Abstract
Objective
Administration of the handle region peptide (HRP), a (pro)renin receptor blocker, decreases body weight gain and visceral adipose tissue (VAT) in high-fat/high-carbohydrate (HF/HC) diet-fed mice. The objective of this study was to elucidate potential mechanisms implicated in these observations.
Methods
Mice were given a normal or a HF/HC diet along with saline or HRP for 10 weeks.
Results
In HF/HC-fed mice, HRP increased the expression of several enzymes implicated in lipogenesis and lipolysis in subcutaneous fat (SCF) while the expression of the enzyme implicated in the last step of lipogenesis decreased in VAT. A reduction was also observed in circulating free fatty acids in these animals which was accompanied by normalized adipocyte size in VAT and increased adipocyte size in SCF. “Beiging“ is the evolution of a white adipose tissue toward a brown-like phenotype characterized by an increased mitochondrial density and small lipid droplets. HRP increased the expression of’ “beiging” markers in SCF of HF/HC diet-fed mice.
Conclusions
HRP treatment may favor healthy fat storage in SCF by activating a triglyceride/free fatty acid cycling and “beiging,” which could explain the body weight and fat mass reduction.
Introduction
Obesity is associated with complications such as type 2 diabetes and cardiovascular disease (1). Augmented fat mass increases the contribution of the local adipose renin-angiotensin system (RAS) to more than 30% to the systemic RAS and contributes to the development of obesity-associated complications (2–5).
The rate limiting step of the RAS is the conversion of angiotensinogen to angiotensin (Ang) I by renin (Supporting Information Figure S1A). Ang I is ultimately converted to Ang II by the angiotensin converting enzyme. Ang II is the main effector of the RAS, and its effects are mediated by its binding to its specific receptors (Supporting Information Figure S1A). While the RAS is well known for its regulation of blood pressure and fluid homeostasis (6), it also favors the development of obesity as Ang II decreases lipolysis and adipogenesis and increases lipogenesis (Supporting Information Figure S1A) (7–10).
When prorenin and renin [collectively known as (pro)renin] bind to the (pro)renin receptor [(P)RR], renin activity increases fourfold while prorenin is non-proteolytically activated. Thus, Ang II production increases which stimulates Ang II-dependent pathways (Supporting Information Figure S1A) (11). Binding to (P)RR also activates Ang II-independent pathways such as mitogen-activated protein kinase (MAPK) signaling and a negative feedback loop implicating the promyelocytic leukemia zinc finger (PLZF) which leads to the downregulation of (P)RR gene expression (Supporting Information Figure S1A) (12),(13).
The handle region peptide (HRP), a (P)RR blocker, encodes part of the prorenin pro-segment. Competitive binding of HRP to the receptor has been shown in vitro (14),(15). In contrast to renin inhibitors, HRP only decreases Ang II production, as unbound renin can still contribute to Ang II production, while blocking the activation of Ang II-independent pathways, although its effect on PLZF is still unclear (Supporting Information Figure S1B) (11). HRP administration has been shown to improve features of different pathologies in animal models, such as diabetic nephropathy and retinopathy (12). Although a certain controversy exists regarding the effects of HRP, this may result from low HRP doses and short treatment duration (16–18).
We previously reported that (P)RR is increased specifically in adipose tissue with obesity in mice (19). We also observed that HRP treatment in mice fed a high-fat/high-carbohydrate (HF/HC) diet reduced both body weight gain and visceral adipose tissue (VAT) mass by 20% and potentially improved insulin sensitivity, while food intake was similar (19). The underlying mechanisms are currently unknown, and we thus hypothesized that HRP could modulate enzymes implicated in lipogenesis, lipolysis, and adipogenesis as a result of its effect on Ang II-dependent and -independent pathways (Supporting Information Figure S1B). Hence, using the same set of mice, we investigated these potential mechanisms in this study.
Methods
An expanded Methods section is available in the Supporting Information.
Animals
Male mice of C57BL/6N genetic background were treated as previously described (Supporting Information Figure S2) (19). Briefly, mice aged 12 to 15 weeks (n = 10–15/group) were fed a normal (N) (2018; Harlan Laboratories, Madison, WI) or a HF/HC diet (F3282; Bio-Serv, Frenchtown, NJ). Mice were concomitantly treated with either saline or HRP (0.1 µg/g/day) for 10 weeks using mini osmotic pumps placed subcutaneously (model #1004; Alzet, Cupertino, CA). All mice received a second pump 5 weeks after starting the treatment to ensure constant administration. The four experimental groups were: N diet treated with saline (N + saline) or HRP (N + HRP) and HF/HC diet treated with saline (HF/HC + saline) or HRP (HF/HC + HRP). Mice had free access to food and water ad libitum. At sacrifice, VAT [perigonadal fat (PGF) and perirenal fat (PRF)], abdominal subcutaneous fat (SCF), and plasma were collected. Care of the mice used in the experiments complied with standards for the care and use of experimental animals set by the Canadian Council for the Protection of Animals, and all procedures were approved by the University Animal Care and Use Committee at the CHUM Research Center.
Real-time polymerase chain reaction
Gene expression was evaluated by qPCR using primers listed in Supporting Information Table S1.
Histological analysis
Embedding, sectioning, and staining of adipose tissue were performed by the histology platform of the Research Institute in Immunology and Cancerology at the Université de Montréal. Slides were stained with hematoxylin-eosin (H&E).
Western blot
Twenty to 50 µg of denatured protein was separated by SDS-PAGE while non-denatured proteins (10 µL) were resolved by native PAGE. Proteins were all transferred to nitrocellulose membranes (162-0115; Bio-Rad). Antibody details are shown in Supporting Information Table S2. Band density was quantified using ImageJ software (20).
Statistical analysis
Data were expressed as the mean ± standard error (SE) and analyzed by two-way ANOVA to assess the effect of diet and treatment. The comparisons between slope or intercepts were performed using Prism 6.0, comparing the residuals with and without any constraint. A post hoc Tukey analysis was conducted if a significant interaction was detected. A P < 0.05 was considered statistically significant.
Results
Lipogenic enzymes are reduced in VAT and increased in SCF following HRP treatment
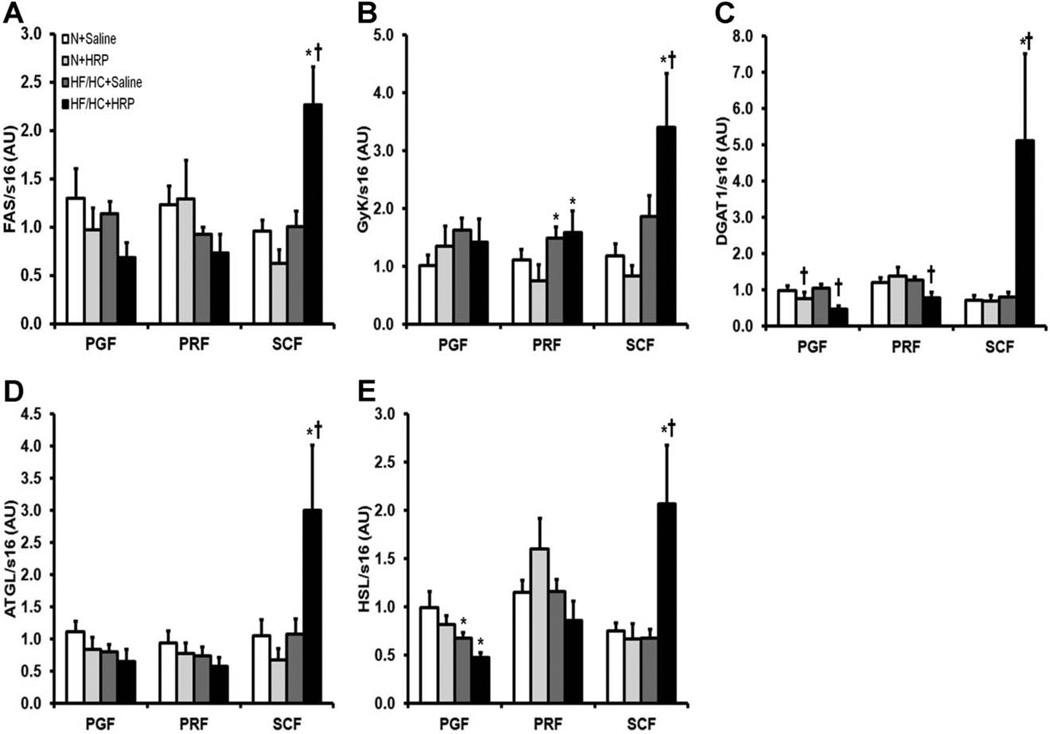
Fatty acid synthase (FAS), glycerol kinase (GyK), and diglyceride acyltransferase 1 (DGAT1) are enzymes implicated in lipogenesis (21). Obesity had no effect on FAS and DGAT1 mRNA levels in all fat pads whereas GyK mRNA levels were increased 1.3-fold in PRF and no changes were observed in PGF and SCF (Figure 1A–C). HRP treatment in HF/HC-fed mice increased FAS and GyK mRNA levels 2.2-fold and 1.8-fold, respectively in SCF while they were unchanged in VAT (Figure 1A, B). In contrast, HRP decreased DGAT1 mRNA levels in PGF of mice on both N and HF/HC diet by 20% and 60%, respectively and by 60% in PRF only in HF/HC-fed mice whereas they were significantly increased by 6.5-fold in SCF of HF/HC-fed mice (Figure 1C).
Figure 1.
HRP treatment increased mRNA levels of enzymes implicated in lipogenesis and lipolysis in SCF of obese mice. (A) FAS, (B) GyK, (C) DGAT1, (D) ATGL, and (E) HSL mRNA levels in adipose tissue. Data are normalized to s16 mRNA levels and are presented as mean ± SE with n = 9–13 per group. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. ATGL, adipose triglyceride lipase; DGAT1, diglyceride acyltransferase 1; FAS, fatty acid synthase; GyK, glycerol kinase; HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; HSL, hormone sensitive lipase; N, normal; PGF, perigonadal fat; PRF, perirenal fat; SCF, abdominal subcutaneous fat.
HRP increases lipolytic enzymes in SCF
Adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) are enzymes involved in lipolysis (21). Obesity had no effect on ATGL mRNA levels in all fat pads and on HSL mRNA levels in PRF and SCF while HSL was decreased by 30% in PGF (Figure 1D, E). While HRP had no effect on ATGL and HSL mRNA levels in VAT of HF/HC-fed mice, these were increased by 3.1-fold and 2.8-fold, respectively in SCF (Figure 1D, E).
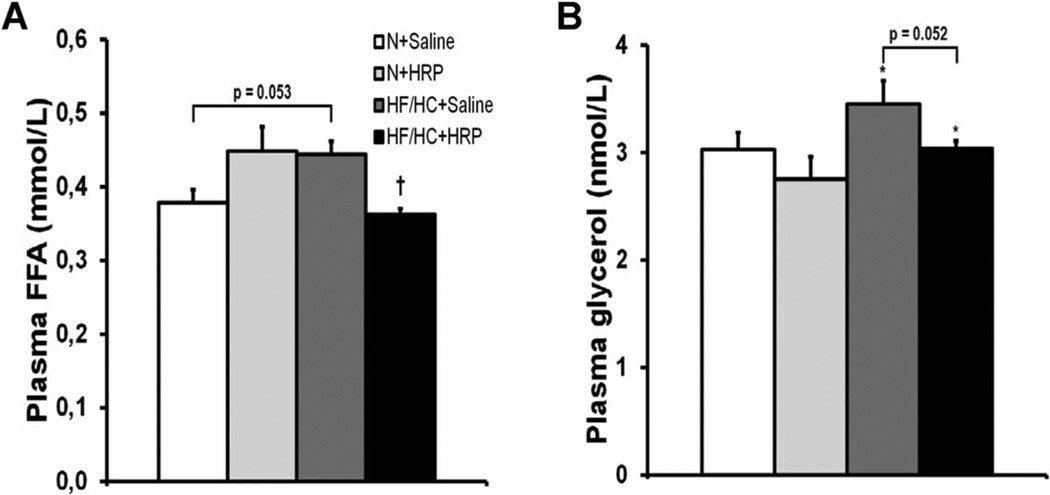
Plasma free fatty acid levels are decreased in obese mice following HRP treatment
Although not statistically significant (P = 0.053), there was a strong trend toward an increase in plasma free fatty acids (FFA) in obese mice while HRP reduced these levels by 20% in HF/HC-fed mice but had no effect in N-fed mice (Figure 2A). Plasma glycerol was increased 1.1-fold with obesity, while HRP treatment had no effect on plasma glycerol, although a strong tendency toward a decrease was observed in HF/HC + HRP mice (P = 0.052) (Figure 2B).
Figure 2.
HRP treatment decreased plasma FFA levels in obese mice. Plasma (A) FFA and (B) glycerol levels. Data are presented as mean ± SE with n = 7–9 per group for plasma FFA and n = 12–15 per group for plasma glycerol. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. FFA, free fatty acid; HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; N, normal.
An adipogenesis marker is normalized in VAT and increased in SCF following HRP treatment
Peroxisome proliferator-activated receptor PPAR-γ1 and 2 are transcriptional factors that induce adipogenesis (22). In all fat pads, neither the diet nor the treatment had any effect on PPAR-γ1 (Figure 3A). In PGF and SCF, obesity had no effect on PPAR-γ2 protein levels while it was decreased by 60% in PRF (Figure 3B). HRP treatment normalized PPAR-γ2 levels in PRF of HF/HC fed mice while it was increased by 1.7-fold and 2.1-fold in SCF mice fed a N or HF/HC diet (Figure 3B).
Figure 3.
HRP treatment increased protein levels of a marker for adipogenesis in SCF of obese mice. (A) PPAR-γ1 and (B) PPAR-γ2 protein levels in adipose tissue. Equal loading of 30 µg of proteins was done in each well for Western blot. Data are normalized to tubulin protein levels and are presented as mean ± SE with n = 4–7 per group for PPAR-γ1 and PPAR-γ2 protein levels. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; N, normal; PGF, perigonadal fat; PPAR-γ, peroxisome proliferator-activated receptor-gamma; PRF, perirenal fat; SCF, abdominal subcutaneous fat.
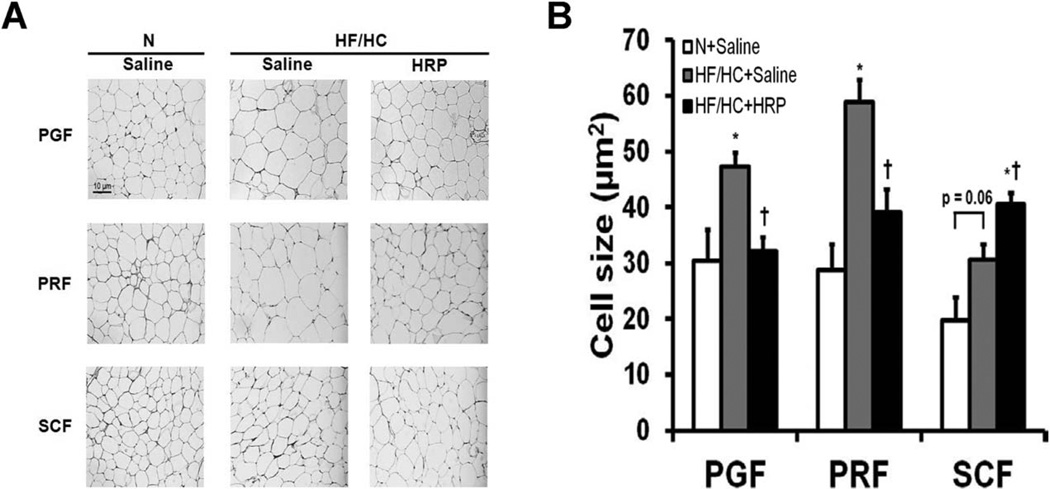
Adipocyte size is decreased in VAT and increased in SCF following HRP treatment
In obese mice, we observed an increase in adipocyte size by 1.6-fold and twofold in PGF and PRF, respectively while only a tendency for an increase was present in SCF (P = 0.060) (Figure 4A, B). In HF/HC + HRP mice, adipocyte size was normalized in PGF and PRF while it was increased 1.3-fold in SCF compared with saline-treated littermates (Figure 4A, B).
Figure 4.
HRP treatment normalized adipocyte size in VAT and increased it in SCF of obese mice. (A) Hematoxylin-eosin staining of adipose tissue (scale bar = 10 µm, magnification = 100×) and (B) adipocyte surface area in adipose tissue. Data are presented as mean ± SE with n = 5–6 per group. For each fat pad, digital pictures were randomly taken from six fields with approximately 60 cells per field. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; N, normal; PGF, perigonadal fat; PRF, perirenal fat; SCF, abdominal subcutaneous fat.
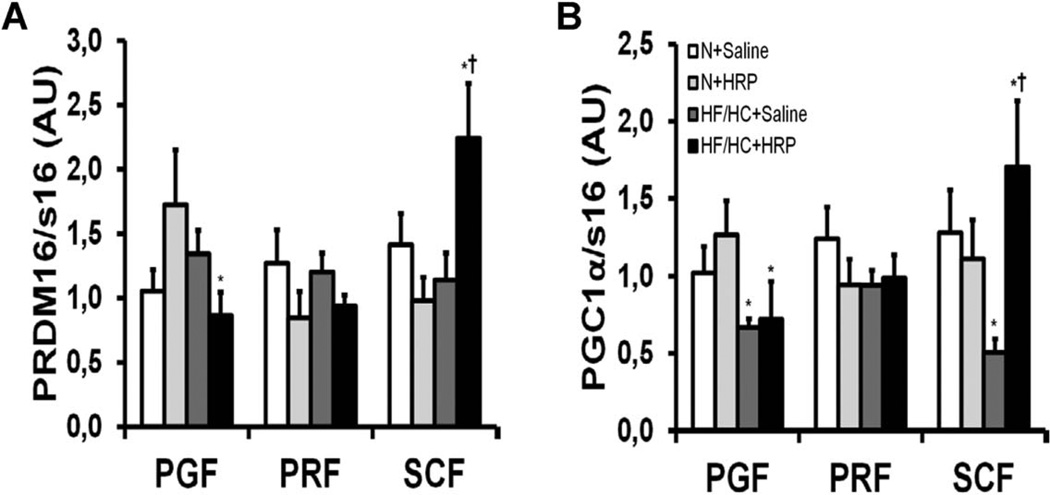
HRP increases “beiging” markers in SCF
“Beiging” is the evolution of a white adipose tissue toward a brown-like phenotype characterized by increased mitochondrial biogenesis and the appearance of numerous small lipid droplets. It has been shown that SCF is more susceptible to “beiging” [measured by an increase in PR domain containing 16 (PRDM16) and PPAR-γ coactivator (PGC)−1α expression] than VAT (23). Obesity produced a 50% and 30% decrease in PRDM16 and PGC-1α mRNA levels, respectively in PGF, but had no effect in PRF and SCF except for PGC-1α which was reduced by 60% in SCF (Figure 5A, B). While no effect of HRP could be observed in VAT, PRDM16, and PGC-1α mRNA levels were increased twofold and 3.4-fold, respectively in SCF of HF/HC-fed mice while there was no change in N-fed mice (Figure 5A, B).
Figure 5.
HRP treatment increased mRNA levels of markers for “beiging” in SCF of obese mice. (A) PRDM16 and (B) PGC-1α mRNA levels in adipose tissue. Data are normalized to s16 mRNA levels and are presented as mean ± SE with n = 9–13 per group. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; N, normal; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1-α; PGF, perigonadal fat; PRDM16, PR domain containing 16; PGF, perigonadal fat; PRF, perirenal fat; SCF, abdominal subcutaneous fat.
HRP increases an angiogenic marker in SCF
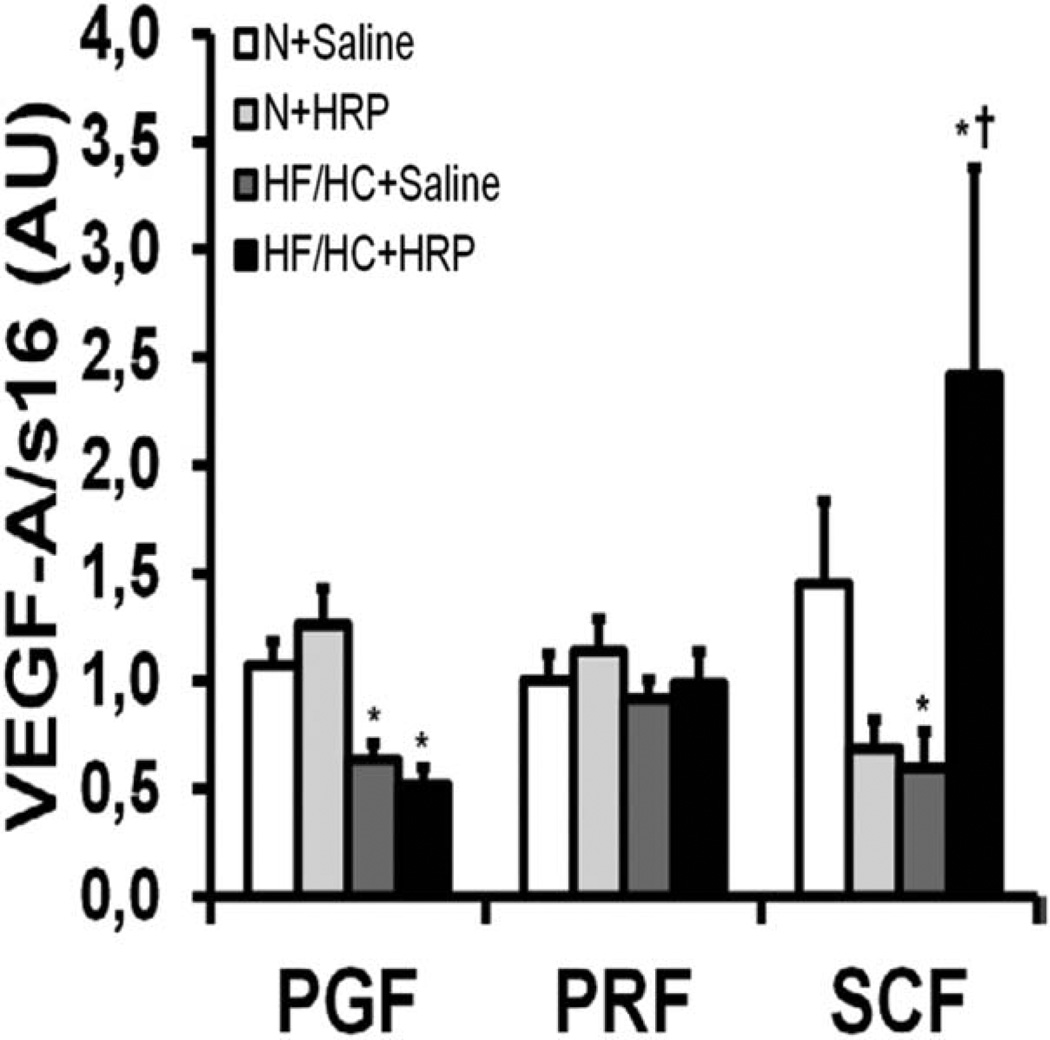
Vascular endothelial growth factor (VEGF)-A promotes angiogenesis and has been linked to adipose tissue function (24). Obesity decreased VEGF-A mRNA levels by 40% and 60% in PGF and SCF, respectively while it had no effect in PRF (Figure 6). HRP had no effect on VEGF-A mRNA levels in VAT while it was increased fourfold in SCF of HF/HC-fed mice with no effect in N-fed mice (Figure 6).
Figure 6.
HRP treatment increased mRNA levels of a marker of angiogenesis in SCF of obese mice. VEGF-A mRNA levels in adipose tissue. Data are normalized to s16 mRNA levels and are presented as mean ± SE with n = 9–13 per group. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; N, normal; PGF, perigonadal fat; PRF, perirenal fat; SCF, abdominal subcutaneous fat; VEGF-A, vascular endothelial growth factor A.
HRP modulates the adiponectin profile in SCF and the circulation
Adiponectin is a well-known insulin sensitizer (25). Obesity decreased adiponectin mRNA levels by 50% in PGF, but had no effect in PRF and SCF (Figure 7A). While we found no effect of HRP treatment in VAT, adiponectin mRNA levels were increased 3.2-fold in SCF of HF/HC-fed mice (Figure 7A). Plasma high molecular weight adiponectin (adipoH) levels are known to be positively correlated with insulin sensitivity and inversely correlated with body weight (26),(27). The correlation between body weight and circulating adipoH levels was similar in N + saline and N + HRP mice (Figure 7B). Surprisingly, the inverse correlation between body weight and circulating adipoH levels in the HF/HC + saline group was modified by HRP as their slopes were significantly different (Figure 7C).
Figure 7.
HRP treatment may increase adiponectin and insulin signaling in obese mice. (A) Adiponectin mRNA levels in adipose tissue. Correlation between plasma adipoH levels and body weight in mice on (B) N and (C) HF/HC diet. (D) Total Akt and (E) p-Akt protein levels in adipose tissue. Equal loading of 10 µL of plasma or 20 µg of proteins was done in each well. Data are normalized to s16 mRNA levels and are presented as mean ± SE with n = 9–13 per group for adiponectin mRNA. n = 8 per group for the correlation analysis between plasma adipoH and body weight. Data are normalized to tubulin protein levels and are presented as mean ± SE with n = 4–7 per group for Akt and p-Akt. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; adipoH, high molecular weight (HMW) adiponectin; N, normal; PGF, perigonadal fat; PRF, perirenal fat; SCF, abdominal subcutaneous fat.
HRP increases insulin signaling in VAT
Once insulin activates its signaling cascade, Akt becomes phosphorylated (p-Akt) and this produces a translocation of glucose transporters to the cellular membrane and allows for glucose to be transported into the cell (28). When normalized to tubulin, obesity decreased total Akt protein levels in PRF and SCF, but had no effect on PGF (Figure 7D). Since total Akt was modulated by the diet, p-Akt levels were normalized to tubulin. Obesity decreased p-Akt levels by 20% in both PGF and PRF, but had no effect in SCF (Figure 7E). p-Akt levels were increased 1.4-fold and 1.5-fold in PGF of N + HRP and HF/HC + HRP mice, respectively compared with their respective control group while HRP had no effect in other fat pads (Figure 7E).
Obesity may increase (P)RR expression by decreasing PLZF
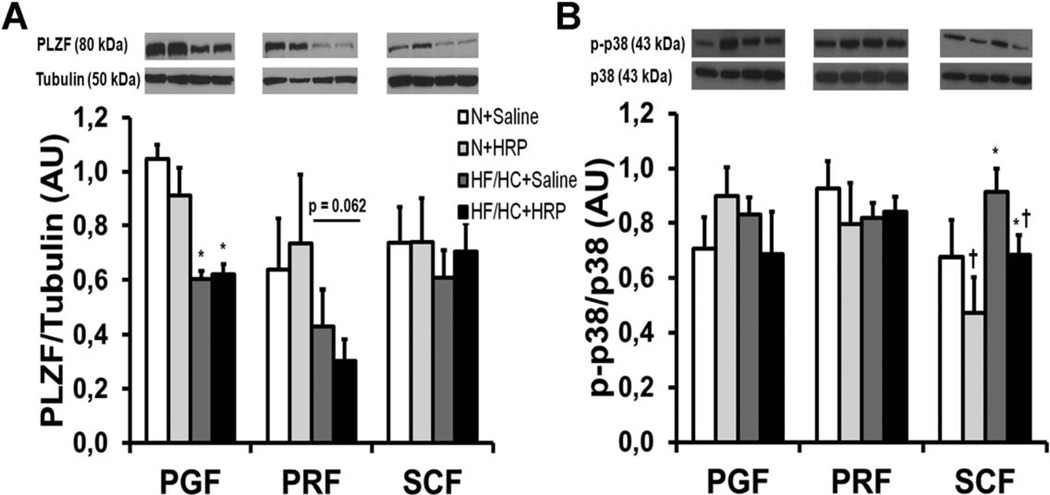
Obesity decreased PLZF protein levels by 40% in PGF and tended to do so in PRF (P = 0.062) but not in SCF while there was no effect of HRP in all fat pads (Figure 8A).
Figure 8.
PLZF was reduced in PGF, and HRP treatment decreased p38 MAPK in SCF of obese mice. (A) PLZF and (B) p-p38 protein levels in adipose tissue. Equal loading of 50 or 20 µg of proteins was done in each well for PLZF and p-p38, respectively. Data are normalized to tubulin protein levels for PLZF and to total p38 protein levels for p-p38 and are presented as mean6SE with n = 4–7 per group. *P < 0.05 compared with N diet; †P < 0.05 compared with saline. HF/HC, high-fat/high-carbohydrate diet; HRP, handle region peptide; MAPK, mitogen-activated protein kinase; N, normal; PGF, perigonadal fat; PLZF, promyelocytic leukemia zinc finger; PRF, perirenal fat; SCF, abdominal subcutaneous fat.
HRP decreases MAPK signaling in SCF
To verify whether HRP is able to decrease MAPK signaling in adipose tissue (Supporting Information Figure S1B), we measured protein levels of a member of the MAPK family, phospho-p38 (p-p38). Diet and treatment had no effect on p-p38 protein levels in VAT. Conversely, obesity produced a 1.3-fold increase in SCF, and HRP decreased p-p38 levels by 25% independently of the diet (Figure 8B).
Discussion
Our data suggest that the reduction in body weight gain previously observed by our group in HF/HC + HRP mice (19), may result from a futile cycle present in SCF, given that we observed an increase in the expression of several lipogenic and lipolytic enzymes, as well as an increase in the expression of “beiging” markers. This may thus lead to an increase in energy expenditure and in FFA uptake which is in line with the reduction in circulating FFA and decreased body weight observed in these mice. Moreover, the lower plasma insulin levels indicative of improved insulin sensitivity previously reported in HF/HC + HRP mice may partly result from an increase in p-Akt levels in VAT (19). In addition, elevation in adiponectin levels in SCF as well as the modified correlation between body weight and circulating adipoH in HF/HC + HRP mice supports the hypothesis of increased insulin sensitivity.
Given that Ang II has been shown to increase lipogenesis and lower lipolysis in human subcutaneous adipocytes (7–9), we had hypothesized that HRP may reduce lipid accumulation in mice fat pads. In line with these studies, we found that ATGL and HSL mRNA levels were increased in SCF of HF/HC + HRP mice, suggesting increased lipolysis. However, HRP was also found to increase FAS, GyK, and DGAT1 mRNA levels in SCF only in HF/HC-fed mice suggesting increased lipogenesis. Put together, our results suggest that HRP may upregulate both lipogenesis and lipolysis in SCF promoting a triglyceride (TG)/FFA futile cycling in HF/HC-fed mice, similarly to what was reported in rats treated with Ang converting enzyme inhibitor or Ang II type 1 receptor (AT1R) blocker (29). This would promote a reduction in FFA and glycerol release into the bloodstream as they would be reutilized inside the cell to synthesize TG. Supporting this hypothesis, plasma FFA was found to be reduced and plasma glycerol tended to decrease in HF/HC + HRP mice compared with HF/HC + saline group. TG/FFA cycling would consume energy and possibly contribute to heat production since each cycle of lipogenesis and lipolysis requires four and seven ATPs, respectively (21). Altogether, our results suggest that activation of TG/FFA cycling in SCF of HF/HC + HRP mice could partly contribute to the decreased body weight gain observed in these animals as well as improved fat deposition (19).
In contrast to the SCF, we found that HRP had no effect on the expression of lipolytic enzymes in VAT. However, lipogenic enzyme DGAT1 was reduced in expression in VAT of HF/HC + HRP mice, similarly to a report in VAT of renin KO mice (30). Therefore, our data suggest that HRP may decrease lipogenesis in VAT as supported by our histological analysis in which HRP normalized VAT adipocyte size in HF/HC-fed mice.
In this study, we observed that adipocyte size was normalized in VAT while it was increased in SCF of HF/HC + HRP mice. This is in line with previous studies which have shown that Ang II inhibits adipogenesis in human adipocytes from SCF (10). We also observed that this may be coupled to an increase in adipocyte number as adipogenesis was found to be activated in both VAT and SCF of HF/HC + HRP mice. Indeed, we found that PPAR-γ2 expression, which is associated with increased adipogenesis (31), was augmented in the fat pads of these mice.
These results suggest that HRP treatment may shift the cellular composition of VAT from an obese fat phenotype (fewer numbers of large cells) to a healthier fat phenotype (a larger number of small cells) in HF/HC-fed mice due to a fat redistribution towards the SCF. FFA storage in SCF is considered healthier compared with VAT because it prevents deposition around visceral tissues, which is associated with insulin resistance (32).
Although it appears counter-intuitive that formation of new adipocytes is considered “healthier,” a larger population of adipocytes may help maintain or increase storage capacity for plasma FFA as well as improve insulin sensitivity compared with fewer adipocytes that have less capacity to store lipids (22).
The potential activation of TG/FFA cycling in SCF observed in HF/HC + HRP mice prompted us to also consider “beiging” in SCF. We observed that PRDM16 and PGC-1α mRNA levels were increased in SCF of HF/HC + HRP mice suggesting that “beiging” may be activated and could also contribute to the reduced body weight gain observed in mice receiving HRP (19).
In this in vivo study, it is difficult to determine if the observed effects of HRP are due to changes in Ang II-dependent or independent pathways (Figure 1B). Nonetheless, our previous data suggest that both pathways are implicated as RAS inhibitors which block Ang II-dependent pathways only decrease circulating TG (33) in obese rats while we observed normalized levels in HF/HC + HRP mice (19). We therefore conclude that Ang II-independent pathways also contribute to the phenotype observed as HRP should produce a milder effect than RAS inhibitors because it only reduces Ang II-dependent pathways, compared with RAS inhibitors which completely block them, while blocking Ang II-independent pathways.
Although it is possible that HRP effects on different metabolic parameters may result from decreased body and VAT weight observed in HF/HC-fed mice, given that these weights were still higher than those in N + saline mice while circulating TG were normalized suggests that there are other factors implicated (19). Furthermore, changes in adipokine levels such as leptin and inflammatory markers measured in HRP-treated mice were more dramatic than those on adiposity and even occurred in N + HRP mice despite having similar body weight compared with N + saline mice (19). Altogether, this suggests that the phenotype observed in our mice is more than just an effect of HRP on the body weight. Moreover, adipose tissue may be central to our observations since we demonstrated that obesity increased (P)RR only in adipose tissue while it was unaltered in the liver, kidneys, and heart (19). Hence, we believe that HRP directly affects adipose tissue and reduced its weight but also its function which modulated its adipokine production as previously demonstrated (19).
In our study, VEGF-A mRNA levels were decreased with obesity in both VAT and SCF suggesting that angiogenesis may be reduced. This is in line with other studies which have found a similar decrease in SCF VEGF mRNA levels of people with obesity compared with lean subjects which could contribute to reduce angiogenesis and cause hypoxia (34). Hypoxia-induced adipose tissue dysfunction is well known (35) and has been associated to glucose intolerance in obese mice (36). In our study, HRP increased VEGF-A mRNA in SCF specifically in HF/HC-fed mice while it was unaffected in VAT. Thus, HRP appears to stimulate angiogenesis specifically in SCF of obese mice, which might be due to differences between SCF and VAT (32). Interestingly, overexpression of VEGF-A specifically in mice white adipose tissue has also been associated with resistance to obesity and “beiging” in SCF (37). Thus, increased VEGF-A mRNA levels in HF/HC + HRP mice may turn on “beiging” genes in SCF as observed in our study.
Given that adipose tissue weight correlates positively with insulin resistance (38) and that we observed reduced plasma insulin levels in HRP-treated mice independently of the diet (19), we hypothesized that HRP may improve insulin sensitivity by reducing VAT mass. Adiponectin is specifically produced and secreted into the blood by white adipose tissue (39). Given that HRP increased adiponectin mRNA levels only in SCF of HF/HC-fed mice, it suggests that SCF may contribute more to plasma total adiponectin levels than VAT. Similar conclusions were reported by others who treated human SCF explants with a PPAR-γ activator (40). Our data showed that a normal correlation between body weight and circulating adipoH was present in HF/HC + saline mice while it was altered in HF/HC + HRP mice. In addition, we found that HRP increased p-Akt protein levels in VAT independently of diet, suggesting improved insulin signaling, while we found no effect of the diet and treatment in SCF. Put together, these mechanisms may contribute to the improved insulin profile observed previously in these mice (19).
We demonstrated that PLZF protein levels were decreased in PGF of HF/HC-fed mice while a similar tendency was observed in PRF. This brought us to consider that (P)RR upregulation previously observed with obesity in VAT (19) may result from decreased PLZF levels. Surprisingly, we did not observe changes in PLZF protein levels in SCF of HF/HC-fed although (P)RR is also upregulated in this fat pad (19). This suggests that other mechanisms are implicated in the regulation of (P)RR expression in the SCF.
Binding of (pro)renin to (P)RR should activate MAPK, for instance p-p38, by stimulating Ang II-dependent and independent pathways (Figure 1A) (12). In line with these studies, we found that the increased (P)RR levels previously reported in SCF was associated with increased p-p38 protein levels in obese mice while it was decreased in HF/HC + HRP mice. In contrast, we observed that pp38 protein levels were unaltered in VAT by the diet or treatment. Therefore, we propose that p38 might not be a pathway implicated in the effects of (P)RR in VAT which could in part explain the discrepancy observed between VAT and SCF.
Future experiments are warranted to confirm activation of futile cycle and “beiging” in adipose tissue, for example, by measuring fatty acid uptake and release in fresh mice adipose tissue explants in the presence of exogenous HRP and by measuring O2 consumption and mitochondrion density in isolated adipocytes.
Conclusion
Overall, our study suggests that HRP treatment may change VAT adipocyte from an obese to a normal phenotype in HF/HC-fed mice as a result of increased adipogenesis and decreased lipogenesis. Moreover, there may be activation of TG/FFA cycling to buffer circulating FFA and adipocyte “beiging” in SCF. This would thus favor circulating lipid uptake into SCF rather than in VAT and would contribute to a healthier phenotype as the latter is associated with multiple cardiometabolic diseases (32).
Supplementary Material
Acknowledgments
We thank Ann-Michele Francoeur for her help with manuscript editing. We also thank Catherine Michel (CRCHUM, Quebec, Canada) for excellent technical assistance in all the mice studies.
Funding agencies: This work was supported by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Diabetes Association, National Institutes of Health (NIH) DA004443, Canadian Institutes of Health Research (CIHR) MOP-89716, and an unrestricted grant from Merck Frosst Canada. JLL was supported by a FRQ-S scholarship. PT was supported by Diabè te Québec and Université de Montréal scholarships.
Footnotes
Disclosure: The authors declared no conflict of interest.
Author contributions: JLL and JG conceived and designed the study. PT, CB, TMDN, and PWS performed the experiments. PT and CB analyzed the data. PT made the figures. PT wrote the manuscript, and all authors edited and revised the manuscript.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab. 2004;286:E891–E895. doi: 10.1152/ajpendo.00551.2003. [DOI] [PubMed] [Google Scholar]
- 3.van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obes Res. 2000;8:337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- 4.Giacchetti G, Faloia E, Mariniello B, et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens. 2002;15:381–388. doi: 10.1016/s0895-7061(02)02257-4. [DOI] [PubMed] [Google Scholar]
- 5.Massiera F, Bloch-Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 6.Montani JP, Van Vliet BN. General physiology and pathophysiology of the renin-angiotensin system. Angiotensin Vol. I. Handb Exp Pharmacol. 2004;163:3–29. [Google Scholar]
- 7.Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology. 1997;138:1512–1519. doi: 10.1210/endo.138.4.5038. [DOI] [PubMed] [Google Scholar]
- 8.Goossens GH, Blaak EE, Arner P, Saris WHM, van Baak MA. Angiotensin II: a hormone that affects lipid metabolism in adipose tissue. Int J Obes. 2007;31:382–384. doi: 10.1038/sj.ijo.0803388. [DOI] [PubMed] [Google Scholar]
- 9.Goossens GH, Blaak EE, Saris WHM, van Baak MA. Angiotensin II-Induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J Clin Endocrinol Metab. 2004;89:2690–2696. doi: 10.1210/jc.2003-032053. [DOI] [PubMed] [Google Scholar]
- 10.Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 2002;51:1699–1707. doi: 10.2337/diabetes.51.6.1699. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed BA, Seda O, Lavoie JL. (Pro)renin receptor as a new drug target. Curr Pharm Des. 2011;17:3611–3621. doi: 10.2174/138161211798220963. [DOI] [PubMed] [Google Scholar]
- 13.Schefe JH, Menk M, Reinemund J, et al. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 14.Nabi AH, Biswas KB, Nakagawa T, Ichihara A, Inagami T, Suzuki F. ’Decoy peptide’ region (RIFLKRMPSI) of prorenin prosegment plays a crucial role in prorenin binding to the (pro)renin receptor. Int J Mol Med. 2009;24:83–89. doi: 10.3892/ijmm_00000210. [DOI] [PubMed] [Google Scholar]
- 15.Nabi AH, Biswas KB, Nakagawa T, Ichihara A, Inagami T, Suzuki F. Prorenin has high affinity multiple binding sites for (pro)renin receptor. Biochim Biophys Acta. 2009;1794:1838–1847. doi: 10.1016/j.bbapap.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Susic D, Lippton H, Knight M, Frohlich ED. Cardiovascular effects of nonproteolytic activation of prorenin. Hypertension. 2006;48:e113. doi: 10.1161/01.HYP.0000247309.36509.7b. [DOI] [PubMed] [Google Scholar]
- 17.Muller DN, Klanke B, Feldt S, et al. (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension. 2008;51:676–681. doi: 10.1161/HYPERTENSIONAHA.107.101493. [DOI] [PubMed] [Google Scholar]
- 18.Feldt S, Maschke U, Dechend R, Luft FC, Muller DN. The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J Am Soc Nephrol. 2008;19:743–748. doi: 10.1681/ASN.2007091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan P, Shamansurova Z, Bisotto S, et al. Impact of the prorenin/renin receptor on the development of obesity and associated cardiometabolic risk factors. Obesity (Silver Spring) 2014;22:2201–2209. doi: 10.1002/oby.20844. [DOI] [PubMed] [Google Scholar]
- 20.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentki M, Madiraju SR. Glycerolipid metabolism and signaling in health and disease. Endocr Rev. 2008;29:647–676. doi: 10.1210/er.2008-0007. [DOI] [PubMed] [Google Scholar]
- 22.Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F. Cross-talk between PPAR gamma and insulin signaling and modulation of insulin sensitivity. Ppar Res. 2009:1–12. doi: 10.1155/2009/818945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 24.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 25.Tschritter O, Fritsche A, Thamer C, et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- 26.Fisher FM, Trujillo ME, Hanif W, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 27.Peterson RM, Beeson L, Shulz E, et al. Impacting obesity and glycemic control using a culturally-sensitive diabetes education program in Hispanic patients with type 2 diabetes. Int J Body Compos Res. 2010;8:85–94. [PMC free article] [PubMed] [Google Scholar]
- 28.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorad S, Dou JT, Benicky J, et al. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi N, Li F, Hua K, et al. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007;6:506–512. doi: 10.1016/j.cmet.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren DL, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPAR gamma knockdown by engineered transcription factors: exogenous PPAR gamma 2 but not PPAR gamma 1 reactivates adipogenesis. Genes Dev. 2002;16:27–32. doi: 10.1101/gad.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol (Oxf) 2012;205:194–208. doi: 10.1111/j.1748-1716.2012.02409.x. [DOI] [PubMed] [Google Scholar]
- 33.Renaud IM, Chainey A, Belair MF, et al. Long-term protection of obese Zucker rat kidneys from fibrosis and renal failure with an angiotensin-converting enzyme inhibitor/diuretic combination. Fundam Clin Pharmacol. 2004;18:437–447. doi: 10.1111/j.1472-8206.2004.00264.x. [DOI] [PubMed] [Google Scholar]
- 34.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 36.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33:904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun K, Wernstedt AI, Kusminski CM, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tulloch-Reid MK, Hanson RL, Sebring NG, et al. Both subcutaneous and visceral adipose tissue correlate highly with insulin resistance in African Americans. Obes Res. 2004;12:1352–1359. doi: 10.1038/oby.2004.170. [DOI] [PubMed] [Google Scholar]
- 39.Phillips SA, Ciaraldi TP, Kong AP, et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–674. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]
- 40.Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab. 2008;295:E842–E850. doi: 10.1152/ajpendo.90359.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.