Abstract
Objective(s):
Many types of human papillomaviruses (HPVs) have been identified, with some leading to cancer and others to skin lesions such as anogenital warts. Studies have demonstrated an association between oncogenic HPV and cervical cancer and many researchers have focused on therapeutic vaccines development. At present, the modulatory effect of opioids on the innate and acquired immune system is characterized. Antagonists of opioid receptors such as naloxone (NLX) can contribute to the shifting Th2 response toward Th1. Herein; we studied the adjuvant activity of NLX/Alum mixture for improvement of the immunogenicity of HPV-16E7d vaccine.
Materials and Methods:
The mice were administered different regimens of vaccine; E7d, E7d-NLX, E7d-Alum, E7d-NLX-Alum, NLX, alum and PBS via subcutaneous route for three times with two weeks interval. Two weeks after the last immunization, the sera were assessed for total antibody, IgG1 and IgG2a with an optimized ELISA method. The splenocytes culture supernatant was analyzed by ELISA for the presence of IL-4, IFN-γ and IL-17 cytokines and lymphocyte proliferation was evaluated with Brdu method.
Results:
Immunization of mice with HPV-16 E7d vaccine formulated in NLX/Alum mixture significantly increased lymphocyte proliferation and Th1 and Th17 cytokines responses compared to other experimental groups. Analysis of humoral immune responses revealed that administration of vaccine with NLX/Alum mixture significantly increased specific IgG responses and also isotypes compared to control groups.
Conclusion:
NLX/Alum mixture as an adjuvant could improve cellular and humoral immune responses and the adjuvant maybe useful for HPV vaccines model for further studies in human clinical trial.
Keywords: Adjuvant, Alum, Naloxone, Papillomavirus, Vaccine
Introduction
Human papillomaviruses (HPVs) is a group of more than 200 related viruses that are non-enveloped, double-stranded DNA viruses with icosahedral nucleocapsid. This virus is the causative agent for cervical cancer, the third most common female cancer, and various other cancers (1). HPV is transmitted from an infected mother to child at the birth time, and causes warts in the mouth and respiratory tract of the newborn, especially throat. Many types of HPV have been identified, just two HPV types, 16 and 18, are responsible for about 70 percent of all cancers (2). Two oncoproteins, E6 and E7, made by certain oncogenic (high-risk) HPVs interfere with cell functions. These proteins bind to and inactivate the tumor suppressor proteins P53 and Rb, respectively, which are helping the cell to grow in an uncontrolled manner and to avoid cell death (3). The E7 gene encodes a 98 amino acid phosphoprotein. This protein maintains differentia-ting cells active in the cell cycle through binding to and mediating the degradation of Rb, as well as the related proteins p107 and p130 (4).
E7 protein is being used as antigen model in the most of researches on therapeutic vaccines (5, 6). Due to the risk of cancer (because of being oncogenic), a mutated form of this protein that contains three amino acid substitution in codons 24, 21 and 26 (called detoxified E7 or E7d), is used. These three amino acid changes prevent this protein binding to Rb protein while the immunogenic property remains so even according to some reports also its immunogenicity will increase (7).
Using adjuvant is one of the ways to increase the immunogenicity of vaccines. An adjuvant is defined as a pharmacological and/or immunological subs-tance that is added to a vaccine to prolong, improve antigen-specific immune responses and make that vaccine more available for macrophages and dendritic cells (DCs) as antigen presenting cells (8). By definition, a successful adjuvant should be able to effectively enhance both cellular (Th1 polarizing) and humoral (Th2 polarizing) immune responses as well as remove or control viral and intracellular pathogen infections (9). The alum is a well-defined adjuvant for induction of the human immune response that is able to trigger Th2-arm of immune response, however alum fails to induce the cellular immune response (Th1) which is important for immune responses against a variety of intracellular pathogens (10). At present, the modulatory effect of opioids on the innate and acquired immune system directly through the opioid receptors on immune cells or indirectly through the hypothalamus pituitary and adrenal (by increasing glucocorticoids) is characterized (11). Endogenous opioids are involved in modulating the balance of Th1/Th2. On the other hand, the antagonists of these opioid receptors such as naloxone (NLX) can react to these receptors and contribute to the shifting Th2 response to Th1, that results in increasing proliferation of T lymphocytes, increasing cytotoxicity of NK cells and is involved in the modulation of inflammatory responses such as IFN-γ responses (12). Our previous studies showed that naloxone can stimulate Th1 immune responses but no Th2 polarization and humoral immune responses (13, 14). Naloxone is a medication called an “opioid antagonist” used to reverse the effects of opioid overdose, for example morphine and heroin overdose. Specifically, naloxone is used in opioid overdoses to counteract life-threatening mental and respiratory depression. Since one of the goals of vaccination against the virus is induction of type-1 cytokines in environment and on the other hand, the design and production of vaccines against HPVs have been focused more on Th1 responses (15).
The HPV infection is a major health problem in the developed and developing countries and protective vaccine against this infection remains elusive (1). Therefore, development of an effective HPV vaccine may control the HPV infection worldwide. Because adjuvants constitute an important component of vaccines, the discovery of more potent adjuvants may allow the development of prophylactic and therapeutic vaccines against infectious diseases such as HPV. The development of new adjuvant formulations can optimize a variety of candidate vaccines. Therefore, there is a critical need for the development of novel and improved vaccine adjuvants. So here, the present study was aimed to put forward an explanation of the possibility of using naloxone and alum as adjuvants for improvement of cellular and humoral immunity in response to HPV-16E7d vaccine in mouse model.
Materials and Methods
Ethics statement
This study was approved by the ethics committee of Pasteur Institute of Iran. All the mouse experiments were carried out in accordance with the Animal Care and Use Protocol of Pasteur Institute of Iran.
Mice
Six to eight-week-old female C57BL/6 mice were purchased from Pasteur Institute of Iran (Karaj, Iran). The mice were housed for one week before the experiments, given free access to food and water and maintained in a light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. The handling of the mice were carried out with an expert technician and in accordance with the Animal Care and Use Protocol of Pasteur Institute of Iran.
HPV-16E7d vaccine preparation
The recombinant detoxified E7 (E7d) has been produced at the Department of Virology of Pasteur Institute of Iran and gifted us by Mr Meysam Gachpazan for this study. Briefly, expression vector E7d protein was prepared and the vector (pET28a-E7d plasmid) was transformed into E. coli BL21 (DE3) and induced by adding 1 mM IPTG to the culture. Expression was confirmed with SDS-page and Western blotting. The recombinant E7d protein was purified with Ni-NTA column and after dialysis versus PBS buffer the sample was filtered and concentration was detected using Bradford method and stored at -20°C until use (Data not shown).
Vaccine formulation
The candidate vaccine was prepared in alum adjuvant (aluminum hydroxide, Pasteur Institute of Iran, Karaj, Iran) with or without NLX (Sigma, Germany) at a concentration of 6 mg/kg in sterile condition. For this purpose, the HPV-16 E7d vaccine was dissolved in sterile PBS and then mixed with alum and NLX. According to our laboratory setup, the mixture was incubated for 60 min at room tempera-ture (RT) condition to absorb the protein on the alum gel matrix. Each dose of vaccine contained 6 mg/kg of NLX and 10 µg of candidate vaccine.
Experimental groups and immunization
The inbred mice were assigned into seven different groups containing 5-6 mice in each one, as described below:
Group I: E7d vaccine (E7d, n= 6)
Group II: E7d adjuvanted in naloxone (E7d-NLX, n= 6)
Group III: E7d adjuvanted in alum (E7d-Alum, n= 6)
Group IV: E7d adjuvanted in naloxone and alum (E7d-NLX-Alum, n= 6)
Group V: naloxone (as control group, n= 5)
Group VI: alum (as control group, n= 5)
Group VII: PBS (as control group, n= 5)
The first four groups of mice were subcutaneously immunized on day 0 with 200 µl containing 10 µg of the vaccine candidate alone or formulated in NLX or alum or both. As control groups, some mice were injected with NLX or alum or PBS buffer in the same condition. Immunized mice were boosted twice with two-week intervals. Two weeks after the last immunization, the blood samples were collected, and sera were taken from all mice in each group by centrifugation and stored at -20°C for further use.
Lymphocyte proliferation assay
Two weeks after third immunization, the mice were killed by cervical dislocation and spleens of the immunized mice were removed under sterile conditions and suspended in sterile cold PBS. RBCs were lysed with lysis buffer and single-cell suspension was adjusted to 3×106 cells per milliliters in RPMI 1640 (Gibco) supplemented with 1 mM sodium pyruvate, 4 mM L-glutamine, 50 µM 2ME, 5% FBS, 100 µg/ml streptomycin and 100 IU/ml penicillin. Then, 100 µl of diluted cell suspensions were dispensed into 96-well flat-bottom culture plates (Nunc) and stimulated with 10 µg/ml of the candidate vaccine. Phytohemagglutinin-A (PHA) (5 μg/ml, Gibco), un-stimulated wells and culture medium were used as a positive control, negative controls and a blank, respectively. After 72 hrs of cell culture, 20 µl of BrdU (Roche, Germany) was added to each well and the plates were further incubated at 37°C for 18 hrs. After incubation, the plates were centrifuged at 300 g for 10 min, the supernatant was aspirated carefully, the plates were dried and subsequently 200 μl of fixation/denaturation buffer was added to each well and incubated for 30 min. The plates were aspirated and 100 μl of anti-BrdU was added for 2 hrs. Afterwards, the plates were washed 5 times with PBS, the TMB substrate was added to wells and incubated for 5 min in the dark at room temperature, and reaction was stopped with adding 100 μl of 2N H2SO4. The absorbance at OD450 was measured for each well. The optical density of the blank wells was subtracted from all other wells and then stimulation index (SI) was calculated according to the formula: SI = A450 of the stimulated wells/A450 of the un-stimulated wells for individual mouse. All experiments were done in triplicate.
ELISA of cytokines
Two weeks after the third immunization, a total number of 3 × 106 spleen cells were seeded on each well of a 24-well plate using complete RPMI 1640, stimulated in vitro with 10 μg/ml of the candidate vaccine and incubated at 37°C in 5% CO2 for three days. After addition of recall antigen, supernatants were removed and the concentration of IFN-γ, IL-4 and IL-17 cytokines was estimated by ELISA Kit (Mabtech, Sweden) according to the manufacturer’s instruction. The pg/ml of each sample was calculated according to the standard curve and the absolute cytokine production of each mouse was used for statistical analysis.
ELISA of antibodies and their isotypes
Specific antibodies were determined by an optimized indirect ELISA method. Briefly, 100 µl of 10 µg/ml of the candidate vaccine in PBS was added into 96-well ELISA Maxisorp plates (Nunc, Naperville, IL) and incubated overnight at 4°C. The wells washed with PBS containing 0.05% Tween 20 (PBS-T as washing buffer) and blocked 1 hr at 37°C with 5% skimmed milk in PBS (blocking buffer). Plates were washed with PBS-T and 100 µl of 1:100 to 1:12800 diluted sera were added to each wells and incubated at 37°C for 90 min. The wells washed five times with washing buffer and incubated for 90 min with 100 µl of 1:10000 dilution of anti-mouse conjugated to HRP (Sigma, USA). The wells washed five times and incubated 30 min with 100 µl of TMB substrate in the dark and reaction was stopped with 2N H2SO4 and color density was measured at OD450 nm with ELISA plate reader. Detection of specific IgG1 and IgG2a subclasses were carried out using goat anti-mouse IgG1 and IgG2a secondary antibodies (Sigma, USA) according to the manufacture’s instruction.
Statistical analysis
The data expressed as means±SD of each experiment. All statistical analyses were carried out by the Graph pad prism V6.01. In all of the cases, P-values less than 0.05 was considered to be statistically significant.
Results
Lymphocyte proliferation
The lymphocyte proliferation of experimental groups was assessed by the BrdU/ELISA-based method. Antigen recall was carried out three days and then lymphoproliferative activity was reported as a SI of individual mice. As shown in Figure 1, the mice immunized with E7d-NLX and E7d-Alum elicited lymphocyte proliferation, demonstrating an increase versus control groups (P≤0.084). Interestingly, the mice immunized with E7d-NLX-Alum increased lymphocyte proliferation in comparison with the E7d-NLX (P=0.061) and E7d (P=0.007) groups. However, there was no significant difference among control groups (P>0.05).
Figure 1.
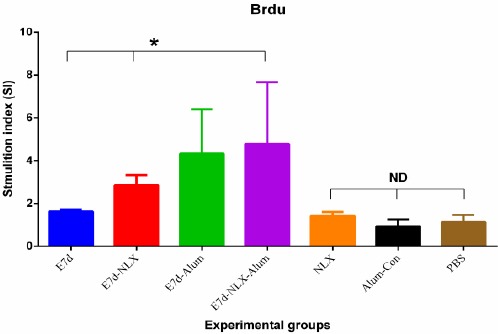
Lymphocyte proliferation response of splenic cells after antigen recall. After immunization course, splenocytes were harvested and re-stimulated in vitro with recombinant protein for 72 hr and lymphocyte proliferation was then quantitated using a commercially available Brdu proliferation kit as described in method section. Proliferation was presented as stimulation index of individual mice and values were represented as mean±SD of experimental mice. Asterisks represent the groups which were statistically significant and ND indicates not detectable differences (P<0.05)
IFN-γ, IL-4 and IL-17 cytokines assay
Cytokine analysis showed that the mice immunized with E7d-NLX-Alum were able to increase IFN-γ (Figure 2a) level in comparison with other test groups (P<0.05). The data demonstrated that there is a significant difference in the IFN-γ level between the group immunized with E7d-NLX and, E7d-Alum and E7d groups (P=0.004). There was no significant difference among control groups (P>0.05). As shown in Figure 2b, the evaluation of IL-4 cytokine in the experimental groups showed that there was no significant difference among test groups and control groups (P>0.05). The mice immunized with E7d-NLX-Alum, E7d-NLX and E7d elicited an increased IL-17 cytokine level (Figure 2c) as compared to control groups (P≤0.016). The group immunized with E7d-Alum showed a decrease in IL-17 cytokine level in comparison with other test groups that was not significant compared to control groups (P>0.05).
Figure 2.
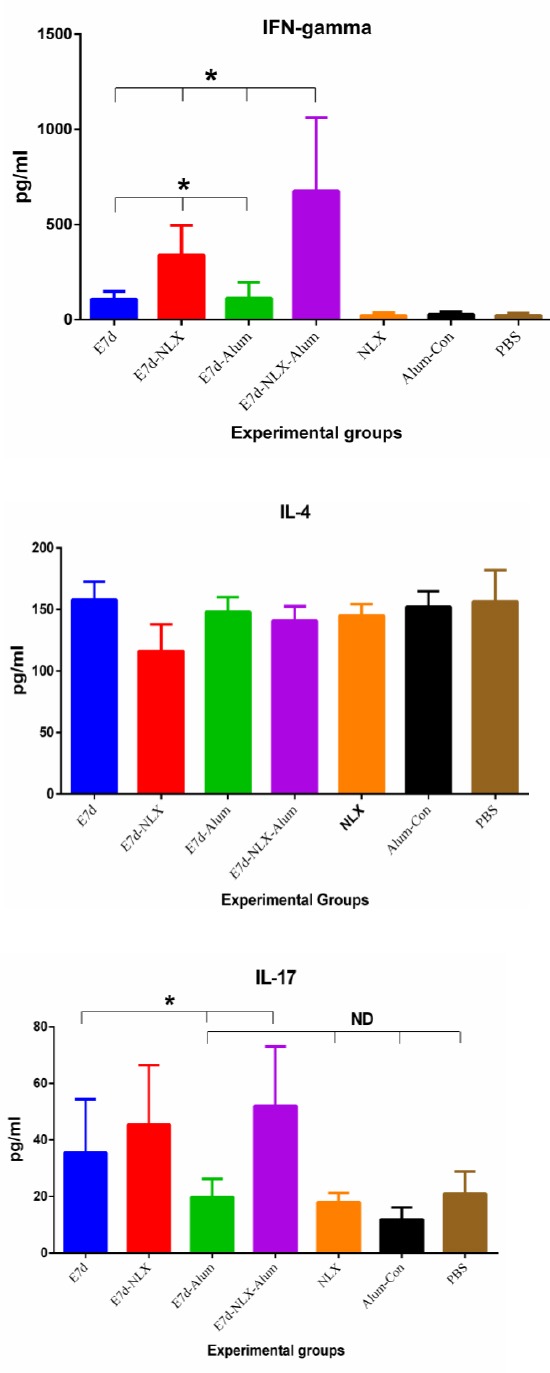
Enzyme-linked immunosorbent assay (ELISA) of IFN-γ (a), IL-4 (b) and IL-17 (c) cytokines in supernatant of spleen cell cultures of experimental mice. Results of IFN-γ, IL-4 and IL-17 cytokines were depicted as mean ± SD of experimental mice (n=5, 6). Asterisks represent the groups which were statistically significant and ND indicates not detectable differences (P<0.05)
Total antibodies and IgG isotypes
Total IgG was investigated by an optimized indirect ELISA test. The results (Figure 3) showed that immunization of mice with E7d-NLX-Alum and E7d-Alum induced higher levels of specific IgG antibody responses compared to other experimental groups (P<0.05). The antibody raised against the E7d-NLX-Alum and E7d-Alum was detectable with 1600 times dilution of the original polyclonal antibody solution. However, the 100 and 200 times dilutions were found significantly (P=0.0001) optimal in comparison with the other vaccinated groups. The level of total specific IgG in the mice immunized with E7d-NLX-Alum was higher than the E7d-Alum-immunized group (P=0.077). There was no significant difference among control groups (P>0.05). Immunization of mice with E7d-NLX-Alum and E7d-Alum increased the IgG1 isotype (Figure 4a) compared to all experimental groups (P=0.082). Significant differences were observed between E7d-NLX-Alum and E7d-Alum groups in the induction of specific IgG1 (P=0.041). Monitoring of IgG2a (Figure 4b) in the all test groups showed a significant increase in comparison with the control groups (P<0.05). Also, immunization with E7d-NLX-Alum significantly increased IgG2a versus E7d/Alum group (P=0.026). However, no significant differences were observed among the E7d, E7d/Alum and E7d/NLX groups in the induction of specific IgG2a isotype (P>0.05), only an increase was observed between E7d-NLX-Alum and E7d groups (P=0.062).
Figure 3.
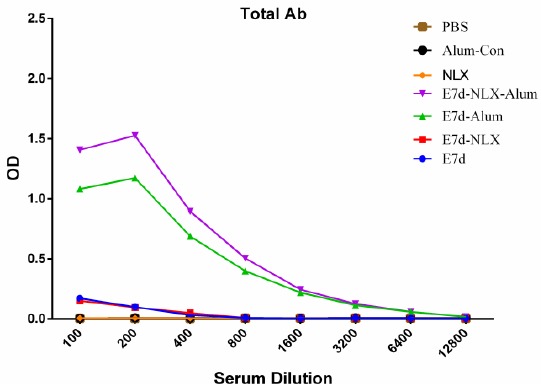
Total antibody responses in experimental groups. Experimental mice were immunized with vaccines with different formulation and total antibody was evaluated with indirect ELISA method. Data are shown as the mean of antibody results at dilutions of 1/100 to 1/12800 for each group
Figure 4.
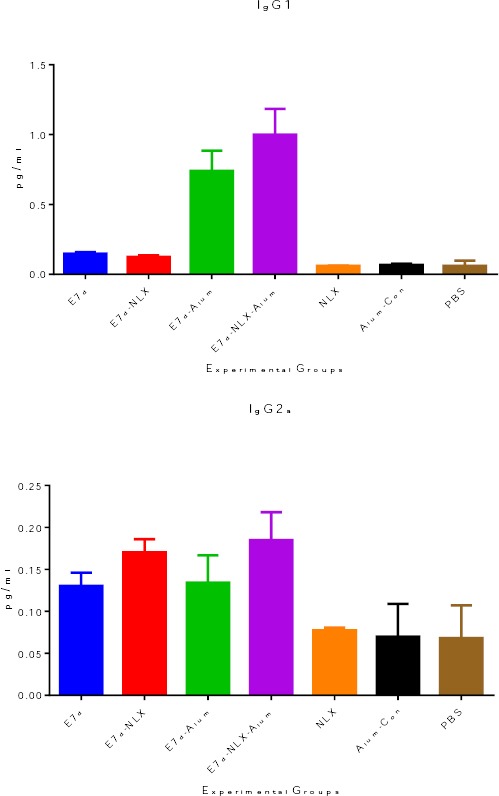
Detection of IgG1 (a) and IgG2a (b) specific antibodies. The measurements were made with sera of experimental mice, and the values represent the means of optical density ± SD. Analysis of IgG1 and IgG2a isotypes have been represented in two distinct graphs. Each mouse sera was evaluated in duplicate by indirect ELISA using anti-IgG1 and anti-IgG2a antibodies and conjugated secondary antibody (HRP). Unlike other immunized groups, the titer of anti E7d-NLX-Alum IgG1 and IgG2a isotypes increased in comparison to other groups. Asterisks represent the groups which were statistically significant and ND indicates not detectable differences (P < 0.05). Error bars represent SD
Discussion
Most vaccines actually have some kind of adjuvant added for increasing vaccine potency and the type of immune response to a vaccine can be greatly modulated through the use of adjuvants (16). Alum is the most commonly used adjuvant in human vaccination. It is found in numerous vaccines, including diphtheria-tetanus-pertussis, Pseudomonas aeruginosa-born infection, herpes simplex virus and hepatitis vaccines (17-20). Alum shifts immune responses toward a Th2 response and enhance humoral immunity, but is rather ineffective against pathogens that require Th1–cell-mediated immunity while the ideal immune response against viral pathogens like HIV-1 and HPVs is induction of both cellular and humoral immune responses (9, 21). In some cases, aluminum as an adjuvant may not stimulate immune system in non-responder cases versus HBs antigen (22).
Recent studies have proved efficacy of NLX as a novel adjuvant that is authorized for use in humans (12). In herpes simplex 1 viral infection (HSV-1) it is demonstrated that during the first seven days, naloxone injection result in increases of cellular immune responses against the virus and higher survival rate (14). Here, we hypothesized that NLX in mixture to alum adjuvant may improve cellular and humoral immune response versus E7d vaccine model. Lymphocyte proliferation is a criterion of cell-mediated immune responses (23) and the results of the present study showed that immunization of mice with E7d-NLX-Alum increases lymphocyte proliferation versus the E7d and E7d/naloxone immunized groups. Also, this group shows a tiny increase of lymphocyte proliferation against E7d-Alum group.
The most important property of NLX as an appropriate adjuvant is its proven safety for human use as approved by the FDA. The study by Jazani et al. shows that the heat-killed Salmonella typhimurium vaccine adjuvanted with NLX/Alum mixture increased lymphocyte proliferation versus an alum formulated vaccine (24, 25). Our recent study demonstrated that immunization of mice with HIV-1 p24-Nef fusion peptide formulated in NLX/Alum mixture significantly increases lymphocyte proliferation versus the Al-Vac immunized group (13). For clearing of infected cells and deletion of viral infection, polarization to Th1 immune response and secretion of IFN-γ is obviously critical. In addition, Th1 cells are thought to mediate the killing of intracellular pathogens while, Th2 induces humoral immunity and result in eradication of extracellular pathogens. The IL-17 is the signature cytokine produced by TH17 cells and promotes inflammation and neutrophil emigration to the sites of infection (26). To detect the immune polarization, IFN-γ, IL-4 and IL-17 cytokines were subsequently evaluated and the results show that, when NLX and alum were added to vaccine formula, a significant increase in the amount of secreted IFN-γ was observed but IL-4 did not show any significant difference. On the other hand, when E7d vaccine was adjuvanted with NLX-Alum and NLX an increased IL-17 cytokine level was shown. Study on HSV-1 DNA vaccine model shows that NLX promote IFN-γ cytokine secretion and strong Th1 polarization (12). In the previous study we found that when NLX was added to the HIV-1 p24-Nef, a significant increase in the amount of secreted IFN-γ would be observed but IL-4 did not show such trend. It is suggested that NLX shifts the immune response toward the Th1 pattern, as well as revealed that increasing IFN-γ production through NLX treatment and this mechanism can lead to an increase in virus clearance, confirming our results for polarization into the Th1 pattern (14).
Antibodies provide the first line of specific defense against infection by microbial pathogens (27). This study demonstrated when E7d vaccine formulated in NLX-Alum and alum, a significant increase in total antibodies would be observed versus other experimental groups. Previously, we showed that the HIV-1 p24-Nef vaccine adjuvanted with NLX/Alum significantly increased total antibodies compared with alum adjuvanted vaccine group (13). Previous reports confirmed our results for the potency of NLX/Alum mixture in the promotion of humoral immune responses (24, 25). Beside cellular immune response, Th2-arm of immune response has critical role in the neutralizing of viruses and clearing of infected cells via antibody-dependent cell-mediated cytotoxicity (ADCC) by natural killer cell, lysis by complement or phagocyting by macrophages (28) and our results show the superiority of NLX-Alum mixture for the induction of humoral immune responses.
Results also revealed that the mice immunized with E7-NLX-Alum significantly increased IgG1 and IgG2a compared with E7-Alum group. In our previous study, analysis of antibody isotypes shows that HIV-1 p24-Nef adjuvanted with NLX/Alum mixture induces the highest level of IgG1, IgG2a, IgG2b, IgG3 and IgM antibodies versus other groups (13). It seems that NLX/Alum mixture is a potent adjuvant for induction of humoral immune responses and this property may be related to the better induction of T helper lymphocytes by NLX that result in better help to B cells for the poly-isotypic humoral response (29).
Conclusion
Overall, the results of the present study demonstrated that NLX-Alum mixture can strongly induce cellular and humoral immune responses versus HPV E7d vaccine and may be a useful choice for human clinical trial study for HPV vaccine research.
Acknowledgment
The results described in this paper were part of student thesis. This work was supported in part by a grant from Pasteur Institute of Iran. We are grateful from Mr Mohammad Choopani, Mr Hamid Reza Moozarmpour and Fatemeh Asgar Halvaee for their technical assistance.
Conflict of interest
The authors of this manuscript have no conflict of interest to disclose.
References
- 1.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol. 2010;2010:326369. doi: 10.1155/2010/326369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avvakumov N, Torchia J, Mymryk JS. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene. 2003;22:3833–3841. doi: 10.1038/sj.onc.1206562. [DOI] [PubMed] [Google Scholar]
- 5.Fan R, Hou WJ, Zhao YJ, Liu SL, Qiu XS, Wang EH, et al. Overexpression of HPV16 E6/E7 mediated HIF-1alpha upregulation of GLUT1 expression in lung cancer cells. Tumour Biol. 2015;28:28. doi: 10.1007/s13277-015-4221-5. [DOI] [PubMed] [Google Scholar]
- 6.Mora-Garcia ML, Monroy-Garcia A. [Immune response in cervical cancer. Strategies for the development of therapeutic vaccines] Rev Med Inst Mex Seguro Soc. 2015;53:S206–211. [PubMed] [Google Scholar]
- 7.Giarre M, Caldeira S, Malanchi I, Ciccolini F, Leao MJ, Tommasino M. Induction of pRb degradation by the human papillomavirus type 16 E7 protein is essential to efficiently overcome p16INK4a-imposed G1 cell cycle Arrest. J Virol. 2001;75:4705–4712. doi: 10.1128/JVI.75.10.4705-4712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim YT. Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease. Clin Exp Vaccine Res. 2015;4:54–58. doi: 10.7774/cevr.2015.4.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 11.Ninkovic J, Roy S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids. 2013;45:9–24. doi: 10.1007/s00726-011-1163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamali A, Mahdavi M, Hassan ZM, Sabahi F, Farsani MJ, Bamdad T, et al. A novel adjuvant, the general opioid antagonist naloxone, elicits a robust cellular immune response for a DNA vaccine. Int Immunol. 2009;21:217–225. doi: 10.1093/intimm/dxn139. [DOI] [PubMed] [Google Scholar]
- 13.Farahani SV, Aghasadeghi MR, Memarnejadian A, Faezi S, Shahosseini Z, Mahdavi M. Naloxone/Alum mixture, a potent adjuvant for HIV-1 vaccine: induction of cellular and poly-isotypic humoural immune responses. Pathog Glob Health. 2015;24 doi: 10.1179/2047773215Y.0000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamali A, Mahdavi M, Shahabi S, Hassan ZM, Sabahi F, Javan M, et al. Naloxone, an opioid receptor antagonist, enhances induction of protective immunity against HSV-1 infection in BALB/c mice. Microb Pathog. 2007;43:217–223. doi: 10.1016/j.micpath.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 16.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faezi S, Safarloo M, Amirmozafari N, Nikokar I, Siadat SD, Holder IA, et al. Protective efficacy of Pseudomonas aeruginosa type-A flagellin in the murine burn wound model of infection. Apmis. 2014;122:115–127. doi: 10.1111/apm.12101. [DOI] [PubMed] [Google Scholar]
- 18.Katz CS. William L, Bradford MD, et al., editors. Humoral antibody formation in infants aged one to three months injected with a triple (diphtheria-tetanus-pertussis) alum-precipitated antigen, Pediatrics, 1949;4:711-718. Pediatrics. 1998;102:207–209. [PubMed] [Google Scholar]
- 19.Moon SH, Shin EC, Noh YW, Lim YT. Evaluation of hyaluronic acid-based combination adjuvant containing monophosphoryl lipid A and aluminum salt for hepatitis B vaccine. Vaccine. 2015;33:4762–4769. doi: 10.1016/j.vaccine.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Morello CS, Kraynyak KA, Levinson MS, Chen Z, Lee KF, Spector DH. Inactivated HSV-2 in MPL/alum adjuvant provides nearly complete protection against genital infection and shedding following long term challenge and rechallenge. Vaccine. 2012;30:6541–6550. doi: 10.1016/j.vaccine.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahdavi M, Ebtekar M, Azadmanesh K, Khorramkhorshid HR, Rahbarizadeh F, Yazdi MH, et al. HIV-1 Gag p24-Nef fusion peptide induces cellular and humoral immune response in a mouse model. Acta Virol. 2010;54:131–136. doi: 10.4149/av_2010_02_131. [DOI] [PubMed] [Google Scholar]
- 22.Tomljenovic L, Shaw CA. Aluminum vaccine adjuvants: are they safe? Curr Med Chem. 2011;18:2630–2637. doi: 10.2174/092986711795933740. [DOI] [PubMed] [Google Scholar]
- 23.Mahdavi M, Ebtekar M, Khorram Khorshid HR, Azadmanesh K, Hartoonian C, Hassan ZM. ELISPOT analysis of a new CTL based DNA vaccine for HIV-1 using GM-CSF in DNA prime/peptide boost strategy: GM-CSF induced long-lived memory responses. Immunol Lett. 2011;140:14–20. doi: 10.1016/j.imlet.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Jazani NH, Parsania S, Sohrabpour M, Mazloomi E, Karimzad M, Shahabi S. Naloxone and alum synergistically augment adjuvant activities of each other in a mouse vaccine model of Salmonella typhimurium infection. Immunobiology. 2011;216:744–751. doi: 10.1016/j.imbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Jazani NH, Sohrabpour M, Mazloomi E, Shahabi S. A novel adjuvant, a mixture of alum and the general opioid antagonist naloxone, elicits both humoral and cellular immune responses for heat-killed Salmonella typhimurium vaccine. FEMS Immunol Med Microbiol. 2011;61:54–62. doi: 10.1111/j.1574-695X.2010.00747.x. [DOI] [PubMed] [Google Scholar]
- 26.O’Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng. 2001;18:69–85. doi: 10.1016/s1389-0344(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 27.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 28.McAndrew EG, Dugast AS, Licht AF, Eusebio JR, Alter G, Ackerman ME. Determining the phagocytic activity of clinical antibody samples. J Vis Exp. 2011:e3588. doi: 10.3791/3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arezoo Shajiei ARM, Ghorbanali Shahabi, Ramin Farhoudi, Sobhan Faezi, Majid Tebianian, Nooshin Sohrabi, Mehdi Mahdavi. Pseudomonas aeruginosa Recombinant Flagellin Induced Poly-Isotypic Humoral Immune Responses in the Balb/C Mic. Jundishapur J Microbiol. 2013:6. [Google Scholar]


