Introduction
Ovarian carcinosarcomas, also called malignant mixed mullerian tumors (MMMTs), are rare tumors with a poor prognosis [1–3]. These tumors can originate anywhere along the female genital tract and in the peritoneum; the most common site is the uterus, but they can also arise from the ovary, vagina, cervix and fallopian tubes. They commonly present in postmenopausal age group. MMMTs are aggressive tumors and respond poorly to treatment both surgical and chemotherapy. It is a poorly understood disease with rapid progression and dismal survival rate. Because of its rarity and the lack of centralized data collection, there is no uniform opinion on the optimal therapy for carcinosarcomas.
Case Report
A 46-year-old woman came to gynecological oncology department of IGIMS in February 2014 with the chief complain of severe pain in lower abdomen and foul-smelling vaginal discharge since 2 months. She had no relevant past medical history. She had undergone total abdominal hysterectomy and left salpingo-oophorectomy on May 31, 2013, for a large left adnexal soft tissue mass (preoperative USG report showed normal size uterus, endometrial thickness of 4 mm, cervix normal, right ovary normal size, 10 × 6.8 cm multiloculated soft tissue mass in left adnexal region.) at a private hospital in the periphery. Histopathology report was unavailable. She was apparently asymptomatic for 3 months and then had excessive bleeding P/V associated with foul-smelling discharge. USG then revealed marked air density in vaginal vault region large, predominantly soft tissue mass with cystic component of 74 × 69 mm adjacent to vault region with no collection in POD. Examination under anesthesia revealed an open vault with friable tissue visible through the vault, adnexa free and vault margins smooth. Biopsy of tissue from the vault revealed soft tissue sarcoma of high malignant potential. On immunohistochemistry, the tumor was copositive for cytokeratin and vimentin favoring possibilities of carcinosarcoma and epithelioid sarcoma. Tumor marker CA-125 was normal. The patient received eight cycles of chemotherapy (cisplatin + cyclophosphamide + doxorubicin + paclitaxel) and radiotherapy at our center. Her USG on February 26 revealed soft tissue of 4.4 × 3.9 cm in vault region with few cystic components. Her CT scan abdomen on March 4 showed approximately 4 × 4 × 4 cm size thick peripheral enhancing wall with central non-enhancing hypodense area in the vaginal vault region suggesting recurrence with central necrotic component. On laparotomy, approximately 5 cm × 4 cm mass was found over the vault adherent to bowel and bladder. There was minimal free fluid. Few deposits were found on omentum or peritoneum. Pelvic and para-aortic lymph nodes were not enlarged. Excision of the growth along with a margin of 1.5 cm of healthy tissue along with infracolic omentectomy and pelvic and para-aortic lymph node sampling was done. Peritoneal biopsies were taken from all quadrants. Histopathology revealed that ovarian sarcoma, omentum, lymph nodes and peritoneal biopsy were negative for malignant cells.
The patient had an uneventful postoperative period and was referred to medical oncology following surgery. Patient is still alive, healthy and in follow-up.
Discussion
MMMTs are considered to be a variant of poor risk, poorly differentiated epithelial ovarian cancer (metaplastic carcinoma). It accounts for 1–3 % of ovarian malignancies The staging system for ovarian and primary peritoneal cancer is also used for staging of MMMTs [3]. The adverse prognostic factors as stated by various studies are advanced age and stage, suboptimal cytoreduction, stromal predominant tumors and tumors with serous epithelial component [4, 5].
The clinical and radiological findings of ovarian carcinosarcoma are practically indistinguishable from other ovarian surface epithelial tumors making their preoperative suspicion or confirmation quite challenging. Tumor markers like CA-125 might not be elevated in all the cases. Even cytological analysis of ascitic fluid may not always reveal malignant component. In a study by Menon et al., preoperative raise of CA-125 was noted in 9 out of the 12 cases of ovarian carcinosarcoma. The hemorrhagic ascitic fluid revealed adenocarcinomatous component in four of their cases [4]. In our case, CA-125 was not elevated and neither was the ascitic fluid cytology positive for malignant cells.
On immunohistochemical examination, anti-cytokeratin monoclonal antibodies and anti-epithelial membrane antigen are useful for the detection of the epithelial component. To detect the mesenchymal components, vimentin monoclonal antibody, CD10, smooth muscle actin, desmin and myoglobin are useful. S100 protein polyclonal antibody is useful to detect chondroid or adipose tissue differentiation within the tumor. In our case, cytokeratin and vimentin were positive on IHC detecting epithelial component and mesenchymal component, respectively (Fig. 1).
Fig. 1.
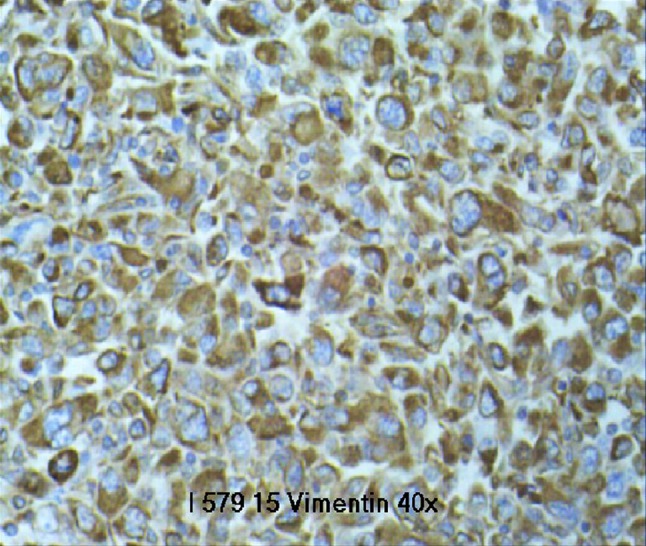
Vimentin positive
Case series of carcinosarcomas of the ovary, present in the literature, agree that maximal cytoreduction correlates with better progression-free survival and overall survival, and complete cytoreduction should be the goal of surgical treatment in such cases. According to the NCCN guidelines of 2015, optimal surgical debulking is recommended for MMMTs [2]. After complete surgical staging, patients with stage I to IV should have postoperative chemotherapy. They are treated with same chemotherapy as epithelial ovarian cancer [6].
Various chemotherapeutic regimes have included cisplatin alone; a combination of doxorubicin, ifosfamide, dacarbazine, cyclophosphamide, taxol; and other combinations. Response rates to chemotherapy are about 20 %.
Brown et al. compared prospectively the clinicopathologic features and outcomes of 65 patients with carcinosarcoma of the ovary to 746 patients with serous adenocarcinoma of the ovary. They reported a median survival of 14.8 months in patients with optimally debulked FIGO stage III ovarian carcinosarcomas as opposed to 3.1 months for suboptimally or non-debulked stage III disease. Their data show a significantly lower objective response rate to platinum therapy [7].
Further multicenter randomized control trial, or well-designed non-randomized studies are needed to compare treatment modalities and improve current knowledge of ovarian carcinosarcoma. Research in genetic and molecular signaling pathways might improve understanding of this tumor subtype and help determine the most effective cytotoxic regimen.
Dr. Sangeeta Pankaj
is interested in the areas of Gynecological Oncology, teaching and research work. She is regularly doing screening for cervical and breast cancers. She has dedicated her services solely to prevent and alleviate sufferings of women with Gynecological Malignancies.
Compliance with Ethical Standards
Conflict of interest
Dr. Sangeeta Pankaj, Dr. Syed Nazneen, Dr. Anjili Kumari, Dr. Simi Kumari, Dr. Vijayanand Choudhary and Dr. Vivek Kumar Roy declare that they have no conflict of interest and they have not received any grant.
Footnotes
Sangeeta Pankaj: Associate Professor, Gynaecological Oncology, RCC, IGIMS, Patna. Syed Nazneen: S.R., Gynaecological Oncology, RCC, IGIMS, Patna. Anjili Kumari: S.R., Gynaecological Oncology, RCC, IGIMS, Patna. Simi Kumari: S.R., Gynaecological Oncology, RCC, IGIMS, Patna. Vijayanand Choudhary: Associate Professor, Pathology, IGIMS, Patna. Vivek Kumar Roy: S.R., Surgery, RCC, IGIMS, Patna.
References
- 1.George EM, Herzog TJ, Neugut AI, et al. Carcinosarcoma of the ovary: natural history, patterns of treatment, and outcome. GynecolOncol. 2013;131:42–45. doi: 10.1016/j.ygyno.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Carmen MG, Birrer M, Schorge JO. Carcinosarcoma of the ovary: a review of the literature. GynecolOncol. 2012;125:271–277. doi: 10.1016/j.ygyno.2011.12.418. [DOI] [PubMed] [Google Scholar]
- 3.Mano MS, Rosa DD, Azambuja E, et al. Current management of ovarian carcinosarcoma. Int J Gynecol Cancer. 2007;17(2):316–324. doi: 10.1111/j.1525-1438.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 4.Menon S, Deodhar K, Rekhi B, et al. Clinico-pathological spectrum of primary ovarian malignant mixed mullerian tumors (OMMMT) from a tertiary cancer institute: a series of 27 cases. Indian J Pathol Microbiol. 2013;56:365–371. doi: 10.4103/0377-4929.125293. [DOI] [PubMed] [Google Scholar]
- 5.Athavale R, Thomakos N, Godfrey K, et al. The effect of epithelial and stromal tumor components on FIGO stages III and IV ovarian carcinosarcomas treated with primary surgery and chemotherapy. Int J Gynecol Cancer. 2007;17:1025–1030. doi: 10.1111/j.1525-1438.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 6.Rauh-Hain JA, Growdon WB, Rodriguez N, et al. Carcinosarcoma of the ovary: a case–control study. Gynecol Oncol. 2011;121:477–481. doi: 10.1016/j.ygyno.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Brown E, Stewart M, Rye T, et al. Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer. 2004;100(10):2148–2153. doi: 10.1002/cncr.20256. [DOI] [PubMed] [Google Scholar]


