Abstract
Background and Objectives:
The mobile cecum is an embryologic abnormality and has been associated with functional colon disease (chronic constipation and irritable bowel syndrome). However, unlike functional disease, the primary treatment is operative, using laparoscopic cecopexy. We compare the epidemiology and pathophysiology of mobile cecum syndrome and functional colon disease and propose diagnostic and treatment guidelines.
Method:
This study was a case–control series of 15 patients who underwent laparoscopic cecopexy. Age, gender, recurrent abdominal pain, and constipation based on Rome III criteria were assessed. Ileocecal–appendiceal unit displacement was graded as follows: I (cecum retroperitoneal or with little mobility); II (wide mobility, crossing the midline); and III (maximum mobility, reaching the left abdomen). Patients with Grades II and III underwent laparoscopic cecopexy. The clinical outcomes were evaluated according to modified Visick's criteria, and postoperative complications were assessed according to the Clavien-Dindo classification.
Results:
The mean age was 31.86 ± 12.02 years, and 13 patients (86.7%) were women. Symptoms of constipation and abdominal pain were present in 14 (93.3%) and 11 (73.3%), respectively. Computed tomography was performed in 8 (53.3%) patients. The mean operative time was 41 ± 6.66 min. There were no postoperative infections. One (7.8%) patient was classified as Clavien Dindo IIIb and all patients were classified as Visick 1 or 2.
Conclusion:
Many patients with clinical and epidemiological features of functional colon disease in common in fact have an anatomic anomaly, for which the treatment of choice is laparoscopic cecopexy. New protocols should be developed to support this recommendation.
Keywords: Cecal volvulus, Laparoscopic cecopexy, Mobile cecum
INTRODUCTION
Mobile cecum syndrome is an embryologic abnormality (agenesis of the cecal mesocolon), resulting from defective right colonic mesenteric attachment at the lateral peritoneum. The cecum and ascending colon become redundant and can move freely in the abdominal cavity, causing partial obstruction (kinking, torsion, or intrusion) and symptoms.1–3 The incidence ranges from 10 to 20%, and there are reports indicating its relationship with functional colon disease, as it presents with chronic constipation (CC) and irritable bowel syndrome (IBS-C). Other symptoms such as recurrent pain, distension, flatulence, and dyspareunia are also present in the syndrome.4–6
Because of the low specificity of symptoms and imaging with computed tomography (CT) and magnetic resonance imaging (MRI),7 the use of McBurney and Rockey-Davis incisions may underestimate the diagnosis. Insufficient knowledge about the pathophysiology and the lack of a laparoscopic ileocecal–appendiceal unit displacement score indicate the need for further study. The criteria for distinguishing between functional disease and an anatomical anomaly that can benefit from laparoscopic cecopexy remain unclear. Thus, patients may be misdiagnosed with a functional disease or undergo nontherapeutic appendectomy, while their true illness remains unmanaged. Minimally invasive surgery is therefore a definite advancement for the in situ recognition of mobile cecum syndrome. A systematic approach to the abdominal cavity, as well as improved visibility and easier ileocecal–appendiceal unit mobilization, has contributed to the understanding of this abnormality.
Accordingly, this report discusses a case–control series of patients with mobile cecum syndrome, with and without acute appendicitis, who underwent laparoscopic cecopexy. The secondary outcome was an analysis of the epidemiologic and pathophysiologic aspects of this malformation. In addition, laparoscopic diagnostic and treatment guidelines are proposed.
METHOD
This case–control series included 13 patients with recurrent abdominal pain, with or without acute appendicitis, who underwent diagnostic laparoscopy and cecopexy at Monte Sinai Hospital, Juiz de Fora, Minas Gerais State, Brazil, between March 2013 and June 2015. Two additional cases were recruited from a literature review, to complete the series of 15 patients. The following clinical data were collected: age, gender, recurrent abdominal pain, and constipation. Constipation was defined according to the Rome III criteria, with at least 2 of the following symptoms over the preceding 3 months: fewer than 3 bowel movements per week, straining, lumpy or hard stools, or manual assistance necessary to defecate. Imaging findings (ultrasound [USG], computed tomography [CT], and nuclear magnetic resonance [NMR]) reported as acute appendicitis, suspected mobile cecum, or inconclusive were recorded in the chart. All participating patients provided signed informed consent.
Patients underwent general anesthesia and received antimicrobial prophylaxis (2 g amoxicillin/clavulanate) as a single injected dose. The procedure was performed with the patient supine in the Trendelenburg position, with slight left laterality (30°). Three ports were placed in the abdominal cavity after pneumoperitoneum was attained, with the standard Veress needle technique. Ports were placed at the umbilicus and in the suprapubic (5 mm) and left lower quadrant (12 mm) regions.
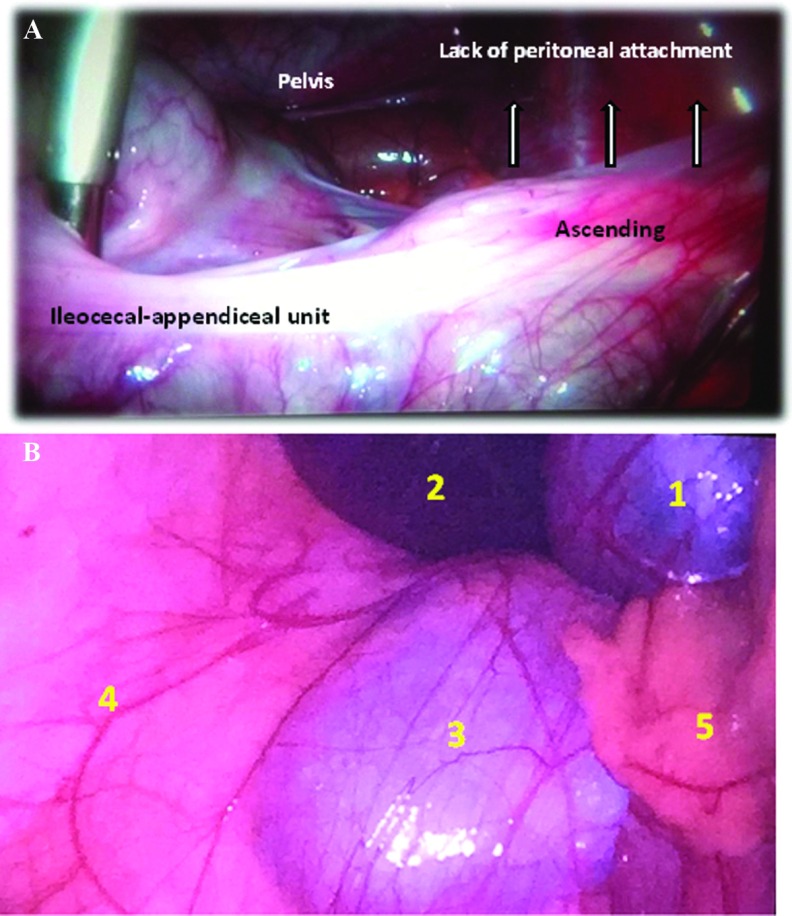
During initial exploration, the appendix was diagnosed as either normal or inflamed (acute appendicitis). Next, the ileocecal–appendiceal unit was mobilized with a grasper, with gripping and traction on the cecum. Displacement was categorized according to right peritoneal fixation and mobility (Figure 1; Table 1).
Figure 1.
A, View of mobile cecum syndrome on laparoscopy. Note the lack of lateral and posterior peritoneal attachments and the mobility of the ileocecal-appendiceal unit, with crossing of the midline (Grade II). B, Laparoscopic view of mobile cecum classified as Grade III (total lack of peritoneal attachment). Anatomic structures are 1 (gallbladder), 2 (liver) 3 (right kidney), 4 (lateral abdominal wall), and 5 (ileocecal–appendiceal unit displaced medially).
Table 1.
Laparoscopic Grading of Mobile Cecum Syndrome
| Grade | Criteria |
|---|---|
| Grade I | Retroperitoneal or little mobility and not reaching the midline |
| Grade II* | Wide mobility, crosses the midline, absence of fixation of the cecum and part of the ascending colon, and able to rotate about its own axis |
| Grade III* | Maximum mobility, reaches the left abdomen, total absence of fixation, and able to rotate about its own axis |
n = 15. Grades were assigned according to ileocecal–appendiceal unit mobilization, peritoneal attachment, and the ability to rotate about its own axis. Proposed grading scheme by Gomes et al. Laparoscopic grading score of mobile cecum syndrome.
Able to rotate about its own axis.
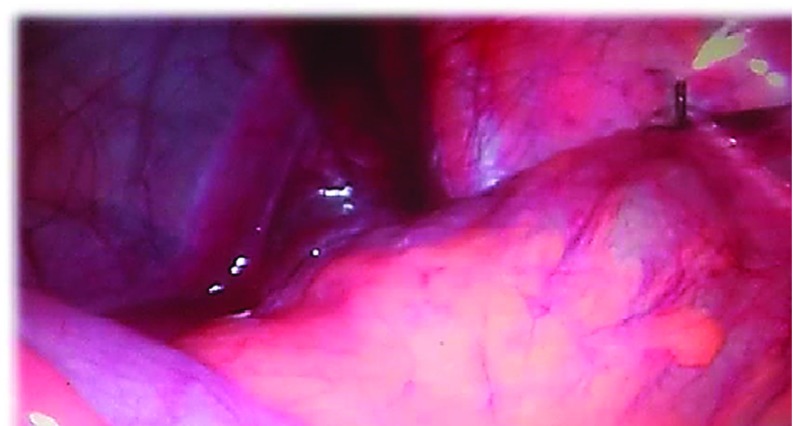
Laparoscopic appendectomy was performed in all patients by use of mesoappendiceal endocoagulation, and the appendiceal stump was closed with T400 metal clips. All patients diagnosed with mobile cecum Grade II or III underwent laparoscopic cecopexy with 2 or 3 knots (PDS 3-0; Ethicon, Somerville, New Jersey, USA) in the right paracolic gutter (midaxillary line) and lateral taenia of the cecum and descending colon (Figure 2). Dixon and Meyer's approach (peritoneal flap) was not performed in this series.8
Figure 2.
Laparoscopic view of cecopexy. Knot position used as reference for the midaxillary line and lateral taenia of the cecum (Grade II).
The patients were discharged in good health, afebrile, and tolerating oral intake. At 7 and 30 d after surgery, all patients were interviewed to evaluate their clinical course, recovery, and postoperative complications. All appendices were sent for anatomic pathology study. Postoperative clinical outcomes were evaluated according to modified Visick's classification: 1, asymptomatic; 2, mild (intermittent symptoms that are easily controlled); 3, moderate (symptoms without substantial interference with lifestyle); and 4, unsatisfactory (recurrent symptoms that greatly interfere with lifestyle).9
The postoperative complications were graded according to the Clavien-Dindo classification (Table 2),10 which was used to assess treatment success and effectiveness. The SPSS database, v. 13.0 for Windows (IBM, Armonk, New York, USA) was used to compile the studied parameters, followed by descriptive exploratory statistical analysis using crossed variables.
Table 2.
Epidemiological Aspects of Patients With Diagnosis of Mobile Cecum Who Underwent Laparoscopic Cecopexy
| Parameter (n = 15) | Gender (%) | Age (y) | Pain (%) | Rome III (%) |
|---|---|---|---|---|
| Female/male age (median-SD) | 13 (86.7 ± 13.3) | 31.86 ± 12.02 | ||
| Recurrent pain, n (%) | 14 (93.3 ± 6.7) | |||
| Constipation (%) | 11 (73.3 ± 26.7) |
RESULTS
Epidemiologic data show that patient ages ranged from 14 to 57 year (mean, 31.86 ± SD 12.02), with predominance in the second and third decades; 13 (86.7%) were women. The symptoms of constipation and recurrent abdominal pain were present in 14 (93.3%) and 11 (73.3%) patients, respectively (Table 2).
Imaging was performed in all patients: CT in 8 (53.3%), followed by MRI in 4 (26.7%). Imaging was suggestive of mobile cecum syndrome in 7 (43.7%) and acute appendicitis in 6 (40%) (Table 3).
Table 3.
Imaging Studies Requested in Patients With Mobile Cecum Who Underwent Laparoscopic Cecopexy
| Parameter | n (%) | Findings, n (%) |
|---|---|---|
| USG | 2 (13.3) | Appendicitis, 6 (40) |
| CT* | 8 (53.3) | Mobile cecum, 7 (43.7) |
| MRI | 4 (26.7) | Inconclusive, 2 (13.3) |
| Enema | 1 (6.7) | |
| Total | 15 (100) |
The operative time was evaluated in 13 of 15 patients and ranged from 28 to 48 min (mean, 41 min ± SD 6.66). No wound or intra-abdominal infections were diagnosed in this series. Only 1 (7.8%) patient was graded as Clavien-Dindo IIIb, because of appendiceal artery bleeding, requiring repeat laparoscopy in the postoperative period. All patients were classified as modified Visick score 1 and 2. i. e. asymptomatic or had only mild symptoms following laparoscopic cecopexy. These results are shown in Table 4.
Table 4.
Postoperative Outcomes in 13 Patients With Diagnosis of Mobile Cecum Who Underwent Laparoscopic Cecopexy
DISCUSSION
Little is known about the impact of laparoscopy in the incidence, diagnosis, and treatment of mobile cecum syndrome. Much of the information available is derived from laparotomy and is mostly based on Wolfer et al11 who found an incidence of 11.2% in autopsy specimens. Even less studied are the neurohormonal mechanisms that could explain the similarity of symptoms in mobile cecum syndrome and 2 other functional disorders that present with constipation (CC, IBS-C), as well as its prevalence in females.4 This study reports the largest series published to date on mobile cecum syndrome managed by laparoscopy. Significant data were recorded, discussed, and new proposals made, most notably the following.
A significant number of patients with anatomical disease (mobile cecum) are diagnosed and treated for functional disease, when in fact, the correct and definitive treatment should be surgical: laparoscopic cecopexy.
The study findings suggest that female patients in the second and third decades of life, with recurrent abdominal pain and constipation, should undergo evaluation for functional disease (CC or IBS), including CT. However, during the examination, the radiologist must “think” (based on the physician's request) and “act” (through positioning) with the presumptive diagnosis of mobile cecum. Thus, bias can be reduced, and imaging accuracy can be increased. Abdominal CT is the method of choice for diagnosis of mobile cecum syndrome, and it was used in 8 (53.3%) patients in this series. However, CT was only able to diagnose the disease in 43.7% of the cases. The most significant findings were redoubled or redundant colon above the iliac crest, deviation toward the midline, and elongated and over-rotated cecocolon in the lower left quadrant or lying transversely across the lower abdomen and crossing the midline to the left upper abdomen.12 MRI offered no advantages over CT. Santos et al,13 using pre-established protocols, diagnosed mobility of the cecum and ascending colon in 72.5% of cases in a series of patients with CC.13
We propose a mobile cecum syndrome score, based on the mobility of the ileocecal–appendiceal unit and the presence of symptoms (Table 1). All patients classified as Grade II or III had recurrent abdominal pain, and 11 (73.3%) also had constipation. It is noteworthy that cecal volvulus, which can be caused by a mobile cecum, is a rare phenomenon, but is more frequent in advanced cases. Moreover, if volvulus occurs, intestinal ischemia can develop and emergency surgery is necessary.1 Despite the small sample size (13 patients) and the addition of 2 other reports in the literature,14,15 all patients underwent laparoscopy. This study therefore added new insights and increased knowledge of the syndrome.
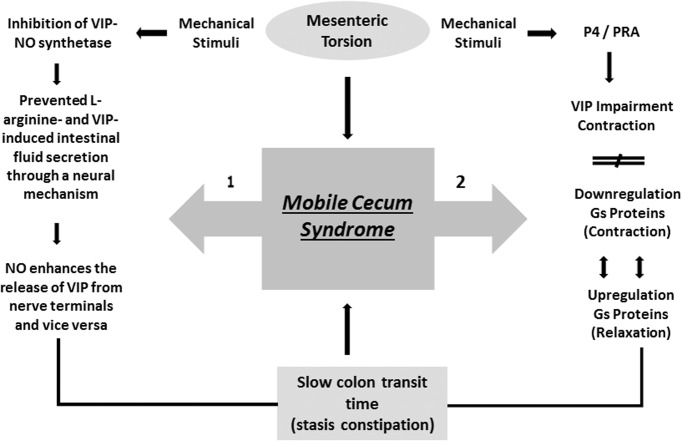
The higher frequency of mobile cecum syndrome in females suggests the likelihood of hormonal factor involvement during fetal formation, particularly during stretching from the proximal portion of the midgut and attachment at the right parietocolic gutter. Cheng et al16 reported that progesterone receptor A, which mediates vasoactive intestinal polypeptide (VIP) inhibition of intestinal contraction (via impairment of agonist-induced contraction), plays an important pathophysiological role in slow intestinal transit and constipation in both women and guinea pigs. Accordingly, the impairment is associated with imbalance between G-proteins that mediate contraction (downregulation) and G-proteins that mediate relaxation (upregulation). In addition, these changes result from overexpression of progesterone (P4) in the large bowel, rendering the smooth muscle cells more responsive to physiologic P4 concentrations. Cheng et al concluded that P4 upregulation of G-proteins increases VIP-induced inhibition of contraction mediated by progesterone receptor A (PR-A) (Figure 3).
Figure 3.
Proposed pathophysiological pathways,1–2 that explain female predominance and frequency of constipation symptoms in mobile cecum syndrome. Note that both pathways converge at the same point (slow colon transit time).15,16
On the other hand, Mourad et al17 in a rat study, reported the interplay between nitric oxide (NO) and VIP in inducing fluid secretion in the small bowel. They concluded that NO and VIP interact both with each other and with the intestinal submucosal neural network and play a role in neuromuscular gut function and fluid secretion. NO and VIP are potent secretagogues; specific stimuli can cause inhibition of VIP and NO synthetase, and block l-arginine- and VIP-induced fluid release. This highlights the fact that NO enhances the reciprocal release of intestinal secretions from terminal nerves (Figure 3).17 In addition, abnormalities in both neurotransmitters and a decreased number of Cajal cells have been described in patients with CC.18 These facts may explain the presence of recurrent abdominal pain (93.3%) and constipation (73.3%) as the predominant symptoms in this series.
It is therefore possible that frequent mechanical stimulation (torsion) and compensatory mechanisms (spasm and increased intraluminal pressure), which allow the passage of enteric content into distal segments, can alter the number and structure of Cajal cells and induce regional modifications in synthesis or receptor activity of VIP and NO. In theory, a decrease in these substrates responds to regional circulatory disorders, spasms, constipation, and pain, and represents a second pathway for the slowing of intestinal transit. It is noteworthy that both mechanisms converge at the same point (ie, reduction of intestinal contractility), leading to enteric secretion and constipation in a vicious cycle. Furthermore, the interaction between P4 and VIP modulates VIP expression in several organs, including the digestive system11 (Figure 3).
Thus, it is possible that mobile cecum syndrome follows one or both pathophysiological pathways and that mechanical stimulation (microtrauma) is a triggering factor. Moreover, the higher frequency of the disease in women may be due to the activation of both pathways (Figure 3). Another possibility is that CC, IBS-C, and mobile cecum syndrome represent a spectrum of the same disease with different anatomic and functional features and can present with symptom variability. Data from the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) study19 showed that few patients with CC fulfill the Rome III criteria for IBS-C or CC. Our study supports this view and showed that a significant proportion of patients with a diagnosis of functional intestinal disease may have an anatomic anomaly subject to operative treatment. Despite a small sample size, all patients who underwent cecopexy were monitored and reported improvement in abdominal pain, constipation, and quality of life (Tables 2, 4).
Last, the study showed that the epidemiological profile of mobile cecum syndrome is similar to that of functional bowel disease (CC and IBS-C). Despite the low specificity of imaging, the characteristic findings enable an increase in diagnostic accuracy. The study also proposed a cause-and-effect relationship between the anatomical anomaly and the neurohumoral pathways involved. Minimally invasive surgery is the procedure of choice for diagnosing, grading, and treating mobile cecum syndrome, and laparoscopic cecopexy is the ultimate key to this complex and difficult diagnosis.
Contributor Information
Carlos Augusto Gomes, Department of Surgery.
Cleber Soares, Jr, Department of Surgery.
Fausto Catena, Department of General Surgery, Maggiore Hospital, Parma, Italy..
Salomone Di Saverio, Department of Surgery, Maggiore Hospital, Bologna, Italy..
Massimo Sartelli, Department of Surgery, Macerata Hospital, Macerata, Italy..
Camila Couto Gomes, Department of Surgery, Israel Pinheiro Governor Hospital, Belo Horizonte, Minas Gerais, Bazil..
Felipe Couto Gomes, Department of Internal Medicine, Therezinha de Jesus University Hospital, Faculty of Medical and Health Sciences of Juiz de Fora (SUPREMA), Minas Gerais, Brazil..
References:
- 1. Bryne WJ, D'Harlingue AE. The gastrointestinal system: general considerations. In: Taeusch W, Ballard RA, Avery ME, eds. Schaffer and Avery's Diseases of the Newborn. 6th ed Philadelphia: WB Saunders Co; 1988;1054–1062. [Google Scholar]
- 2. Smith WR, Goodwin JN. Cecal volvulus. Am J Surg. 1973;126:215–222. [DOI] [PubMed] [Google Scholar]
- 3. Rogers RL, Harford FJ. Mobile cecum syndrome. Dis Colon Rectum. 1984;27:399–402. [DOI] [PubMed] [Google Scholar]
- 4. Santos JCM., Junior Alteração congênita de fixação do segmento ceco ascendente dá sintomas iguais ao da síndrome do cólon irritável. Rev Bras Coloproct 2005;25361–369. [Google Scholar]
- 5. Meyers JR, Heifetz CJ, Baue AE. Cecal volvulus. Arch Surg. 1972;104:594–599. [DOI] [PubMed] [Google Scholar]
- 6. Ingelfinger FJ. Intermittent volvulus of the mobile cecum. Arch Surg. 1942;45:156–163. [Google Scholar]
- 7. Heller MT, Bhargava P. MDCT of acute cecal conditions. Emerg Radiol. 2014;21:75–82. [DOI] [PubMed] [Google Scholar]
- 8. Dixon CF, Meyer AC. Volvulus of the cecum. Surg Clin North Am. 1948;28:953–963. [DOI] [PubMed] [Google Scholar]
- 9. Rijnhart-De Jong HG1, Draaisma WA, Smout AJ, Broeders IA, Gooszen HG. The Visick score: a good measure for the overall effect of antireflux surgery? Scand J Gastroenterol. 2008;43:787–793. [DOI] [PubMed] [Google Scholar]
- 10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolfer JA, Beato LE, Anson BJ. Volvulus of the cecum: anatomical factors in its etiology: report of a case. Surg Gynecol Obstet. 1942;74:882–894. [Google Scholar]
- 12. Tirol FT. Recurrent cecocolic torsion: radiological diagnosis and treatment. JSLS. 2003;7:23–31. [PMC free article] [PubMed] [Google Scholar]
- 13. Santos CHM, Marão RF, Bezera FMM. Síndrome do ceco móvel-manifestações clínicas e avaliação radiológica da mobilidade do ceco e cólon ascendente em pacientes com queixa de constipação crônica. Rev Bras Coloproct. 2007;27:174–178. [Google Scholar]
- 14. Kakizoe S. Laparoscopic cecoplication for mobile cecum. Endoscopy. 1997;29:227–228. [DOI] [PubMed] [Google Scholar]
- 15. Tsushimi T, Kurazumi H, Takemoto Y, et al. Laparoscopic cecopexy for mobile cecum syndrome manifesting as cecal volvulus: report of a case. Surg Today. 2008;38:359–362. [DOI] [PubMed] [Google Scholar]
- 16. Cheng L, Biancani P, Behar J. Progesterone receptor a mediates VIP inhibition of contraction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G433–G439. [DOI] [PubMed] [Google Scholar]
- 17. Mourad FH, Barada KA, Abdel-Malak N, et al. Interplay between nitric oxide and vasoactive intestinal polypeptide in inducing fluid secretion in rat jejunum. J Physiol. 2003;550:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao JS, Tong WD. [Pathophysiology of slow transit constipation]. (In Chinese). Zhonghua Wei Chang Wai Ke Za Zhi [Chin J Gastrointest Surg.]. 2012;15:758–760. [PubMed] [Google Scholar]
- 19. Enck P, Leinert J, Smid M, Köhler T, Schwille-Kiuntke J. Functional constipation and constipation-predominant irritable bowel syndrome in the general population: data from the GECCO Study. Gastroenterol Res Pract. 2016;2016:3186016. [DOI] [PMC free article] [PubMed] [Google Scholar]