Abstract
Purpose
The purpose of this study was to correlate the clinical findings, RMI-4 index and frozen section, in cases of ovarian fibroma with the final histopathology.
Methods
This is a retrospective study of clinical and pathological features of 23 patients of ovarian fibroma. The patient’s age ranged from 34 to 66 years (mean—49 years). The most common presenting symptom was abdominal pain. On clinical examination, the mean size of ovarian tumor was 9.5 cm, CA-125 levels were found to be raised in 14 patients, and it was associated with ascites in 10 patients. USG showed a well-circumscribed mass (with a mean size of 14 cm), on the left side in 14 cases and on the right side in 9 patients. RMI-4 was calculated in all the patients, and it revealed the possibility of a benign histology in 17 patients. All patients underwent exploratory laparotomy with the removal of ovarian tumor followed by frozen section examination. All but one (22/23) patient had positive correlation among frozen section and final histopathological findings.
Result
Ovarian fibroma generally tends to occur in post-menopausal women. All the patients in our study of ovarian fibroma were symptomatic, with the presence of palpable mass in majority of patients. RMI-4 Index correlated very well with benign nature of disease. Frozen section has an invaluable role at surgery; fertility-conserving surgery is the choice in young women.
Conclusion
Clinical findings, RMI-4 Index and frozen section, play vital roles before and during surgery in cases of benign ovarian tumors.
Keywords: Ovarian fibroma, RMI-4 index, Frozen section, Histopathological findings
Introduction
Ovarian fibromas are solid tumors that belong to sex-cord stromal cell tumors of the ovary and comprise spindle shape fibroblastic cells and abundant collagen. They are the most common benign solid tumors of the ovary, accounting for 1–4 % of all ovarian tumors. Ovarian fibroma is often difficult to diagnose preoperatively and usually misdiagnosed as uterine myoma, because of the solid nature of the mass on clinical examination, and the ultrasound similarities between the two anomalies. They are seldom bilateral, and their masses vary in size from microscopic to extremely large ones. Although infrequently diagnosed prior to the age of 30, they can occur in any age group; however, the average age of diagnosis is the latter half of the fifth decade of life. It rarely occurs in prepubertal age group [1–3]. About 10–15 % of large ovarian fibroma can be associated with ascites due to escape of large amount of fluid from the edematous surface. Furthermore, 1 % of ovarian fibromas can present with Meigs syndrome, a condition which refers to triad of benign ovarian tumor, ascites, and pleural effusion (usually unilateral and right sided) [4–6].
In the minority of patients with ovarian fibroma, the serum level of CA125 is raised, which may lead to misdiagnosis of a malignant ovarian tumor. Risk of malignancy index (RMI index) is a valuable tool to differentiate between benign and malignant ovarian tumors based on Ca-125 levels, menopausal status, and ultrasonographic features. RMI-4 Index was developed by Yamamoto et al. in 2009, where the tumor size was added as the fourth variant to increase the accuracy of this modality [7].
The standard of treatment for ovarian fibroma is surgical removal of tumor followed by intraoperative frozen section. In all post-menopausal women and those who have completed their family, total abdominal hysterectomy (TAH) is the treatment of choice; however, owing to benign nature of the disease, a fertility-preserving surgery with unilateral salpingo-oophorectomy in young premenopausal women can be contemplated [8, 9].
With this background in mind, we conducted a study on clinico-pathological features and the surgical management of all patients presenting with ovarian fibroma treated at a tertiary care center.
Materials and Methods
This is a retrospective study of patients with ovarian fibroma, treated in single unit over a period of 8 years from January 1, 2007 till January 1, 2015. All medical records were reviewed. During the same period, 904 patients underwent surgery for ovarian mass. The frequency of ovarian fibroma is 2.5 %. We studied the demographic profile, clinical features, Ultrasonography (USG) findings, operative findings, and histopathological characteristics (both frozen and final histopathology). Risk of malignancy index—(RMI)-4—scores were calculated in all patients as follows:
where M denotes the Menopausal/post-menopausal status (premenopause-1, post-menopause-4) and U denotes the Ultrasonography. Features included bilaterality, multilocularity, solid component, ascites, intraabdominal metastasis (≤1 feature—1, >1 feature—4). S denotes the Single largest tumor diameter (<7 cm−1, >7 cm−2).
Cutoff value of 450 was taken for RMI-4 index, with score >450 being considered as malignant.
Data were analyzed by means of SPSS DATA, version 17.
Results
Mean age of patients was 49 years (ranging from 34 to 66 years). Seventeen patients (64 %) were post-menopausal. Patients presented with pain in abdomen (100 %), vomiting (17.4 %), dysmenorrhea (13 %), and post-menopausal bleeding (8.7 %). One patient presented with acute emergency in view of torsion of ovarian mass. In 20 cases (87 %), the adnexal mass could be identified as a firm-solid mass on bimanual examination, with sizes varying from 8 to 27 cm (mean-9.5 cm). Ascites was detectable on clinical examination in 10 (43.4 %) cases; however, in 2 patients (8.7 %), cytologically negative pleural effusion was also present, constituting Meigs syndrome (Figs. 1, 2).
Fig. 1.
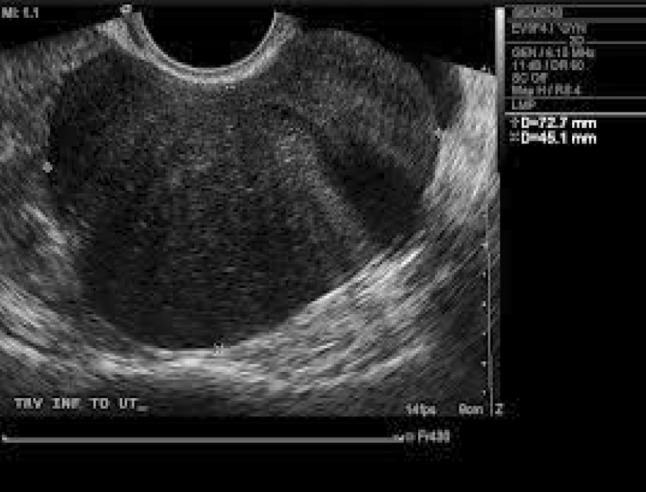
Abdominal sonography—left circumscribed ovarian hypoechoic tumor
Fig. 2.
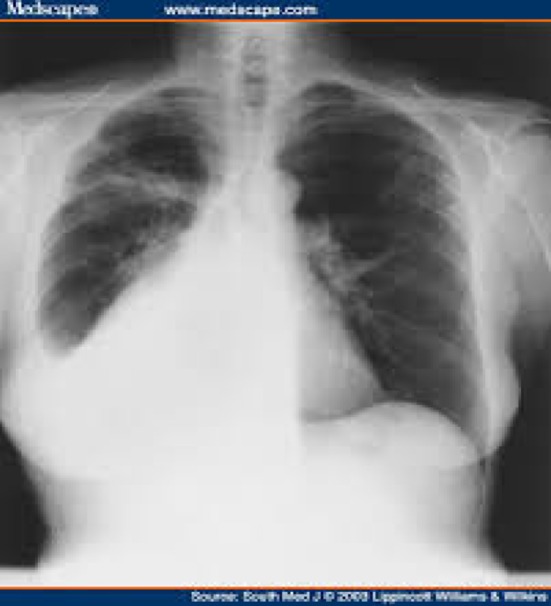
X-ray chest showing R-sided pleural effusion
CA-125 levels were raised in 14 cases (61 %): 11 were post-menopausal (11/17, and 3 were premenopausal (3/6).
USG was done in all cases, showing a unilateral well-circumscribed large hypoechoic tumor, on the left side in 14 cases (60.8 %) and on the right side in 9 (39.2 %) cases (Fig. 1). Ovarian masses were generally large, greater than 10 cm in 12 cases (52.3 %) and less than 10 cm in 11 cases (47.7 %) with a mean size of 14 cm. Solid-cystic components were seen in 16 (70 %) cases, cystic component was seen in four cases, and only solid component was seen in three cases. USG showed ETs (endometrial thicknesses) of 11 and 10 mm, respectively, for two post-menopausal patients who had presented with post-menopausal bleeding; however, endometrial biopsy was normal in both the patients.
On RMI-4 calculation, the score was <450 (cut off value) in 17 patients (74 %) with a mean score of 230; however, in 6 patients (26 %), the score was >450 with the maximum value being 970.
All patients underwent laparotomy, with removal of ovarian mass, followed by frozen section in all cases. After frozen section confirmation of benign tumor in all the patients, all post-menopausal women (n = 17) and 3 premenopausal women who had completed their family, underwent TAH with BSO. In the remaining three young premenopausal women, fertility sparing unilateral salpingo—oophorectomy was done. All patients had an uneventful post-operative period. In two patients with Meigs syndrome, an intercostal drainage (ICD) was inserted preoperatively and then removed on the 9th post-operative day after complete resolution of pleural effusion (Fig. 3).
Fig. 3.
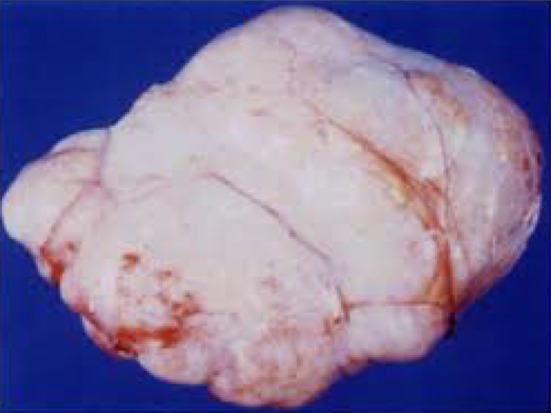
Operative specimen-ovarian fibroma
Eighteen cases (78.2 %) of ovarian fibroma and four cases (17.4 %) of fibrothecoma diagnosed on frozen section were confirmed on final histopathology; however, one case of fibrothecoma on frozen section turned out to be cystadenofibroma on final histology.
Discussion
Fibromas are rare benign tumors of gonadal—stromal cell origin, growing from connective tissue of ovarian cortex. They account for 1–4.7 % of ovarian tumors [10, 11]. In our study, the incidence was 2.5 %. These endocrine-inert tumors are reported to be seldom bilateral; however, all the patients in our study had unilateral tumor with 60.8 % being left sided [12]. Average age group for ovarian fibroma has been reported to be in later half of the fifth decade of life [12]. Our study has once again confirmed the findings with 73.9 % of women in this age group. All the patients were symptomatic in our study with a palpable mass in 87 % of women. Reported series in the literature has suggested the absence of palpable mass in up to 50 % of cases [10]. Ascites were detected in 43.4 % cases on clinical examination. Similar incidence of 37–40 % of ascites with large ovarian fibroma has been reported in the literature [13]. In our study, the average tumor size was 14 cm. Also, Two patients (8.7 %) in our study had Meigs Syndrome, while the reported incidence in the literature is in the range of 1–10 %. Broad spectrum of sonographic findings are observed, with the common one being the finding of solid-cystic mass. Echogenic mass with posterior shadowing is known to occur with fibrothecoma as reported by Stephenson and Lang.
Occasionally, high serum levels of CA-125 can be seen with ovarian fibroma, but they do regress after removal of the tumor. In our study, 64 % (11/17) of post-menopausal and 50 % (3/6) of premenopausal patients had CA-125 levels >35 IU/ml, and in all patients, CA-125 level regressed after surgery.
Our study has shown that the use of RMI-4 demonstrated a benign nature of the disease in 74 % (17 patients) of cases [7]. As per the literature, the diagnostic accuracy of RMI-4, compared to other 3 RMI indices is 71 %, and our study shows 74 % [14]. This has an important clinical implication for the centers where frozen section is not available, and still, the decision for fertility preserving surgery is warranted.
The treatment of ovarian fibroma has always been surgical, which comprises removal of tumor followed by intraoperative frozen section [8, 9]. Our study has demonstrated a very good correlation between frozen section and final histopathological diagnosis of ovarian fibroma. This is again clinically relevant as a conservative approach in young women in order to preserve the reproductive function that is essential.
Histopathological Varieties
Fibroma-spindly fibroblastic cells with abundant cytoplasm produce collagen. Fibroma–thecoma lipid-laden thecal cells are seen. Thecomas are composed only of theca cells; these lipid-laden cells may produce estrogens/androgens associated with endometrial CA in 21 % cases. Cellular fibromas are composed of cellular fibroblasts containing cytonuclearatypia and high number of mitosis—benign if <3 mitosis/hpf. Our study had 18 fibromas and 4 fibrothecomas (Figs. 4, 5, 6, 7).
Fig. 4.
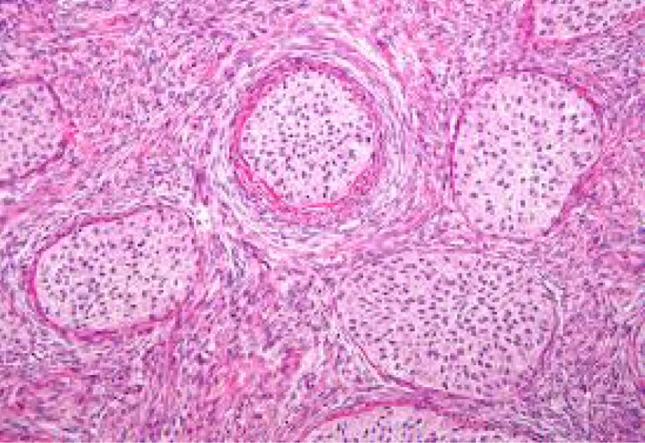
Fibroma of ovary—microscopic appearance
Fig. 5.
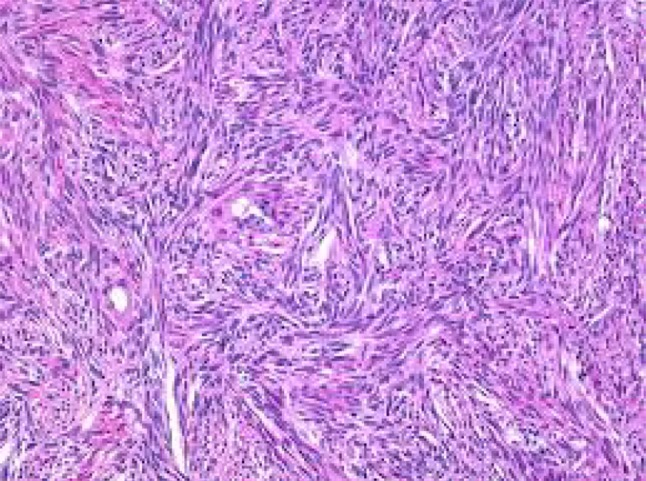
Fibrothecoma of ovary
Fig. 6.
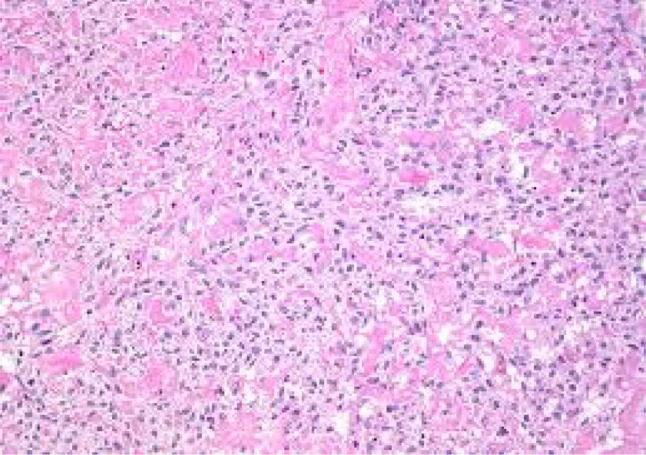
Thecoma of ovary
Fig. 7.
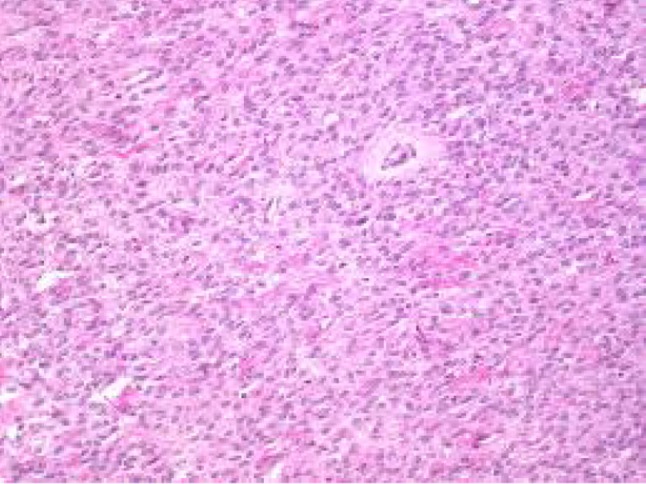
Fibrosarcoma of ovary
Conclusion
Ovarian fibromas are uncommon. Ovarian fibromas are solid tumors that belong to sex-cord stromal cell tumors of the ovary. They are mostly seen in post-menopausal women. Clinical and USG data remain the current available approach to form a diagnosis, followed by RMI-4 Index. In our study, RMI-4 Index values correlated with the findings of the final histopathological report in 17 patients (73.9 %). This can have important clinical implications for centers where frozen section facilities are not available. Frozen section helps in deciding the extent of surgery. Fertility-preserving surgery in young patients, TAH with BSO in menopausal women, is the current standard treatment.
Acknowledgments
Compliance with ethical requirements and Conflict of interest
Dr Nikhil S. Parwate, Dr Shilpa M. Patel, Dr Ruchi Arora, and Dr Monisha Gupta declare that they have no conflict of interest as far as this manuscript is concerned. Since this is a retrospective study of cases in the institution over 8 years, there was no trial on human subject. This article does not contain any studies with human or animal subjects.
Dr Nikhil S. Parwate
is a fellow in Gynaecologic Oncology in the Department of Gynaecologic Oncology, Gujarat Cancer and Research Institute, Ahmedabad, Gujarat. He is a colposcopist and a member of the American Society of Colposcopy and Cervical Pathology. He is also a certified and trained physician in pain and palliative care. He completed his post-graduate training in OBGY from KEM Hospital, Mumbai in 2004. He has been a managing committee member of Association of Fellow Gynaecologist (AFG) during 2008–2009.
Footnotes
Dr Nikhil S. Parwate is a Fellow in the Department of Gynaecologic Oncology at GCRI, Ahmedabad; Dr Shilpa M. Patel is a Professor and Head of Department in the Gynaecologic Oncology at GCRI, Ahmedabad; Dr Ruchi Arora is a Associate Professor in the Department of Gynaecologic Oncology at GCRI, Ahmedabad; Dr Monisha Gupta is a Associate Professor in the Department of Gynaecologic Oncology at GCRI, Ahmedabad.
References
- 1.Chen YJ, Hsieh CS, Eng HL, et al. Ovarian fibroma in a 7-month old infant: a case report and review of literature. Pediatr Surg Int. 2004;20:894–897. doi: 10.1007/s00383-004-1284-6. [DOI] [PubMed] [Google Scholar]
- 2.Howell C, Rogers D, Gable D, et al. Bilateral ovarian fibromas in children. J Pediatr Surg. 1990;25:690–691. doi: 10.1016/0022-3468(90)90366-H. [DOI] [PubMed] [Google Scholar]
- 3.Quint E, Smith YR. Ovarian surgery in premenarchal girls. J Pediatr Adolesc Gynecol. 1999;12:27–29. doi: 10.1016/S1083-3188(00)86617-6. [DOI] [PubMed] [Google Scholar]
- 4.Gargano G, De Lina M, Zito F, et al. Ovarian fibroma: our experience of 34 cases. Eur Radiol. 2004;14:798–804. doi: 10.1007/s00330-003-2060-z. [DOI] [PubMed] [Google Scholar]
- 5.Rouzier R, Berger A, Cugnenc PH. Meigssyndrome: is it possible to make preoperative diagnosis? J Gynecol Obstet Biol Reprod (Paris) 1998;27:517–522. [PubMed] [Google Scholar]
- 6.Novoa VA, Tenorio GF, Gomez PJ, et al. Meigs syndrome. A clinical case and review of literature. Ginecol Obstet Mexico. 1994;62:217–222. [PubMed] [Google Scholar]
- 7.Yamamoto Y, Yamada R, Oguri H, et al. Comparison of four malignancy risk indices in the preoperative evaluation of patients with pelvic masses. Eur J Obstet Gynaecol Reprod Biol. 2009;144:163–167. doi: 10.1016/j.ejogrb.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Sivanesaratnam V, Dutta R, Jayalakshmi P. Ovarian fibroma—clinical and histopathological characteristics. Int J Gynecol Obstet. 1990;33:243–247. doi: 10.1016/0020-7292(90)90009-A. [DOI] [PubMed] [Google Scholar]
- 9.Eltabbakh GH. Laparoscopic management of ovarian cyst : before choosing appropriate surgical procedure, a two-pronged approach—transvaginal ultrasonography and CA-125 assessment is best way to determine malignant or benign nature of ovarian mass. Ob Gyn. 2003;48:37–38. [Google Scholar]
- 10.Dockerty MB, Masson JC. Ovarian fibromas : clinical and pathologic study of 283 cases. Am J Obstet Gynecol. 1944;47:741. [Google Scholar]
- 11.Govan A. Ovarian tumors: clinical and pathological features. Clin Obstet Gynecol. 1976;3:89–157. [PubMed] [Google Scholar]
- 12.Martins SM, Klinger OJ. Bilateral ovarian fibromas occurring before menarche. Am J Obstet Gynecol. 1964;89:386–391. doi: 10.1016/0002-9378(64)90698-2. [DOI] [PubMed] [Google Scholar]
- 13.Young RH, Scully RE. Ovarian sex-cord stromal tumors: recent advances and current status. Clin Obstet Gynecol. 1984;11:93–134. [PubMed] [Google Scholar]
- 14.Yenen MC, Alanbay I, Aktürk E, et al. Comparison of risk of malignancy indices; RMI 1-4 in borderline ovarian tumor. Eur J Gynaecol Oncol. 2012;33(2):168–173. [PubMed] [Google Scholar]


