Abstract
Objective(s):
Cerebral ischemia is often associated with cognitive impairment. Oxidative stress has a crucial role in the memory deficit following ischemia/reperfusion injury. α-Terpineol is a monoterpenoid with anti-inflammatory and antioxidant effects. This study was carried out to investigate the effect of α-terpineol against memory impairment following cerebral ischemia in rats.
Materials and Methods:
Cerebral ischemia was induced by transient bilateral common carotid artery occlusion in male Wistar rats. The rats were allocated to sham, ischemia, and α-terpineol-treated groups. α-Terpineol was given at doses of 50, 100, and 200 mg/kg, IP once daily for 7 days post ischemia. Morris water maze (MWM) test was used to assess spatial memory and in vivo extracellular recording of long-term potentiation (LTP) in the hippocampal dentate gyrus was carried out to evaluate synaptic plasticity. Malondialdehyde (MDA) was measured to assess the extent of lipid peroxidation in the hippocampus.
Results:
In MWM test, α-terpineol (100 mg/kg, IP) significantly decreased the escape latency during training trials (P<0.01). In addition, α-terpineol increased the number of crossings over the platform location and decreased average proximity to the target in probe trial (P<0.05). In electrophysiological recording, α-terpineol (100 mg/kg) facilitated the induction of LTP in the hippocampus which was persistent over 2 hr. α-Terpineol (100 and 200 mg/kg) also significantly lowered hippocampal MDA levels in rats subjected to cerebral ischemia.
Conclusion:
These findings indicate that α-terpineol improves cerebral ischemia-related memory impairment in rats through the facilitation of LTP and suppression of lipid peroxidation in the hippocampus.
Keywords: α-Terpineol, Cerebral ischemia, Long-term potentiation, Memory, Oxidative stress, Rats
Introduction
Cognitive impairments and memory deficits are common pathological conditions contributing to several neurological diseases such as vascular dementia, Alzheimer’s disease, and ischemic stroke (1). Cerebral ischemia which is caused by an obstruction within cerebral arteries is often associated with a high incidence of cognitive impairment and memory deficit. There are several reports indicating that cerebral hypoperfusion followed by brain ischemia leads to progressive cognitive impairments (2).
Reduction in cerebral blood flow which result in disturbances of energy metabolism in the brain, may lead to structural and functional damage to neurons in vulnerable regions of the brain especially the cerebral cortex and hippocampus (3). It has been well established that the integrity of the hippo-campal formation is required for normal spatial memory and navigation (4).
Many lines of evidence indicate that the induction of hippocampal long-term potentiation (LTP) have a crucial role for the acquisition of spatial memory (5). Evidence of synaptic plasticity impairment in the hippocampus following global cerebral ischemia provides further insight into the association between brain ischemia and memory deficit (6).
When ischemia occurs, oxygen free radicals are produced in the mitochondria as a result of anaerobic metabolism. This may lead to the oxidative stress and damage to the hippocampal formation (7). Since the oxidative stress and overproduction of free radicals have a crucial role in the memory impairment following ischemia/reperfusion injury, using strategies focused on these underlying mechanisms have been shown to be efficacious to improve memory deficit (8).
Many studies have reported that antioxidants and anti-inflammatory agents could prevent damage to neuronal cells and could be considered a part of clinical treatment of memory impairment following cerebral ischemia (9, 10).
α-Terpineol is a monoterpenoid alcohol with several pharmacological properties including antinociceptive (11, 12), anticonvulsant (13), antioxidant (14, 15), and acetylcholinesterase enzyme inhibitory effects (16). α-Terpineol has been found in many medicinal plants such as Abies koreana Wilson (17) and Salvia spp. (16, 18). It has been shown that A. koreana Wilson extract can be useful in treating diseases that accompany dementia such as Alzheimer’s disease and vascular dementia (17). In addition, Salvia spp. extract (Sage) is used in European traditional medicine to boost memory and to cure dementia (18). Considering the pharmacological properties of α-terpineol especially its antioxidant effect, along with clinical application of α-terpineol -rich medicinal plants (Sage and A. koreana), it can be hypothesized that α-terpineol may be effective in attenuating memory impairment and neuronal damage following cerebral ischemia. As the protective effect of α-terpineol against memory impairment has not been previously elucidated, we investigated the effects of α-terpineol on spatial memory, synaptic plasticity, and lipid peroxidation following cerebral ischemia/reperfusion in rats.
Materials and Methods
Chemicals
α-Terpineol was purchased from Sigma-Aldrich Chemical Co. Phosphoric acid, TBA (2-thiobarbituric acid), n-butanol, Tween 80 (polysorbate 80), urethane, potassium chloride, and 1,1,3,3-tetramethoxypropane were obtained from Merck (Germany). Ketamine and xylazine were purchased from Rotexmedica, GmbH (Germany) and Loughrea Co, Galway (Ireland), respectively. α-Terpineol was dissolved in normal saline and suspended in 0.4% (v/v) Tween 80. The rats in ischemia group received the vehicle (normal saline + Tween 80).
Animals
Male Wistar rats weighing 270±30 g were used in this study. The animals were kept on a 12/12 hr light/dark cycle at 22±2ºC and had free access to tap water and food. The rats were allocated into 5 groups (each consisting of 8 rats). Group 1 (Sham): the animals were subjected to the operation but their common carotid arteries were not occluded; Group 2 (ischemia): the animals underwent bilateral occlusion of common carotid arteries and then treated with vehicle (normal saline + Tween 80); Groups 3 to 5: the rats underwent cerebral ischemia by transient bilateral occlusion of common carotid arteries and then treated with 3 different doses of α terpineol (50, 100, and 200 mg/kg, repectively). All experiments complied with the guidelines of University of Shahid Beheshti (School of Medicine) for laboratory animal care and use.
Induction of global cerebral ischemia
The global brain ischemia was induced by transient bilateral common carotid artery occlusion (19, 20). Briefly, rats were first anesthetized with ketamine and xylazine at doses of 100 and 10 mg/kg, IP, respectively. An incision was then made along the anterior surface of the neck and common carotid arteries on both sides were exposed and carefully separated from nerves and tissues. After that, common carotid arteries were occluded using the microvascular clamps for 20 min. At the end of ischemia, the microvascular clamps were released and cerebral blood flow through common carotid arteries was re-established. α-Terpineol or vehicle were injected IP immediately after reperfusion and the administration was continued once daily for 7 days. Overall, 5 rats died due to cerebral ischemia which were replaced by new animals.
Morris water maze test
In order to evaluate the reference memory through a spatial search strategy, Morris water maze test was performed 7 days after the induction of cerebral ischemia/reperfusion injury. The Morris water maze was consisted of a round tank of water, 120 cm in diameter and 60 cm in height, and was filled with water (25±0.5°C) to a depth of 40 cm. The tank was divided into four equal imaginary quadrants and four positions for starting (N: north, W: west, S: south, E: east,). A circular platform (diameter: 12 cm) was installed into the water tank in the center of NW quadrant (target quadrant), 1.5 cm below the surface of the water. Non-fat dry milk was used to make the water opaque. The rats were given 4 training trials each day for 3 consecutive days. In each trial, the rats were randomly placed into the water maze at one of starting positions of N, S, E, or W, facing the wall of the tank. Once the rats reached the platform the training trial was over. Otherwise, the animals were allowed to swim/search for the platform for a maximum of 60 sec. The rats were gently guided to the platform by the experimenter, if they failed to reach the platform within 60 Sec. In either case, the experimenter allowed the rats to stay over the platform for 30 sec. The inter-trial interval was at least 10-15 min. After all animals had completed 4 trials for 3 consecutive days, they were given one probe trial on the 4th day in which the platform was removed from the tank. Swimming paths of the rats were monitored by an infrared camera linked to a tracking system. For each training trial, the swimming time to find the platform (escape latency) was recorded. For probe trial, time spent in the target quadrant, distance travelled in the target quadrant, number of crossings over the platform location, average proximity to the platform location, and average speed were recorded and analyzed. In addition, the average speed of animals in each day was compared between different groups. A trial by trial comparison of escape latency on day 1 was also carried out. At the end of the trials, the animals were dried using a clean towel and placed under a heating lamp in a holding cage before they were returned to their home cages (21).
In vivo recording of hippocampal long-term potentiation (LTP)
Electrophysiological procedures were conducted one day after the end of Morris water maze test. Rats were first anesthetized with urethane (1.5 g/kg, IP) and fixed in a stereotaxic table (Stoelting Co USA) for surgery and recording. Supplementary doses of urethane (0.2-0.5 g/kg, IP) were given when necessary to ensure maintenance of an adequate level of anesthesia. The skull was exposed and two 1 mm holes were drilled on the right side of the skull overlying the perforant pathway (PP) and dentate gyrus (DG) for inserting the stimulating and recording electrodes. Twisted bipolar electrodes were used in this study. The electrodes were made from stainless steel wire (125 µm diameter, Teflon-coated, A-M Systems, USA). The stimulating electrode was placed in the PP at the coordinates of AP: -8.1 mm, ML: +4.3 mm, and DV: 3.2 mm, and the recording electrode was positioned in the DG granular cell layer at the coordinates of AP: -3.8 mm, ML: +2.3 mm, and DV: 2.7-3.2 mm. All coordinates were determined based on the Paxinos and Watson atlas of the rat brain (22). The stimulating and recording electrodes were slowly lowered at the selected positions until a field excitatory post-synaptic potential (fEPSP) was appeared. After determining the final position of the electrodes, test pulses (0.2 ms in duration) were delivered at the frequency of 0.033 Hz to elicit fEPSPs. Extracellular fEPSPs were recorded via a 2-channel Electromodule amplifier (model R12, ScienceBeam, Tehran, Iran). The signals were amplified (×1000) and filtered with the input frequency band set between 1-3000 Hz before digitization at 1 kHz (Electromodule R12, ScienceBeam). eTrace software (ScienceBeam) was used for acquisition of data and preliminary analysis. At the beginning of each recording trial, an input-output curve was constructed from responses (mean of 5 consecutive sweeps) obtained at 10 stimulus intensities (ranging from 100 to 1000 µA) to determine the maximum fEPSP amplitude. The stimulation intensity of test pulses was adjusted to evoke a fEPSP of 50% of maximal amplitude. Responses were allowed to stabilize for at least 20 min. Then, a 30 min stable baseline was recorded prior to induction of LTP to make sure that the synaptic response is stable. Following baseline recording, a high frequency stimulation (HFS) consisting of 10 bursts of 4 shocks (each delivered at 100 Hz) with an inter-burst interval of 200 msec was applied to PP for the induction of LTP. The stimulus intensity used for HFS was the same as for test pulses. After HFS, the recording of the baseline fEPSPs was continued for 2-4 hr. The amplitude of fEPSPs were averaged over 5 min intervals and normalized to the average amplitude value measured during the 30-min baseline period prior to LTP induction. The induction of LTP was considered to be successful if the amplitude of fEPSP was increased more than 20% for at least 30 min after the HFS (20, 23).
Measurement of malondialdehyde (MDA) in the hippocampus
In order to assess the extent of lipid peroxidation in the hippocampus, MDA levels as a by-product of lipid peroxidation were measured by detecting substances which react with thiobarbituric-acid. At the end of each LTP recording session, the brains of anesthetized rats were removed and the hippocampus was dissected out in a cold petri dish. The hippocampus samples were weighed and homogenized in ice-cold KCl solution (1.5%) to give a 10% (w/v) homogenous tissue suspension. Then, 3 ml of phosphoric acid (1%) and 1 ml of thiobarbituric acid (6%) were added to 0.5 ml of the tissue homogenates in centrifuge tubes and the mixture was heated for 45 min in boiling water bath. After cooling, n-butanol (4 ml) was added to the tissue homogenates and then vortexed vigorously for 30-60 sec. The samples were then centrifuged for 20 min (20,000×g) and finally the supernatants were collected and used for MDA assay. The absorbance of n-butanol phase was read at 535 nm and the concentration of MDA in the tissue samples of the hippocampus was measured using 1,1,3,3-tetramethoxypropane as the standard (24).
Statistical analysis
The mean (±SEM) was used to express data in each group (n=8). The data were analyzed using GraphPad Prism software (Version 6.01). To compare the differences among groups, the one-way ANOVA (analysis of variance) was used followed by Tukey’s post-hoc test. Also, two-way repeated measure analysis of variance (2-way RM-ANOVA) was used to evaluate the differences within groups. The P-value less than 0.05 were considered as the level of statistical significance.
Results
Effect of α-terpineol on Morris water maze test
Cerebral ischemia/reperfusion injury caused by transient bilateral common carotid artery occlusion impaired spatial memory in ischemic rats. The animals in ischemia group showed a significant longer escape latency compared to sham group (P<0.05). However, the administration of α-terpineol (100 mg/kg, IP) significantly improved spatial learning during training trials (P<0.01). As it is shown in Figure 1, the escape latency (the mean latency time to find the hidden platform) was decreased in α-terpineol-treated rats compared to ischemia group. In addition, treatment of animals with α-terpineol at the dose of 200 mg/kg led to a non-significant decrease in time latency in comparison with ischemia group (P=0.99). When comparing the escape latencies between groups (Figure 1), a significant treatment effect on escape latency was revealed (2-way RM-ANOVA; F4,105=13.36, P<0.0001). Moreover, a significant day effect on escape latency was found within groups (2-way RM-ANOVA; F2,105=35.11, P<0.0001), indicating that α-terpineol -treated and sham-operated animals improved their spatial learning during the 3-day training period (Figure 1).
Figure 1.
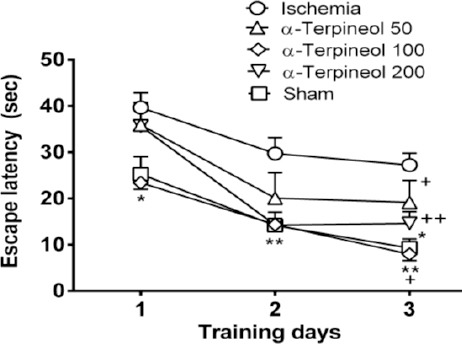
Effect of α-terpineol on escape latency in Morris water maze test in rats subjected to cerebral ischemia. Administration of α-terpineol (100 mg/kg) significantly decreased escape latency during training trials. Each symbol indicates the mean±SEM for 8 rats. Two-way ANOVA was used for data analysis. *P<0.05, **P<0.01 (compared to ischemia); +P<0.05, ++P<0.01(compared to day 1)
In the probe trial on the 4th day, statistical analysis showed a significant difference regarding time spent (one-way ANOVA; F4,35 =5.838, P<0.001) and distance swam (one-way ANOVA; F4,35 =3.995, P=0.009) in the target quadrant between groups (Figure 2 and 3). The rats in the sham group spent more time (P<0.01; Figure 2) and swam more distance in the target quadrant (P<0.05; Figure 3). Compared with the ischemia group, α-terpineol (100 mg/kg) successfully improved spatial memory in rats, so that the time spent in the target quadrant was increased (P<0.01; Figure 2) and distance travelled in the target quadrant was prolonged (P<0.05, Figure 3). Moreover, the number of crossing over the platform location was significantly different between groups (one-way ANOVA; F4,35 =5.838, P<0.001; Figure 4). The data also indicated that the number of crossing over the platform location in sham group was significantly further than ischemia group (P<0.05, Figure 4). In addition, the number of crossings over the platform location was increased in α-terpineol-treated rats in comparison with ischemia groups (P<0.05; Figure 4). Also, average proximity was significantly different between groups (one-way ANOVA; F4,35 =6.128, P=0.0008; Figure 5). In sham-operated group, a significant decrease in the average proximity to the platform location in comparison with ischemia animals was obtained (P<0.01; Figure 5). Figure 5 also shows that α-terpineol (100 mg/kg) significantly decreased the average proximity of animals to the platform location compared to ischemia group (P<0.05). There was no significant difference between the average speed between groups during 4 days (2-way RM-ANOVA; treatment effect: F4,35 =1.274, P=0.2986; day effect: F3,105 =0.8978, P=0.4450; treatment effect × day effect: F12,105 =1.021, P=0.4358; Figure 6).
Figure 2.
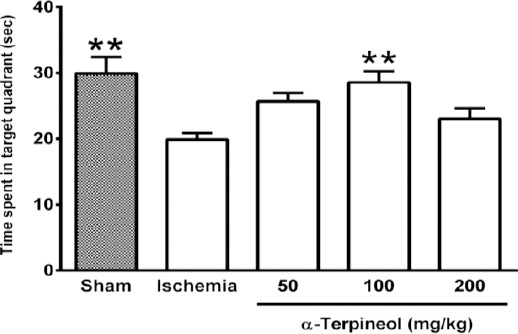
Effect of α-terpineol on time spent in target quadrant in Morris water maze test in rats subjected to cerebral ischemia. Administration of α-terpineol (100 mg/kg) significantly prolonged time spent in target quadrant in probe trial. Each column indicates the mean±SEM for 8 rats. One-way ANOVA and Tukey’s post-hoc test were used for data analysis. **P<0.01 (compared to ischemia)
Figure 3.
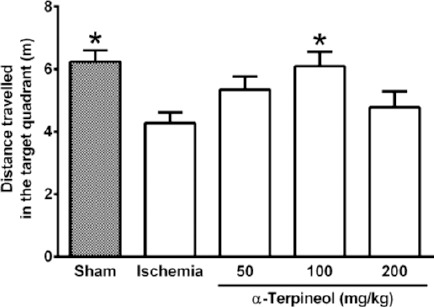
Effect of α-terpineol on distance travelled in the target quadrant in Morris water maze test in rats subjected to cerebral ischemia. Administration of α-terpineol (100 mg/kg) significantly prolonged distance moved by rats in target quadrant in probe trial. Each column indicates the mean±SEM for 8 rats. One-way ANOVA and Tukey’s post-hoc test were used for data analysis. *P<0.05 (compared to ischemia)
Figure 4.
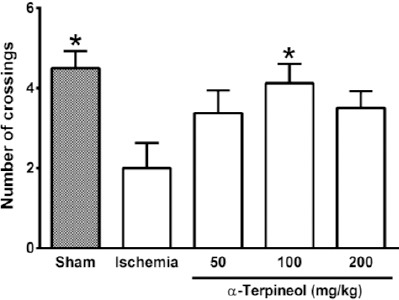
Effect of α-terpineol on number of crossings in the target quadrant in Morris water maze test in rats subjected to cerebral ischemia. Administration of α-terpineol (100 mg/kg) significantly increased the number of crossings of rats over the platform location in probe trial. Each column indicates the mean±SEM for 8 rats. One-way ANOVA and Tukey’s post-hoc test were used for data analysis. *P<0.05 (compared to ischemia)
Figure 5.
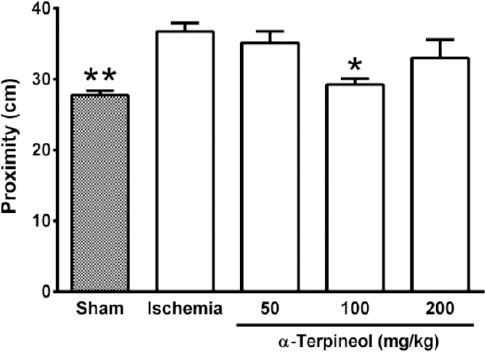
Effect of α-terpineol on proximity to the target in the target quadrant in Morris water maze test in rats subjected to cerebral ischemia. Administration of α-terpineol (100 mg/kg) significantly decreased the mean proximity of rats to the platform location in probe trial. Each column indicates the mean±SEM for 8 rats. One-way ANOVA followed by Tukey’s post-hoc test were used for data analysis. *P<0.05, **P<0.01 (compared to ischemia).
Figure 6.
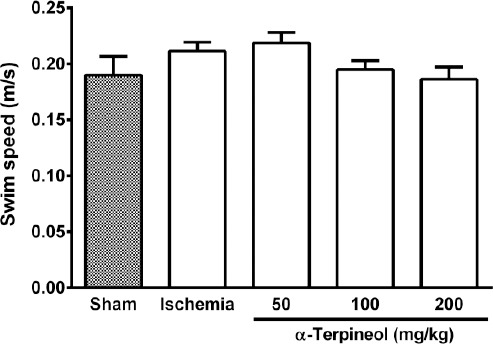
The average swim speed of rats in Morris water maze test. No significant difference was found between groups with regard to the average speeds of the rats subjected to cerebral ischemia/reperfusion injury. Administration of α-terpineol (50, 100, and 200 mg/kg) did not make any change to the swimming speed of rats in probe trial. Each column indicates the mean±SEM for 8 rats. One-way ANOVA was used for data analysis
A comparison of speed in each day showed that there is no significant difference between the swimming speeds of different groups in each day (Figure 7). In addition, no significant difference was seen between escape latency of different groups in trials 1, 2, and 3 on day 1 (Figure 8).
Figure 7.
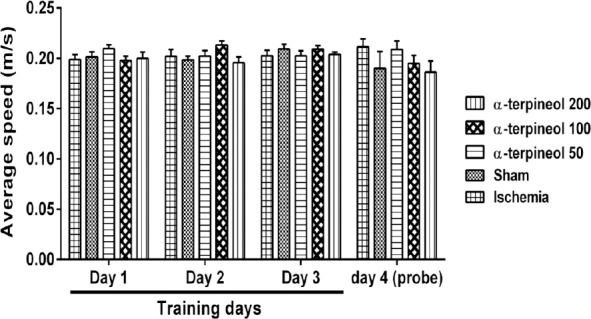
The average swim speed of rats in each day in Morris water maze test. No significant difference was observed between groups with regard to the average speeds of the rats in each day. Each column indicates the mean±SEM for 8 rats. Two-way ANOVA was used for data analysis
Figure 8.
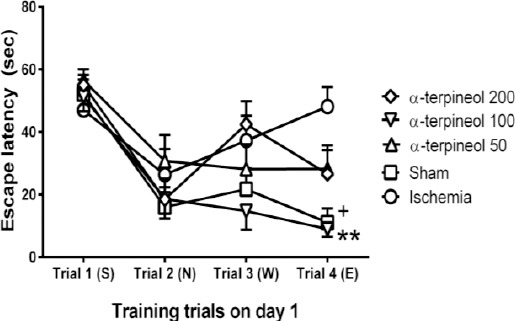
The effect of α-terpineol on escape latency in Morris water maze test in rats on day 1. A trial by trial comparison of escape latency was carried out in different groups. Administration of α-terpineol (100 mg/kg) significantly decreased escape latency during trial 4. Each symbol indicates the mean±SEM for 8 rats. S, N, W, and E represent starting positions (South, North, West, and East, respectively). Two-way ANOVA was used for data analysis. **P<0.01, comparison between α-terpineol (100 mg/kg) and ischemia group in trial 4; +P<0.05, comparison between sham-operated and ischemia group in trial 4
Effect of α-terpineol on LTP induction in the hippocampus
In the electrophysiological experiments, LTP (long-term potentiation) was recorded to assess how memory is formed at the cellular level. To this purpose, the synaptic transmission was evaluated in the hippocampus before and after high-frequency stimulation (HFS). In ischemia group, in which the animals underwent transient occlusion of common carotid arteries and then treated with vehicle for a week, HFS failed to induce LTP (Figure 9). Data analysis showed that there was no significant difference between the amplitude of field excitatory post-synaptic potentials (fEPSPs) before and after HFS in ischemia group. However, in sham-operated and α-terpineol-treated groups, HFS significantly increased the amplitude of fEPSP (2-way RM-ANOVA; F2,60 =90.55, P<0.0001). As it is shown in the Figures 10 and 11, the administration of α-terpineol (100 mg/kg, IP, for 7 days) in rats subjected to the cerebral ischemia, restored LTP, so that a successful induction of LTP was obtained following HFS. There was no significant difference between the amplitude of basic responses between the different groups (Figures 10 and 11).
Figure 9.
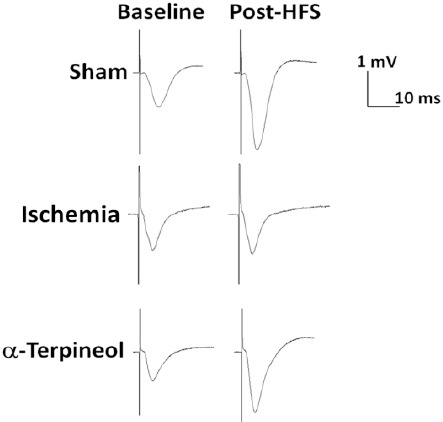
Effect of α-terpineol on hippocampal long-term potentiation (LTP) in rats subjected to cerebral ischemia/reperfusion injury. Representative traces (average of 5 sweeps) have been recorded from dentate gyrus before and after high-frequency stimulation (HFS) in anesthetized rats. The administration of α-terpineol (100 mg/kg) clearly increased the amplitude of field excitatory post-synaptic potentials following HFS
Figure 10.
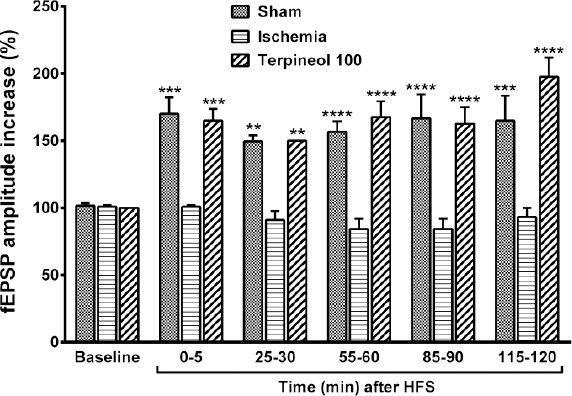
Comparison of the amplitude of field excitatory post-synaptic potentials (fEPSPs) between ischemia, sham, and α-terpineol groups. Administration of α-terpineol (100 mg/kg) significantly increased the fEPSPs amplitudes after HFS. Each column shows the mean±SEM for 10 sweeps. Two-way ANOVA and followed by Tukey’s post-hoc test were used for data analysis. **P<0.01, ***P<0.001, ****P<0.0001 (compared to ischemia)
Figure 11.
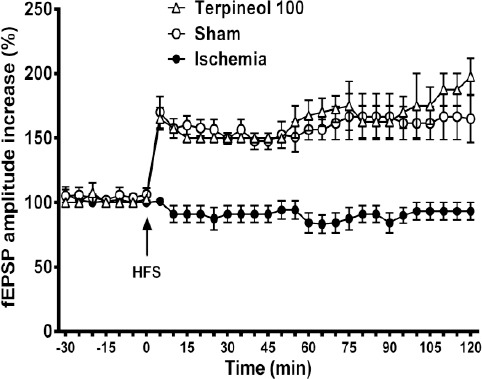
Time course of changes in the amplitude of field excitatory post-synaptic potentials (fEPSPs) recorded from the hippocampus in rats subjected to cerebral ischemia/reperfusion injury. Each symbol represents the mean±SEM for 10 sweeps. Administration of α-terpineol (100 mg/kg) facilitated the induction of LTP in the hippocampus which was persistent over 2 hours. Differences between the fEPSPs amplitudes of ischemia group and the α-terpineol -treated group were statistically significant all the time after HFS (0-5 min after HFS, P<0.001, 5-50 min after HFS, P<0.01, 55-120 min after HFS, P<0.0001; Two-way ANOVA)
When HFS was applied in α-terpineol-treated animals, the percent increase of fEPSP amplitude was 65% immediately after (P<0.001), 50% 30 min after (P<0.01), 67% 60 min after (P<0.0001), 62% 90 min after (P<0.0001) and 97% (P<0.0001) 120 min after HFS (Figure 10). No significant difference was observed between the percent increase in the amplitude of fEPSP in α-terpineol-treated animals at different post-tetanic times (Figure 10).
Effect of α-terpineol on malondialdehyde (MDA) levels in the hippocampus
In order to assess the extent of lipid peroxidation resulting from free radical-induced neuronal damage following ischemia/reperfusion injury, MDA levels were measured in the rat hippocampus. MDA level was significantly different between groups (one-way ANOVA; F4,35=7.035, P=0.0003; Figure 12). A significant increase in the mean level of MDA was found in the ischemia group compared with the sham group (182.6±7.77 vs 127.1±6.01 nmol/g tissue, P<0.001). As it is shown in Figure 12, the administration of α-terpineol at doses of 100 and 200 mg/kg for a 7-day period in rats subjected to cerebral ischemia, significantly lowered MDA levels in the hippocampus (145.1±9.9 and 145.2±8.25 nmol/g tissue, respectively; P<0.05).
Figure 12.
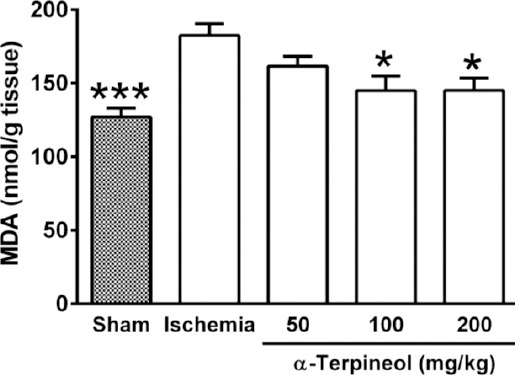
Effect of α-terpineol on hippocampal malondialdehyde (MDA) levels in rats subjected to cerebral ischemia. MDA was measured to assess the extent of lipid peroxidation in the hippocampus. Administration of α-terpineol (100 and 200 mg/kg) significantly decreased the mean MDA levels in the rat hippocampus. Each column represents the mean±SEM for 8 rats. One-way ANOVA followed by Tukey’s post-hoc test were used for data analysis. *P<0.05, ***P<0.001 (compared to ischemia)
Discussion
In the present study, cerebral ischemia/-reperfusion caused spatial memory impairment in rats; however, α-terpineol attenuated memory deficit through inhibiting lipid peroxidation and enhancement of LTP in the hippocampus.
Learning and memory impairments are consider-ed commonly reported pathological conditions contributing to cerebral ischemia, which can adversely affect everyday life activities and social functioning (2). Many attempts have been made to improve cognitive function in patients experienced one or more episodes of cerebral ischemia. During the last decade, several medicinal plants and their active constituents have been investigated for possible protective effect against cerebral ischemia and subsequent memory impairment (24-26). There is some evidence showing that α-terpineol-rich medicinal plants such as A. koreana Wilson extract and Salvia spp. extract (Sage) are useful in patients with Alzheimer’s disease and vascular dementia (17, 18). Kim et al (2006) showed that A. koreana Wilson extract which contains high levels of α-terpineol could enhance memory in scopolamine-induced amnesia in mice (17). In addition, Kennedy et al (2011) assessed the effects of monoterpenoid extract of sage in a double-blind, placebo-controlled, balanced crossover study. They reported that sage extract can improve of cognitive performance and mood in healthy adults (18). Consistent with these reports, the results obtained from our study demonstrates that α-terpineol improves memory deficit after transient global ischemia in rats. Our findings showed that the neuroprotective effect of α-terpineol could be due to its inhibitory effects on lipid peroxidation and enhancement of LTP in the hippocampus. In the present study, impaired learning performance in the Morris water maze test was observed in rats seven days after the induction of ischemia/reperfusion injury. The available evidence shows a significant decrease in blood flow in the hippocampus and cerebral cortex after transient cerebral ischemia (27). Since hippocampus is among the most vulnerable region of the brain to cerebral ischemia/reperfusion injury (3), impaired learning performance in the Morris water maze test reflects deficits in hippocampal-dependent spatial cognition (26-28). The results obtained from the present study indicate an obvious impairment in the spatial learning and reference memory in ischemia group. Our results are consistent with previous studies showing that transient bilateral common carotid artery occlusion correlates with impaired learning performance in the Morris water maze task (26-28). For example, Hartman et al (2005) demonstrated that the bilateral common carotid artery occlusion model in rat could be useful for evaluating the efficacy of therapeutic procedures designed to manage neurodegeneration and/or promote recovery of cognitive functions (27). Similar results have been reported by Dabaghian et al (2015) and Schiavon et al (2014) who studied protective effects of Cyperus rotundus and cannabidiol on cerebral ischemia-induced memory dysfunction, respectively (26, 28).
Besides, our finding showed that α-terpineol-treated animals represented improved spatial memory by decreasing the escape latency and average proximity of rats to the platform location. It also increased the swimming time and distance travelled by rats in the target quadrant, as well as number of crossing over the platform location. Maei et al (2009) showed that the mean proximity to the platform location can be considered the most sensitive and accurate measure to evaluate in the Morris water maze performance (29).
Our findings showed that α-terpineol at dose of 100 mg/kg, improved spatial memory in rats subjected to cerebral ischemia. In contrast, higher dose of α-terpineol (200 mg/kg) did not result in a significant change in the performance of rats in the Morris water maze test. This may reflect the adverse effects of α-terpineol on spatial learning and reference memory at doses higher than 100 mg/kg. However, this effect needs to be assessed in further studies. Nevertheless, the effects of different doses of α-terpineol on spatial navigation do not seem to be related to alteration in motor ability, because the average speeds of the animals in each day were similar in all experimental groups. On the other hand, in the trial by trial comparison of escape latency on day 1, we found out that the escape latency in trial 4 in the rats received α-terpineol (100 mg/kg) was significantly shorter than that of ischemia group (P<0.01). This finding, together with the results obtained from the comparison of speed on each day further revealed that the better learning performance of animals treated with α-terpineol (100 mg/kg) could very likely related to the improvement of their spatial learning and reference memory.
Several studies have indicated that cerebral ischemia adversely affect synaptic plasticity in the brain (6). There are even evidences showing that detrimental alterations in the synaptic structure and function begin prior to obvious neuronal damage (30). In order to evaluate the earliest stages in the synaptic dysfunction, basal synaptic transmission and LTP are often studied (6).
There is a strong relationship between cerebral ischemia and the blockade of tetanic-induced LTP in the hippocampus, which contributes to memory deficits. It has been well established that the induction of hippocampal LTP is impaired following global cerebral ischemia, although the amplitude of basic responses in rats subjected to cerebral ischemia may not significantly altered compared to sham-operated animals (6, 31, 32). Consistent with these reports, our results showed that in ischemia group, in which the animals underwent cerebral ischemia and received the vehicle alone, tetanic stimulation failed to induce LTP. These observations reinforce the notion that cerebral ischemia compromises synaptic transmission and impairs the induction of LTP. In contrast, our findings showed that the administration of α-terpineol (100 mg/kg) in the rats subjected to cerebral ischemia could enhance the induction of LTP in the hippocampus which was persistent over 2 hr. Previous studies have provided evidence that cognitive function of animals in Morris water maze test is strongly correlated with the synaptic plasticity in the hippocampus (5, 6). Thus, the ability of α-terpineol to enhance the induction and maintenance of LTP may explain the attenuation of memory impairment in Morris water maze test.
Some studies have indicated a link between oxidative stress and neuronal damage following cerebral ischemia (7, 8). When cerebral blood flow is significantly reduced, extensive amounts of free radicals are produced in the brain which may lead to structural and functional damage to neural cells. Chen et al (2011) showed that cerebral ischemia could significantly increase the amount of lipid peroxidation products, including MDA in the ischemic brain tissues (8). The results of many studies have indicated that antioxidant agents with natural origin may attenuate neuronal damage following cerebral ischemia (9, 24, 25). In this regard, α-terpineol has shown potential as an antioxidant and a free radical scavenger (14, 15). The results of the present study provide further evidence indicating that α-terpineol attenuates oxidative stress by inhibiting lipid peroxidation. In this study we assessed the lipid peroxidation level by measuring the contents of malondialdehyde as an end-product of lipid peroxidation in the hippocampal homogenates. The results showed a significant increase in MDA content in ischemia group in comparison with sham group. In marked contrast to ischemia group, treatment of rats with α-terpineol (100, 200 mg/kg) resulted in significant reduction in hippocampus MDA levels. The inhibitory action of α-terpineol on lipid peroxidation was comparable to that of sham-operated animals. These findings demonstrate for the first time that α-terpineol is a strong inhibitor of lipid peroxidation in the neural cells and propose the ability of α-terpineol in attenuating oxidative stress.
Several studies have reported oxidative stress positively correlates to the impairment of synaptic plasticity in the hippocampus. There is evidence that increased levels of free radicals and reduced antioxidant enzyme activity can result in the impairment of LTP (33, 34). It is well known that enhanced free radical generation in the cell membranes leads to considerable increase in lipid peroxidation and subsequent neuronal damage (8). Karimi et al (2013) in an in vivo study showed that supplementation with antioxidants could reverse the impairment of synaptic plasticity induced by oxidative stress in rats (35). Based on these observations we suggest that the facilitation of LTP observed in α-terpineol-treated rats may be related to the inhibitory effect of α-terpineol on neuronal lipid peroxidation in the hippocampus.
Recent findings show that α-terpineol may increase nitric oxide (NO) production and causes more NO to be released (36). There is also evidence suggesting that α-terpineol exerts a positive regulatory effect on NO-cGMP pathway (37). Since NO-cGMP pathway has been shown to play a key role on the establishment of synaptic plasticity (38), it is possible that the facilitation of LTP in rats may be related to the positive regulatory effect of α-terpineol on NO-cGMP pathway. However, this effect needs to be assessed in further studies.
Conclusion
Collectively, the current study provides evidence for the effectiveness of applying α-terpineol to improve cerebral ischemia-related learning and memory impairment. Our findings demonstrate, for the first time, that the memory-improving effects of α-terpineol may be mediated through the facilitation of LTP and suppression of lipid peroxidation in the hippocampus.
Acknowledgment
This work was financially supported by “Research Department of the School of Medicine, Shahid Beheshti University of Medical Sciences” (Grant No. 13/1191).
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- 1.Lucke-Wold BP, Turner RC, Logsdon AF, Simpkins JW, Alkon DL, Smith KE, et al. Common mechanisms of Alzheimer’s disease and ischemic stroke: the role of protein kinase C in the progression of age-related neurodegeneration. J Alzheimers Dis. 2015;43:711–724. doi: 10.3233/JAD-141422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan L, Bushnell C, Bell CL, Espeland MA. Global cognitive function before, surrounding, and after ischemic stroke: the role of risk and protective factors varies with time among ischemic stroke survivors. Aging Neuropsychol Cogn. 2016;23:117–131. doi: 10.1080/13825585.2015.1058323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron JC, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab. 2014;34:2–18. doi: 10.1038/jcbfm.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 6.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 7.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62:712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korani MS, Farbood Y, Sarkaki A, Moghaddam HF, Mansouri MT. Protective effects of gallic acid against chronic cerebral hypoperfusion-induced cognitive deficit and brain oxidative damage in rats. Eur J Pharmacol. 2014;733:62–67. doi: 10.1016/j.ejphar.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Zhang JJ, Xiong L, Zhang L, Sun D, Liu H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J Nutr Biochem. 2010;21:741–748. doi: 10.1016/j.jnutbio.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira MG, Marques RB, de Santana MF, Santos AB, Brito FA, Barreto EO, et al. α-Terpineol reduces mechanical hypernociception and inflammatory response. Basic Clin Pharmacol Toxicol. 2012;111:120–125. doi: 10.1111/j.1742-7843.2012.00875.x. [DOI] [PubMed] [Google Scholar]
- 12.Quintans-Júnior LJ, Oliveira MG, Santana MF, Santana MT, Guimarães AG, Siqueira JS, et al. α-Terpineol reduces nociceptive behavior in mice. Pharm Biol. 2011;49:583–586. doi: 10.3109/13880209.2010.529616. [DOI] [PubMed] [Google Scholar]
- 13.de Sousa DP, Quintans L, de Almeida RN. Evolution of the anticonvulsant activity of α-terpineol. Pharm Biol. 2007;45:69–70. [Google Scholar]
- 14.El-Ghorab A, El-Massry KF, Shibamoto T. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of Egyptian Corn Silk (Zea mays L.) J Agric Food Chem. 2007;55:9124–9127. doi: 10.1021/jf071646e. [DOI] [PubMed] [Google Scholar]
- 15.Bicas JL, Neri-Numa IA, Ruiz ALTG, De Carvalho JE, Pastore GM. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Toxicol. 2011;49:1610–1615. doi: 10.1016/j.fct.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK. In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol. 2000;52:895–902. doi: 10.1211/0022357001774598. [DOI] [PubMed] [Google Scholar]
- 17.Kim K, Bu Y, Jeong S, Lim J, Kwon Y, Cha DS, et al. Memory-enhancing effect of a supercritical carbon dioxide fluid extract of the needles of Abies koreana on scopolamine-induced amnesia in mice. Biosci Biotechnol Biochem. 2006;70:1821–1826. doi: 10.1271/bbb.50608. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy DO, Dodd FL, Robertson BC, Okello EJ, Reay JL, Scholey AB, et al. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J Psychopharmacol. 2011;25:1088–1100. doi: 10.1177/0269881110385594. [DOI] [PubMed] [Google Scholar]
- 19.Hosseinzadeh H, Asl MN, Parvardeh S. The effects of carbenoxolone, a semisynthetic derivative of glycyrrhizinic acid, on peripheral and central ischemia-reperfusion injuries in the skeletal muscle and hippocampus of rats. Phytomedicine. 2005;12:632–637. doi: 10.1016/j.phymed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Wu CP, Cheung G, Rakhshani N, Parvardeh S, Asl MN, Huang HL, et al. CA3 neuronal activities of dorsal and ventral hippocampus are differentially altered in rats after prolonged post-ischemic survival. Neuroscience. 2005;130:527–39. doi: 10.1016/j.neuroscience.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Hosseinzadeh H, Asl MN, Parvardeh S, Mansouri SM. The effects of carbenoxolone on spatial learning in the Morris water maze task in rats. Med Sci Monit. 2005;11:BR88–94. [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- 23.Bortolotto ZA, Amici M, Anderson WW, Isaac JT, Collingridge GL. In: Current protocols in neuroscience. John Wiley & Sons, Inc; 2011. Synaptic plasticity in the hippocampal slice preparation. [DOI] [PubMed] [Google Scholar]
- 24.Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Guven M, Aras AB, Akman T, Sen HM, Ozkan A, Salis O, et al. Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran J Basic Med Sci. 2015;18:356–363. [PMC free article] [PubMed] [Google Scholar]
- 26.Dabaghian FH, Hashemi M, Entezari M, Movassaghi S, Goushegir SA, Kalantari S, et al. Effect of Cyperus rotundus on ischemia-induced brain damage and memory dysfunction in rats. Iran J Basic Med Sci. 2015;18:199–204. [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman RE, Lee JM, Zipfel GJ, Wozniak DF. Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res. 2005;1043:48–56. doi: 10.1016/j.brainres.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Schiavon AP, Soares LM, Bonato JM, Milani H, Guimarães FS, de Oliveira RM. Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox Res. 2014;26:307–316. doi: 10.1007/s12640-014-9457-0. [DOI] [PubMed] [Google Scholar]
- 29.Maei HR, Zaslavsky K, Wang AH, Yiu AP, Teixeira CM, Josselyn SA. Development and validation of a sensitive entropy-based measure for the water maze. Front Integr Neurosci. 2009;3:33. doi: 10.3389/neuro.07.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 31.Xu B, Li XX, He GR, Hu JJ, Mu X, Tian S, et al. Luteolin promotes long-term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur J Pharmacol. 2010;627:99–105. doi: 10.1016/j.ejphar.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 32.Osada N, Kosuge Y, Oguchi S, Miyagishi H, Ishige K, Ito Y. Protective action of mithramycin against neurodegeneration and impairment of synaptic plasticity in the hippocampal CA1 area after transient global ischemia. Neurochem Int. 2012;60:47–54. doi: 10.1016/j.neuint.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: Contributory or inhibitory? J Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 34.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Karimi SA, Salehi I, Komaki A, Sarihi A, Zarei M, Shahidi S. Effect of high-fat diet and antioxidants on hippocampal long-term potentiation in rats: an in vivo study. Brain Res. 2013;1539:1–6. doi: 10.1016/j.brainres.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 36.Parvardeh S, Moghimi M, Eslami P, Masoudi AR. α-Terpineol attenuates morphine-induced physical dependence and tolerance in mice: role of nitric oxide. Iran J Basic Med Sci. 2016;19:201–208. [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro T, Porto D, Menezes C, Antunes A, Silva D, De Sousa D, et al. Unravelling the cardiovascular effects induced by α-terpineol: a role for the nitric oxide-cGMP pathway. Clin Exp Pharmacol Physiol. 2010;37:811–816. doi: 10.1111/j.1440-1681.2010.05383.x. [DOI] [PubMed] [Google Scholar]
- 38.Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


