Abstract
Objective(s):
Endometriosis is a complex gynecologic disease with unknown etiology. Noscapine has been introduced as a cancer cell suppressor. Endometriosis was considered as a cancer like disorder, The aim of present study was to investigate noscapine apoptotic effect on human endometriotic epithelial and stromal cells in vitro.
Materials and Methods:
In this in vitro study, endometrial biopsies from endometriosis patients (n=9) were prepared and digested by an enzymatic method (collagenase I, 2 mg/ml). Stromal and epithelial cells were separated by sequential filtration through a cell strainer and ficoll layering. The cells of each sample were divided into five groups: control (0), 10, 25, 50 and 100 micromole/liter (µM) concentration of noscapine and were cultured for three different periods of times; 24, 48 and 72 hr. Cell viability was assessed by colorimetric assay. Nitric oxide (NO) concentration was measured by Griess reagent. Cell death was analyzed by Acridine Orange (AO)–Ethidium Bromide (EB) double staining and Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay. Data were analyzed by one-way ANOVA.
Results:
Viability of endometrial epithelial and stromal cells significantly decreased in 10, 25, 50 and 100 µM noscapine concentration in 24, 48, 72 hr (P<0.05) and apoptotic index increased in 25, 50 and 100 µM noscapine concentrations in 48 hr significantly (P<0.05). Concentrations of NO didn’t show a significant decrease.
Conclusion:
Noscapine increased endometriotic epithelial and stromal cell death and can be suggested as a treatment for endometriosis.
Keywords: Apoptosis, Endometriosis, Nitric oxide, Noscapine
Introduction
Endometriosis is a complex and enigmatic gynecologic disease with incompletely understood etiology and is defined as the presence of endometrial tissue outside the uterine cavity which causes an inflammatory reaction, chronic pelvic pain, and infertility. Endometriosis prevalence is estimated to be 5–15% in women of reproductive age and 3–5% in postmenopausal women (1). The morphologic appearance of endometriosis is marked by proliferation, infiltration, angiogenesis and severe adhesions to the surrounding tissues. Researchers have focused on its pathogenesis, including anatomic, hormonal, immunologic and genetic factors (2, 3).
The blood vessels supplying oxygen and nutrients are essential for the development and survival of endometriosis (4). It has been shown that angiogenesis is necessary for the survival of tumors larger than 2-3 mm (5) and angiogenesis is found in endometriosis tissue (6) and it is considered as an angiogenic disease. Previous study has shown increased incidence of cancer in women with endometriosis, and high prevalence of endometriosis has been seen in women with ovarian cancer (7).
Endometriosis is often diagnosed by laparoscopy, and a high percentage of recurrences of endometriosis happen. It is suggested that surgical and drug treatments may provide better results (8). Drug treatments used for endometriosis are Gonadotropin-releasing hormone analogs (GnRH) agonists (1) and oral contraceptives (9), progestin antagonists such as mifepristone (RU 486) (10) and new suggestions such as statin (11) and cyclooxygenase-2 (COX-2) inhibitor (celecoxib) (12). The main purpose of medical treatment of endometriosis is inhibition of the growth and activity of endometriosis tissue, although, their side effects may limit the use of these drugs (8).
Programmed cell death or apoptosis plays an important role in the physiology of endometrial tissue. Studies have shown that during the secretory phase of the menstrual cycle, the stromal and epithelial cells of endometrium undergo apoptosis (13). On the other hand, programmed cell death (apoptosis) significantly is reduced in endometriosis compared to normal endometrial tissue, and there is possibly an inverse relationship between apoptosis and endometriosis (14).
Noscapine, a phthalideisoquinoline alkaloid, is composed of 1-10% opium alkaloids and is used as an antitussive in humans and experimental animals (15). Also, it has anti-cancer activity (16). This drug has high safety, low or no toxicity in the tumor suppression dosage and has been shown in liver, heart, bone marrow, spleen, and small intestine (17). Furthermore, noscapine solubility in water makes it more suitable than many other drugs to administer orally to treat cancer (15). Noscapine interacts with alpha-tubulin and induced apoptosis in cancer cells (18, 19). Also, it has been shown to reduce neo-angiogenesis and tumor size and to stop apoptosis of tumor cells (20, 21). Due to lack of definitive medical treatments for endometriosis and the need to identify new pharmaceutical compounds influencing this disease; this study was conducted to identify the in vitro effect of noscapine on endometrial epithelial and stromal cells of endometriosis patients.
Materials and Methods
In this in vitro experimental study, endometrial biopsies (n = 9) were prepared from women in the reproductive age (25-40 years old) and secretory phase of menstruation. The work on the human tissue was accepted by Ethics Committee of Kermanshah University of Medical Sciences and all patients signed informed consent. The samples with polyps, endometrial hyperplasia and cancer, and hormone therapy in the last three months were excluded.
Stromal and epithelial cells isolation
Endometrial cells were separated according to our previous studies (11, 22). After washing with phosphate buffer solution (PBS) containing 2% antibiotics-antimycotic solution, the biopsy was chopped using a sterile scalpel in a Petri dish and the chopped tissue was transferred to collagenase I solution (2 mg/ml) (Sigma, Germany) in medium: Dulbecco’s Modified Eagle Medium/Nutrient Mixture F12 (DMEM/F12) (Gibco, Denmark) supplemented with 5% fetal bovine serum (FBS), (Gibco, Denmark) and incubated at 37°C for 60 - 90 min. Cell suspensions were passed through 70 and 40 micrometer (μm) filter mesh (cell strainer; Becton Dickenson Company, USA). The 40 μm filter mesh was washed back to collect endometrial glands. These glands were dissociated to single cells by 0.02% trypsin and were cultured in DMEM/F12 with 10% FBS.
The filtrate was centrifuged (2500 g) for 15 minutes and the cell pellet was broken by the media and the layering on the ficoll (Amersham-Abrahamson) and centrifuged (1500 g) for 30 min. Then, the supernatant was collected and rinsed with saline solution (0.15 mM) and was centrifuged (100 g) for 10 minutes. Stromal cells were cultured in DMEM/F12 with FBS 5%. According to our previous work (11, 22), DAKO standard methods were done for cytokeratin as an epithelial cell marker and vimentin for stromal cell marker.
Experimental design
Isolated stromal and epithelial cells of each sample were divided into five groups; control (0), 10, 25, 50 and 100 µM noscapine concentrations (Sigma) and were cultured for three separate periods (24, 48 and 72 hr), noscapine (MW = 413.4) is water soluble and these doses were selected based on Tayarani-Najaran study (23). Noscapine (0.0413 g) was dissolved in Deionized water to made 0.1 millimole/liter (100 µM). The other doses were prepared from 100 µM concentration.
After treatment, the cells were examined for viability by colorimetric assay (MTT) assay. Dead cell and its variants were stained with acridine orange and ethidium bromide (AO/EB) and Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay. The amount of nitric oxide (NO) release in the medium was measured by using Griess reagent (24, 25).
Cell viability
Viability was assessed by MTT (MTT [3-(4, 5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide]) (Roche, GmbH, Germany). After treatment of the cells, 20 µl of MTT solution (0.5 mg / ml in PBS) was added to each well of the 96 wells culture plate and incubated in a 5% CO2 at 37°C for 4 hr. After incubation, the supernatant of each well was gently removed and 100 µlit Dimethyl sulfoxide (DMSO) was added to wells and placed at room temperature for 30 min to dissolve produced formazan crystals. Absorbance was read at a wavelength of 570 nm against a reference wavelength of 630 nm using ELISA reader (Stat fax, USA) and the percentage of cell viability was calculated by dividing the absorbance of treated group to the absorption of control group × 100 (25).
Acridine orange (AO)–ethidium bromide (EB) double staining cell morphological analysis
Cell death detection was performed by acridine orange/ethidium bromide (AO/EB) double staining (26). Thus, 100 µliter of the mixture of two colors (100 µg/mL AO and 100 µg/mL EB in PBS) was added to the cells in 96-well culture plates, which were then washed with PBS and cells were observed by fluorescence microscopy (Nikon, Germany). At least 200 cells were counted in each sample and the percentage of cell death was determined.
TUNEL staining
Apoptosis (nuclear DNA fragmentation) was detected by the TUNEL method using an in situ cell death detection Kit (Roche Diagnostics Corporation Mannheim, Germany) according to the manufacturer’s instructions. Briefly, After 48 hr treatment of epithelial and stromal cells with Noscapine, the cells were fixed in 4 % PBS-buffered paraformaldehyde for 30 min at room temperature and then permeabilized with 0.1% Triton X-100 in sodium citrate for 2 min on ice. Then, the cells were incubated with 50 μl of terminal deoxynucleotidyl transferase end-labeling solution for 60 min at 37°C in a humidified chamber in the dark. Then, the cells were counterstained in PI staining solution for 4 min at room temperature in the dark. After each step, the stained cells were washed 1-2 times with PBS and were then observed by fluorescence microscopy (Nikon, Germany). The percentage of positively stained cells was calculated as the percentage of the apoptotic cells relative to the total number of the cells (27).
Nitric oxide assay
The supernatants of epithelial and stromal cells were used for NO measurement in 24, 48 and 72 hr culture periods. NOx (total nitrite and nitrate) levels were measured by Griess method (24). The supernatant was deproteinized by adding 400 µl of the supernatant to 6 mg zinc sulfate powder and centrifuged for 12 min (5°C) at 12000 rpm. Then, 100 µl of deproteinized samples and 100 µl of vanadium chloride (III) (to convert nitrate to nitrite) were added to microplate wells. A mixture of sulfonamides 2% (50 µl) and naphthyl ethylenediamine dihydrochloride (NEED) 0.1% (50 µl) was added to wells and incubated for 30 min at 37°C. Concentrations of 6.25, 12.5, 25, 50, 100 and 200 µl sodium nitrate were used as the standard. The absorbance of wells was measured at 540 and 630 nm by ELISA reader (Stat fax 100. USA). Each experiment was repeated at least three times.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) to compare different groups. The analysis was carried out using Statistical Package for the Social Science (SPSS) version 16 (Chicago, IL, USA). Results were expressed as mean ± SEM and P < 0.05 was considered significant.
Results
The viability indices of stromal cells for control, 10, 25, 50 and 100 µM noscapine concentrations were 97.9%, 91.7%, 90.3 %, 87.2 % and 85.7 % in 24 hr, 94.3%, 88.4%, 86.7%, 83.6% and 76.9% in 48 hr, and 95.7%, 84.3%, 82.7%, 79.6% and 72.9% in 72 hr, respectively. Survival rate of stromal cells at concentrations of 10, 25, 50 and 100 µM noscapine decreased compared to the control group in 24-, 48- and 72-hr time periods in time- and dose- dependent manner. The differences were significant at 50 and 100 µM concentrations (P< 0.05) (Figure 1).
Figure 1.
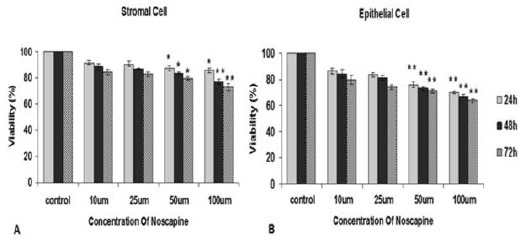
The effect of different concentrations of noscapine on the human endometrial stromal (A) and epithelial cells (B) viability from endometriosis patients by MTT assay in 24, 48 and 72 hr. Data were analyzed by one-way ANOVA. The results showed as mean ± SEM (*P< 0.05; **P< 0.001)
The viability indices of epithelial cells were 93.7%, 86.2%, 83.4%, 75.7% and 69.7% for control, 10, 25, 50 and 100 µM noscapine concentrations in 24 hr, 91.0%, 84.1%, 80.8%, 72.7% and 67.1% in 48 hr and 86.8%, 79.4%, 74.1%, 70.9% and 63.5% in 72 hr. The differences were significant at 50 and 100 µM concentrations (P< 0.05).
Types of cell death (acridine orange and ethidium bromide (AO/EB) staining
Epithelial cells death increased significantly in 10, 25, 50 and 100 µM noscapine concentrations (11.8%, 15.7%, 20.5% and 26.4%, respectively) compared to control group (7.2%) in 24 hr. Stromal cell death increased significantly in 10, 25, 50 and 100 µM noscapine concentrations (10.6%, 14.3%, 19.9% and 23.2%, respectively) compared to control group (5.6%) in 24 hr (P< 0.05) (Figure 2). Epithelial and stromal cells comparison showed noscapine more prominently affects epithelial cells than stromal cells.
Figure 2.
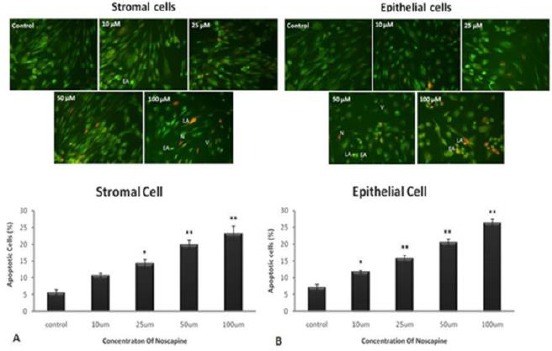
The effect of different concentrations of noscapine on cell death in human endometrial stromal (A) and epithelial cells (B) of endometriosis patients by acridine orange and ethidium bromide staining (AO/EB) in 48 hr. L= live cells (Opaque Green), EA= early apoptotic cells (bright green), LA= late apoptotic cells (Orange), N= necrotic cells (Reddish orange). Magnification: ×200. Data were analyzed by one-way ANOVA. The results showed as mean ± SEM (*P< 0.05, **P<0.001)
Cell death (TUNEL Assay)
Epithelial and stromal cells death increased significantly in high concentrations of noscapine.
Apoptotic epithelial and stromal (TUNEL positive) cells in 10, 25, 50 and 100 µM noscapine concentrations increased to 9.04%, 13.07%, 15.15% and 17.82%, and 7.48%, 12.56%, 14.44% and 16.11%, respectively compared to control groups (4.96% and 4.46%) in 48 hr (P< 0.05), (Figure 3).
Figure 3.
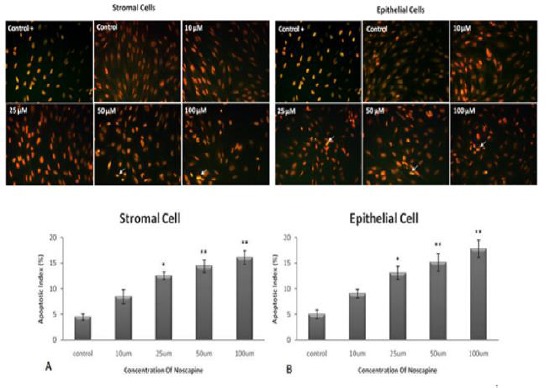
The effect of different concentrations of noscapine on cell death in human endometrial stromal (A) and epithelial cells (B) of endometriosis patients by TUNEL staining in 48 hr. Apoptotic cells: arrowhead. Magnification: ×200. Data were analyzed by one-way ANOVA. The results showed as mean ± SEM (*P< 0.05; **P< 0.001)
Nitric oxide concentration
NO secretions by stromal cell were 29.12±5.8, 28.13±1.89, and 24.39±5.13 in 100 µM concentration at 24, 48 and 72 hr treatments, respectively compared to their controls: 66.85±16.77, 52.17±11.47, and 53.28±13.5. Epithelial cells NO secretions were 32.25±11.8, 27.44±12.97 and 22.17±8.13 in 100 µM concentration at 24, 48 and 72 hr treatments respectively compare to their controls: 59.76±11.62, 49.97±12.87, and 46.5±2.57. Nitric oxide secretion of stromal and epithelial cells in the concentrations of 10, 25, 50 and 100 µM noscapine decreased time- and dose- dependent manner, but there is no significant difference compared to control group in 24, 48 and 72 hr. (Figure 4).
Figure 4.
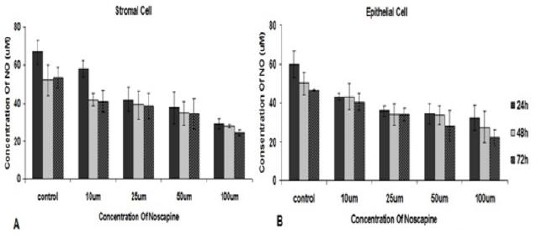
The effect of different concentrations of noscapine on NO secretion by human endometrial stromal (A) and epithelial cells (B) from endometriosis patients by Griess reagent in 24, 48 and 72 hr. Data were analyzed by one-way ANOVA. The results showed as mean ± SEM
Discussion
Noscapine significantly decreased the viability of endometriotic epithelial and stromal cells in a dose- and time-dependent manner, which was more prominent in the epithelial cells (higher apoptotic index). Also, noscapine induced significant apoptosis in these cells and reduced their nitric oxide secretion. The inhibitory effect of noscapine on endometrial epithelial and stromal cells of endometriosis patients was similar to its inhibitory effect on breast cancer cells; MDA-MB-468, MDA-MB-231(28), and melanoma cells (B16LS9) in mice (29). Despite the different types of cells, the results of these studies were similar to our finding.
In the present study, we used pure noscapine while noscapine derivatives such as brominated noscapine 5-bromonoscapine (5-Br-nosc), reduced 5-bromonoscapine (Rd 5-Br-nosc), inhibited cell proliferation effectively in lower concentrations in prostate, breast, ovary, and cervix cancer (30). Further, noscapine reduced the survival of liver cancer cells in a dose- and time-dependent manner and had no effect on non-malignant cells (23) which is in accordance with our results.
Heidari et al (2007) showed that noscapine increased the ratio of Bax/Bcl-2 expression and induced apoptosis in two myeloid cell lines. In this study, noscapine as a new therapeutic agent with potential anticancer activity was suggested for glioblastoma multiform patients (31). Our study also showed that noscapine induced apoptosis in endometriotic cells and higher concentrations of noscapine caused cell death by apoptosis.
In the present study, high concentrations of noscapine inhibited the secretion of NO from both endometriotic stromal and epithelial cells of endometriosis patients. The previous study has shown increased levels of NO in the peritoneal fluid of women with endometriosis compared to women without endometriosis (32). Several studies have shown high levels of NO and NO synthetase in cancerous tissues. For instance, NO levels increased in patients with gastric cancer (33). NO has antioxidant activity and prevents ROS-induced apoptosis leading to tumor growth by removing the oxygen free radicals (ROS) (34). On the other hand, NO induced apoptosis in endometrial cells and macrophages (35). There is a high NO produced by macrophages in women with endometriosis (36) which correlates to increased expression of NO synthetase and subsequent NO production (37).
In the present study, we showed that nitric oxide production from endometriotic stromal and epithelial cells was lower in the experimental groups compared to the control group in time- and dose-dependent manner. However, this reduction did not show significant differences and this decrease was higher in epithelial cells than in stromal cells, which can be attributed to the amount of cell death which was higher in epithelial cells. So, noscapine increased epithelial and stromal cells apoptosis and reduced their nitric oxide secretion, and can be considered in endometriosis treatment.
The study of cell death in epithelial and stromal cells of endometriosis has shown that cell death is varied in different phases of the menstrual cycle and the highest rate of cell death occurs at later stages of the secretory phase. Apoptosis occurs in both epithelial and stromal cells in the functional layers of the endometrium of endometriosis patients. On the other hand, the comparison between normal endometrium and endometriosis has shown that the cell death rate is reduced in stromal and epithelial cells from endometriotic tissue (35). The resistance of these cells to apoptosis and cell death has been demonstrated in previous studies (38). Therefore, induction of apoptosis in endometrial cells can prevent cell growth which was visible in our results.
What is clear is that apoptosis is a mechanism that controls the promotion and development of endometriosis (39). In both normal and endometriotic endometrium, the apoptosis index in epithelial glands is higher than stromal cells (40). This suggests that these cells do not have an equal contribution in the creation and development of endometriosis. Also, in our study, the rate of cell death was higher in epithelial cells than stromal cells. Noscapine decreased cell proliferation and increased the apoptosis of endometriotic epithelial and stromal cells. It also reduced nitric oxide secretion.
The present study was a preliminary two-dimensional cell culture study with a limited number of patients. It needs to examine noscapine effect on endometriosis tissue in three-dimensional culture which is a better model for endometriosis study (41), and even animal model of endometriosis. Also, we didn’t survey apoptosis-related genes which may be affected by noscapine. In future study we plan to complete the present study by three-dimension culture of endometrial tissue of endometriosis patients.
Conclusion
These results suggest that the inhibitory effects of noscapine on the endometriotic stromal and epithelial cells may be useful to treat and suppress the growth and proliferation of endometriosis tissue.
Acknowledgment
The results described in this paper were part of a PhD thesis (92383). This work was supported by the Voice Chancellor of Research, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Conflict of interest
The authors declare that there is no conflict of interest in this study.
References
- 1.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. European Society of Human Reproduction and Embryology. Hum Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 2.Vignali M, Infantino M, Matronem R, Chiodo I, Somigliana E, Busacca M, et al. Endometriosis: novel etiopathogenetic concepts and clinical perspectives. Fertil Steril. 2002;78:665–678. doi: 10.1016/s0015-0282(02)03233-8. [DOI] [PubMed] [Google Scholar]
- 3.Nap AW, Groothuis PG, Demir AY, Evers JL, Dunselman GA. Pathogenesis of endometriosis. Best Practice and Research Clinical Obstetrics and Gynaecolgy. 2004;18:233–244. doi: 10.1016/j.bpobgyn.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 5.Yadav L, Puri N, Rastogi V, Satpute P, Sharma V. Tumour Angiogenesis and Angiogenic Inhibitors: A Review. J Clin Diagn Res. 2015;9:XE01–XE05. doi: 10.7860/JCDR/2015/12016.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Annals of New York Academy Sciences. 2002;955:89–100. doi: 10.1111/j.1749-6632.2002.tb02769.x. [DOI] [PubMed] [Google Scholar]
- 7.Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF, Nikrui N, Goff BA. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol. 1996;60:238–244. doi: 10.1006/gyno.1996.0032. [DOI] [PubMed] [Google Scholar]
- 8.Huang HY. Medical Treatment of Endometriosis. Chang Gung Med J. 2008;31:431–440. [PubMed] [Google Scholar]
- 9.Vercellini P, De Giorgi O, Mosconi P, Stellato G, Vicentini S, Crosignani PG. Cyproterone acetate versus a continuous monophasic oral contraceptive in the treatment of recurrent pelvic pain after conservative surgery for symptomatic endometriosis. Fertil Steril. 2002;77:52–61. doi: 10.1016/s0015-0282(01)02951-x. [DOI] [PubMed] [Google Scholar]
- 10.Kettel LM, Murphy AA, Morales AJ, Yen SS. Preliminary report on the treatment of endometriosis with low-dos mifepristone (RU 486) Am J Obstet Gynecol. 1998;178:1151–1156. doi: 10.1016/s0002-9378(98)70316-3. [DOI] [PubMed] [Google Scholar]
- 11.Esfandiari N, Khazaei M, Jafar A, Bielecki R, Gotlieb L, Ryan E, et al. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2007;87:257–262. doi: 10.1016/j.fertnstert.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Rezavand N, Khazaei M, Oliapanah E, Nikzad H, Khazaei MR. Low doses of celecoxib stimulate human endometrium growth in a three-dimensional culture model. Int J Fertil Steril. 2013;7:7–12. [PMC free article] [PubMed] [Google Scholar]
- 13.Kokawa K, Shikone T, Nakano R. Apoptosis in the human uterine endometrium during the menstrual cycle. J Clinic Endocrin Met. 1996;81:4144–4147. doi: 10.1210/jcem.81.11.8923873. [DOI] [PubMed] [Google Scholar]
- 14.Meresman GF, Bilotas MA, Lombardi E, Tesone M, Sueldo C, Baranao R. Effect of GnRH analogues on apoptosis and release of interleukin-1b and vascular endothelial growth factor in endometrial cell cultures from patients with endometriosis. Hum Reprod. 2003;18:1767–1771. doi: 10.1093/humrep/deg356. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoudian M. Recent progress in clinical application of noscapine: a review. Cur Topics Pharmacology. 2006;10:81–86. [Google Scholar]
- 16.Ebrahimi SA, Zareie-Rostami P, Mahmoudian M. Interaction of noscapine with the bradukinin mediation of cough response. Acta Physiolo. 2003;90:147–155. doi: 10.1556/APhysiol.90.2003.2.7. [DOI] [PubMed] [Google Scholar]
- 17.Landen JW, Lang R, McMahon SJ, Rusan NM, Yvon AM, Adams AW, et al. Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Research. 2002;62:4109–4114. [PubMed] [Google Scholar]
- 18.Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. P53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Research. 2007;67:3862–3870. doi: 10.1158/0008-5472.CAN-06-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, et al. Opium alkaloid noscapine is an antitumor agent that arrest metaphase and induces apoptosis in dividing cells. Proceeding of the National Academy of Sciences USA. 1998;95:1601–1606. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoudian M, Rahimi-Moghaddam P. The anti-cancer activity of noscapine: a review. Recent Patents on Anticancer Drug Discovery. 2009;4:92–97. doi: 10.2174/157489209787002524. [DOI] [PubMed] [Google Scholar]
- 21.Barken I, Geller J, Rogosnitzky M. Noscapine inhibits human prostate cancer progression and metastasis in a mouse model. Anticancer Research. 2008;28:3701–3704. [PubMed] [Google Scholar]
- 22.Khazaei M, Chobsaz F, Khazaei S. The effect of different doses of clomiphene citrate on morphology and proliferation of endometrial stromal cells in in-vitro culture. J Bab Uni Med Sci. 2010;12:7–12. [Google Scholar]
- 23.Tayarani-Najaran Z, Parsaee H, Hoseini A, Mousavi SH. Study of noscapine-induced cell death in hepatocellular carcinoma cell Line. Pharmacology online. 2009;3:522–530. [Google Scholar]
- 24.Khazaei M, Roshankhah Sh, Ghorbani P, Chobsaz F. Sildenafil effect on nitric oxide secretion by normal human endometrial epithelial cells cultured in vitro. Int J Fertil Steril. 2011;5:142–147. [PMC free article] [PubMed] [Google Scholar]
- 25.Mirzapur P, Rashidi Z, Rezakhani L, Khazaei M. In vitro inhibitory effect of crab shell extract on human umbilical vein endothelial cell In Vitro. Cellular & Developmental Biology – Animal. 2015;51:36–41. doi: 10.1007/s11626-014-9810-x. [DOI] [PubMed] [Google Scholar]
- 26.Baskic D, Popovic S, Ristic P, Nebojsa N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Intern. 2006;30:924–932. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Rashidi Z, Azadbakht M, Khazaei M. Hydrostatic pressure improves in-vitro maturation of oocytes derived from vitrified-warmed mouse ovaries. Iran J Reprod Med. 2012;10:257–64. [PMC free article] [PubMed] [Google Scholar]
- 28.Chougule MB, Apurva RP, Jackson T, Patel R. Antitumor activity of noscapine in combination with doxorubicin in triple negative breast cancer. Mandip Singh PLoS ONE. 2011;3:1–12. doi: 10.1371/journal.pone.0017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronald l, Steve JM, Jaren W, McMahon J. Noscapine alters microtubule dynamics and inhibits the progression of melanoma in living cells. Cancer Res. 2002;62:4109–4114. [PubMed] [Google Scholar]
- 30.Zhou J, Gupta K, Aggarwal S, Aneja R, Chandra R, Panda D, et al. Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Molecular Pharmacology. 2003;63:799–807. doi: 10.1124/mol.63.4.799. [DOI] [PubMed] [Google Scholar]
- 31.Heidari N, Goliaei B, Moghaddam PR, Rahbar-Roshandel N, Mahmoudian M. Apoptotic pathway induced by noscapine in human myelogenous leukemic cells. Anticancer Drugs. 2007;18:1139–1147. doi: 10.1097/CAD.0b013e3282eea257. [DOI] [PubMed] [Google Scholar]
- 32.Wu MY, Chao KH, Yang JH, Lee TH, Yang YS, Ho HN. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Hum Reprod. 2003;18:2668–2671. doi: 10.1093/humrep/deg484. [DOI] [PubMed] [Google Scholar]
- 33.Bakan E, Taysi S, Polat MF, Dalga Z, Bakan N, Gumus M. Nitric oxide levels and lipid peroxidation in plasma of patients with gastric cancer. Clinical Oncology. 2002;32:162–166. doi: 10.1093/jjco/hyf035. [DOI] [PubMed] [Google Scholar]
- 34.Tong X, Li H. eNOS protects prostate cancer cells from TRAIL-induced apoptosis. Cancer Letters. 2004;210:63–71. doi: 10.1016/j.canlet.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Vaskivuo TE, Stenbäck F, Karhumaa P, Risteli J, Dunkel L, Tapanainen JS. Apoptosis and apoptosis-related proteins in human endometrium. Molecular Cellular Endocrinology. 2000;165:75–83. doi: 10.1016/s0303-7207(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 36.Dahmoun M, Boman K, Cajander S, Westin P, Bäckström T. Apoptosis, proliferation and sex hormone receptors in superficial parts of human endometrium at the end of the secretory phase. J Clin Endocrinol Meta. 1999;84:1737–1743. doi: 10.1210/jcem.84.5.5706. [DOI] [PubMed] [Google Scholar]
- 37.Osborn BH, Haney AF, Misukonis MA, Weinberg JB. Inducible nitric oxide synthase expression by peritoneal macrophages in endometriosis-associated infertility. Fertil Steril. 2002;77:46–51. doi: 10.1016/s0015-0282(01)02940-5. [DOI] [PubMed] [Google Scholar]
- 38.Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil Steril. 1998;69:1042–1047. doi: 10.1016/s0015-0282(98)00073-9. [DOI] [PubMed] [Google Scholar]
- 39.Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB, Braun DP. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum Reprod. 2001;16:1802–1808. doi: 10.1093/humrep/16.9.1802. [DOI] [PubMed] [Google Scholar]
- 40.Dunselman GA, Hendrix MG, Bouckaert PX, Evers JL. Functional aspects of peritoneal macrophages in endometriosis of women. J Reprod Fertil. 1988;82:707–710. doi: 10.1530/jrf.0.0820707. [DOI] [PubMed] [Google Scholar]
- 41.Fasciani A1, Bocci G, Xu J, Bielecki R, Greenblatt E, Leyland N, et al. Three-dimensional in vitro culture of endometrial explants mimics the early stages of endometriosis. Fertil Steril. 2003;80:1137–1143. doi: 10.1016/s0015-0282(03)02164-2. [DOI] [PubMed] [Google Scholar]


