Abstract
As insufficient access to clean water is expected to become worse in the near future, water purification is becoming increasingly important. Membrane filtration is the most promising technologies to produce clean water from contaminated water. Although there have been many studies to prepare highly water-permeable carbon-based membranes by utilizing frictionless water flow inside the carbonaceous pores, the carbon-based membranes still suffer from several issues, such as high cost and complicated fabrication as well as relatively low salt rejection. Here, we report for the first time the use of microporous carbonaceous membranes via controlled carbonization of polymer membranes with uniform microporosity for high-flux nanofiltration. Further enhancement of membrane performance is observed by O2 plasma treatment. The optimized membrane exhibits high water flux (13.30 LMH Bar−1) and good MgSO4 rejection (77.38%) as well as antifouling properties. This study provides insight into the design of microporous carbonaceous membranes for water purification.
Carbon-based membranes have been extensively studied because of their unique characteristics, such as high physicochemical stability, fast mass transport behavior, large surface area, biocidal property, and narrow pore size distribution1,2,3,4,5,6,7,8,9,10,11. Based on these properties, they have been utilized in diverse applications including gas or liquid separation1,2,3,4, catalytic reactions5, chemical sensing6,7,8, energy storage9, and tissue engineering10. In particular, water treatment membranes consisting of carbon nanomaterials, such as carbon nanotube (CNT) and graphene derivatives, have a unique advantage of fast water permeation by the low frictional water flow through their carbonaceous pores12,13,14,15,16,17,18,19. For example, CNT array membranes with aligned 1D carbonaceous nanochannels exhibit ultrahigh water flux values, which are several orders of magnitude higher than those exhibited by conventional ultrafiltration (UF) membranes13,14,15,16. Graphene oxides (GO) membranes with 2D carbonaceous nanochannels have been reported to exhibit fast water flux with controlled separation performance for sub-10 nm particles and molecules17,18,20. However, practical applications of these membranes are still limited by several issues, such as high cost and complicated fabrication for CNT-based membranes13,14,15,16,17, as well as poor stability under hydrated conditions and difficult pore size control for graphene-based membranes4,20. In addition, both membranes often suffer from relatively low salt rejection rates, attributed to the large pore size of CNT-based membranes13 and the deterioration of integrity of graphene-based membranes by the hydration4,20, which in turn hampers the application of the membranes for nanofiltration (NF) or reverse osmosis (RO). Hence, a more convenient and efficient method for preparing carbonaceous membranes with a high flux and salt rejection rate is required for the water treatment applications.
Microporous polymers are of great interest as promising next-generation molecular sieving and storage materials for the applications of gas sorption, separation and storage, pervaporation, and catalytic supports21,22,23,24,25. Recently, polymers of intrinsic microporosity (PIMs), a novel class of microporous polymers, have attracted considerable attention because of good solubility and processability, different available functional groups, high glass transition temperature, good thermal stability, and excellent mechanical and film-forming properties26,27,28,29,30,31,32,33,34. As PIMs contain fused-ring and ladder-like structures integrated with contortion sites, they have uniform interconnected micropores (<2 nm) and a high surface area (300–1000 m2 g−1)30,31,32. Several studies have reported the use of PIM membranes for gas separation by exploiting their high gas permeability and selectivity26,27,28,30,31,33,34; however, only a few studies have reported the use of PIM membranes for the filtration of organic solutions32. Moreover, thus far, a PIM membrane for water treatment applications has not been reported because it is difficult to utilize the hydrophobic micropores of PIMs for transporting water molecules. Considering the low frictional water flow through the pores of carbonaceous membrane, it might be possible to prepare microporous, carbonized PIM membranes with high water flux and selectivity by carbonization of the PIM membranes.
Previously, we have reported the preparation of 2–15 nm thick, graphene-like carbonaceous thin films on a quartz substrate by the carbonization of thin films of a polymer of intrinsic microporosity (PIM-1)35. Herein, we report the fabrication of a new type of free-standing carbonaceous membrane based on PIM-1 via controlled carbonization; this membrane exhibits interconnected, sub-1 nm pores with a narrow size distribution. These characteristics result in high flux and a good salt rejection rate for the filtration of an MgSO4 aqueous solution, thus making the membrane attractive for NF applications. In addition, the water flux and antifouling property of the membrane can be further enhanced without sacrificing the salt rejection rate by subjecting the membrane to O2 plasma treatment.
Results
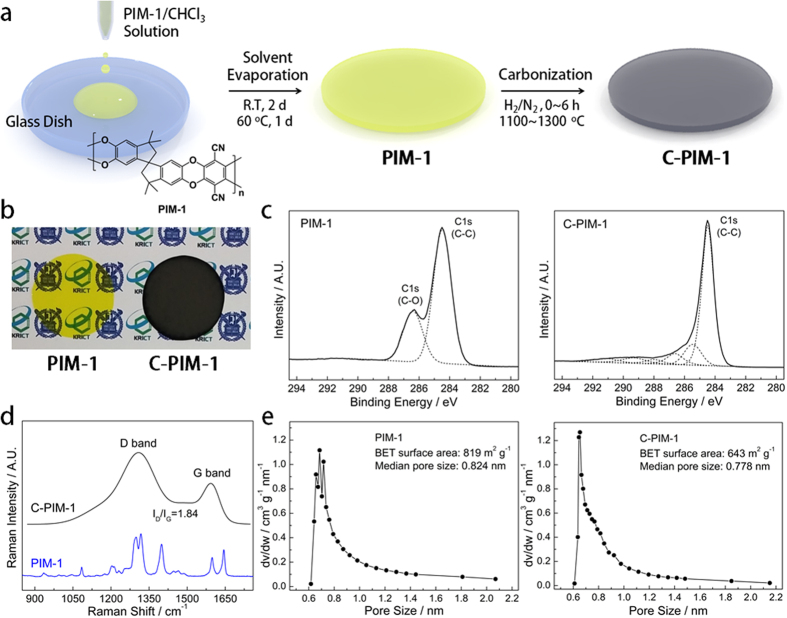
PIM-1 was synthesized by polycondensation of 5,5′,6,6′-tetrahydroxy-3,3,3′,3′-tetramethyl-1,1′-spirobisindane (TTSBI) and 2,3,5,6-tetrafluoroterephthalonitrile (TFTPN), as previously reported29,35,36,37. 1H NMR and elemental analysis (EA) revealed that the polymer was successfully synthesized (see Methods, Supplementary Information). The number-average molecular weight (Mn) and molecular weight distribution (Đ) of PIM-1, obtained by gel-permeation chromatography (GPC), are 50,100 g mol−1 and 1.87, respectively. A PIM-1 membrane was prepared by a simple solution casting method (Fig. 1a); a solution of PIM-1 in CHCl3 was poured into a glass dish (diameter = 10 cm), followed by the slow evaporation of the solvent at room temperature. The thickness of the PIM-1 membrane was controlled by changing the concentration (0.5–2.0 wt%) and amount of the PIM-1 casting solution. After the PIM-1 membrane was completely dried under vacuum at 60 °C, controlled thermal treatment under N2/H2 atmosphere (95/5 vol%) was conducted to fabricate a carbonaceous PIM-1 membrane (C-PIM-1). The yellow transparent PIM-1 membrane changed into a glittering-grey opaque C-PIM-1 membrane after carbonization (Fig. 1b; Fig. S1, Supplementary Information). The degree of carbonization, defined as the membrane weight loss (%) during thermal treatment, was controlled by changing the temperature (1,100–1,300 °C) and time (1–6 h). As shown in Table S1 (Supplementary Information), the degree of carbonization for the C-PIM-1 membranes was controlled from 37.5% to 60%. Unfortunately, it was difficult to prepare C-PIM-1 membranes with a degree of carbonization below ≈35% due to the abrupt weight loss of PIM-1 from 0% to ≈35%. The abrupt weight loss of PIM-1 could be also observed by TGA under N2 flow (Fig. S2, Supplementary Information), although the actual decomposition temperature under N2/H2 flow (95/5 vol%) might be different from the TGA result. In addition, the C-PIM-1 membranes with a degree of carbonization higher than 60% were prepared, however, they were too fragile to be used as the pressure-driven filtration membranes. Thus, C-PIM-1 membranes with a degree of carbonization from 37.5% to 60% were used because they are sufficiently robust, maintaining their free-standing film state from the filtration even under an applied pressure of 10 bar.
Figure 1. Preparation and characteristics of PIM-1 and C-PIM-1 membranes.
(a) Preparation procedure of PIM-1 and C-PIM-1 membranes. (b) Photographs of the PIM-1 and C-PIM-1 membranes. (c) XPS C 1s spectra, (d) Raman spectra, and (e) pore size distributions of the PIM-1 and C-PIM-1 (40% carbonization) membranes.
The carbonization the PIM-1 membrane to the C-PIM-1 membrane via the thermal treatment could be monitored by X-ray photoelectron spectroscopy (XPS) analysis; the carbon content of the membrane increases from 82.60 at% to 96.82 at% upon the carbonization process, while the content of oxygen and nitrogen decreases (Table S2, Supplementary Information). In addition, the content of carbon in the C–C bond (284.4 eV) of the C-PIM-1 membrane was found to be much larger than that of the PIM-1 membrane (Fig. 1c). The atomic composition results, obtained from EA and XPS experiments, indicate the uniform carbonization from surface to inside part of the membrane (Table S2, Supplementary Information). Raman spectroscopy clearly shows the D (1310 cm−1) and G (1595 cm−1) band peaks, corresponding to the graphitic carbon structures of the C-PIM-1 membrane (Fig. 1d)38,39,40, while such graphitic carbon structural peaks were not observed for the PIM-1 membrane. In addition, the relative intensity of D3 peak at 1500 cm−1, compared to that of G peak at 1595 cm−1, decreases with increasing the degree of carbonization; D3 and G peaks correspond to amorphous carbon and graphitic carbon lattice, respectively (Fig. S3, Supplementary Information). Therefore, C-PIM-1 membrane with a high degree of carbonization has low amorphous carbon content38,39. The degree of crystallinity, calculated from the integrated intensity ratio of the D and G bands (ID/IG), is 1.84 for the C-PIM-1 membrane with 40% carbonization; this is typical value for the carbonaceous materials prepared by the thermal treatment of polymer precursors41,42. The change of surface morphology of the membranes could be observed from scanning electron microscopy (SEM) and atomic force microscopy (AFM) analyses; a quite flat surface (root-mean-square roughness, Rq = 0.85 ± 0.26) of the PIM-1 membrane was found to be changed to a relatively rough surface (Rq = 15.51 ± 2.10) for the C-PIM-1 membrane, attributed to the nanoscale thermal shrinkage by the carbonization (Figs S4 and S5, Supplementary Information)43,44. Still the interconnected micropore characteristics of the PIM-1 membrane having median pore size of 0.824 nm and surface area of 819 m2 g−1 are preserved for some degree after the carbonization for the C-PIM-1 membrane having median pore size of 0.778 nm and surface area of 643 m2 g−1 (Figs 1e and S6, Supplementary Information).
Dead-end filtration test was performed to evaluate the pure water permeability behavior of the C-PIM-1 membrane with a thickness of 30 μm. Figure 2a clearly shows the very large increase of the pure water flux after the carbonization; pure water flux increases from 0.23 LMH bar−1 for the PIM-1 membrane to 6.43 LMH bar−1 for the C-PIM-1 membrane with 60% carbonization, which is a 28-fold increase in the water flux as a result of carbonization. The increase in water flux by carbonization is attributed to the low frictional water flow inside the carbonaceous pores rather than the pore size and surface area of the membranes12,13,14,15,16,17,18,19. A solution-diffusion model, which is widely used to explain mass transport through dense membranes with sub-1 nm pores, was employed in order to elucidate the increase of water permeability by the carbonization12,45,46. Water flux (Jw, g cm−2 s−1) in the solution-diffusion model is expressed as follows:
Figure 2. Water flux and salt rejection performance of the PIM-1 and C-PIM-1 membranes.
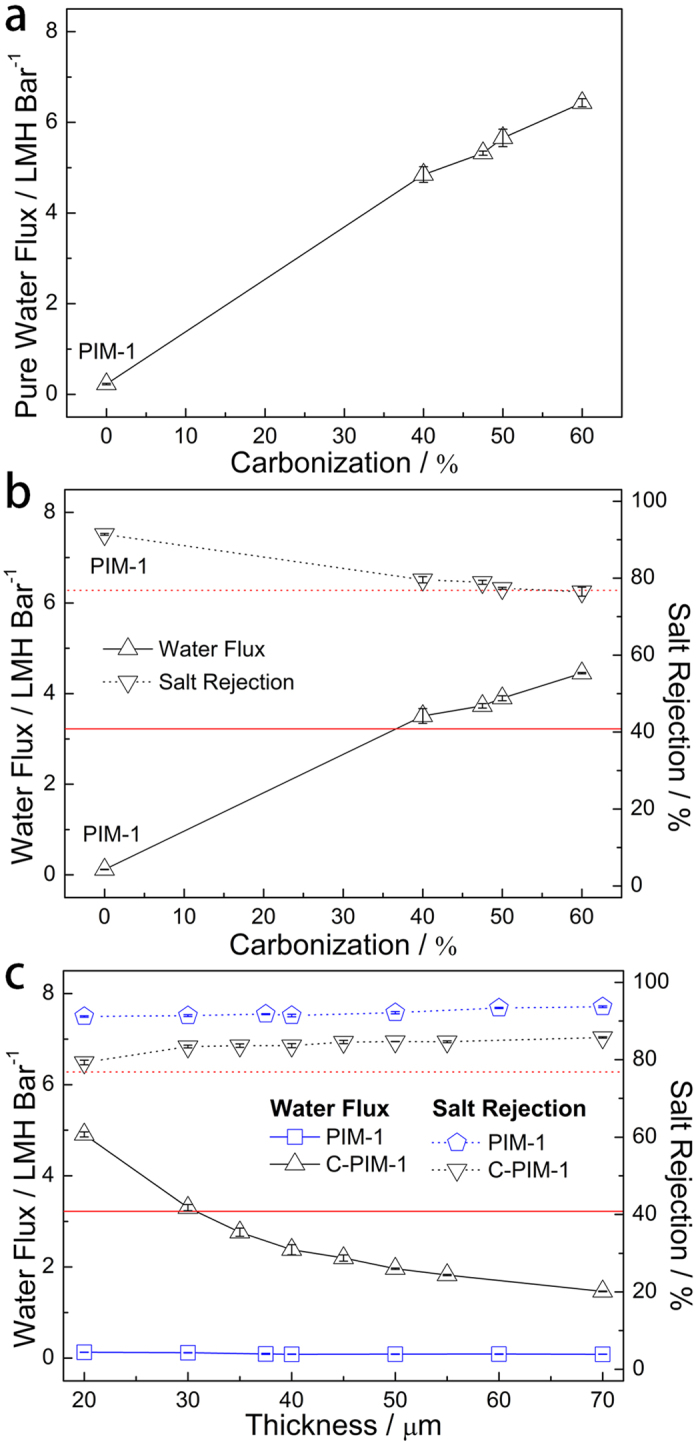
The effect of degree of carbonization on water flux and salt rejection of the membranes with a thickness of 30 μm upon (a) pure water and (b) MgSO4 solution (2,000 ppm) filtrations. (c) The effect of membrane thickness on water flux and salt rejection of the membranes with the degree of carbonization of 37.5% upon MgSO4 solution (2,000 ppm) filtration. The red solid and dotted lines indicate the water flux and salt rejection of a commercial polyamide NF membrane (NF2A), respectively.
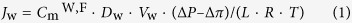 |
where CmW,F is the equilibrium water concentration in the membrane (g H2O in a 1 cm−3 swollen membrane), Dw is the average water diffusion coefficient in the membrane (cm2 s−1), Vw is the partial molar volume of water (18.0 cm3 mol−1), which is typically approximated by the molar volume of pure water45,46, ΔP is the difference in pressure between feed and permeate (bar), Δπ is the osmotic pressure difference across the membrane (bar), L is the membrane thickness (cm), R is the gas constant (83.1 cm3 bar mol−1 K−1), and T is the absolute temperature (298 K). Two parameters, CmW,F and Dw, should be the key factors in determining the water flux behavior for the PIM-1 and C-PIM-1 membranes because all the other parameters are identical. The CmW,F of the membranes was evaluated by the measurement of the equilibrium water uptake of the membranes in pure water (Fig. S7, Supplementary Information). The CmW,F of the C-PIM-1 membrane with 40% carbonization (5.52 × 10−2 g H2O in a 1 cm3 swollen membrane) is approximately 4.7 times larger than that of the PIM-1 membrane (1.18 × 10−2 g H2O in a 1 cm3 swollen membrane). CmW,F was also found to increase with the degree of carbonization. A membrane with a large CmW,F is known to exhibit high water permeability because the larger amount of water in the membrane pores can provide the pathways for water molecules (i.e., convective frame of reference effect)45,46. The calculated Dw values of the C-PIM-1 membranes (7.08 × 10−3–8.90 × 10−3 cm2 s−1) are approximately 4.8–6.1 times larger than that of the PIM-1 membrane (1.47 × 10–3 cm2 s−1), which are close to those of other carbon-based membranes (5 × 10−3–8 × 10−3 cm2 s−1)47,48. Those of conventional polymeric membranes are in the range of 1 × 10−4 to 1 × 10−7 cm2 s−1 45,46. Therefore, the water diffusion behavior of the C-PIM-1 membrane is similar to that in the carbon-based membranes. The carbon-based membranes containing CNT and graphene derivatives have well-defined micropores and exhibit low frictional water flow inside the carbonaceous pores via the formation of agglomerated hydrogen bonds between water molecules, thus resulting in the high water permeability47,48. The much larger CmW,F and Dw values of the C-PIM-1 membrane than those of the PIM-1 membrane can be explained for some degree by water contact angle study (Fig. S8, Supplementary Information). It is well known that membranes with high water wettability exhibit large water sorption and diffusion coefficients45,46. The C-PIM-1 membrane shows smaller water contact angle and higher water wettability than PIM-1 membrane possibly due to its graphitic carbon structure49 and rough surface morphology50,51, as presented in the Raman spectroscopy and AFM results, respectively (Figs 1d and S5, Supplementary Information). It has been reported that clean graphene surface exhibited quite low water contact angle value (37 °), °riginated from the strong interaction between graphene surface and water molecules49. Higher water wettability of membrane could also be obtained by introducing the rough surface morphologies50,51.
Subsequently, the NF performance of the C-PIM-1 membrane was investigated using an aqueous MgSO4 solution. The pure water flux behavior of the C-PIM-1 membrane is mirrored in Fig. 2b for the MgSO4 solution filtration, where the C-PIM-1 membrane also shows an increase of water flux with increasing degree of carbonization, and exhibits much larger water flux (3.51–4.45 LMH bar−1) than the PIM-1 membrane (0.12 LMH bar−1). Although the salt rejection rates of the C-PIM-1 membranes (78.76–82.94%) are somewhat smaller than that of PIM-1 membrane (91.41%) due to the typical trade-off behavior between water diffusion coefficient and water/salt selectivity45,46, those are still comparable to or slightly larger than that of a commercial polyamide (PA) NF membrane (NF2A) (76.86%) measured in this study. The NF performance of NF2A is worse than that in the technical specification provided by the company, however, such discrepancy has been also reported by others, which is attributed to the effect of the membrane filtration condition52. The high salt rejection rate of the high-flux C-PIM-1 membrane is consistent with the BET results, which demonstrate the sub-1 nm sized, interconnected carbonaceous pores present in the membrane (Figs 1e and S6, Supplementary Information). Figure 2c shows that the C-PIM-1 membranes as thin as 20 μm can be easily prepared, yielding water flux as high as 4.91 LMH bar–1 for the MgSO4 solution filtration, when the degree of carbonization of the membrane is 37.5%. The increase in the water flux of the C-PIM-1 membrane with decreasing membrane thickness is attributed to the reduction of thickness resistance (Equation (1))17,46. The salt rejection rate is almost independent of the membrane thickness, indicating that membranes are substantially free from micro- or several nanometer-scale defects. 20 μm was found to be the minimum thickness for the free-standing C-PIM-1 membrane to have the physical and mechanical stability under the high pressure of NF. The water flux behavior of PIM-1 membranes with different thicknesses is similar to that of C-PIM-1 membrane (Table S1, Supplementary Information). However, because of their small values, the changes in water flux of the PIM-1 membrane were not clearly seen in Fig. 2c.
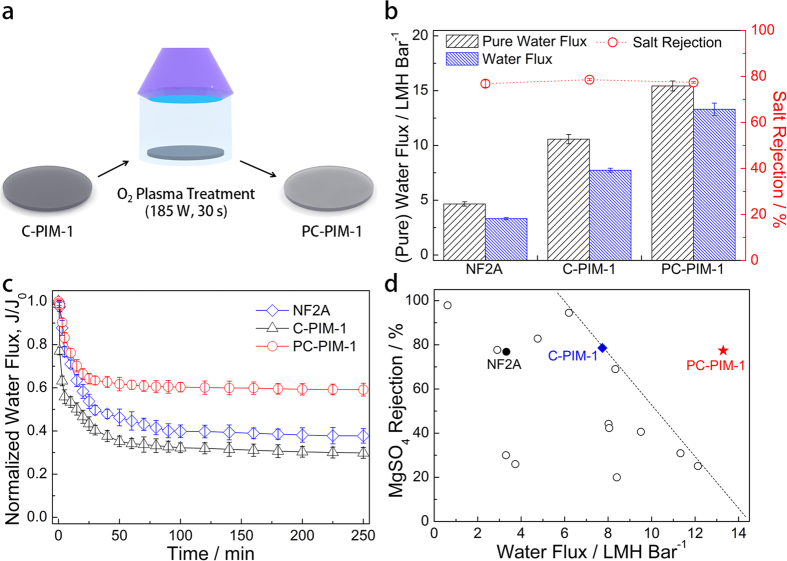
Carbon-based membranes, such as CNT array membranes, are known to exhibit a large entrance/exit resistance for water molecules to pass through the inner pores of the membranes13,53. For example, the entrance and exit resistances are larger than 120 bar and 1,000 bar, respectively, for the CNT array membrane, calculated by the molecular dynamic simulations13,53. As compared to the CNT array membranes, the C-PIM-1 membrane possibly exhibits a relatively smaller entrance/exit resistance13,53, as expected from its better water wettability (Fig. S8, Supplementary Information). Still, the water permeability of the C-PIM-1 membrane can be further improved by hydrophilic surface modification for decreasing the entrance/exit resistance. Both surfaces of the C-PIM-1 membrane were subjected to O2 plasma for preparing the O2 plasma-treated C-PIM-1 membrane (PC-PIM-1), as illustrated in Fig. 3a. The oxygen content on the membrane surface, analyzed by XPS, significantly increases by the O2 plasma treatment (Table S2, Supplementary Information), thereby increasing the water wettability on the membrane surface (Fig. S8, Supplementary Information), while the bulk atomic composition of the membrane does not change much as observed from EA measurement. This clearly demonstrates that hydrophilic oxygen functional groups are formed on the membrane surface by the O2 plasma treatment without changing the inner carbonaceous structure of the membrane. Furthermore, ID/IG ratios of C-PIM-1 and PC-PIM-1 membranes were found to be close from the Raman spectroscopy, indicating that the graphitic carbon structures on the C-PIM membrane are not damaged during O2 plasma treatment (Fig. S9, Supplementary Information). The effect of O2 plasma treatment on membrane surface morphologies was also investigated by SEM and AFM (Figs S10 and S11, Supplementary Information); any distinct change was not observed after the O2 plasma treatment, indicating that the O2 plasma treatment does not change the surface morphologies much. The hydrophilic functional groups imparted by the O2 plasma treatment were found to stably remain even after exposed to air for a week. The overall water permeability behavior of the PC-PIM-1 membranes with different degrees of carbonization and thicknesses is close to that of the C-PIM-1 membranes, while the water permeability of the PC-PIM-1 membranes is about 1.5 times higher than that of the C-PIM membranes due to the decreased entrance/exit resistance (Table S1, Supplementary Information). We could obtain the highest water flux from a PC-PIM-1 membrane with a thickness of 20 μm and 60% carbonization; 15.43 LMH bar–1 and 13.30 LMH bar−1 for the filtration of pure water and MgSO4 solution, respectively, as shown in Fig. 3b.
Figure 3. Preparation and performance of O2 plasma-treated C-PIM-1 membrane (PC-PIM-1).
(a) Preparation procedure of PC-PIM-1 membrane. (b) Pure water flux, water flux, and salt rejection performance of NF2A, and C-PIM-1 and PC-PIM-1 membranes with a thickness of 20 μm and a degree of carbonization of 60%. (c) Time-dependent normalized water flux variations of the NF2A, C-PIM-1, and PC-PIM-1 (20 μm, 60% carbonization) membranes during BSA solution (1 g L−1) filtration. (d) MgSO4 rejection rate and water flux performance of optimized C-PIM-1 and PC-PIM-1 membranes in this study and other NF membranes in the literature.
The salt rejection rate is generally assumed to decrease with increasing water flux of filtration membranes45,46. However, both C-PIM-1 and PC-PIM-1 membranes exhibit similar salt rejection performance, despite the significant increase in water flux for the membranes after O2 plasma treatment (Fig. 3b; Table S1, Supplementary Information). This result could be attributed to the presence of negatively charged oxygen functional groups on the PC-PIM-1 membrane (Fig. S12, Supplementary Information), which can improve the salt rejection rate by electrostatic repulsion (i.e., Donnan exclusion ability)54,55. To investigate the Donnan exclusion ability of the PC-PIM-1 membrane, filtration experiments were conducted with various salt solutions having different ion valences under a relatively low feed pressure (5 bar) and low salt concentration (10 mM) for minimizing the transport of ions by convection and diffusion, respectively (Fig. S13, Supplementary Information)54,55. Considering the hydrated salt size and charge effects, the rejection (R) of salt solutions should follow the orders of R(MgSO4) > R(MgCl2) > R(Na2SO4) > R(NaCl) and R(Na2SO4) > R(MgSO4) ≈ R(NaCl) > R(MgCl2), respectively (Table S3, Supplementary Information)17,54. The rejection of salt solutions of the C-PIM-1 membrane follows the order of R(MgSO4) > R(MgCl2) > R(Na2SO4) > R(NaCl), indicating that the salt rejection of the C-PIM-1 membrane is mainly determined by the size effect. However, the rejection of the PC-PIM-1 membrane follows the order of R(MgSO4) > R(Na2SO4) > R(MgCl2) > R(NaCl); the change of the rejection order and the significant increase for R(Na2SO4) and R(NaCl) are observed for the PC-PIM-1 membrane, demonstrating that the salt rejection of the PC-PIM-1 membrane is determined by both of charge and size. Therefore, the PC-PIM-1 membrane shows increased water flux without decreasing the salt rejection compared to the C-PIM-1 membrane due to the Donnan exclusion from the negatively charged surface functional groups.
Antifouling properties of the membranes were also evaluated using bovine serum albumin (BSA) as a model foulant, which is the most commonly used protein foulant for the antifouling tests56,57,58,59,60,61,62,63. Figure 3c presents the time-dependent normalized water flux variations of the NF2A, C-PIM-1, and PC-PIM-1 membranes during the filtration of a BSA solution. The NF2A and C-PIM-1 membranes show larger flux decreases as compared to the PC-PIM-1 membrane, especially in the initial filtration stage. Upon reaching a steady state after 250 min of filtration, the flux decline ratio (DR) of the PC-PIM-1 membrane (40.8%) is much smaller than those of NF2A (62.3%) and C-PIM-1 (70.1%) membranes; interestingly, the C-PIM-1 membrane shows the largest DR possibly due to its non-polar and uncharged surface (Fig. S12, Supplementary Information)62,63. Thus, the treatment of the C-PIM-1 membrane by O2 plasma further imparts antifouling properties to the membrane against BSA, which would be another advantage of the O2 plasma treatment. A hydrophilic and charged membrane surface can provide an energetic barrier for the adhesion of foulants on the membrane surface via favorable water-surface interaction and electrostatic repulsion between foulants and the surface60,62,63.
Figure 3d displays the salt rejection and water fluxes of various NF membranes for the filtration of MgSO4 aqueous solutions (Table S4, Supplementary Information, for details). Most of the membranes reported previously have been found to exhibit a typical trade-off phenomenon. For example, a PA membrane exhibits a high salt rejection rate (94.5%) but low water flux (6.20 LMH bar−1) for the filtration of a 3,000 ppm MgSO4 solution64. In contrast, a graphene/CNT composite membrane shows the highest water flux (12.13 LMH bar−1) but a poor salt rejection rate (25.1%) for the filtration of a 1,200 ppm MgSO4 solution19. As compared with representative results across recently published studies, the C-PIM-1 membrane exhibits a comparable water flux and salt rejection rate. Furthermore, the high flux and good salt rejection rate of the PC-PIM-1 membrane clearly exceed the upper limit of state-of-the-art NF membrane performance. Although the reported MgSO4 rejection rate and water flux data were obtained under different conditions (Table S4, Supplementary Information), at least, such a comparison has demonstrated that the carbonaceous PIM-1 membrane with an O2 plasma-treated surface (PC-PIM-1) could act as a high-performance NF membrane.
Discussion
We have demonstrated that a carbonaceous NF membrane (C-PIM-1) can be prepared by the controlled carbonization of a PIM-1 membrane. Sub-1 nm-sized, interconnected, low frictional carbonaceous pores of the C-PIM-1 membrane facilitate the permeation of water molecules through the membrane, leading to a high water flux and good salt rejection rate. Moreover, the O2 plasma treatment of the C-PIM-1 membrane results in water flux enhancement without decreasing the salt rejection rate, as well as high fouling resistance against proteins. These properties are attributed to the negatively charged hydrophilic membrane surface that decreases the entrance/exit resistance of the carbonaceous pores while facilitating the Donnan exclusion and reduces the interaction of proteins with the membrane surface. This study provides insight into the design and preparation of carbonaceous PIM membranes for versatile applications including the filtration. In particular, the modification of the chemical structure of PIMs can possibly control the pore characteristics of the corresponding carbonaceous PIM membranes. Currently, studies for the further improvement of these membranes, such as fabrication of a thin, selective layer of carbonized PIMs on a supporting membrane for increasing water flux, are underway in our laboratory.
Methods
Materials and methods including membrane preparation details are described in the Supplementary Information.
Additional Information
How to cite this article: Kim, H. J. et al. A Carbonaceous Membrane based on a Polymer of Intrinsic Microporosity (PIM-1) for Water Treatment. Sci. Rep. 6, 36078; doi: 10.1038/srep36078 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation (NRF-2010-C1AAA01-0029061), the R&D Convergence Program of NST (National Research Council of Science and Technology), and Korea Research Institute of Chemical Technology (KRICT) core project (KK1602-D00).
Footnotes
Author Contributions H.J.K. and D.-G.K. contributed equally to this study. H.J.K., D.-G.K., B.G.K. and J.-C.L. designed the study and co-wrote the paper. H.J.K. and D.-G.K. conducted most of the experiments and analysed the data. K.L. synthesized the PIM-1 and prepared the PIM-1 and C-PIM-1 membranes. Y.B. discussed the solution-diffusion model of the membranes. Y.Y. and Y.S.K. conducted the membrane analysis. H.J.K. and D.-G.K. drafted the manuscript and B.G.K. and J.-C.L. critically reviewed the manuscript. B.G.K. and J.-C.L. are the guarantors of the paper. All authors agreed with the results and conclusions.
References
- Karan S., Samitsu S., Peng X. S., Kurashima K. & Ichinose I. Ultrafast viscous permeation of organic solvents through diamond-like carbon nanosheets. Science 335, 444–447 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang D.-E., Cooper V. R. & Dai S. Porous graphene as the ultimate membrane for gas separation. Nano Lett. 9, 4019–4024 (2009). [DOI] [PubMed] [Google Scholar]
- Huang L., Li Y. R., Zhou Q. Q., Yuan W. J. & Shi G. Q. Graphene oxide membranes with tunable semipermeability in organic solvents. Adv. Mater. 27, 3797–3802 (2015). [DOI] [PubMed] [Google Scholar]
- Hung W.-S. et al. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing. Chem. Mater. 26, 2983–2990 (2014). [Google Scholar]
- Lv R. T. et al. Open-ended, N-doped carbon nanotube-graphene hybrid nanostructures as high-performance catalyst support. Adv. Funct. Mater. 21, 999–1006 (2011). [Google Scholar]
- Salehi-Khojin A. et al. On the sensing mechanism in carbon nanotube chemiresistors. ACS Nano 5, 153–158 (2011). [DOI] [PubMed] [Google Scholar]
- Liu Y. X., Dong X. C. & Chen P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 41, 2283–2307 (2012). [DOI] [PubMed] [Google Scholar]
- Sippel-Oakley J. et al. Carbon nanotube films for room temperature hydrogen sensing. Nanotechnology 16, 2218–2221 (2005). [DOI] [PubMed] [Google Scholar]
- Che G. L., Lakshmi B. B., Fisher E. R. & Martin C. R. Carbon nanotubule membranes for electrochemical energy storage and production. Nature 393, 346–349 (1998). [Google Scholar]
- Harrison B. S. & Atala A. Carbon nanotube applications for tissue engineering. Biomaterials 28, 344–353 (2007). [DOI] [PubMed] [Google Scholar]
- Liu S. B. et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5, 6971–6980 (2011). [DOI] [PubMed] [Google Scholar]
- Paul D. R. Creating new types of carbon-nased membranes. Science 335, 413–414 (2012). [DOI] [PubMed] [Google Scholar]
- Lee B. et al. A carbon nanotube wall membrane for water treatment. Nat. Commun. 6, 7109 (2015). [DOI] [PubMed] [Google Scholar]
- Hinds B. J. et al. Aligned multiwalled carbon nanotube membranes. Science 303, 62–65 (2004). [DOI] [PubMed] [Google Scholar]
- Holt J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006). [DOI] [PubMed] [Google Scholar]
- Sholl D. S. & Johnson J. K. Making high-flux membranes with carbon nanotubes. Science 312, 1003–1004 (2006). [DOI] [PubMed] [Google Scholar]
- Han Y., Xu Z. & Gao C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 23, 3693–3700 (2013). [Google Scholar]
- Huang H. B. et al. Ultrafast viscous water flow through nanostrand-channelled graphene oxide membranes. Nat. Commun. 4, 2979 (2013). [DOI] [PubMed] [Google Scholar]
- Han Y., Jiang Y. Q. & Gao C. High-flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes. ACS Appl. Mater. Interfaces 7, 8147–8155 (2015). [DOI] [PubMed] [Google Scholar]
- Yeh C.-N., Raidongia K., Shao J. J., Yang Q.–H. & Huang J. X. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 7, 166–170 (2015). [DOI] [PubMed] [Google Scholar]
- Ghanem B. S., Swaidan R., Ma X. H., Litwiller E. & Pinnau I. Energy-efficient hydrogen separation by ab-type ladder-polymer molecular sieves. Adv. Mater. 26, 6696–6700 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang P. F., Li H. Y., Veith G. M. & Dai S. Soluble porous coordination polymers by mechanochemistry: from metal-containing films/membranes to active catalysts for aerobic oxidation. Adv. Mater. 27, 234–239 (2015). [DOI] [PubMed] [Google Scholar]
- Pandey P. et al. Imine-linked microporous polymer organic frameworks. Chem. Mater. 22, 4974–4979 (2010). [Google Scholar]
- Carta M. et al. An efficient polymer molecular sieve for membrane gas separations. Science 339, 303–307 (2013). [DOI] [PubMed] [Google Scholar]
- Guiver M. D. & Lee Y. M. Polymer rigidity improves microporous membranes. Science 339, 284–285 (2013). [DOI] [PubMed] [Google Scholar]
- Bezzu C. G. et al. A spirobifluorene-based polymer of intrinsic microporosity with improved performance for gas separation. Adv. Mater. 24, 5930–5933 (2012). [DOI] [PubMed] [Google Scholar]
- Lau C. H. et al. Gas-separation membranes loaded with porous aromatic frameworks that improve with age. Angew. Chem. Int. Edit. 54, 2669–2673 (2015). [DOI] [PubMed] [Google Scholar]
- Song Q. L. et al. Controlled thermal oxidative crosslinking of polymers of intrinsic microporosity towards tunable molecular sieve membranes. Nat. Commun. 5, 4813 (2014). [DOI] [PubMed] [Google Scholar]
- Budd P. M. et al. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv. Mater. 16, 456–459 (2004). [Google Scholar]
- McKeown N. B. & Budd P. M. Polymers of intrinsic microporosity (PIMs): organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 35, 675–683 (2006). [DOI] [PubMed] [Google Scholar]
- Du N. Y. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 10, 372–375 (2011). [DOI] [PubMed] [Google Scholar]
- Gorgojo P. et al. Ultrathin polymer films with intrinsic microporosity: anomalous solvent permeation and high flux membranes. Adv. Funct. Mater. 24, 4729–4737 (2014). [Google Scholar]
- Yong W. F. et al. Molecular engineering of PIM-1/Matrimid blend membranes for gas separation. J. Membr. Sci. 407–408, 47–57 (2012). [Google Scholar]
- Yong W. F., Kwek K. H. A., Liao K.-S. & Chung T.-S. Suppression of aging and plasticization in highly permeable polymers. Polymer 77, 377–386 (2015). [Google Scholar]
- Son S.-Y. et al. One-step synthesis of carbon nanosheets converted from a polycyclic compound and their direct use as transparent electrodes of ITO-free organic solar cells. Nanoscale 6, 678–682 (2014). [DOI] [PubMed] [Google Scholar]
- Kim B. G. et al. Sulfonation of PIM-1 towards highly oxygen permeable binders for fuel cell application. Macromol. Res. 22, 92–98 (2014). [Google Scholar]
- Song J. et al. Linear high molecular weight ladder polymers by optimized polycondensation of tetrahydroxytetramethylspirobisindane and 1,4-dicyanotetrafluorobenzene. Macromolecules 41, 7411–7417 (2008). [Google Scholar]
- Li P. et al. Characterization of carbon nanofiber composites synthesized by shaping process. Carbon 43, 2701–2710 (2005). [Google Scholar]
- Sadezky A., Muckenhuber H., Grothe H., Niessner R. & Poschl U. Raman micro spectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 43, 1731–1742 (2005). [Google Scholar]
- Moon I. K., Lee J., Ruoff R. S. & Lee H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 1, 73 (2010). [DOI] [PubMed] [Google Scholar]
- Li Z. et al. Carbonized chicken eggshell membranes with 3d architectures as high-performance electrode materials for supercapacitors. Adv. Energy Mater. 2, 431–437 (2012). [Google Scholar]
- Xu H. X., Guo J. R. & Suslick K. S. Porous carbon spheres from energetic carbon precursors using ultrasonic spray pyrolysis. Adv. Mater. 24, 6028–6033 (2012). [DOI] [PubMed] [Google Scholar]
- Amato L. et al. Pyrolysed 3D-carbon scaffolds induce spontaneous differentiation of human neural stem cells and facilitate real-time dopamine detection. Adv. Funct. Mater. 24, 7042–7052 (2014). [Google Scholar]
- Zhi L. et al. From well-defined carbon-rich precursors to monodisperse carbon particles with hierarchic structures. Adv. Mater. 19, 1849–1853 (2007). [Google Scholar]
- Geise G. M., Park H. B., Sagle A. C., Freeman B. D. & McGrath J. E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 369, 130–138 (2011). [Google Scholar]
- Geise G. M., Paul D. R. & Freeman B. D. Fundamental water and salt transport properties of polymeric materials. Prog Polym Sci 39, 1–42 (2014). [Google Scholar]
- Striolo A. The mechanism of water diffusion in narrow carbon nanotubes. Nano Lett. 6, 633–639 (2006). [DOI] [PubMed] [Google Scholar]
- Ma M., Tocci G., Michaelides A. & Aeppli G. Fast diffusion of water nanodroplets on graphene. Nat. Mater. 15, 66–72 (2016). [DOI] [PubMed] [Google Scholar]
- Li Z. T. et al. Effect of airborne contaminants on the wettability of supported graphene and graphite. Nat. Mater. 12, 925–931 (2013). [DOI] [PubMed] [Google Scholar]
- Sheng Y.-J., Jiang S. Y. & Tsao H.-K. Effects of geometrical characteristics of surface roughness on droplet wetting. J. Chem. Phys. 127, 234704 (2007). [DOI] [PubMed] [Google Scholar]
- Huh C. & Mason S. G. Effects of surface-roughness on wetting (theoretical). J. Colloid Interf. Sci. 60, 11–38 (1977). [Google Scholar]
- Van Wagner E. M., Sagle A. C., Sharma M. M. & Freeman B. D. Effect of crossflow testing conditions, including feed pH and continuous feed filtration, on commercial reverse osmosis membrane performance. J. Membr. Sci. 345, 97–109 (2009). [Google Scholar]
- Walther J. H., Ritos K., Cruz-Chu E. R., Megaridis C. M. & Koumoutsakos P. Barriers to superfast water transport in carbon nanotube membranes. Nano Lett. 13, 1910–1914 (2013). [DOI] [PubMed] [Google Scholar]
- Schaep J., Van der Bruggen B., Vandecasteele C. & Wilms D. Influence of ion size and charge in nanofiltration. Sep. Purif. Technol. 14, 155–162 (1998). [Google Scholar]
- Fornasiero F. et al. Ion exclusion by sub-2-nm carbon nanotube pores. P. Natl. Acad. Sci. USA 105, 17250–17255 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-G., Kang H., Han S. & Lee J.-C. Dual effective organic/inorganic hybrid star-shaped polymer coatings on ultrafiltration membrane for bio- and oil-fouling resistance. ACS Appl. Mater. Interfaces 4, 5898–5906 (2012). [DOI] [PubMed] [Google Scholar]
- Kim H. J. et al. Polyphenol/FeIII complex coated membranes having multifunctional properties prepared by a one-step fast assembly. Adv. Mater. Interfaces 2, 1500298 (2015). [Google Scholar]
- Kim D.-G., Kang H., Choi Y.-S., Han S. & Lee J.-C. Photo-cross-linkable star-shaped polymers with poly(ethylene glycol) and renewable cardanol side groups: synthesis, characterization, and application to antifouling coatings for filtration membranes. Polym. Chem. 4, 5065–5073 (2013). [Google Scholar]
- Sun X. H., Wu J., Chen Z. Q., Su X. & Hinds B. J. Fouling characteristics and electrochemical recovery of carbon nanotube membranes. Adv. Funct. Mater. 23, 1500–1506 (2013). [Google Scholar]
- Kim D.-G., Kang H., Han S. & Lee J.-C. The increase of antifouling properties of ultrafiltration membrane coated by star-shaped polymers. J. Mater. Chem. 22, 8654–8661 (2012). [Google Scholar]
- Kim D.-G., Kang H., Han S., Kim H. J. & Lee J.-C. Bio- and oil-fouling resistance of ultrafiltration membranes controlled by star-shaped block and random copolymer coatings. RSC Adv. 3, 18071–18081 (2013). [Google Scholar]
- Banerjee I., Pangule R. C. & Kane R. S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23, 690–718 (2011). [DOI] [PubMed] [Google Scholar]
- Yang R., Jang H., Stocker R. & Gleason K. K. Synergistic prevention of biofouling in seawater desalination by zwitterionic surfaces and low-level chlorination. Adv. Mater. 26, 1711–1718 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y. H. et al. Improved antifouling properties of polyamide nanofiltration membranes by reducing the density of surface carboxyl groups. Environ. Sci. Technol. 46, 13253–13261 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.