemic globin gene. Further evidence for the role of distal promoter element ACACCC. J Biol Chem. 1984 Jul 25;259(14):8679–8681. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Poncz M., Ballantine M., Solowiejczyk D., Barak I., Schwartz E., Surrey S. beta-Thalassemia in a Kurdish Jew. Single base changes in the T-A-T-A box. J Biol Chem. 1982 Jun 10;257(11):5994–5996. [PubMed] [Google Scholar]
- Ramsdale C. D., Haas E. Some aspects of epidemiology of resurgent malaria in Turkey. Trans R Soc Trop Med Hyg. 1978;72(6):570–580. doi: 10.1016/0035-9203(78)90005-6. [DOI] [PubMed] [Google Scholar]
- Rosatelli M. C., Oggiano L., Battista Leoni G., Tuveri T., Di Tucci A., Scalas M. T., Dore F., Pistidda P., Massa A., Longinotti M. Thalassemia intermedia resulting from a mild beta-thalassemia mutation. Blood. 1989 Feb;73(2):601–605. [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]