Abstract
There is no accepted clinical imaging modality for concussion, and current imaging modalities including fMRI, DTI, and PET are expensive and inaccessible to most clinics/ patients. Functional near-infrared spectroscopy (fNIRS) is a non-invasive, portable, and low-cost imaging modality that can measure brain activity. The purpose of this study was to compare brain activity as measured by fNIRS in concussed and age-matched controls during the performance of cognitive tasks from a computerized neurocognitive test battery. Participants included nine currently symptomatic patients aged 18–45 years with a recent (15–45 days) sport-related concussion and five age-matched healthy controls. The participants completed a computerized neurocognitive test battery while wearing the fNIRS unit. Our results demonstrated reduced brain activation in the concussed subject group during word memory, (spatial) design memory, digit-symbol substitution (symbol match), and working memory (X’s and O’s) tasks. Behavioral performance (percent-correct and reaction time respectively) was lower for concussed participants on the word memory, design memory, and symbol match tasks than controls. The results of this preliminary study suggest that fNIRS could be a useful, portable assessment tool to assess reduced brain activation and augment current approaches to assessment and management of patients following concussion.
Keywords: Concussion, Mild Traumatic Brain Injury, Neurocognitive Testing, Near Infrared Spectroscopy
Introduction
Sport-related concussions affect approximately 1.6 to 3.8 million people each year in the United States (Langlois et al. 2006). A concussion (or mild traumatic brain injury) is a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces secondary to direct or indirect forces to the head (McCrory et al. 2013). Alteration of mental status is related to neurometabolic dysfunction rather than structural brain injury per se; which may or may not involve a loss of consciousness (McCrory et al. 2013). Concussions differ from other traumatic brain injuries in their initial milder presentation (e.g., Glasgow Coma Scale score ≥13) and do not typically include structural alterations detectable using standard clinical imaging techniques (i.e., CT, MRI). In sports, concussions typically result from head-to-head, head-to-body part (i.e., shoulder or knee), or head-to-ground contact. These injuries can result in myriad physical, psychiatric, and sleep-related symptoms as well as cognitive, vestibular, and other deficits that can impair one’s ability to function in sport and in everyday life (Alsalaheen et al. 2010; McCrory et al. 2009; McGrath 2010). Previously, researchers have hypothesized that there is decreased cerebral blood flow during a period of vulnerability in the days immediately following concussion (Giza and Hovda 2001). This decrease in cerebral blood flow (CBF) may in part explain the performance deficits on cognitive tasks that have been documented following concussion. Assessment of changes in CBF during cognitive performance following concussion may help clinicians to better assess and manage this injury. Surprisingly, only a handful of studies have examined hemodynamic changes during performance of cognitive tasks in patients following concussion (Len et al. 2011; Maugans et al. 2012). Current methods of quantifying CBF include transcranial Doppler ultrasonography (TCD), positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional magnetic resonance imaging (fMRI). Although effective in detecting cerebral blood flow changes, these methods are limited in their portability, cost, and utility in the confines of clinical space. A novel portable brain imaging technology called functional near-infrared spectroscopy (fNIRS) is well suited to assessing cerebral blood oxygenation during the performance of cognitive tasks, and may provide information that enhances current concussion assessment and management. Researchers have yet to employ fNIRS to measure evoked changes in cerebral blood flow during cognitive and attentional tasks in patients following concussion.
Computerized neurocognitive test batteries are commonly used to assess and manage cognitive impairment following concussion (Lovell and Fazio 2008; Van Kampen et al. 2006). These tests typically include varying combinations of memory, learning, processing speed, and reaction time tasks. Consensus statements on concussion in sports have advocated for the use of such tests as one tool in a comprehensive concussion assessment and management approach that includes on-field, balance, and other assessments (McCrory et al. 2013). The premise of computerized neurocognitive testing is that performance on these tests is reflective of functional impairment in the brain following concussion. Initial research by Lovell and colleagues (2007) comparing concussed athletes to age-matched controls using fMRI during the N-back working memory task supported the premise for neurocognitive testing following concussion. Specifically, these researchers reported that concussed patients with hyper-activation were more likely to have a longer recovery than those without. Similarly, Jantzen et al. (2004) reported increased brain activation using fMRI from baseline to post-concussion in spite of a concurrent return to normal cognitive performance. More recently, Slobounov and colleagues (Slobounov et al. 2011) reported that brain activation increased as measured by fMRI. They also reported that concussed and control participants differed in brain activation in the acute phase (i.e., 10 days post injury) of concussion in the absence of symptom and neuropsychological test findings.
There is also evidence to suggest that even at rest, the injured brain behaves differently. Researchers investigating functional connectivity of the quiescent brain have demonstrated acute post-injury changes in the strength but not the number of connections (e.g., Nakamura et al. 2009, Slobounov et al. 2011) and overall reduced inter-hemispheric connectivity (e.g., Johnson et al. 2012). Researchers who examined the default mode network reported a similar reduction in connection strength as well as number, and a moderating effect of the number of injuries sustained (Johnson et al. 2012). Together this evidence suggests that brain function may be altered both during cognition and at rest.
Together these findings suggest that brain activation patterns and recovery may not mirror those of neurocognitive testing following concussion. However, it is important to note that the preceding studies either did not use and/or did not report concurrent neurocognitive performance on commonly used computerized neurocognitive test batteries (e.g., ANAM, AXON, CogSport, ImPACT, Headminders). Instead, both studies used traditional paper and pencil tests including Trail Making, Stroop, and Digit Span tests, which are characterized by more substantial practice effects relative to computerized tests; furthermore, computerized assessments after a concussive injury are more commonly used owing to their cost and time effectiveness among other things (Collie et al. 2001; Erlanger et al. 2003). As such, research comparing functional brain activation patterns in patients following concussion with concurrent completion of computerized neurocognitive test batteries that are sensitive to the effects of concussion is warranted.
Brain imaging techniques such as fMRI have been developed to measure local changes in cerebral oxygenation and blood flow evoked during functional tasks. Researchers have begun to establish the utility of fMRI in detecting functional changes in individuals following concussion during several of the popular neurocognitive tests (McAllister et al. 2001; Ptito et al. 2007; Slobounov et al. 2011; Slobounov et al. 2010). To date, several fMRI studies have demonstrated hyper-activation particularly in the prefrontal cortex and dorsolateral prefrontal cortex (reviewed in Slobounov et al. 2012). However, other researchers have reported reduced activation during working memory tasks (Chen et al. 2004; Chen et al. 2008). The equivocal findings may relate to the different demands of the tasks being performed. For example, a more resource intensive task such as recall of a specific item may result in bilateral or diffused activation following concussion. Regardless, these discrepant findings highlight the need for further research into brain activation during cognitive performance following concussion. Functional neuroimaging is a potentially valuable tool for assessing brain activation and informing clinical decision making following concussion (Ptito et al. 2007) that warrants additional investigation.
Although fMRI can provide images of task-evoked blood flow changes in the brain, this technology requires expensive equipment, technical personnel, and a large dedicated space. As such, the use of fMRI in assessing concussion is only practical in large hospital/clinic and research settings. In contrast to fMRI, fNIRS instruments are small, portable, and relatively inexpensive. Functional NIRS is a non-invasive technology that uses low-levels of near-infrared light to measure changes in blood oxygenation in the brain (Jobsis 1977; Obrig and Villringer 2003). Several research groups and commercial companies have developed portable and even wireless and wearable fNIRS devices. Functional NIRS also has a high temporal resolution allowing for insight into the dynamics of the brain and relationships between regions. The primary disadvantage of fNIRS is that it is restricted to measurements of events that are vascular in nature and is therefore limited to the cortex. In the 1970s Jöbsis (1977) first demonstrated optical measurements of evoked cerebral changes. Since that time, fNIRS has been applied to study a variety of brain regions including the frontal, visual, motor, auditory, and somatosen-sory cortices (see Huppert et al. 2008 for review).
Functional NIRS is based on the non-invasive, spectroscopic measurement of optical absorption of cerebral blood. Biological tissues, such as the scalp, skull, and brain, have low intrinsic optical absorption in the range of 650–900 nm; a region often termed the “biological optical window”. In this range of colors, light can travel through centimeters of tissue, allowing measurements of the spectroscopic properties of underlying structures such as the brain. Because tissues vary in their saturation points, light will undergo multiple scattering events as it travels through them, which can be accurately modeled as a diffusion process. In fNIRS, a set of light emitters (sources) and detectors is placed non-invasively on the surface of the scalp at a separation distance of around 3–3.5 cm. Light from the emitter will diffuse through the tissue before either reaching the detector or being lost due to absorption or escaping from the scalp. For typical fNIRS configurations, the measurement of the light passing between an emitter and detector pair is sensitive to changes in the optical properties of the underlying scalp and brain to a depth of about 5–8 mm into the outer cortex of the brain. This depth is sufficient enough to reach many, though not all, of the frontal and temporal neocortical areas involved in cognition. Because NIRS is portable, it has the potential to be used as an on-field assessment tool for detecting blood flow deficits from concussion. Functional NIRS has been previously shown to detect changes in traumatic brain injury (Adelson et al. 1998; Haitsma and Maas 2007; Weatherall et al. 2012). However, to date, no studies have employed fNIRS with patients following concussion.
The purpose of the present study was to compare brain activation as measured by fNIRS during the performance of a computerized neurocognitive test battery in a sample of concussed patients compared to age-matched healthy controls. Based on findings by McAllister et al. (2001), we hypothesized that the concussed group would have altered cognitive resource allocation and display more bilateral activation patterns. More specifically, for less demanding cognitive tasks (e.g., word memory, design memory, symbol-match), we predicted that the concussed group’s activation would be similar to or slightly higher than the control group. For more demanding cognitive tasks (e.g., color-match, 3-letters, X’s and O’s), we expected that the concussed group would have a lower cognitive capacity and demonstrate decreased activity compared to the controls. We predicted that this decreased capacity would correspond to poorer performance of memory, learning, processing speed, and reaction time tasks.
Materials and methods
Design
The design for this study involved an age- and sex-matched, experimental-control group comparison. The University of Pittsburgh institutional review board for human subject research approved the study. All participants completed informed written consent forms prior to being enrolled in the study.
Participants
A total of 14 participants were included in the study. Participants included nine patients (5 males, 4 females) aged 18–45 years with a recent (15–45 days from time of injury) sport-related concussion who were symptomatic at the time of testing and five age-matched controls. The concussion group was comprised of nine participants with an average age of 22.73 years (±1.32) and average time since injury of 21.02 days (±12.00). The control group included five participants (1 male and 4 females) with an average age of 22.00 years (±0.28). The control group reported one previous concussion, whereas the concussion group reported 0.67 (±1.32) concussions previous to the presenting injury with a range of 0 – 3 previous concussions. The two groups did not differ significantly on age (t(9)=0.11, p=0.91) or the number of previous concussions (t(12)=1.11, p=0.29). Inclusion criteria included: age 18–45 years, sport-related concussion within the past 15–45 days (for concussed group only). Exclusion criteria included a history of any one of the following: learning disability, ADHD, color blindness, substance abuse, major psychological disorder, brain surgery, TBI, or major neurological condition. Control participants with a current, symptomatic or recent (<6 months) concussion were also excluded.
Procedures
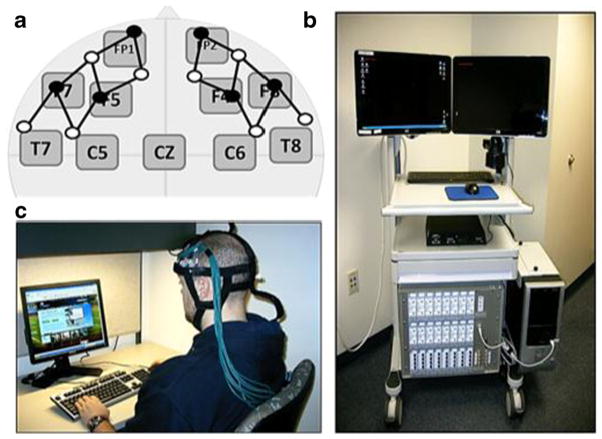
Concussed participants who met the inclusion/exclusion criteria were referred to the researchers by clinicians for testing. Age-matched control participants were recruited for the study from a large urban university campus. After completing the informed consent forms, participants then completed the computerized neurocognitive test battery (described below) while wearing the fNIRS fiber optics head cap (see Fig. 1). All testing was completed in a private exam room and took approximately 35–50 min total per participant.
Fig. 1.
FNIRS signals were recorded using a custom-built optical head cap (b) covering the frontal cortex bilaterally as shown in a. The closest points on the International 10–20 system are shown relative to the optical sensor locations. Open and filled circles indicate optical detector and source positions respectively. A 32-channel TechEn Inc fNIRS system (c) was used in this study, which was contained on a portable cart moved into the patient/subject’s neurocognitive testing area
Neurocognitive test battery
The Immediate Post-concussion Assessment and Cognitive Test (ImPACT) test was used to measure computerized neurocognitive performance. The ImPACT neurocognitive battery includes three sections: 1) demographics, 2) self-reported concussion symptoms, and 3) neurocognitive modules. The Post-concussion Symptom Scale (PCSS) contains 22 self-report symptoms that comprise cognitive, somatic, affective, and sleep-related items that are rated on a 0 (non) to 6 (severe) -point Likert scale (McCrory et al., 2009). The neurocognitive test component of ImPACT comprises six modules: 1) word memory- involves the presentation of random words with immediate and delayed word recall; 2) design memory- involves the presentation of abstract single line designs with immediate and delayed design recall; 3) symbol matching- involves the presentation of representative symbols positioned on the screen in numerical positions- 1 through 9, symbols are then “hidden”, and the subject must recall the hidden symbol-digit matches; 4) color match- involves the presentation of words representing colors (e.g., “RED”) in different colors such that the participant must respond when the word and the color match (the word RED in red-colored font); 5) three letters memory- involves the presentation of three random letters, followed by a number counting reaction time distractor task, and subsequent recall of the three letters; and 6) X’s and O’s- involves the presentation of three highlighted X’s and O’s on a screen full of X’s and O’s, followed by a distractor choice reaction time task, and recall of the location of the three X’s and Os. Typically, these modules are collapsed into composite scores (i.e., verbal and visual memory, processing speed, reaction time) for analysis; however, the current testing paradigm required time-marked events in order to analyze the fNIRS data, and the composite scores include data from different modules. Therefore, only the raw module scores were used in the fNIRS analysis. The composite scores were used in the behavioral data analysis to compare the concussed and control groups. All participants were given the post-injury 1 test version of the ImPACT test.
Behavioral data analysis
A series of independent sample t-tests with Bonferroni correction for multiple comparisons were performed on selected subtest scores for each of six neurocognitive tasks, overall neurocognitive composite scores, and total reported symptoms. All statistical analyses were conducted using the software program SPSS (version 19) and statistical significance was set at p≤0.05.
Functional near-infrared spectroscopy
A 32-channel continuous wave (CW) fNIRS system was used in this study (CW6; TechEn Inc, Milford MA). A custom-made head cap constructed from plastic and Velcro materials embedded in a neoprene head cap was used for holding the fNIRS sensors. A photograph of this head cap and the fNIRS instrument is shown in Fig. 1. The fNIRS instrument and cart is about 80 cm wide by 60 cm deep by 150 cm high and could be transported between the clinical examination rooms and controlled by a single operator. In this study, the fNIRS cart (Fig. 1c) was discretely positioned behind the participant and across the room where they were taking the ImPACT. A total of 6 detectors and 8 source positions on the head were recorded using 3-m fiber optic cables between the cap and the fNIRS instrument. The sources and detectors were arranged in a 3.2 cm nearest neighbor geometry as shown in Fig. 1a allowing a total of 32 measurement combinations. The probe extended from above the ear (international 10/20 point FT7 on the left and FT8 on the right) up to and including the frontal lobe (international 10/20 points F3 and F4 respectively) as shown in Fig. 1a. Each measurement pair samples the optical properties of the tissue within a diffuse volume with maximum sensitivity along a line between the source and detector positions, roughly corresponding to the labeled international 10/20 positions shown in Fig. 1a. The sensitivity of the fNIRS measurements is roughly 2.5 cm into the head, which provides sensitivity to the outer approximately 5–8 mm of cortex in these areas. The probe was designed to cover bilateral areas of the inferior frontal, dorsal-lateral prefrontal, and frontal regions of the brain including Brodmann areas [BA] 44, 45, 46, 47, and 10. Because fNIRS measurements only sample the limited area beneath the position of sensors, other areas of the cortex were not examined in this study. Each source position emitted light at 690 nm (~12 mW) and 830 nm (~8 mW), which provides sensitivity to both oxy-and deoxy-hemoglobin. Data were collected at 4Hz by the system.
Prior to the start of the recording, the NIRS cap was placed on the head of the subject and the amplification (gain) of the detectors was adjusted to maximize the signal-to-noise level for all measurement pairs. If needed, the head cap was adjusted to create better contacts between the NIRS sensors and the head.
FNIRS brain signals were recorded during the unmodified ImPACT test. In order to synchronize the timing of ImPACT modules with the fNIRS data, the acquisition software allowed the fNIRS operator to use a manual button press input. This timing was accurate to about 1 s, which was a negligible timing error compared to the length of the blocks of the task (30–90s). In order to analyze the fNIRS data as a block-design task, the participant was asked to pause for about 30s in between modules to create rest periods for analysis.
NIRS data analysis
A review of the steps of analysis for fNIRS studies can be found in Huppert et al. (2009). In brief, after collection the raw fNIRS data for each source-detector pairing and each wavelength was converted to changes in the optical density (absorption) and then converted to oxy- and deoxy-hemoglobin changes via the modified Beer-Lambert relationship (Cope et al. 1988). The channels of data are then visually inspected for quality and the entire channel is removed if the data quality indicates that there was bad coupling between the sensors and the scalp (e.g. extremely high temporal variance or no visible physiological fluctuations such as cardiac pulsation). In general, only 1–3 channels (out of 16) were removed. First level statistical analysis is done using a canonical general linear model (Ye et al. 2009) based on the timing of the modules of the ImPACT was constructed. This approach is identical to the general linear model approach used in most fMRI studies (e.g., (Friston 2007)).
A hemodynamic response based on a gamma-variant function with parameters taken from published fMRI methods was used for this study (Friston 2007). In addition, low-pass filtering is done inside the GLM by projection of drift (pre-whitening) from both the data and design matrix using a discrete cosine transform basis (0–0.02Hz) (e.g. S.Y= S.X.β where Y is the data vector, X is the GLM design matrix, β is the coefficients of the model describing the amplitude of brain activity, and S is the filtering matrix created from the cosine transform). This approach, which is the method of filtering used in fMRI analysis (e.g. SPM8), is preferable to a direct filtering on the data alone, which can over-correct and remove brain activation in the context of the longer-duration or self-paced epoch timing inherent to the tasks used in this study. A restricted maximum likelihood (ReML) model was used to solve the regression model using weighted least-squares (e.g. the Gauss-Markov equation) (Friston 2007). The magnitude of changes (e.g. coefficients in the GLM regression model; β as denoted in the equation above) was estimated for each optical source-detector pair and for each task condition.
In order to visualize the maps of brain activation, the optical images were reconstructed from the estimated GLM model coefficients based on a template brain (Colin-27 atlas; (Holmes et al. 1998)) as described by Abdelnour and colleagues (Abdelnour et al. 2010; Abdelnour and Huppert 2010). Prior to data collection, the location of the fNIRS headcap was measured relative to the international 10/20 coordinate system (Fig. 1a). Based on this registration, a sensitivity model for the fNIRS measurements was calculated using a finite element model (NIRFAST; (Dehghani et al. 2008)) of light diffusion in the head using the segmented brain atlas model. Although individual differences in brain anatomy will alter the sensitivity of the fNIRS measurements, previous publications have shown that atlas-based fNIRS analysis still provide a close approximation to individualized models (Custo et al. 2006). The fNIRS measurement sensitivity model for each subject was inverted using a random-effects inverse model (Abdelnour and Huppert 2011) to produce an estimate of the brain activation map for each subject and the weighted average of the two subject groups (controls and concussed). In this random-effects model (Abdelnour and Huppert 2011), the forward models obtained from the finite element model for each subject’s registration are collected into a single linear matrix operator such that the estimate of each subject’s brain activity is the sum of the group average and a random-effects perturbation term for that subject. Thus, instead of performing multiple independent image reconstructions (one for each subject), the larger combined forward model is inverted in order to estimate an image of brain activity that is most simultaneously consistent with all participants’ data. This approach was shown to be less susceptible to artifacts and errors introduced by outlier measurements in only a few participants. Restricted maximum likelihood (ReML) is an empirical Bayesian method, which is used to provide stabilization of the inverse (image reconstruction) model and for simplified models is mathematically equivalent to an L-curve technique to optimize regularization (Abdelnour et al. 2010). This approach has been widely used in fMRI analysis (Cox 1996; Friston 2007) and in the implementation of the similar image reconstruction problem for electroencephalography (EEG) and magnetoencephalography (MEG) within the software SPM-8 (Friston 2007; Mattout et al. 2006). The use of ReML for fNIRS was described in Abdelnour et al. (2010). The ReML based random-effects image reconstruction method used in this work is very similar to the implementation of the random-effects MEG reconstruction model used in SPM-8 (Mattout et al. 2006). A k-means clustering algorithm (SPM-8) was used to define regions of significant activation (p<0.05 corrected; minimum cluster 10voxels based on oxy-hemoglobin) and the mean hemoglobin contrast relative to baseline and center of the cluster in MNI coordinates was reported in the results. Based on the MNI coordinates, we also report the nearest Brodmann area to the center of the activation.
Results
The fNIRS data were recorded during the completion of the computerized neurocognitive test battery and analyzed using a canonical general linear model. Statistical parametric maps for individual participants and the two participants groups were reconstructed. The group-level results for the word memory, design memory, symbol-match, color-match, three letters memory, and X’s and O’s tasks are presented in this section. The reaction time and percentage correct for all of the neurocognitive test battery components are provided in Table 1. As expected, the concussed group reported significantly more symptoms and lower neurocognitive composite scores than the control group. The neurocognitive results are explained further in the results section for each task.
Table 1.
The module scores for the six parts of the neurocognitive test are provided above. The mean, standard deviation, and range over the number of participants are provided for each test part
| Concussed (N=9)
|
Control (N=5)
|
||||||
|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Range | Mean | Standard deviation | Range | Group difference | |
| Word memory | |||||||
| Total % correct | 77.1 % | 15.0 % | 60–97 % | 95.6 % | 3.5 % | 89–100 % | t(12)=−2.29, p=0.04 |
| Design memory | |||||||
| Total % correct | 68.5 % | 15.4 % | 50–94 % | 85.8 % | 8.7 % | 73–94 % | t(12)=−2.44, p=0.03 |
| Symbol match visible | |||||||
| Total correct | 26.6 | 0.7 | 25–27 | 27.0 | 0.0 | 27 | t(12)=−1.23, p=0.23 |
| Symbol match hidden | |||||||
| Total correct | 4.0 | 2.5 | 2–9 | 7.1 | 2.5 | 3–9 | t(12)=−2.25, p=0.04 |
| Three letter | |||||||
| Average total correct | 11.6 | 4.0 | 6–15 | 14.3 | 0.5 | 14–15 | t(12)=−1.79, p=0.09 |
| X&O’s | |||||||
| Total correct memory | 8.3 | 2.7 | 5–12 | 9.1 | 2.1 | 6–12 | t(12)=−0.66, p=0.52 |
Word memory task
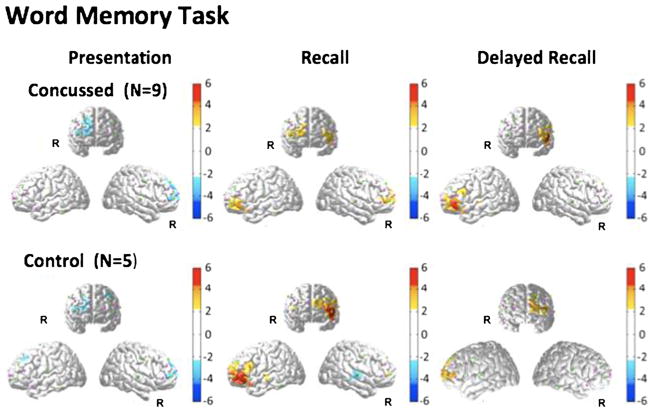
Reconstructed fNIRS brain activation images of oxy-hemoglobin changes for the three phases (word presentation, word recall, and delayed word recall) of this task are shown in Fig. 2. During the word presentation phase, a slight decrease in oxy-hemoglobin in the right frontal cortex during stimulus presentation compared to baseline. This change was not statistically different between the concussed and control research groups. During the word recall phase of the task, an increase in oxy-hemoglobin was observed in the left inferior frontal region of the brain (close to BA 45/46/47) in both subject groups. The magnitude of this change was much larger (about two-fold) in the control group compared to the concussed participants (Table 2). Additionally, in the concussed subject group, activation of the right inferior frontal gyrus region was also observed. No significant activation was observed in the right inferior frontal cortex of the control group. During the delayed recall phase of the test, activation in the left inferior frontal region was observed in the same location as during the initial recall phase. In the control group, activation during the delayed recall phase was less than during the initial recall phase. By contrast, within the concussed population, activation during delayed recall was slightly higher than during the initial recall phase. No additional activation of the right inferior frontal cortex was observed in either group during delayed recall.
Fig. 2.
FNIRS activation maps (oxy-hemoglobin effect size; T-score) were reconstructed for the presentation, recall, and delayed recall parts of the word memory module of the neurocognitive test. The color maps indicate the effect size. Only significant areas (p<0.05; corrected) are shown. Because fNIRS has limited depth penetration and spatial coverage (see Fig. 1), only cortical areas of the frontal cortex are accessible in this study
Table 2.
The mean change in brain activity from regions-of-interest within the left and right frontal cortices for the Word Memory and Design Memory modules of the neurocognitive test. Regions were obtained from cluster analysis (k-means clustering) of the oxy-hemoglobin reconstructions with a threshold of p<0.05 and minimum cluster size of 10 voxels. The deoxy-hemoglobin values within this same region are also provided. For each region, the anatomical label and Brodmann area [if applicable] is provided based on the MNI coordinate of the center of the region. Abbreviations used IFG [inferior frontal gyrus], MFG [middle frontal gyrus], MTG [middle temporal gyrus], STG [superior temporal gyrus]
| Concussed (N=9)
|
Control (N=5)
|
Difference (Concussed-Controls)
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | [X | Y | Z] | Cluster size (mm^2) |
Oxy- hemoglobin |
Deoxy- hemoglobin |
[X | Y | Z] | Cluster size (mm^2) |
Oxy- hemoglobin |
Deoxy- hemoglobin |
[X | Y | Z] | Cluster size (mm^2) |
Oxy- hemoglobin |
Deoxy- hemoglobin |
|||
| Word memory presentation | |||||||||||||||||||||
| Right | IFG[BA 47] | 48 | 20 | −4 | 53 | −6.2 | 0.9 | MTG[BA 22] | 60 | 8 | −4 | 38 | −4.5 | - | IFG[BA 47] | 56 | 16 | −2 | 36 | −06 | 0.0 |
| Left | IFG[BA 47] | −21 | 30 | −8 | 18 | −1.2 | MFG | −22 | 35 | −13 | 17 | −2.1 | 1.1 | - | - | - | - | - | - | - | |
| Word memory recall | |||||||||||||||||||||
| Right | - | - | - | - | - | - | MFG | 29 | 57 | 7 | 41 | 3.9 | −1.2 | - | - | - | - | - | - | ||
| Left | MFG | −6 | 55 | −4 | 90 | 6.9 | −1.8 | IFG | −51 | 30 | −10 | 61 | 12.3 | −2.9 | IFG | −46 | 32 | −10 | 61 | −1.7 | 0.1 |
| Word memory delayed recall | |||||||||||||||||||||
| Right | MFG | 30 | 51 | 3 | 50 | 5.0 | −1.4 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Left | IFG | −53 | 22 | −6 | 74 | 10.6 | −1.4 | MFG [BA 10] | −3 | 60 | −3 | 85 | 7.9 | −3.1 | MFG | −41 | 51 | −6 | 42 | 1.7 | −0.1 |
| Design memory presentation | |||||||||||||||||||||
| Right | STG | 61 | 7 | −8 | 29 | 2.9 | −2.2 | IFG [BA 47] | 56 | 23 | −10 | 29 | 2.4 | −1.4 | MTG [BA 21] | 60 | 3 | −16 | 35 | −0.2 | 0.0 |
| Left | IFG | −46 | 43 | 0 | 34 | − | −1.6 | IFG | −49 | 26 | −8 | 9 | 1.9 | −1.5 | - | - | - | - | - | - | |
| Design memory recall | |||||||||||||||||||||
| Right | MFG | 37 | 43 | −3 | 55 | - | −1.5 | MFG | 28 | 50 | 8 | 43 | 2.4 | −1.3 | MFG | 32 | 43 | −5 | 51 | −0.5 | 0.0 |
| Left | IFG | −40 | 27 | −15 | 13 | 2.1 | − | MFG | −9 | 55 | −4 | 24 | 2.3 | - | - | - | - | - | - | - | |
| Design memory delayed recall | |||||||||||||||||||||
| Right | IFG | 54 | 22 | −13 | 36 | 4.7 | −2.0 | IFG [BA 47] | 53 | 35 | −13 | 26 | 2.0 | −1.7 | STG | 44 | 3 | −12 | 53 | 1.2 | 0.0 |
| Left | MFG [BA 11] | −44 | 35 | −13 | 16 | 2.8 | −1.6 | IFG [BA 47] | −50 | 24 | −2 | 6 | 2.1 | −1.6 | - | - | - | - | - | - | |
Results from a series of independent samples t-test with Bonferroni correction for multiple comparisons revealed several significant differences on the ImPACT modules between the concussed and control groups (Table 1). Specifically the concussed group demonstrated significantly lower performance on Word Memory Percent Correct for the delayed recall condition (t (12)=−2.32, p=0.04) and a lower overall percentage correct for the Word Memory module (t (12)=−2.29, p=0.04).
Design memory task
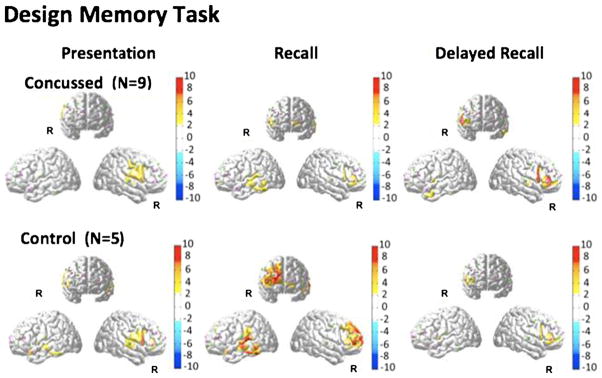
The brain activation images (oxy-hemoglobin T-maps) for the design memory task are shown in Fig. 3. During the initial recall and delayed recall phases, activation was seen in the right frontal area of the brain in both groups. Activation of this region in the control group was significantly greater (p<0.05; see Table 2) during the initial recall task compared to the concussed group. Similar to the observations in the verbal memory task, the delayed recall produced less brain activity but in the same region (right inferior frontal) as the initial recall task in the control population. In the concussed population, the delayed recall showed a larger change compared to the initial recall task in the same population, but less than the initial recall response in the control group. The delayed recall responses in both groups were not significantly different from each other although the concussed group showed a statistical trend to be higher compared to the controls. Compared to controls, the concussed group identified fewer Correct “No” Responses (t (12)=−2.14, p=0.05) and demonstrated lower Percent Correct (t (12)=−2.44, p=0.03) for the immediate recall condition of the Design Memory module.
Fig. 3.
FNIRS activation maps (oxy-hemoglobin effect size; T-score) were reconstructed for the presentation, recall, and delayed recall parts of the design memory module of the neurocognitive test. The color maps indicate the effect size. Only significant areas (p<0.05; corrected) are shown
Symbol-match task
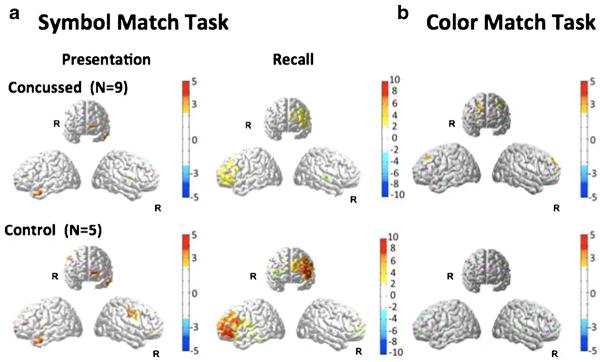
The fNIRS results for this task are shown in Fig. 4. During the presentation phase, both subject groups showed slight activation of the superior frontal cortex (near BA 10). Activity was also observed in the left and right temporal regions. Neither of these regions differed significantly between the two groups. During the recall phase, activation was observed in the left frontal cortex. Activation in the control group was significantly larger than that of the concussed group (p<0.05; see Table 3). In the behavioral data, the concussed group also had fewer total correct responses on the symbol-match module (t (12)=−2.25, p=0.04).
Fig. 4.
FNIRS activation maps (oxy-hemoglobin effect size; T-score) were reconstructed for the presentation and recall parts of the symbol match module of the neurocognitive test (panel a). The activation maps for the color match task is shown in panel b. The color maps indicate the effect size. Only significant areas (p<0.05; corrected) are shown. There was no significant activation in the control group for the color match task (panel b bottom)
Table 3.
The mean change in brain activity from regions-of-interest within the left and right frontal cortices for the Symbol Match and Color Match modules of the neurocognitive test. Regions were obtained from cluster analysis (k-means clustering) of the oxy-hemoglobin reconstructions with a threshold of p<0.05 and minimum cluster size of 10 voxels. The deoxy-hemoglobin values within this same region are also provided. For each region, the anatomical label and Brodmann area [if applicable] is provided based on the MNI coordinate of the center of the region. Abbreviations used IFG [inferior frontal gyrus], SFG [superior frontal gyrus], MFG [middle frontal gyrus], MTG [middle temporal gyrus], STG [superior temporal gyrus]
| Concussed (N=9)
|
Control (N=5)
|
Difference (Concussed-Controls)
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | [X | Y | Z] | Cluster size (mm^2) |
Oxy- hemoglobin |
Deoxy- hemoglobin |
[X | Y | Z] | Cluster size (mm^2) |
Oxy- hemoglobin |
Deoxy- hemoglobin |
[X | Y | Z] | Cluster size (mm^2) |
Oxy- hemoglobin |
Deoxy- hemoglobin |
|||
| Symbol match presentation | |||||||||||||||||||||
| Right | IGF | 59 | 12 | 6 | 19 | 2.6 | −0.2 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Left | SFG | −26 | 43 | −17 | 17 | 2.2 | −0.1 | MFG | −8 | 55 | −4 | 24 | 2.3 | −0.1 | - | - | - | - | - | - | |
| Symbol match recall | |||||||||||||||||||||
| Right | MTG [BA 21] | 62 | −2 | −21 | 12 | 2.6 | −0.3 | SFG | 26 | 69 | −2 | 36 | 4.2 | −0.1 | - | - | - | - | - | - | |
| Left | SFG [BA 10] | −23 | 62 | −6 | 53 | 5.4 | −0.4 | IFG | −51 | 27 | −5 | 74 | 14.3 | −0.4 | ITG | −63 | −9 | −20 | 16 | −0.4 | 0.0 |
| Color match | |||||||||||||||||||||
| Right | SFG [BA 10] | 27 | 67 | 2 | 34 | 3.6 | −0.1 | IFG | 53 | 29 | −18 | 22 | 1.8 | −0.2 | 44 | 47 | −2 | 50 | 0.3 | 0.0 | |
| Left | IFG | −53 | 22 | 6 | 34 | 3.6 | −0.1 | IFG [BA 47] | −45 | 36 | −10 | 11 | 2.0 | −0.2 | MFG [BA 10] | - | - | - | - | - | - |
| Three letter presentation | |||||||||||||||||||||
| Right | MFG [BA 10] | 36 | 48 | 14 | 27 | 2.4 | −0.1 | STG [BA 22] | 60 | 7 | −2 | 34 | 3.6 | −0.1 | MFG | 42 | 50 | −7 | 38 | −0.1 | 0.0 |
| Left | IFG | −47 | 37 | 1.7 | 46 | 2.8 | −0.1 | SFG [BA 10] | −33 | 58 | −4 | 36 | 2.6 | −0.2 | - | - | - | - | - | - | |
| Three letter recall | |||||||||||||||||||||
| Right | STG [BA 10] | 30 | 65 | 1 | 41 | 6.6 | −0.2 | SFG | 36 | 48 | 15 | 28 | 2.3 | −0.1 | MFG | 40 | 51 | −4 | 49 | 0.2 | 0.0 |
| Left | IFG [BA 45] | −54 | 18 | 6 | 39 | 3.9 | −0.2 | MFG | −46 | 38 | −7 | 60 | 5.7 | −0.2 | - | - | - | - | - | - | |
| X&O’s presentation | |||||||||||||||||||||
| Right | IFG [BA 47] | 50 | 16 | −2 | 27 | 1.5 | −0.1 | MFG [BA 10] | 33 | 63 | 1 | 34 | 2.1 | −0.2 | - | - | - | - | - | - | |
| Left | MTG [BA 22] | −61 | −2 | −4 | 37 | 4.8 | 0.0 | MTG | −56 | 9 | −19 | 11 | 1.6 | −0.2 | ITG | −61 | −3 | −15 | 32 | −0.9 | 0.0 |
| X&O’s recal | |||||||||||||||||||||
| Right | IFG | 41 | 28 | −2 | 23 | 1.9 | −0.1 | IFG | 53 | 24 | 10 | 29 | 3.2 | −0.2 | - | - | - | - | - | - | |
| Left | MTG | −65 | −14 | −12 | 8 | 1.3 | −0.1 | MFG [BA 11] | −45 | 42 | −13 | 43 | 4.1 | −0.3 | MFG | −48 | 37 | −4 | 60 | −0.3 | 0.0 |
Color-match task
Brain activation signals for the color-match task are shown in Fig. 4. In the control group, we failed to detect any significant activation during color-match. The concussed group showed a very slight significant activation in the left frontal region (p<0.05; Table 3), and a slower reaction time for correct responses on the module (t (12)=2.35, p=0.04) relative to controls.
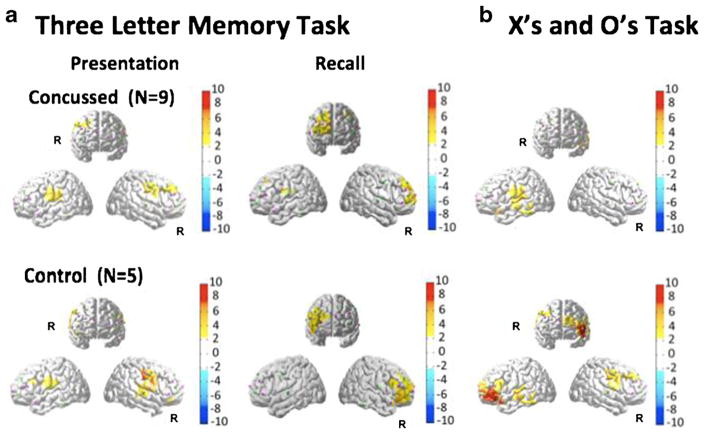
Three-letters memory task
The fNIRS results for this task are shown in Fig. 5 and Table 3. In the initial presentation phase, both the control and concussed groups showed similar bilateral activation patterns. The control group showed more activation on the right hemisphere. In the recall phase of the task, both groups showed strong activation of the right hemisphere. There were no statistically significant between-group differences for the behavioral response data for the three-letters task (Table 1).
Fig. 5.
FNIRS activation maps (oxy-hemoglobin effect size; T-score) were reconstructed for the presentation and recall parts of the Three-Letters Memory module of the neurocognitive test (panel a). The activation maps for the X’s and O’s task is shown in panel b. The color maps indicate the effect size. Only significant areas (p<0.05; corrected) are shown
X’s and O’s task
The fNIRS results for this task are shown in Fig. 5. In the control group, activation of left frontal cortex was observed. In the concussed group, a less significant activation of the left frontal region was also observed. There were no statistically significant between-group reaction time differences on the X’s and O’s module (Table 1).
Discussion
In the current study, fNIRS was applied to imaging cerebral blood oxygenation changes in the brain during the performance of a commonly used computerized neurocognitive test battery in participants with concussion and healthy age-matched controls. The results suggest that fNIRS was sensitive to changes in neural haemodynamic activity between these two groups. Specifically, in the word memory, design memory, symbol match, and X’s & O’s tasks the concussed group demonstrated decreased activation compared to the controls. In the three letters and color match tasks we found similar levels of activation in both groups. With the exception of the X’s and O’s task, the behavioral results corroborate the fNIRS results, in that performance was lower for concussed participants on the word memory, design memory, and symbol match modules, but not significantly different on the three letters and X’s & O’s tasks.
Previous research involving fMRI data for patients with concussion has often reported increased brain activity in comparison to controls. The most consistent finding among these studies is hyper-activation of the prefrontal cortex (PFC) and dorsolateral prefrontal cortex (DLPFC). Slobounov and colleagues (Slobounov et al. 2010) conducted fMRI imaging during the performance of a complex virtual reality maze task paradigm with concussed athletes and controls. Their results supported increased amplitude and more diffuse brain responses (i.e., fMRI-BOLD response) in both visual and cognitive areas (specifically the DLPFC) in the concussed group. Similarly, McAllister and colleagues demonstrated significantly more activation in concussed patients during cognitive tasks (McAllister et al. 2006; McAllister et al. 2001). More specifically, they showed that concussion was associated with a decreased capacity for increasing working memory load (McAllister et al. 2001). These researchers also reported that at low cognitive load (i.e., specifically 0- and 1-back conditions of an N-back memory task), the concussed group showed significantly increased brain activity compared to controls. However, for more difficult tasks (2-back and 3-back tasks), the control group showed stepwise increases in activation associated with increased cognitive load, which was diminished in the concussed group. This finding suggested that the concussed patients had altered cognitive resource allocation and were using more reserved resources at lower task load compared to the control group.
Our findings using fNIRS in the current study indicated reduced brain activation in the concussed group, which is in contrast with the studies noted above. However, this hypo-activation has been reported in several other fMRI studies. In a study by Chen and colleagues (Chen et al. 2004), symptomatic concussed athletes had decreased brain activation and more bilateral brain involvement compared to control participants during the performance of visual and verbal working memory tasks. Hypo-activation during a Stroop task (similar to the color-match task in the current study) was reported by Soeda and colleagues (Soeda et al. 2005) in TBI patients. Using fNIRS, León-Carrion and colleagues (León-Carrion et al. 2008) reported that healthy controls with faster reaction times had higher levels of oxy-Hb concentration in the superior DLPFC. Similarly, a recent study by Mayer et al. (2009) examined attentional effects of concussion in an auditory reaction time task using fMRI. These researchers reported decreased activation in several subcortical regions known to be involved in the maintenance of attention- specifically, the bilateral striatum, medial dorsal nucleus of the thalamus, pons, midbrain nuclei and cerebellum. Mayer et al. (2009) also reported decreased activity in the cortical attention networks comprised of frontal visual fields, ventrolateral prefrontal cortex, and the posterior parietal lobe. These researchers suggested that their findings supported disengagement of attentional focus during the initial recovery phase of concussion. Stulemeijer et al. (2010) reported no group-level differences between control and concussed patients during an N-back task. However, subsequent voxel-based analysis revealed an inverse correlation between medial temporal lobe activity and injury severity with reduced activation or negative BOLD responses in the most severe patients.
Discrepancies in the observation of hyper- or hypo-activation could be the result of several experimental factors including type or severity of injury, time since injury, and nature of the tasks being performed. The homogeneity of the patient population may also play a role, as variability in brain responses between participants or even within an individual would diminish the statistical effect size of activation in either fMRI or fNIRS. Variability could be the result of inherent differences in the responses between participants (e.g., due to variability associated with the injury) or the result of the effect of attention deficits and reproducibility in the performance of the cognitive tests. For example, the finding by Mayer et al. (2009) suggested that concussed patients have diminished activity in the attentional network. It is possible that these patients may have more difficulty maintaining attention during longer duration tasks, such as those used in the current study’s test paradigms.
Experimental design, timing, and engagement in the task may also play a factor. For example, in the Stulemeijer study (2010) the researchers used a 60-s duration N-back block and showed decreased brain activity, whereas, the work of McAllister et al. (2001) study involved a shorter 27-s block and showed increased brain activity. Likewise, the Chen et al. study (2004) used a longer (60 s) blocked design during working memory similar to the current study and demonstrated decreased brain activation in the concussed group. In our study, we recorded brain activity during neurocognitive tasks, which are self-paced and last approximately 1 min in duration, similar to Chen et al. (2004). Changes in attention over the course of this time may introduce variability in the brain response and appear as reduced activation in the concussed group.
An alternative explanation is that the pattern of hypo-activation observed in the concussed patients may be the result of impaired neuronal function, impaired neuro-vascular coupling, impaired metabolic function, and/or direct damage to the cerebrovascular system. Both fMRI and fNIRS record blood oxygenation changes. During brain activity, these blood oxygenation changes (e.g., the BOLD signal) are the result of a mismatch between blood flow changes (oxygen supply) and cerebral oxygen metabolism (oxygen demand). Proper functioning of the brain relies on neural-metabolic-vascular coupling. Concussion is known to affect both neural and metabolic function that can last from seconds to weeks after the trauma (Giza and Hovda 2001). Both fNIRS and fMRI are relative measurements, and as such, the amplitude of changes is also sensitive to baseline conditions. For example, a decrease in baseline CBF has been shown to result in a reduced change in the BOLD signal for the exact same level stimulation (Sicard and Duong 2005). Functional NIRS has a similar dependence on baseline blood flow and oxygenation (Abdelnour and Huppert 2009; Huppert et al. 2009). Given that baseline CBF is postulated to go down over the first week after a concussion (Giza and Hovda 2001), this could also influence the results of previous fMRI work and our current fNIRS findings.
The current study is not without limitations. The sample size (N=14) is small for this preliminary study and therefore limits its clinical utility. Moreover, the control group consisted of more females than the concussed group. Given the differential effects reported for males and females (Broshek et al. 2005; Dick 2009; Frommer et al. 2011), it is possible that the lower number of females in the concussed group for the current study may have muted the current study’s findings. Future research should match controls and concussed groups on both age and sex. We conducted fNIRS measures only once for each participants. Multiple assessments across the different phases of concussion, from acute to sub-acute to return to play, are needed to quantify intra-individual changes in brain activation following concussion. Separating issues specific to time since injury would require more thorough investigation where groups are demarcated by time since injury. The current uncoupling is likely due to symptoms presentation and neurocognitive dysfunction secondary to injury. The fNIRS cap and sensors and sources used in the current study did not assess changes in activation of the parietal and occipital lobes, and only provided partial coverage of the temporal lobes. Consequently, we cannot infer anything about activation in these areas of the brain from the current findings. Further, NIRS is limited to neocortical activity as it lacks sufficient penetration depth to reach subcortical structures. Accordingly, future studies employing more powerful methods such as fMRI might be useful in delineating the changes in subcortical brain areas that may augment the initial findings presented here.
The results of this study have potential implications for concussion assessment and management. Currently, there is no accepted clinical imaging modality for concussion. More significant brain injuries such as intracranial bleeding and skull fractures can be detected by CT and MRI scans; however, these approaches cannot detect the subtle presentation of concussion. As mentioned earlier, fMRI, DTI, PET and other imaging modalities that have demonstrated positive findings related to concussion are expensive, inaccessible to most clinics/patients, and require large spaces. The portable fNIRS device from the current study may offer a portable and effective method for measuring changes in cerebral blood flow following concussion. Similar to fMRI, fNIRS provides a temporal as well as spatial assessment of changes in cerebral blood flow. As such, fNIRS can provide a dynamic measure of cerebral blood flow during the performance of cognitive and other tasks as opposed to a static measure at rest. However, in order for fNIRS to be clinically useful, clinical cut-offs need to be established to allow for identification and management of concussion.
Conclusion
The findings of this preliminary study supported the use of fNIRS as a potential non-invasive, portable optical imaging technique for assessing changes in oxygenated cerebral blood flow associated with concurrent performance of neurocognitive tasks in patients following concussion. Our results indicated reduced brain activity in the concussed participants compared to the control group, particularly during the performance of more demanding tasks. We believe the fNIRS may provide a physiological component to augment current concussion assessment approaches involving clinical examination, neurocognitive testing, vestibularocular evaluation, and other approaches. Functional NIRS provides visual temporal-spatial data that are intuitive and easy to interpret and quantify. The fNIRS technique can be applied to concussions occurring in sports, military, and other dynamic environments wherein a portable, non-invasive imaging tool is warranted. Initial evidence suggests that group differences in brain activation between concussed and control participants may be detectable using fNIRS approach from the current study. However, in order for fNIRS to be clinically useful in assessing patients following concussion individual patient differences and clinical specificity and sensitivity need to be established. Additionally, further research involving a larger sample, with better-matched controls, and multiple assessments across acute, sub-acute, and return to play phases of concussion is needed to corroborate our initial findings.
Acknowledgments
Funding for this study was provided by the University of Pittsburgh Department of Radiology.
Footnotes
Conflicts of interest statement A. P. Kontos, T. J. Huppert, N. H. Beluk, R. J. Elbin, L. C. Henry, J. French, S. M. Dakan & M. W. Collins declare that they have no conflict of interest. Dr. Collins is a shareholder in ImPACT Applications, Inc. Dr. Collins involvement in the current manuscript involved interpretation of data. He did not have direct access to the raw data or participate in the analysis of the data
Informed consent statement All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Contributor Information
A. P. Kontos, Email: akontos@pitt.edu, UPMC Sports Medicine Concussion Program/Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA, UPMC Sports Medicine Concussion Program, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, UPMC Center for Sports Medicine, 3200 South Water Street, Pittsburgh, PA 15203, USA
T. J. Huppert, Department of Radiology, University of Pittsburgh, Pittsburgh, PA, USA, Department of Biomedical Engineering, University of Pittsburgh, Pittsburgh, PA, USA
N. H. Beluk, Department of Radiology, University of Pittsburgh, Pittsburgh, PA, USA
R. J. Elbin, UPMC Sports Medicine Concussion Program/Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA
L. C. Henry, UPMC Sports Medicine Concussion Program/Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA
J. French, UPMC Sports Medicine Concussion Program/Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA
S. M. Dakan, UPMC Sports Medicine Concussion Program/Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA
M. W. Collins, UPMC Sports Medicine Concussion Program/Department of Orthopaedic Surgery, University of Pittsburgh, Pittsburgh, PA, USA
References
- Abdelnour F, Huppert TJ. Group analysis for functional optical brain imaging using a random effects model. Biomedical Optics Express. 2010;2:1–25. doi: 10.1364/BOE.2.000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour AF, Huppert T. Real-time imaging of human brain function by near-infrared spectroscopy using an adaptive general linear model. NeuroImage. 2009;46:133–143. doi: 10.1016/j.neuroimage.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour AF, Huppert TJ. A random-effects model for group-level analysis of diffuse optical brain imaging. Biomedical Optics Express. 2011;2:1–25. doi: 10.1364/BOE.2.000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour F, Genovese C, Huppert T. Hierarchical Bayesian regularization of reconstructions for diffuse optical tomography using multiple priors. Biomedical Optics Express. 2010;1:1084–1103. doi: 10.1364/BOE.1.001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson PD, Nemoto E, Colak A, Painter M. The use of near infrared spectroscopy (NIRS) in children after traumatic brain injury: a preliminary report. Acta Neurochirurgica Supplement. 1998;71:250–254. doi: 10.1007/978-3-7091-6475-4_72. [DOI] [PubMed] [Google Scholar]
- Alsalaheen BA, Mucha A, Morris LO, Whitney SL, Furman JM, Camiolo-Reddy CE, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. Journal of Neurologic Physical Therapy. 2010;34:87–93. doi: 10.1097/NPT.0b013e3181dde568. [DOI] [PubMed] [Google Scholar]
- Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT. Sex differences in outcome following sports-related concussion. Journal of Neurosurgery. 2005;102:856–863. doi: 10.3171/jns.2005.102.5.0856. [DOI] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. NeuroImage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Petrides M, Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch General Psychology. 2008;65:81–89. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. British Journal of Sports Medicine. 2001;35:297–302. doi: 10.1136/bjsm.35.5.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Advances in Experimental Medicine and Biology. 1988;222:183–189. doi: 10.1007/978-1-4615-9510-6_21. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Custo A, Wells WM, 3rd, Barnett AH, Hillman EM, Boas DA. Effective scattering coefficient of the cerebral spinal fluid in adult head models for diffuse optical imaging. Applied Optics. 2006;45:4747–4755. doi: 10.1364/ao.45.004747. [DOI] [PubMed] [Google Scholar]
- Dehghani H, Eames ME, Yalavarthy PK, Davis SC, Srinivasan S, Carpenter CM, et al. Near infrared optical tomography using NIRFAST: algorithm for numerical model and image reconstruction. Communications in Numerical Methods in Engineering. 2008;25:711–732. doi: 10.1002/cnm.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick RW. Is there a gender difference in concussion incidence and outcomes? British Journal of Sports Medicine. 2009;43:46–50. doi: 10.1136/bjsm.2009.058172. [DOI] [PubMed] [Google Scholar]
- Erlanger D, Feldman D, Kutner K, Kaushik T, Kroger H, Fes-ta J, et al. Development and validation of a Web-based neuro-psychological test protocol for sports-related return-to-play decision-making. Archives of Clinical Neuropsychology. 2003;18:293–316. [PubMed] [Google Scholar]
- Friston K. Statistical parametric mapping: the analysis of functional brain images. London: Academic; 2007. [Google Scholar]
- Frommer LJ, Gurka KK, Cross KM, Ingersoll CD, Comstock SA. Sex differences in concussion symptoms of high school athletes. Journal of Athletic Training. 2011;46:76–84. doi: 10.4085/1062-6050-46.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The Neurometabolic Cascade of Concussion. Journal of Athletic Training. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- Haitsma IK, Maas AI. Monitoring cerebral oxygenation in traumatic brain injury. Progress in Brain Research. 2007;161:207–216. doi: 10.1016/S0079-6123(06)61014-5. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Boas DA. Direct estimation of evoked hemoglobin changes by multimodality fusion imaging. Journal of Biomedical Optics. 2008;13:054031. doi: 10.1117/1.2976432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics. 2009;48:D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Anderson B, Steinberg FL, Kelso JA. A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR American Journal of Neuroradiology. 2004;25:738–745. [PMC free article] [PubMed] [Google Scholar]
- Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovits S, Hallett M, Sebastianelli W, et al. Alteration of brain default network in subacute phase of injury in concussed individuals: resting state fMRI study. Neuroimage. 2012;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of Head Trauma Rehabilitation. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Len TK, Neary JP, Asmundson GJ, Goodman DG, Bjornson B, Bhambhani YN. Cerebrovascular reactivity impairment after sport-induced concussion. Medicine and Science in Sports and Exercise. 2011;43:2241–2248. doi: 10.1249/MSS.0b013e3182249539. [DOI] [PubMed] [Google Scholar]
- León-Carrion J, Damas-López J, Martín-Rodríguez JF, Domínguez-Roldán JM, Murillo-Cabezas F, Barroso Y, et al. The hemodynamics of cognitive control: the level of concentration of oxygenated hemoglobin in the superior prefrontal cortex varies as a function of performance in a modified Stroop task. Behavioral Brain Research. 2008;193:248–256. doi: 10.1016/j.bbr.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Fazio V. Concussion management in the child and adolescent athlete. Current Sports Medicine Reports. 2008;7:12–15. doi: 10.1097/01.CSMR.0000308671.45558.e2. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Pardini JE, Welling J, Collins MW, Bakal J, Lazar N, et al. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61:352–359. doi: 10.1227/01.NEU.0000279985.94168.7F. discussion 359–360. [DOI] [PubMed] [Google Scholar]
- Mattout J, Phillips C, Penny WD, Rugg MD, Friston KJ. MEG source localization under multiple constraints: an extended Bayesian framework. NeuroImage. 2006;30:753–767. doi: 10.1016/j.neuroimage.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129:28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Elgie R, Gasparovic C, Phillips JP, et al. Auditory orienting and inhibition of return in mild traumatic brain injury: a FMRI study. Human Brain Mapping. 2009;30:4152–4166. doi: 10.1002/hbm.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. NeuroImage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. Journal of Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuqisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Clinical Journal of Sport Medicine; Consensus statement on concussion in sport 3rd international conference on concussion; Zurich. November 2008; 2009. pp. 185–200. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. British Journal of Sports Medicine. 2013;47:250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- McGrath N. Supporting the student-athlete’s return to the classroom after a sport-related concussion. Journal of Athletic Training. 2010;45:492–498. doi: 10.4085/1062-6050-45.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hillary FG, Biswal BB. Resting network plasticity following brain injury. PLoS One. 2009:4. doi: 10.1371/journal.pone.0008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Beyond the visible—imaging the human brain with light. Journal of Cerebral Blood Flow and Metabolism. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Ptito A, Chen JK, Johnston KM. Contributions of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. NeuroRehabilitation. 2007;22:217–227. [PubMed] [Google Scholar]
- Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. NeuroImage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov SM, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Experimental Brain Research. 2010;202:341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov SM, Gay M, Zhang K, Johnson B, Pennell D, Sebastianelli W, et al. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage. 2011;55:1716–1727. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Gay M, Johnson B, Zhang K. Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging and Behavior. 2012;6:224–243. doi: 10.1007/s11682-012-9167-2. [DOI] [PubMed] [Google Scholar]
- Soeda A, Nakashima T, Okumura A, Kuwata K, Shinoda J, Iwama T. Cognitive impairment after traumatic brain injury: a functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47:501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Stulemeijer M, Vos PE, van der Werf S, van Dijk G, Rijpkema M, Fernandez G. How mild traumatic brain injury may affect declarative memory performance in the post-acute stage. Journal of Neurotrauma. 2010;27:1585–1595. doi: 10.1089/neu.2010.1298. [DOI] [PubMed] [Google Scholar]
- Van Kampen DA, Lovell MR, Pardini JE, Collins MW, Fu FH. The “value added” of neurocognitive testing after sports-related concussion. The American Journal of Sports Medicine. 2006;34:1630–1635. doi: 10.1177/0363546506288677. [DOI] [PubMed] [Google Scholar]
- Weatherall A, Skowno J, Lansdown A, Lupton T, Garner A. Feasibility of cerebral near-infrared spectroscopy monitoring in the pre-hospital environment. Acta Anaesthesiologica Scandinavica. 2012;56:172–177. doi: 10.1111/j.1399-6576.2011.02591.x. [DOI] [PubMed] [Google Scholar]
- Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2009;44:428–447. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]