Recent work has demonstrated a potentially symbiotic relationship between oral commensal bacteria and humans through the salivary nitrate-nitrite-nitric oxide pathway (C. Duncan et al., Nat Med 1:546–551, 1995, http://dx.doi.org/10.1038/nm0695-546). Oral nitrate-reducing bacteria contribute physiologically relevant levels of nitrite and nitric oxide to the human host that may have positive downstream effects on cardiovascular health (V. Kapil et al., Free Radic Biol Med 55:93–100, 2013, http://dx.doi.org/10.1016/j.freeradbiomed.2012.11.013). In the work presented here, we used 16S rRNA Illumina sequencing to determine whether a connection exists between oral nitrate-reducing bacteria, nitrates for cardiovascular disease, and migraines, which are a common side effect of nitrate medications (U. Thadani and T. Rodgers, Expert Opin Drug Saf 5:667–674, 2006, http://dx.doi.org/10.1517/14740338.5.5.667).
KEYWORDS: headaches, microbiome, migraines, nitrate reductases
ABSTRACT
Nitrates, such as cardiac therapeutics and food additives, are common headache triggers, with nitric oxide playing an important role. Facultative anaerobic bacteria in the oral cavity may contribute migraine-triggering levels of nitric oxide through the salivary nitrate-nitrite-nitric oxide pathway. Using high-throughput sequencing technologies, we detected observable and significantly higher abundances of nitrate, nitrite, and nitric oxide reductase genes in migraineurs versus nonmigraineurs in samples collected from the oral cavity and a slight but significant difference in fecal samples.
IMPORTANCE Recent work has demonstrated a potentially symbiotic relationship between oral commensal bacteria and humans through the salivary nitrate-nitrite-nitric oxide pathway (C. Duncan et al., Nat Med 1:546–551, 1995, http://dx.doi.org/10.1038/nm0695-546). Oral nitrate-reducing bacteria contribute physiologically relevant levels of nitrite and nitric oxide to the human host that may have positive downstream effects on cardiovascular health (V. Kapil et al., Free Radic Biol Med 55:93–100, 2013, http://dx.doi.org/10.1016/j.freeradbiomed.2012.11.013). In the work presented here, we used 16S rRNA Illumina sequencing to determine whether a connection exists between oral nitrate-reducing bacteria, nitrates for cardiovascular disease, and migraines, which are a common side effect of nitrate medications (U. Thadani and T. Rodgers, Expert Opin Drug Saf 5:667–674, 2006, http://dx.doi.org/10.1517/14740338.5.5.667).
Observation
Nitrate associations with headaches and migraines.
Nitrate-containing compounds have been identified as common headache triggers. Food preservatives are frequently identified triggers for those who suffer from migraines (1). Also, cardiac medications containing nitrates may cause severe headaches, which occur in over 80% of patients taking them. Indeed, approximately 10% of patients cannot tolerate nitrate therapies due to unbearable headaches (2). Nitrate-induced headaches typically manifest in one of two ways: “immediate” headaches with mild to medium severity developing within an hour of medication ingestion and “delayed” headaches occurring 3 to 6 h after nitrate intake that are much more severe, with migrainelike symptoms (3, 4). Delayed migraines appear to be dose dependent and are more likely to occur in individuals with a family history of migraines (5). The primary literature suggests two differing mechanisms behind these two headache types. Immediate headaches appear to be connected to nitric oxide (NO)-mediated vasodilation; in contrast, delayed migraines, similarly to migraines triggered by foods, stress, and other factors, appear to be activated by the release of calcitonin gene-related peptide (CGRP), glutamate, cyclic GMP (cGMP), or S-nitrosylation-mediated changes in ion channel function (5). Notably, S-nitrosylation is dependent on the presence of NO.
Nitrate-reducing bacteria in the oral and fecal samples of the AGP.
Because only bacteria, and not human cells, can reduce nitrate to nitrite (6), this may represent a symbiotic relationship by which our oral microbes maintain cardiovascular health using molecules present in our food. It has also been reported that in murine macrophages in vitro, the bacterial nitric oxide reductase NorB increases the decomposition rate of S-nitrosothiol (SNO) (7). This represents a potential connection between nitric oxide reductases and nitrate-induced migraines. Therefore, we determined the presence and abundance of nitrate, nitrite, and nitric oxide reductase genes in predicted metagenomes from stool and oral samples in the American Gut Project (AGP) cohort and correlated these genes with self-reported migraine status.
Using a subset of 16S rRNA data from sequencing rounds 1 to 25 of the public American Gut Repository (ftp://ftp.microbio.me/AmericanGut/rounds-1-25; subset details are described in Text S1 in the supplemental material), we used analysis of composition of microbiomes (ANCOM) (8) to identify GreenGenes (GG, 97% similarity) operational taxonomic units (OTUs) that were differentially abundant between migraineurs and nonmigraineurs. We next predicted the metagenome functional content of the entire group of differentially abundant OTUs using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (9). To assess the accuracy of the predictions, we calculated the nearest sequenced taxon index (NSTI), which calculates the average branch length separating each OTU in a sample from a reference bacterial genome, weighted by the abundance of that OTU in the sample. The NSTI scores were good (below 0.10) for both stool and oral samples, indicating appropriate metagenome predictions, which were stronger in oral samples than in stool samples (see Fig. S1 in the supplemental material).
Nearest sequenced taxon index (NSTI). Fecal and oral NSTI per migraine status, which shows predictions of gene composition range from quite good to good. Download Figure S1, EPS file, 1.8 MB (1.8MB, eps) .
Copyright © 2016 Gonzalez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Detailed analytical steps for all analyses. Additionally, all commands and analyses performed in the manuscript can be found at: https://github.com/knightlab-analyses/mSystems00105-16. Download Text S1, DOCX file, 0.1 MB (26.6KB, docx) .
Copyright © 2016 Gonzalez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
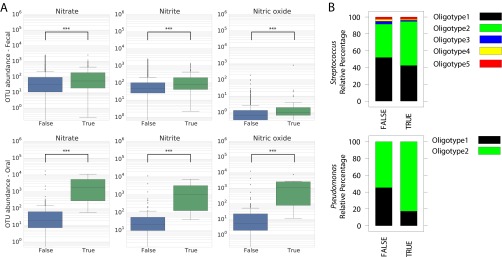
Given the role of the oral microbiome in nitrate reduction and the association between nitrates and headaches, we hypothesized that the abundances of nitrate, nitrite, and NO reductase genes in the predicted metagenomes in oral and stool samples would differ significantly between migraineurs and nonmigraineurs. As seen by the results in Fig. 1A, there were small but significant increases (Kruskal-Wallis: nitrate, P ≤ 0.001; nitrate, P ≤ 0.001; and nitric oxide, P ≤ 0.001) in nitrate, nitrite, and nitric oxide reductase genes in stool samples collected from migraineurs, and in oral samples, nitrate, nitrite, and nitric oxide reductase genes were all significantly (Kruskal-Wallis: nitrate, P ≤ 0.001; nitrite, P ≤ 0.001; and nitric oxide, P ≤ 0.001) more abundant (based on ANCOM) in migraineurs.
FIG 1 .
Nitrate-, nitrite-, and nitric oxide-reducing bacteria. (A) Differential abundances of OTUs as detected by ANCOM that have nitrate-, nitrite-, and nitric acid-producing KEGG orthologies (KOs) as reported by PICRUSt by body site. Oral samples show obvious differences between migraineurs (True) and nonmigraineurs (False) in nitrate, nitrite, or NO reductase genes, while there are no obvious differences in stool samples. However, both nitrate and nitrite are significant in stool samples, but not nitric oxide, as sample groups are too small. (B) Relative abundance profiles of oligotypes (sub-OTUs) in Streptococcus (two oligotypes) and Pseudomonas (five oligotypes). Intergroup analysis revealed no major differences in the Streptococcus distribution profiles. Pseudomonas oligotype 2 was highly enriched in the “TRUE” group (FALSE = 54%, TRUE = 82%).
The dominant oral OTUs (>10% of the reads in the data set) that were significantly different between migraineurs and nonmigraineurs belonged to the genera Streptococcus and Pseudomonas, both of which have species with the potential to reduce nitrate (10, 11). Additionally, while Pseudomonas has not previously been reported in the context of oral nitrate reduction, Streptococcus did increase in the oral cavities of rats supplemented with nitrate in their drinking water (12). To explore whether there were strain-level differences within these genera across the populations, we performed oligotyping (13). The genera Streptococcus and Pseudomonas were decomposed into 5 and 2 oligotypes, respectively (Fig. 1B). There was no significant difference in the relative abundance patterns of Streptococcus oligotypes across both populations. Two-group analysis (Fisher’s exact t test, P < 0.005) suggested that Pseudomonas (10% of the total reads) has differential abundance patterns in the oral microbiome of migraineurs and nonmigraineurs. Pseudomonas oligotypes 1 and 2 were both present in both populations, but oligotype 2 was significantly more abundant in migraineurs. These results indicate that host preference patterns in the genus Pseudomonas are driven by host physiology and that migraineurs share similar strains of Pseudomonas.
Finally, based on the extrapolation of the strain level profiles of Pseudomonas oligotypes and the genomic variations found via PICRUSt, it is likely that these strains comprise a genetic repertoire selected for genetic adaptation in this host environment (migraine).
We next determined the taxonomic classification of OTUs contributing nitrate, nitrite, and nitric oxide reductase genes to the predicted metagenomes in our datasets. From this list, two bacterial taxa (Rothia mucilaginosa and Haemophilus parainfluenzae) have previously been reported as some of the main nitrate reducers in the human oral cavity (12, 14), and some have also been reported to be associated with headaches (Table 1).
TABLE 1 .
GreenGenes identification numbers and taxonomy assignments of the five most common OTUs that were found to be differentially abundant between migraineur status and whether they contain nitrate-, nitrite-, and/or nitric oxide-reducing KEGG orthologies
| GG IDa | GG taxonomy | OTU contributes: |
Referenceb | ||
|---|---|---|---|---|---|
| Nitrate reductase(s) | Nitrite reductase(s) | Nitric oxide reductase(s) | |||
| 903426 | k__Bacteria; p__Actinobacteria; c__Actinobacteria; o__Actinomycetales; f__Micrococcaceae; g__Rothia; s__mucilaginosa | X | X | X | 15 |
| 926526 | k__Bacteria; p__Actinobacteria; c__Actinobacteria; o__Actinomycetales; f__Micrococcaceae; g__Rothia; s__mucilaginosa | X | X | X | 15 |
| 538000 | k__Bacteria; p__Proteobacteria; c__Gammaproteobacteria; o__Enterobacteriales; f__Enterobacteriaceae; g__; s__ | X | X | ||
| 960871 | k__Bacteria; p__Actinobacteria; c__Actinobacteria; o__Actinomycetales; f__Actinomycetaceae; g__Actinomyces; s__ | X | |||
| 4448331 | k__Bacteria; p__Proteobacteria; c__Gammaproteobacteria; o__Enterobacteriales; f__Enterobacteriaceae; g__; s__ | X | X | X | |
| 4299925 | k__Bacteria; p__Proteobacteria; c__Gammaproteobacteria; o__Pasteurellales; f__Pasteurellaceae; g__Haemophilus; s__parainfluenzae | X | 16 | ||
| 821562 | k__Bacteria; p__Proteobacteria; c__Gammaproteobacteria; o__Pseudomonadales; f__Pseudomonadaceae; g__; s__ | X | |||
| 4128270 | k__Bacteria; p__Proteobacteria; c__Gammaproteobacteria; o__Pseudomonadales; f__Pseudomonadaceae; g__Pseudomonas; s__ | X | |||
GG, GreenGenes; ID, identification number.
For those taxonomies with a species name, we provide references to reports of their relationship with headache studies.
Conclusions.
These results show for the first time a potential link between bacterial nitrate, nitrite, and nitric oxide reducers and migraines, by reporting their higher abundances in the oral cavities of people with migraines than in the oral cavities of those who do not suffer from migraines. Future studies should focus on further characterizing the connection between oral bacterial nitrate, nitrite, and nitric oxide reducers and migraines.
ACKNOWLEDGMENTS
We thank Jamie Morton for his help with ANCOM and Jesse Zaneveld for his help with PICRUSt.
A.G. and N.S. were involved in data analysis and interpretation; E.H. was involved in data interpretation and manuscript writing; R.K., J.A.G. and E.V. were involved in data interpretation and manuscript editing.
REFERENCES
- 1.Sun-Edelstein C, Mauskop A. 2009. Foods and supplements in the management of migraine headaches. Clin J Pain 25:446–452. doi: 10.1097/AJP.0b013e31819a6f65. [DOI] [PubMed] [Google Scholar]
- 2.Thadani U, Rodgers T. 2006. Side effects of using nitrates to treat angina. Expert Opin Drug Saf 5:667–674. doi: 10.1517/14740338.5.5.667. [DOI] [PubMed] [Google Scholar]
- 3.Bellantonio P, Micieli G, Buzzi MG, Marcheselli S, Castellano AE, Rossi F, Nappi G. 1997. Haemodynamic correlates of early and delayed responses to sublingual administration of isosorbide dinitrate in migraine patients: a transcranial Doppler study. Cephalalgia 17:183–187. doi: 10.1046/j.1468-2982.1997.1703183.x. [DOI] [PubMed] [Google Scholar]
- 4.Juhász G, Zsombók T, Gonda X, Bagdy G. 2004. Nitroglycerin-induced headaches. Orv Hetil 145:2323–2328. (In Hungarian.) [PubMed] [Google Scholar]
- 5.Bagdy G, Riba P, Kecskeméti V, Chase D, Juhász G. 2010. Headache-type adverse effects of NO donors: vasodilation and beyond. Br J Pharmacol 160:20–35. doi: 10.1111/j.1476-5381.2010.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. 2004. Nitrate, bacteria and human health. Nat Rev Microbiol 2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 7.Laver JR, Stevanin TM, Messenger SL, Lunn AD, Lee ME, Moir JW, Poole RK, Read RC. 2010. Bacterial nitric oxide detoxification prevents host cell S-nitrosothiol formation: a novel mechanism of bacterial pathogenesis. FASEB J 24:286–295. doi: 10.1096/fj.08-128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal S, Van Treuren W, White RA, Eggesbo M, Knight R, Peddada SD. 2015. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langston CW, Williams PP. 1962. Reduction of nitrate by streptococci. J Bacteriol 84:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida JS, Reis MA, Carrondo MJ. 1995. Competition between nitrate and nitrite reduction in denitrification by Pseudomonas fluorescens. Biotechnol Bioeng 46:476–484. doi: 10.1002/bit.260460512. [DOI] [PubMed] [Google Scholar]
- 12.Hyde ER, Luk B, Cron S, Kusic L, McCue T, Bauch T, Kaplan H, Tribble G, Petrosino JF, Bryan NS. 2014. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic Biol Med 77:249–257. doi: 10.1016/j.freeradbiomed.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Eren AM, Maignien L, Sul WJ, Murphy LG, Grim SL, Morrison HG, Sogin ML. 2013. Oligotyping: differentiating between closely related microbial taxa using 16S rRNA gene data. Methods Ecol Evol 4:1111–1119. doi: 10.1111/2041-210X.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. 2005. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci 113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoang A, Wisler K, Rico A. 2016. Bacteremia and mycotic aneurysm due to Rothia mucilaginosa. Clin Microbiol Case Rep 2:026. [Google Scholar]
- 16.Latyshev Y, Mathew A, Jacobson JM, Sturm E. 2013. Purulent pericarditis caused by Haemophilus parainfluenzae. Tex Heart Inst J 40:608–611. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nearest sequenced taxon index (NSTI). Fecal and oral NSTI per migraine status, which shows predictions of gene composition range from quite good to good. Download Figure S1, EPS file, 1.8 MB (1.8MB, eps) .
Copyright © 2016 Gonzalez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Detailed analytical steps for all analyses. Additionally, all commands and analyses performed in the manuscript can be found at: https://github.com/knightlab-analyses/mSystems00105-16. Download Text S1, DOCX file, 0.1 MB (26.6KB, docx) .
Copyright © 2016 Gonzalez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.