Abstract
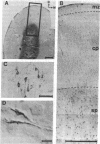
In the mammalian cerebral cortex, neurons destined for layers 2-6 are generated only after the period of genesis for a group of transient neurons that populate the subplate and marginal zones. Although a number of molecular markers for the subplate zone exist, most are also expressed by other cell populations in the cortical plate. To begin to study molecular properties of the subplate, we generated monoclonal antibodies against homogenates of cat cortical subplate zone. One monoclonal antibody, termed subplate 1 (SP1), recognized a polypeptide of 56 kDa. This antigen was strongly expressed within the subplate neurons only during a 3-week period beginning at birth and extending until 3 weeks after birth. From postnatal day 1, the number of SP1-immunoreactive neurons below the visual cortex increased until the end of second postnatal week and then declined thereafter. This period coincides with the period when a majority of the subplate neurons undergo naturally occurring cell death. The antigen was not expressed by subplate neurons surviving in the adult white matter. At the peak of antigen expression, 14% or less of the immunoreactive neurons also coexpressed gamma-aminobutyric acid, somatostatin, or neuropeptide Y. Biochemical and immunocytochemical properties of the SP1 antigen were also compared with the Alz-50 antigen (A68), a marker for dying neurons. On Western blots, SP1- and Alz-50-reactive polypeptides were selectively enriched in cytosolic fractions of kitten cerebral cortex, but each marker recognized different molecular weight polypeptides. In tissue sections many subplate, cortical plate, and layer 1 neurons were Alz-50 immunoreactive. In contrast, a rarer subpopulation of neurons restricted to the subplate was labeled by SP1. We propose that the SP1 antigen is a protein expressed within dying cortical subplate neurons, at the commencement of cell death.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ghoul W. M., Miller M. W. Transient expression of Alz-50 immunoreactivity in developing rat neocortex: a marker for naturally occurring neuronal death? Brain Res. 1989 Mar 6;481(2):361–367. doi: 10.1016/0006-8993(89)90815-9. [DOI] [PubMed] [Google Scholar]
- Allendoerfer K. L., Shelton D. L., Shooter E. M., Shatz C. J. Nerve growth factor receptor immunoreactivity is transiently associated with the subplate neurons of the mammalian cerebral cortex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):187–190. doi: 10.1073/pnas.87.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimatsu Y., Naegele J. R., Barnstable C. J. Molecular markers of neuronal subpopulations in layers 4, 5, and 6 of cat primary visual cortex. J Neurosci. 1987 Apr;7(4):1250–1263. doi: 10.1523/JNEUROSCI.07-04-01250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980 Jul 17;286(5770):231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Blum A. S., Barnstable C. J. O-acetylation of a cell-surface carbohydrate creates discrete molecular patterns during neural development. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8716–8720. doi: 10.1073/pnas.84.23.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Chun J. J., Nakamura M. J., Shatz C. J. Transient cells of the developing mammalian telencephalon are peptide-immunoreactive neurons. Nature. 1987 Feb 12;325(6105):617–620. doi: 10.1038/325617a0. [DOI] [PubMed] [Google Scholar]
- Chun J. J., Shatz C. J. A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons. J Cell Biol. 1988 Mar;106(3):857–872. doi: 10.1083/jcb.106.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J. J., Shatz C. J. Interstitial cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J Comp Neurol. 1989 Apr 22;282(4):555–569. doi: 10.1002/cne.902820407. [DOI] [PubMed] [Google Scholar]
- Chun J. J., Shatz C. J. The earliest-generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter immunohistochemistry during fetal life. J Neurosci. 1989 May;9(5):1648–1667. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall J. E., Jacobson M., Kosik K. S. Ontogenesis of microtubule-associated protein 2 (MAP2) in embryonic mouse cortex. Brain Res. 1986 Jul;393(1):127–133. doi: 10.1016/0165-3806(86)90072-6. [DOI] [PubMed] [Google Scholar]
- Friauf E., McConnell S. K., Shatz C. J. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990 Aug;10(8):2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushiki S., Schachner M. Immunocytological localization of cell adhesion molecules L1 and N-CAM and the shared carbohydrate epitope L2 during development of the mouse neocortex. Brain Res. 1986 Jan;389(1-2):153–167. doi: 10.1016/0165-3806(86)90183-5. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Jr, Johnson H. G., Binder L. I. Expression of microtubule-associated protein 2 by reactive astrocytes. Proc Natl Acad Sci U S A. 1990 May;87(10):3967–3971. doi: 10.1073/pnas.87.10.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golumbeski G. S., Jr, Dimond R. L. The use of tolerization in the production of monoclonal antibodies against minor antigenic determinants. Anal Biochem. 1986 May 1;154(2):373–381. doi: 10.1016/0003-2697(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Hockfield S. A Mab to a unique cerebellar neuron generated by immunosuppression and rapid immunization. Science. 1987 Jul 3;237(4810):67–70. doi: 10.1126/science.3603010. [DOI] [PubMed] [Google Scholar]
- Koh S., Loy R. Localization and development of nerve growth factor-sensitive rat basal forebrain neurons and their afferent projections to hippocampus and neocortex. J Neurosci. 1989 Sep;9(9):2999–0318. doi: 10.1523/JNEUROSCI.09-09-02999.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I., Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980 Apr;9(2):219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Love S., Saitoh T., Quijada S., Cole G. M., Terry R. D. Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, Pick bodies and Lewy bodies. J Neuropathol Exp Neurol. 1988 Jul;47(4):393–405. doi: 10.1097/00005072-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Luca F. C., Bloom G. S., Vallee R. B. A monoclonal antibody that cross-reacts with phosphorylated epitopes on two microtubule-associated proteins and two neurofilament polypeptides. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1006–1010. doi: 10.1073/pnas.83.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin M. B., Shatz C. J. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985 Dec 22;242(4):611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Luskin M. B., Shatz C. J. Studies of the earliest generated cells of the cat's visual cortex: cogeneration of subplate and marginal zones. J Neurosci. 1985 Apr;5(4):1062–1075. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. Early prenatal ontogenesis of the cerebral cortex (neocortex) of the cat (Felis domestica). A Golgi study. I. The primordial neocortical organization. Z Anat Entwicklungsgesch. 1971;134(2):117–145. doi: 10.1007/BF00519296. [DOI] [PubMed] [Google Scholar]
- Matus A., Bernhardt R., Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci U S A. 1981 May;78(5):3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. K., Ghosh A., Shatz C. J. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989 Sep 1;245(4921):978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- Meyer G., Ferres-Torres R. Postnatal maturation of nonpyramidal neurons in the visual cortex of the cat. J Comp Neurol. 1984 Sep 10;228(2):226–244. doi: 10.1002/cne.902280209. [DOI] [PubMed] [Google Scholar]
- Naegele J. R., Arimatsu Y., Schwartz P., Barnstable C. J. Selective staining of a subset of GABAergic neurons in cat visual cortex by monoclonal antibody VC1.1. J Neurosci. 1988 Jan;8(1):79–89. doi: 10.1523/JNEUROSCI.08-01-00079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduzzi J. D. Genesis of GABA-immunoreactive neurons in the ferret visual cortex. J Neurosci. 1988 Mar;8(3):920–931. doi: 10.1523/JNEUROSCI.08-03-00920.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Early developmental events: cell lineages, acquisition of neuronal positions, and areal and laminar development. Neurosci Res Program Bull. 1982 Apr;20(4):439–451. [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976 Jun 10;261(5560):467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Stewart G. R., Pearlman A. L. Fibronectin-like immunoreactivity in the developing cerebral cortex. J Neurosci. 1987 Oct;7(10):3325–3333. doi: 10.1523/JNEUROSCI.07-10-03325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F., Facal-Valverde M. V. Postnatal development of interstitial (subplate) cells in the white matter of the temporal cortex of kittens: a correlated Golgi and electron microscopic study. J Comp Neurol. 1988 Mar 8;269(2):168–192. doi: 10.1002/cne.902690203. [DOI] [PubMed] [Google Scholar]
- Wahle P., Meyer G. Early postnatal development of vasoactive intestinal polypeptide- and peptide histidine isoleucine-immunoreactive structures in the cat visual cortex. J Comp Neurol. 1989 Apr 8;282(2):215–248. doi: 10.1002/cne.902820206. [DOI] [PubMed] [Google Scholar]
- Wahle P., Meyer G., Wu J. Y., Albus K. Morphology and axon terminal pattern of glutamate decarboxylase-immunoreactive cell types in the white matter of the cat occipital cortex during early postnatal development. Brain Res. 1987 Nov;433(1):53–61. doi: 10.1016/0165-3806(87)90064-2. [DOI] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Davies P. Alzheimer-related neuronal protein A68: specificity and distribution. Ann Neurol. 1987 Oct;22(4):521–526. doi: 10.1002/ana.410220412. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Scicutella A., Davies P. Reexpression of a developmentally regulated antigen in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6202–6206. doi: 10.1073/pnas.85.16.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]