Abstract
Acrolein, a highly toxic α, β–unsaturated aldehyde, has been a longstanding key biomarker associated with a range of disorders related to oxidative stresses. One of the most promising methods for detecting acrolein involves the use of antibodies that can recognize the acrolein–lysine conjugate, 3-formyl-3, 4-dehydropiperidines (FDP), within oxidatively stressed cells and tissues from various disease states. We have uncovered here that FDP could reduce nitroarenes in high yields at 100 °C in the presence of excess CaCl2 as a Lewis acid promoter. This unique transformation allowed for the development of a de novo method for detecting levels of FDPs generated from proteins in urine or blood serum samples. Thus we successfully converted a non-fluorescent and inexpensive 4-nitrophthalonitrile probe to the corresponding fluorescent aniline, thereby constituting the concept of fluorescent switching. Its sensitivity level (0.84 nmol/mL) is more than that of ELISA assays (3.13 nmol/mL) and is already equally reliable and reproducible at this early stage of development. More importantly, this method is cost effective and simple to operate, requiring only mixing of samples with a kit solution. Our method thus possesses potential as a future alternative to the more costly and operatively encumbered conventional antibody-based methods.
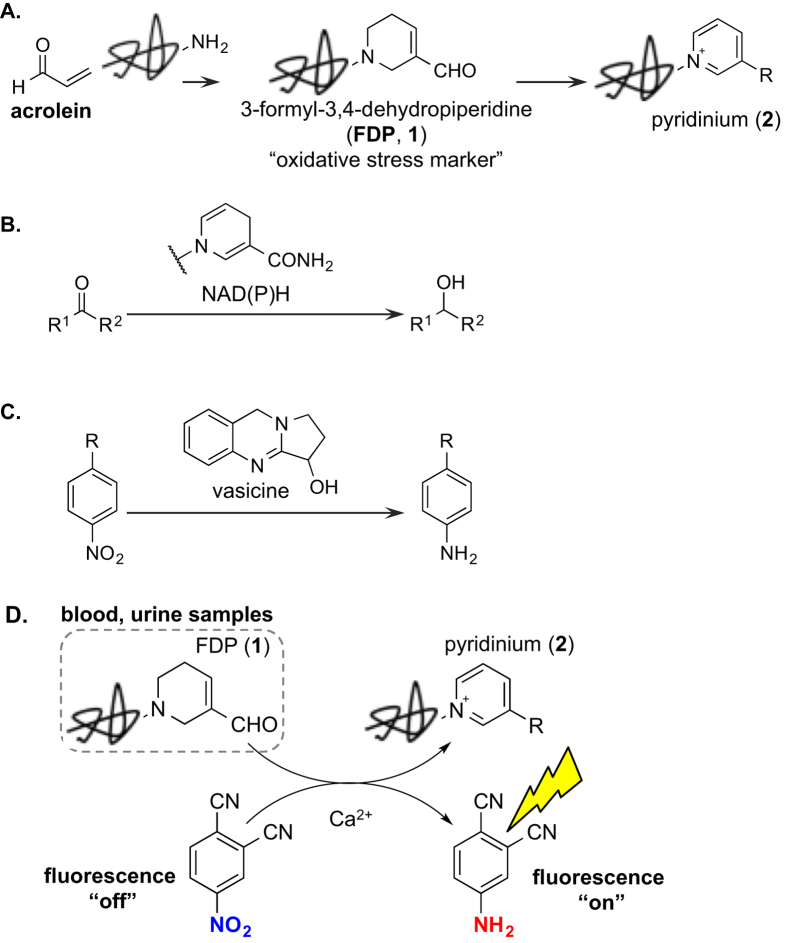
Acrolein is a highly toxic unsaturated aldehyde generated from an array of sources ranging from tobacco smoke to incomplete combustion of oil, charcoal, wood, plastic and other organic substances (Fig. 1A)1. It can also be produced endogenously under oxidative stresses from polyamines, lipids, amino acids and other biomolecules in the presence of reactive oxygen species1,2,3,4. Chemically, acrolein is well known to be highly reactive and have become recognized as a real hazard to the human health. More specifically, while reacting mainly with amino, thiol, and hydroxyl groups of proteins or nucleobases5,6, these intrinsic reactivities in biological settings often lead to many immunological disorders7,8 and inflammations5. Therefore, it remains critically important to understand relationships between acrolein and various diseases at the molecular level. Among these endeavors, developing new and improved detection of acrolein ranks at the very top in significance and urgency.
Figure 1.
(A) Production of acrolein-conjugates (FDP, 1) and their pyridinium derivatives (2). (B) Reduction by NAD(P)H. (C) Reduction by vacisine. (D) Detection of FDP using reduction of non-fluorescent nitroarenes to fluorescent anilines.
Detections of acrolein have traditionally been analyzed through chemical derivatizations with nucleophiles, i.e., 3-aminophenol9,10 or fluorescently labeled hydrazines11,12, although such methods require harsh conditions and often suffer from poor selectivity in the presence of other aldehydes. More sensitive and efficient methods that do not rely on HPLC, including a solid-supported two-reaction system, have been developed recently for detecting acrolein in mouse serum13. In our own work, we have managed to carry out the imaging of acrolein in oxidatively stressed cancer cells by means of unique acrolein/azide click reaction14. Acrolein exogenously or endogenously generated from oxidatively stressed cells could be directly visualized by simply treating cells with a fluorescent-azide probe. Alternatively, it has been known to diagnosis acrolein related diseases through detections of acrolein-amine conjugates6,15,16,17,18,19,20,21,22,23,24 such as 3-formyl-3,4-dehydropiperidine (or FDP; see 1 in Fig. 1A). Consequently, detection of FDP as biomarkers can indirectly indicate the biological concentration of acrolein25.
In addition to Igarashi’s elegant work7,8,26,27,28,29, the most notable method to date was reported by the Uchida’s laboratory involving immunochemical method (ELISA kit, Takara Bio Inc.)6,15,25,30,31. It is currently accepted to use monoclonal antibodies that recognize FDP-type structures in proteins. In particular, antibodies have been utilized for detecting of FDP-type structures to assess the disease state such as arterial sclerosis15,32,33,34, Alzheimer’s disease35,36, tumors37,38,39,40,41, diabetes42,43,44,45,46, autoimmune diseases47,48, hypertensions27, and many other types of diseases49,50,51,52. Uchida’s group also reported that oxidative stress markers include not only FDP (1) but also the pyridinium species such as 2, which could be transformed from FDP via aromatic oxidation (Fig. 1A)15,30. This important finding implies that FDP also possess the reduction potential via hydrogen-transfer. Such assessment could find numerous precedents. For instance, nicotinamide adenine dinucleotide (NADH), a natural redox biomolecule that contains a similar dihydropyridine ring, can reduce ketones enzymatically (Fig. 1B)53. Similarly, vasicine is another natural product that has the reduction potential for nitro compounds through its own molecular oxidation (Fig. 1C)54. Given such reductive potential of FDP (1), we envisioned a possible fluorescent switch rendered by FDP during the conversion from non-fluorescent nitroarene probes to fluorescent anilines (Fig. 1D). This idea could make it possible to detect the acrolein-biomarkers easily without the use of the antibody. Herein, we wish to report a convenient and efficient method for detecting the oxidative stress marker FDP.
Results and Discussion
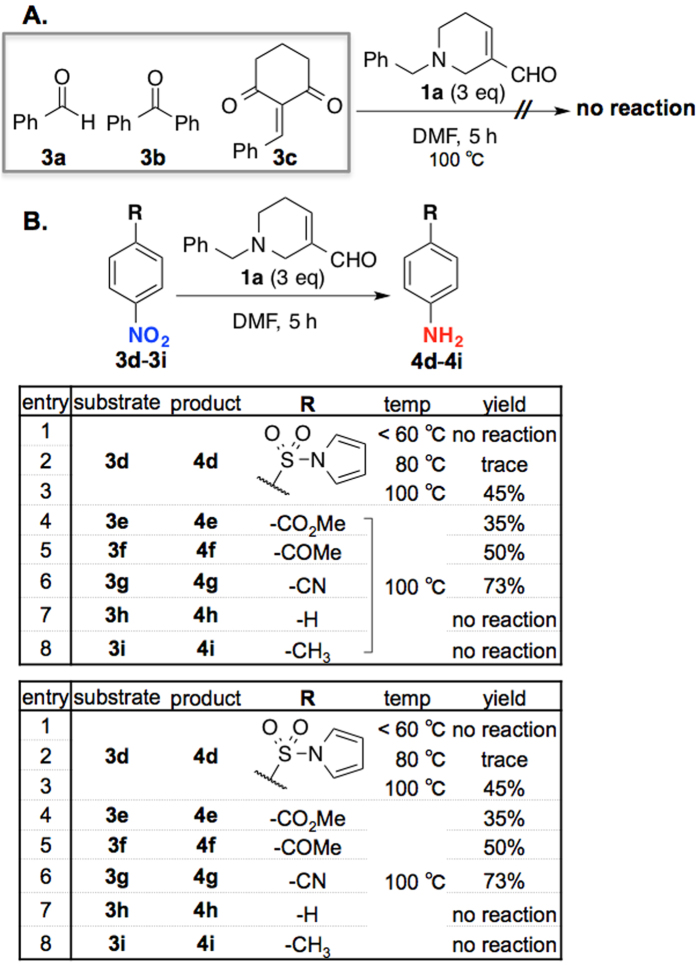
To commence our investigation, we attempted to use the readily accessible N-Bn-FDP (1a)55 as a reducing reagent against various substrates (Fig. 2). However, for examples containing carbonyl groups or unsaturated bond activated by an electron-withdrawing group (3a–c), reductions did not take place (Fig. 2A). On the other hand, we discovered that the nitroarene 3d containing the sulfonyl group could be selectively reduced to the corresponding aniline 4d using 1a (Fig. 2B). While the reduction did not proceed under 60 °C (entry 1) initially with only trace amount of aniline 4d was observed in the trial at 80 °C (entry 2), we found that 100 °C was the most optimal temperature for this reduction (entry 3, 45%). To examine the scope of this reduction, we proceeded with several commercially available nitroarenes as substrates. We found that like 3d activated with an electron withdrawing sulfonyl group, nitrobenzene 3e with an ester group could also reduced in 35% yield (entry 4), and that 4-nitroacetophenone 3f and 4-nitrobenzonitrile 3g were reduced in 50% and 73% yield, respectively (entries 5–6). On the other hand, nitrobenzenes 3h and 3i without an electron-withdrawing group were not reduced at all under the optimized conditions (entries 7–8).
Figure 2. Initial attempts of reduction by N-Bn FDP 1a.
(A) Reductions of simple aldehydes and ketones (3a–c). (B) Reductions of nitroarenes 3d–i.
To improve the yield, we explored possibility of Brønsted acid or Lewis acid assisted reduction. Given that the NAD(P)H reduction can be promoted using a metal ion as catalyst53, we screened for a suitable acid using nitroarene 3d as a model substrate (Fig. 3). Unfortunately, Brønsted acids such as HCl, H2SO4, CF3COOH, and H3BO3 (entry 2–8) led to decreased yields relative to our initial result (entry 1, 45%). Fortuitously, one equiv of transition metal-based Lewis acidic (e.g. Cu2+, In3+) additives led to a remarkable increase of the yields (entries 9–19, up to 76%). We particularly pleased to find that focused on alkaline earth metal-based Lewis acid (Mg2+, Ca2+), inspired by NADH reduction catalyzed by metal ion53, were equally effective (entries 20–25). With 5 equiv of CaCl2 being the most effective for this reduction at 89% yield (entry 26). Counter anion dependency on reactivity (among MgCl2, MgBr2, MgSO4, Ca(OH)2, CaCO3 and CaCl2) is most probably due to the solubility of metal species in reaction media; While MgCl2 and CaCl2 are soluble in either DMF or DMF/H2O, other species are hardly soluble or insoluble (see Fig. S1 in Supplementary Information). We also checked pH of the reaction in the presence of these metal species (Fig. S1). Although additives slightly affected solution pH, the observed reactivity in Fig. 3 could be well explained by the solubility of additives. For detecting FDPs in biological samples, i.e., urine (vide infra), very little amount of samples were diluted with excess H2O and then subjected to excess nitrobenzene and metal additives. Hence pH of sample solution should not be significantly concerned for our FDP detection.
Figure 3. Evaluating additives for reduction.
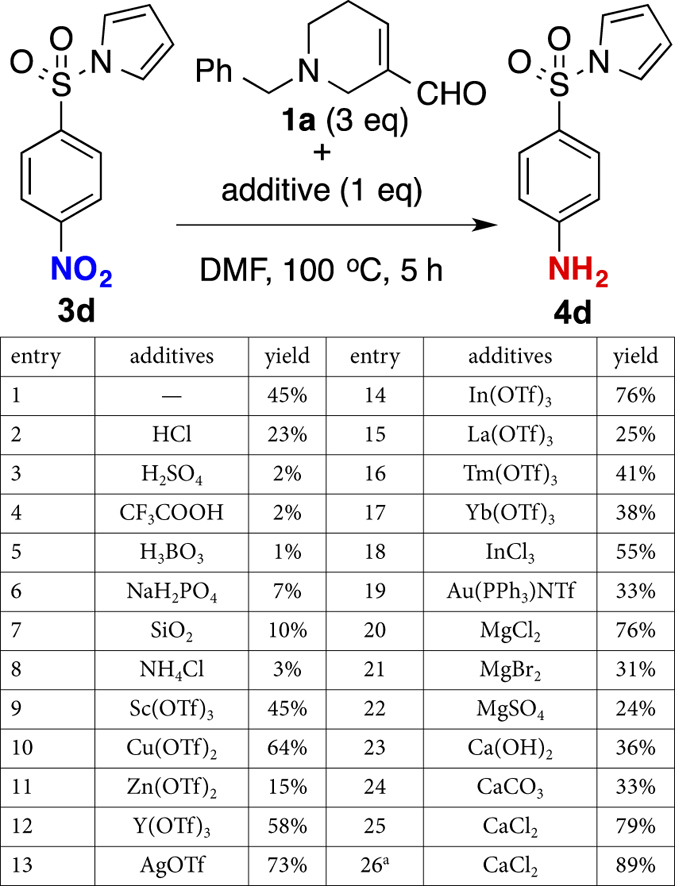
a5 equiv of CaCl2 was added.
Having established the optimized conditions (5 equiv of CaCl2, 100 °C, and 5 h), we proceeded to examine scope and limitation of this reduction with various nitroarenes. As shown in Fig. 4A, in comparison with the initial trial shown in Fig. 2B, nitrobenzenes containing sulfonyl (3d), ester (3e), ketone (3f), and nitrile (3g) as electron withdrawing groups were all reduced to corresponding anilines in higher yields (Fig. 4A, entries 1–4, 4d: 89%, 4e: 69%, 4f: 88%, 4g: 82%), thereby accentuates the importance of CaCl2. More notably, while simple nitrobenzene 3h was not reduced at all in previous trial (Fig. 2B, entry 7), it could now be reduced to the corresponding aniline 4h in 18% yield in the presence of CaCl2 (entry 5). On the other hand, 4-nitrotoluene 3i containing an electro-donating group was still not reduced under these optimized conditions (entry 6). Nitrobenzene 3j containing an indolyl sulfone motif, which is an intermediate in the indole alkaloid synthesis, could be reduced to 4j in 80% yield (entry 7). Predictably, 4-nitrobenzoic acid 3k and 4-nitrobenzaldehyde 3l were reduced in 61% and 40%, respectively (entries 8–9). Similarly, 4-fluoronitrobenzene 3m were naively reduced without any substitution (entry 10, 73%). The reduction yield was significantly lower when using 4-chloro-nitrobenzene 3n with just an inductive electron-withdrawing group, although unreacted 3n could be mostly recovered without any other isolable byproducts (entry 11, 21%). The unique substituent effect observed here is further supported by the linear Hammett plot shown in Fig. 4B.
Figure 4. Substitution effects of reduction of nitrobenzene.
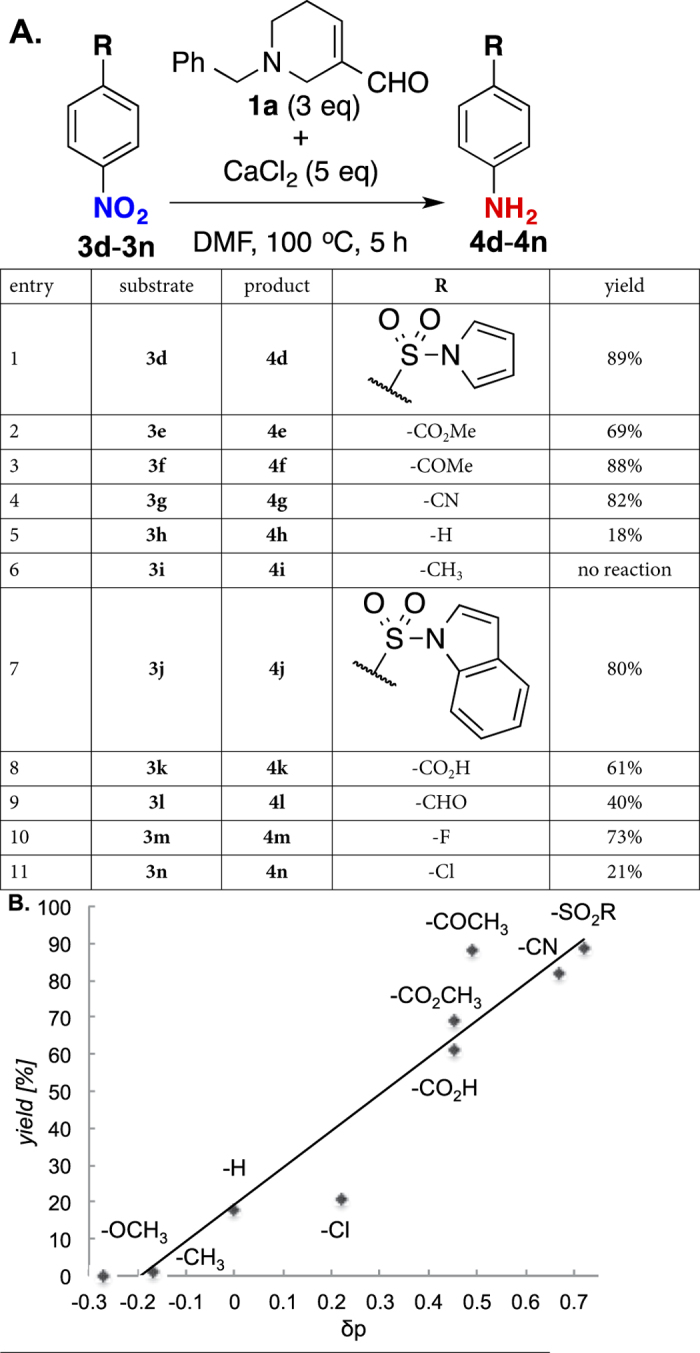
(A) Conversion from nitroarenes 3d–n to corresponding anilines 4d–n. (B) Hammett plot of reduction yields against substituent groups.
To develop this reduction into an effective and simple method for FDP detection, we investigated possible reactions of FDP in biological sample with a non-fluorescent nitroarene probe affording a reaction mixture that contains fluorescence of the converted corresponding aniline. Conceptually, we envisioned that the fluorescence intensity should be proportional to the amount of the aniline, which is related with the amount of FDP at a constant rate via the reduction, and thus, the exact amount of FDP in biological sample could be estimated. Most critically for this endeavor, an adequate nitroarene probe is to detect the level of FDP in the presence of various kinds of biological materials. We focused on two points for selecting an appropriate nitroarene probe: (a) fluorescence property for switching; and (b) efficient reactivity.
With these assessments in mind, we proceeded to screen for nitroarenes that could be reduced to fluorescent anilines. Typical fluorescent anilines are classified electronically as being pull-push. That is, the aromatic ring is substituted with both electro-withdrawing and electron-donating groups such as the combination of nitrile and amino group, which is widely known to fluoresce (Fig. 5A)56. It is equally important that excitation and emission wavelengths are sufficiently resolved (non-overlapping), and that the quantum yield is sufficiently high for to an accurate measurement. After screening dozens of candidates including 4g and 4o, we found that 4-nitrophthalonitrile 3p to be the most suitable nitroarene probe. As shown in Fig. 5B, while the reduction of 3p is 78% yield and is complete in 5 h, the overlaid fluorescent spectrum of 3p and 4p suggested a clear switch at emission λmax of 404 nm (Fig. 5C). Moreover, time-dependent measurement reveals a distinct correlation between the fluorescence intensity and the reaction progress (Fig. 5D).
Figure 5.
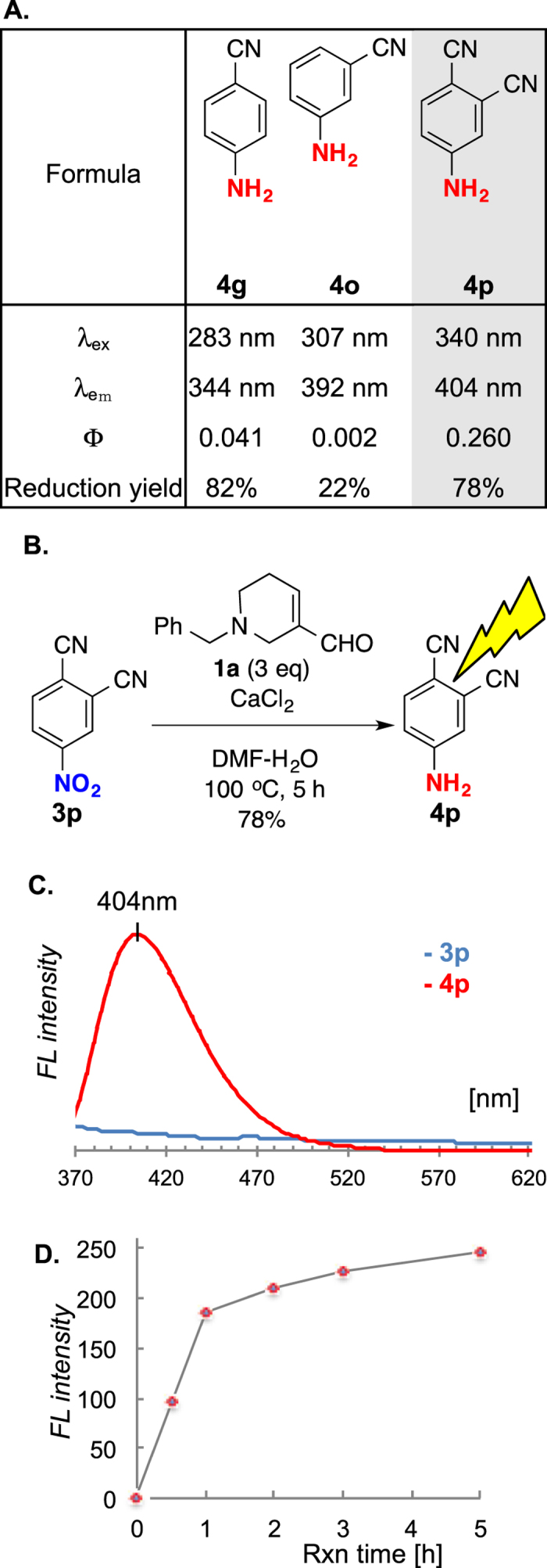
(A) Fluorescence and reactivity profiles of cyano-substituted anilines. (B) Reduction of 4-nitrophthalonitrile (3p). (C) Fluorescent spectrum of 3p (blue) and 4p (red) at 340 nm excitation. (D) Time-dependent analysis of fluorescent intensity by FDP reduction. Mean values with standard deviations are indicated. λex = excitation wavelength, λem = emission wavelength, Φ = quantum yield.
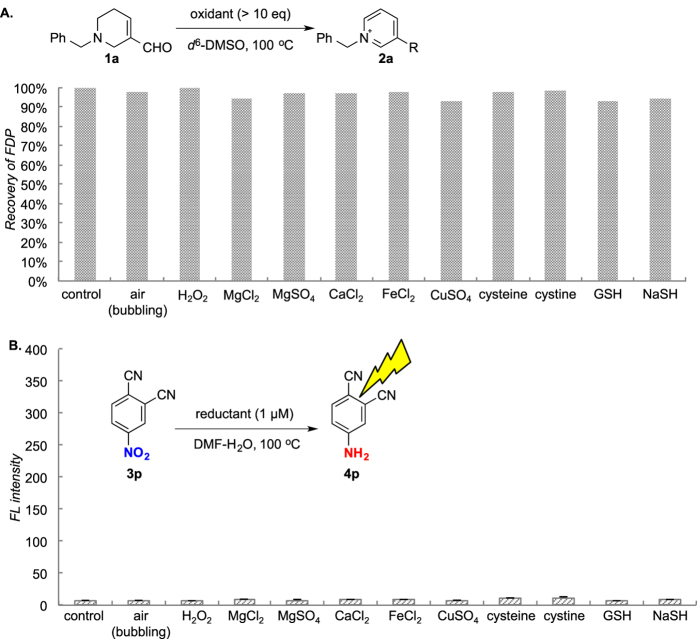
To confirm the reduction-based “fluorescent on” concept for FDP, and to verify chemoselectivity of such concept under biological conditions, we tested robustness of FDP and nitroarene probe in the presence of biologically relevant metals and redox reagents (Fig. 6). Addition of air or hydrogen peroxide afforded no oxidation of FDP 1a (Fig. 6A) and, in the presence of biologically abundant or redox metal species (Mg2+, Ca2+, Fe2+, Cu2+), FDP was recovered quantitatively. For the nitroarene probe, 3p did not react with cystein, cystine, glutathione (GSH) or sodium hydrogen sulfide (NaSH) at 1 μM, which is supposed as biological concentration57 (Fig. 6B). Therefore, any biologically relevant metal species and redox agents gave little influence on FDPs and 4-nitrophthalonitrile 3p themselves. Hence undesired consumption of FDPs or background fluorescence increase can be avoided under the established conditions. Namely, Ca2+ or Mg2+ selectively activate the reduction in the presence of both substrates. We do not think that very small amount of metal species existing in biological systems, in comparison to excess CaCl2 used in this research, can efficiently mediate the reaction, but even if so, this is advantageous for our reduction-based sensor.
Figure 6.
(A) Stability of FDP in the presence of various metals and redox reagents (air, H2O2, MgCl2, MgSO4, CaCl2, FeCl2, CuSO4, cysteine, cystine, GSH, NaSH). Recovery ratio was determined by 1H NMR using an internal standard (dimethylsulfone). (B) Stability of nitrobenzene 3p in the presence of various metals and redox reagents. Conversion was estimated by aniline fluorescence. Mean values with standard deviations are indicated.
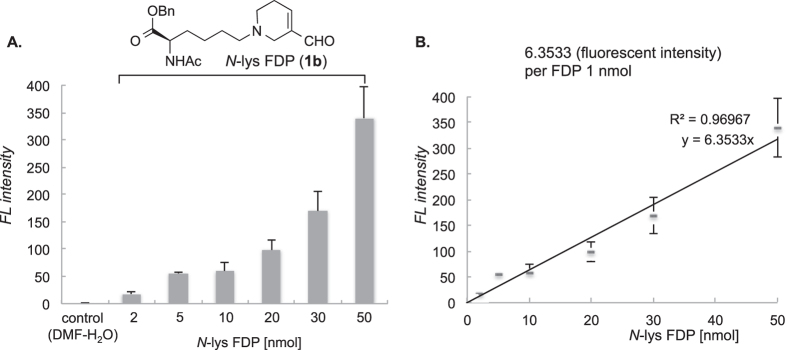
To further demonstrate the reliability of this new detection method, we demonstrated biological application using N-lys FDP 1b under established conditions as shown above. We established the precise correlation between the amount of FDP and the fluorescent intensity using authentic N-lys FDP as the standard (Fig. 7A). The delta values between each fluorescence and control are proportional to that of FDP amount (Fig. 7B). The slope of the linear plot in Fig. 7B indicates fluorescent intensity per FDP unit. According to statistical processing (see calculation in Supplementary Information, Fig. S2), detection limit (LOD) is determined to be 0.84 nmol/mL using this method, which is more sensitive than that of ELISA kit (3.13 nmol/mL).
Figure 7.
(A) Fluorescence intensity by prepared N-lys FDP 1b solution (0, 2, 5, 10, 20, 30, 50 nmol/mL) as authentic standard in detection protocol. (B) Fluorescence intensity per FDP unit was calculated. Reaction was conducted under optimized kit condition (3p: 1.7 mg; CaCl2: 5.5 mg; temp: 100 °C; time: 5 h). Mean values with standard deviations are indicated.
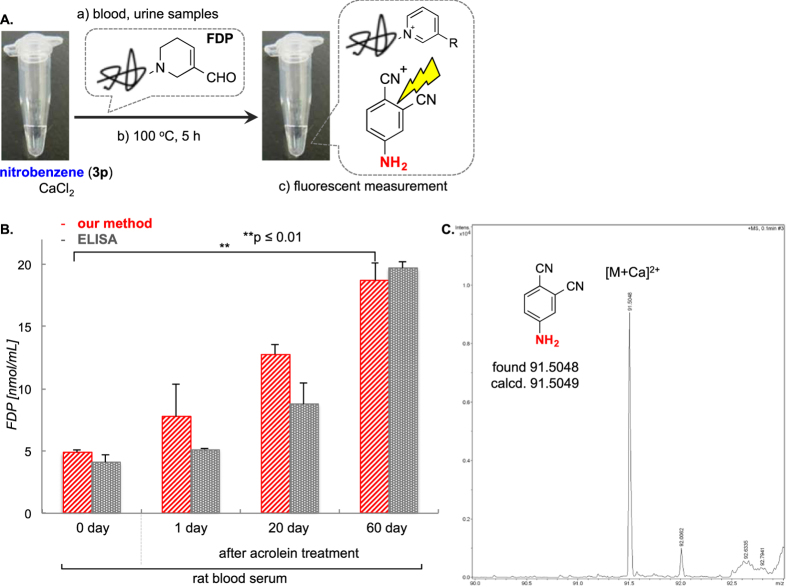
With these validations in hand, we simplified the method by constructing a kit based on the optimized conditions. The procedure of detection is quite simple with three steps: (a) Mixing the sample and the pre-prepared kit solution containing 1.7 mg of nitroarene probe, and 5.5 mg of CaCl2 in 50 μL of DMF-H2O; (b) heating at 100 °C for 5 h; and c) measuring the resulting fluorescence (Fig. 8A).
Figure 8.
(A) Protocol of our detection method to mouse and rat blood samples. (B) Detection using rat blood serum treated with acrolein over 0 (normal), 1, 20, or 60 days and compared with the ELISA method. Mean values with standard deviations are indicated. **p < 0.01; p values were determined by using two-tailed Student t test. (C) Detection of 4p by ESI-MS after reduction by 60 days’ sample. HRESI-MS m/z calcd for C8H5N3Ca [M+Ca]2+ 91.5049, found 91.5048.
We first evaluated a normal blood serum and prepared serum samples that contain artificially generated FDP via pre-treatment of excess acrolein over 0, 1, 20, and 60 days (Fig. 8B). We would measure the amount of FDP estimated by standard values as calculated in Fig. 7B. The rat blood serum normally has 4.9 ± 0.2 nmol/mL of FDP, which is what we find here. In comparison with this control, 1-day treatment of excess acrolein led to a little increase of FDP (7.7 ± 2.6 nmol/mL), but it the level was 12.7 ± 0.7 nmol/mL in the sample after 20-d treatment. In addition, a 60-d sample shows further increase of FDP level to 18.7 ± 1.4 nmol/mL. Fluorescent aniline 4p, which was obtained by reduction with FDP in rat blood serum (pretreated with aclorein over 60 days, Fig. 8B), was successfully identified by ESI-MS (Fig. 8B). These results are in good agreement with ELISA assay of antibody recognizing FDP.
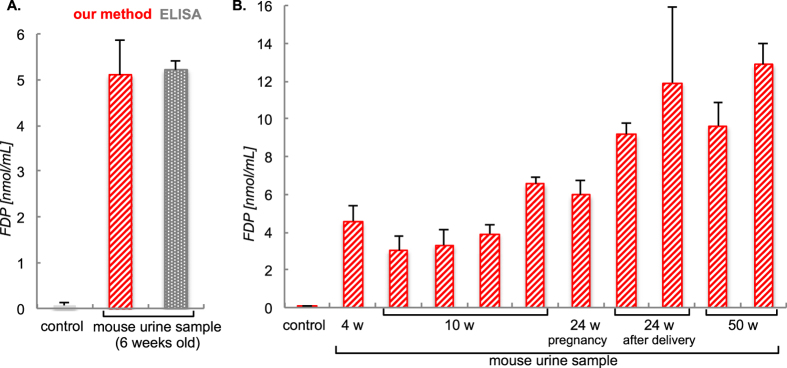
We then pursued a more practical experiment by using a real urine sample from a 6-week old mouse. After preparation of 20-fold diluted urine sample and dividing the sample into three sample-lots, we attempted the FDP-detection using both our method in comparison with the ELISA protocol. As shown in Fig. 9A, the diluted urine sample showed a level of FDP of 5.2 ± 0.8 nmol/mL, which is in excellent statistical agreement with the ELISA assay15.
Figure 9.
(A) Detection of FDP using the 20-fold diluted urine sample of a 6-week old mouse and compared with the ELISA method. (B) Simultaneous detection urine samples from ten mice (4, 10, 24, or 50 weeks old). Mean values with standard deviations are indicated.
Finally, we conducted the detection urine samples from 10 mice (Fig. 9B). Although we conducted these experiment in triplicates (totally 30 lots), the entire set of urine test was carried out smoothly and swiftly using the kit solution within 6 h unlike any medical/laboratory services currently in practice. Our results show that levels of FDP in diluted urines from the 10-mice set are within the 3.4–13.1 nmol/mL range. We have thus succeeded in achieving a sensitive and inexpensive detection of FDP from biological samples extracted from mammalian models through the use of an under explored reaction of FDP.
Conclusion
We have uncovered here N-Bn-3-formyl-3,4-dehydropiperidine, an analog of oxidative stress marker FDP, could reduce nitroarene in high yields using CaCl2 as a Lewis acid promoter. Based on this unique transformation, we developed a de novo detection method for FDP levels via converting a non-fluorescent nitroarene probe to the corresponding fluorescent aniline, thereby constituting the concept of fluorescent switching. This new method is amenable for actual biological samples; its sensitivity level is comparable to that of ELISA assays and is already equally reliable and reproducible at this early stage of development. More importantly, it is easy to handle, practical to operate, and cost effective. Biological samples containing specific nitrobenzenes, i.e., drugs with strongly electron-withdrawing groups, may react with FDP and affect the analysis. While method should be used with cautions, excess 4-nitrophthalonitrile 3p with CaCl2 is otherwise preferentially reduced, hence could be applied in most cases for detecting oxidatively stressed diseases. Thus, our method possesses potential as a future alternative to the more costly and operatively encumbered conventional methods. Efforts are underway to further develop this novel detection method.
Experimental Methods
General information
All solvents were of reagent grade. All commercially purchased chemicals were used as received1. H and 13C NMR spectra were obtained from a JEOL RESONANCE AL400 NMR and a JEOL RESONANCE AL300 NMR spectrometer. Signals were internally referenced to solvent residues. High-resolution mass spectral analyses were carried out using micrOTOF-Q III-HCTM (BRUKER). All fluorescent measurement was carried out using JASCO FP6500 spectrofluorometer with 96-well flat-bottomed plates from Corning Inc. Each value of amount is calculated from intensity of authentic standards. All procedures involving experiment animals was approved by the Ethics Committee of RIKEN. The experiments were performed in accordance with the institutional and national guidelines.
A General Procedure for the Reduction of Nitroarenes with FDP
To a solution of nitoroarene 3d (25.0 mg, 0.10 mmol) and CaCl2 (55.0 mg, 0.50 mmol) in DMF (0.2 mL) was added N-benzyl 3‒formyl‒3,4-dehydropiperidine (N-Bn FDP, 60.0 mg, 0.30 mmol) at 100 °C. After stirring for several hours at this temperature, the mixture was concentrated in vacuo to give a crude mixture as sticky gum. The crude residue was monitored in NMR or purified by either preparative TLC or silica gel flash column chromatography with the hexane-EtOAc solvent mixture as the eluting system to give the desired aniline product 4d (19.6 mg, 89% yield).
Sample Preparation and Detection Procedure
Normal rat serum (100 μL) purchased from Wako Pure Chemical Industry Ltd. was treated with acrolein 100 μL (>100-fold equivalent to serum proteins) for a specific number of days (0, 1, 20, or 60 d). After which, the sample was diluted to 1 mL with distilled water. Fresh urine samples (100 μL) were supplied from C57BL/6 mouse of RIKEN bio-resource center and diluted 20-fold with distilled water. To a solution of nitroarene probe 3k (1.7 mg, 10.0 μmol) and CaCl2 (5.5 mg, 50.0 μmol) in DMF-H2O (50 μL) was added a given urine sample. After stirring for 5 h at 100 °C, the crude reaction mixture was filtered, and the resulting filtrate was measured by spectrofluorometer at 340 nm/404 nm.
ELISA Assay
The measurement was conducted with the Enzyme Linked Immunosorbent Assay (ELISA) kit system (TAKARA, Acrolein-Lysine adduct competitive ELISA kit)15 and followed by attached instructions. The absorbance at 450 nm was measured using micro plate reader (ImmunoMini NJ1000). Data represent averages of more than twice assay with standard deviations from individual experiments.
Characterizations
1-((4-aminophenyl)sulfonyl)-1H-pyrrole (4d, 89%): 1H NMR (400 MHz, CDCl3) δ 7.63 (dd, 2H, J = 6.7, 2.0 Hz), 7.13 (apparent t, 2H, J = 2.3 Hz), 6.63 (dd, 2H, J = 6.8, 2.0 Hz), 6.26 (apparent t, 2H, J = 2.3 Hz), 4.21 (brs, NH2); 13C NMR (100 MHz, CDCl3)δ 151.6, 129.2(2C), 126.8, 120.6(2C), 114.1(2C), 113.1(2C); HRESI-MS m/z calcd for C10H10N2O2S [M + H]+ 223.0536, found 223.0534.
Methyl 4-aminobenzoate (4e, 69%): 1H NMR (400 MHz, CDCl3) δ 7.85 (dd, 2H, J = 8.7, 2.8 Hz), 6.64 (dd, 2H, J = 8.7, 2.8 Hz), 4.10 (brs, NH2), 3.87 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 167.2, 150.8, 131.2(2C), 119.7, 113.9(2C), 51.6; HRESI-MS m/z calcd for C8H10N1O2 [M + H]+ 152.0706, found 152.0709.
4-aminoacetophenone (4f, 88%): 1H NMR (400 MHz, CDCl3) δ 7.79 (dd, 2H, J = 8.6, 1.4 Hz), 6.63 (dd, 2H, J = 8.7, 1.4 Hz), 4.23 (brs, NH2), 2.49 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 196,7, 151.3, 130.9(2C), 127.7, 119.7, 113.7(2C), 26.1; HRESI-MS m/z calcd for C8H9N1O1 [M + H]+ 136.0762, found 136.0757.
4-aminobenzonitrile (4g, 82%): 1H NMR (400 MHz, CDCl3) δ 7.41 (dd, 2H, J = 8.6, 1.2 Hz), 6.64 (dd, 2H, J = 8.6, 1.2 Hz), 4.17 (brs, NH2); 13C NMR (100 MHz, CDCl3) δ 150.5, 133.9(2C), 120.2, 114.6(2C), 100.4; HRESI-MS m/z calcd for C7H6N2 [M + H]+ 119.0604, found. 119.0605.
aniline (4h, 18%): 1H NMR (400 MHz, CDCl3) δ 7.13 (ddd, 2H, J = 7.5, 7.5, 1.2 Hz), 6.74 (tt, 1H, J = 7.5, 1.2 Hz), 6.65 (dd, 2H, J = 7.5, 1.2 Hz), 3.51(brs, NH2); 13C NMR (100 MHz, CDCl3) δ 146.3, 129.2(2C), 118.4, 115.0(2C).
1-((4-aminophenyl)sulfonyl)-1H-indole (4j, 80%): 1H NMR (400 MHz, CDCl3) δ 7.97 (dd, 1H, J = 8.3, 1.2 Hz), 7.66 (dd, 2H, J = 9.1, 2.4 Hz), 7.55(d, 1H, J = 3.6 Hz), 7.52 (d, 1H, J = 8.3 Hz), 7.29 (ddd, 1H, J = 8.3, 8.3, 1.2 Hz), 7.20 (ddd, 1H, J = 8.3, 8.3, 1.2 Hz), 6.62 (d, 1H, J = 3.6 Hz), 6.55 (dd, 2H, J = 9.1, 2.4 Hz), 4.11(brs, NH2); 13C NMR (100 MHz, CDCl3) δ 153.5, 151.3, 134.8, 130.7, 129.1 (2C), 126.3 (2C), 124.2, 122.9, 121.2, 113.9, 113.5, 108.4; HRESI-MS m/z calcd for C14H13N2O2S [M + H]+ 273.0692, found 273.0691.
4-aminobenzoic acid (4k, 61%): 1H NMR (400 MHz, d6-DMSO) δ 7.62 (d, 2H, J = 8.4 Hz), 6.54 (dd, 2H, J = 8.4 Hz), 5.89 (brs, NH2); 13C NMR (100 MHz, CDCl3) δ 167.6, 153.2, 131.3(2C), 116.9, 112.6(2C); HRESI-MS m/z calcd for C7H7N1O2 [M + H]+ 138.0550, found 138.0539.
4-aminobenzaldehyde (4l, 40%): 1H NMR (400 MHz, d6-DMSO) δ 9.56 (s, 1H), 7.54 (d, 2H, J = 8.7 Hz), 6.62 (d, 2H, J = 8.7 Hz), 4.14; 13C NMR (100 MHz, d6-DMSO) δ 189.7, 155.3(2C), 132.2, 124.8, 113.1(2C); HRESI-MS m/z calcd for C7H7N1O1 [M + H]+ 122.0600, found 122.0610.
4-fluoroaniline (4m, 73%): 1H NMR (400 MHz, CDCl3) δ 6.84–6.78 (m, 2H), 6.56–6.51 (m, 2H), 3.51(brs, NH2); 13C NMR (100 MHz, CDCl3) δ 156.1(d, J = 235 Hz, C-F), 142.4, 115.8(2C), 115.3(2C); HRESI-MS m/z calcd for C6H6F1N1 [M + H]+ 112.0557, found 112.0558.
4-chloroaniline (4n, 21%): 1H NMR (400 MHz, CDCl3) δ 7.09 (d, 2H, J = 8.6 Hz), 6.58 (d, 2H, J = 8.6 Hz), 3.45(brs, NH2); 13C NMR (100 MHz, CDCl3) δ 145.4, 129.2(2C), 123.2, 116.3(2C); HRESI-MS m/z calcd for C6H6N1Cl1 [M + H]+ 128.0262, found 128.0263.
3-aminobenzonitrile (4o, 22%): 1H NMR (300 MHz, CDCl3) δ 7.16 (dd, 1H, J = 7.2, 7.2 Hz), 6.94 (d, 1H, J = 7.2 Hz), 6.83–6.77 (m, 2H), 3.82 (brs, NH2); 13C NMR (75 MHz, CDCl3) δ 147.0, 130.2(2C), 122.1, 119.3, 117.6, 113.1; HRESI-MS m/z calcd for C7H6N2 [M + H]+ 119.0604, found. 119.0612.
4-aminophthalonitrile (4p, 78%): 1H NMR (400 MHz, d6-DMSO) δ 7.64 (d, 1H, J = 8.7 Hz), 7.03 (d, 1H, J = 2.4 Hz), 6.68 (dd, 1H, J = 8.7, 2.4 Hz), 6.72 (brs, NH2); 13C NMR (100 MHz, d6-DMSO) δ 153.2, 135.1, 117.6, 117.3, 117.1, 116.5, 115.6, 97.9; HRESI-MS m/z calcd for C8H5N3 [M + Na]+ 166.0400, found 166.0380. Fluorescent excitation/emission: 304/404 nm, quantum yield Φ = 0.26.
Additional Information
How to cite this article: Takamatsu, M. et al. A Reduction-Based Sensor for Acrolein Conjugates with the Inexpensive Nitrobenzene as an Alternative to Monoclonal Antibody. Sci. Rep. 6, 35872; doi: 10.1038/srep35872 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers JP16H03287, JP16K13104 and JP15H05843 in Middle Molecular Strategy, RIKEN Junior Research Associate Program, and SUNBOR SCHOLARSHIP. This work was also performed with the support of the Russian Government Program for Competitive Growth granted to Kazan Federal University. We are grateful to Prof. R. Hsung (University of Wisconsin-Madison), Dr. Ambara R. Pradipta, and Mr. Kazuki Tsubokura for carefully reading the manuscript and for giving valuable comments.
Footnotes
Author Contributions K.T. directed the whole research project. M.T. performed experiments and analysis. M.T. and K.T. performed fluorescence measurements. M.T., R.O., S.K. and N.T. performed ELISA assays. M.T. K.F. and K.T. analyzed and discussed the data. M.T. and K.T. wrote the paper.
References
- Alarcon R. A. Acrolein. IV. Evidence for the formation of the cytotoxic aldehyde acrolein from enzymatically oxidized spermine or spermidine. Arch Biochem Biophys 137, 365–372 (1970). [DOI] [PubMed] [Google Scholar]
- Houen G., Bock K. & Jensen A. L. HPLC and NMR investigation of the serum amine oxidase catalyzed oxidation of polyamines. Acta Chem Scand 48, 52–60 (1994). [DOI] [PubMed] [Google Scholar]
- Kimes B. W. & Morris D. R. Preparation and stability of oxidized polyamines. Biochim Biophys Acta 228, 223–234 (1971). [DOI] [PubMed] [Google Scholar]
- Hensley K., Robinson K. A., Gabbita S. P., Salsman S. & Floyd R. A. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 28, 1456–1462 (2000). [DOI] [PubMed] [Google Scholar]
- West J. D. & Marnett L. J. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem Res Toxicol. 19, 173–194 (2006). [DOI] [PubMed] [Google Scholar]
- Uchida K. et al. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem 273, 16058–16066 (1998). [DOI] [PubMed] [Google Scholar]
- Sharmin S. et al. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem Biophys Res Commun. 282, 228–235 (2001). [DOI] [PubMed] [Google Scholar]
- Yoshida M. et al. Acrolein toxicity: Comparison with reactive oxygen species. Biochem Biophys Res Commun. 378, 313–318 (2009). [DOI] [PubMed] [Google Scholar]
- Alarcon R. A. Fluorometric determination of acrolein and related compounds with m-aminophenol. Anal Chem. 40, 1704–1708 (1968). [DOI] [PubMed] [Google Scholar]
- Bohnenstengel F., Eichelbaum M., Golbs E. & Kroemer H. K. High-performance liquid chromatographic determination of acrolein as a marker for cyclophosphamide bioactivation in human liver microsomes. J Chromatogr B Biomed Sci Appl. 692, 163–168 (1997). [DOI] [PubMed] [Google Scholar]
- Boor P. J. & Ansari G. A. High-performance liquid chromatographic method for quantitation of acrolein in biological samples. J Chromatogr. 375, 159–164 (1986). [DOI] [PubMed] [Google Scholar]
- Büldt A. & Karst U. 1-Methyl-1-(2,4-dinitrophenyl)hydrazine as a New Reagent for the HPLC Determination of Aldehydes. Anal Chem 69, 3617–3622 (1997). [DOI] [PubMed] [Google Scholar]
- Togashi M. et al. Practical fluorescence detection of acrolein in human plasma via a two-step tethering approach. Chem Commun (Camb) 50, 14946–14948 (2014). [DOI] [PubMed] [Google Scholar]
- Rachmat P. A. et al. Uncatalyzed Click Reaction between Phenyl Azides and Acrolein: 4-Formyl-1,2,3-Triazolines as “Clicked” Markers for Visualizations of Extracellular Acrolein Released from Oxidatively Stressed Cells. ACS Sensors 1, 623–632 (2016). [Google Scholar]
- Uchida K. et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA 95, 4882–4887 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradipta A. & Tanaka K. SYNTHESIS OF 3,7,9-AND 2,6,9-TRIAZABICYCLO[3.3.1]NONANE DERIVATIVES. Heterocycles 87, 2001–2014 (2013). [Google Scholar]
- Tsutsui A. & Tanaka K. 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein. Org Biomol Chem 11, 7208–7211 (2013). [DOI] [PubMed] [Google Scholar]
- Takamatsu M., Fukase K., Kurbangalieva A. & Tanaka K. Imino [4+4] cycloaddition products as exclusive and biologically relevant acrolein-amine conjugates are intermediates of 3-formyl-3,4-dehydropiperidine (FDP), an acrolein biomarker. Bioorg Med Chem 22, 6380–6386 (2014). [DOI] [PubMed] [Google Scholar]
- Tanaka K. et al. Facile Preparation of 1,5-Diazacyclooctanes from Unsaturated Imines: Effects of the Hydroxyl Groups on [4+4] Dimerization. Synlett 25, 1026–1030 (2014). [Google Scholar]
- Pradipta A. et al. Microfluidic Mixing of Polyamine with Acrolein Enables the Detection of the [4+4] Polymerization of Intermediary Unsaturated Imines: The Properties of a Cytotoxic 1,5-Diazacyclooctane Hydrogel. Synlett 25, 2442–2446 (2014). [Google Scholar]
- Tsutsui A. et al. Polyamine modification by acrolein exclusively produces 1,5-diazacyclooctanes: a previously unrecognized mechanism for acrolein-mediated oxidative stress. Org Biomol Chem. 12, 5151–5157 (2014). [DOI] [PubMed] [Google Scholar]
- Tsutsui A., Pradipta A., Saigitbatalova E., Kurbangalieva A. & Tanaka K. Exclusive formation of imino[4+4]cycloaddition products with biologically relevant amines: plausible candidates for acrolein biomarkers and biofunctional modulators. Medchemcomm 6, 431–436 (2015). [Google Scholar]
- Pradipta A. & Tanaka K. Unexplored Reactivity of N-Alkyl Unsaturated Imines: A Simple Procedure for Producing Optically Active 1,3-Diamines via a Stereocontrolled Formal [4 + 2] and [4 + 2 + 2] Iminocycloaddition. Bulletin of the Chemical Society of Japan 89, 337–345 (2016). [Google Scholar]
- Ayumi T. et al. 1,5-Diazacyclooctanes, as Exclusive Oxidative Polyamine Metabolites, Inhibit Amyloid-β(1–40) Fibrillization. Adv. Sci. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. et al. Evaluation of N (epsilon)-(3-formyl-3,4-dehydropiperidino)lysine as a novel biomarker for the severity of diabetic retinopathy. Diabetologia 51, 1723–1730 (2008). [DOI] [PubMed] [Google Scholar]
- Sakata K., Kashiwagi K., Sharmin S., Ueda S. & Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans 31, 371–374 (2003). [DOI] [PubMed] [Google Scholar]
- Tomitori H. et al. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke 36, 2609–2613 (2005). [DOI] [PubMed] [Google Scholar]
- Yoshida M. et al. Identification of acrolein-conjugated protein in plasma of patients with brain infarction. Biochem Biophys Res Commun. 391, 1234–1239 (2010). [DOI] [PubMed] [Google Scholar]
- Tomitori H. et al. Augmented glutathione synthesis decreases acrolein toxicity. Biochem Biophys Res Commun. 418, 110–115 (2012). [DOI] [PubMed] [Google Scholar]
- Maeshima T. et al. Quantitative analysis of acrolein-specific adducts generated during lipid peroxidation-modification of proteins in vitro: identification of N(τ)-(3-propanal)histidine as the major adduct. Chem Res Toxicol. 25, 1384–1392 (2012). [DOI] [PubMed] [Google Scholar]
- Tran T. N. et al. Acrolein modification impairs key functional features of rat apolipoprotein E: identification of modified sites by mass spectrometry. Biochemistry 53, 361–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindis C. et al. Desensitization of platelet-derived growth factor receptor-beta by oxidized lipids in vascular cells and atherosclerotic lesions: prevention by aldehyde scavengers. Circ Res. 98, 785–792 (2006). [DOI] [PubMed] [Google Scholar]
- Vindis C. et al. Lipid oxidation products and oxidized low-density lipoproteins impair platelet-derived growth factor receptor activity in smooth muscle cells: implication in atherosclerosis. Redox Rep. 12, 96–100 (2007). [DOI] [PubMed] [Google Scholar]
- Shao B. et al. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-I. J Biol Chem. 280, 36386–36396 (2005). [DOI] [PubMed] [Google Scholar]
- Calingasan N. Y., Uchida K. & Gibson G. E. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. J Neurochem. 72, 751–756 (1999). [DOI] [PubMed] [Google Scholar]
- Hamann K. et al. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 107, 712–721 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K. et al. A 1-hour enzyme-linked immunosorbent assay for quantitation of acrolein- and hydroxynonenal-modified proteins by epitope-bound casein matrix method. Anal Biochem. 270, 323–328 (1999). [DOI] [PubMed] [Google Scholar]
- Kawai Y., Furuhata A., Toyokuni S., Aratani Y. & Uchida K. Formation of acrolein-derived 2′-deoxyadenosine adduct in an iron-induced carcinogenesis model. J Biol Chem. 278, 50346–50354 (2003). [DOI] [PubMed] [Google Scholar]
- Glass C. K. & Witztum J. L. Atherosclerosis. the road ahead. Cell 104, 503–516 (2001). [DOI] [PubMed] [Google Scholar]
- Akatsuka S. et al. Contrasting genome-wide distribution of 8-hydroxyguanine and acrolein-modified adenine during oxidative stress-induced renal carcinogenesis. Am J Pathol 169, 1328–1342 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkovic K., Uchida K., Kolenc D., Hlupic L. & Zarkovic N. Tissue distribution of lipid peroxidation product acrolein in human colon carcinogenesis. Free Radic Res. 40, 543–552 (2006). [DOI] [PubMed] [Google Scholar]
- Suzuki D. et al. Expression of megsin mRNA, a novel mesangium-predominant gene, in the renal tissues of various glomerular diseases. J Am Soc Nephrol. 10, 2606–2613 (1999). [DOI] [PubMed] [Google Scholar]
- Schaefer S. et al. Aberrant utilization of nitric oxide and regulation of soluble guanylate cyclase in rat diabetic retinopathy. Antioxid Redox Signal 5, 457–465 (2003). [DOI] [PubMed] [Google Scholar]
- Iuchi Y. et al. Carbonyl stress and detoxification ability in the male genital tract and testis of rats. Histochem Cell Biol. 121, 123–130 (2004). [DOI] [PubMed] [Google Scholar]
- Cameron-Schaefer S. et al. Maintaining the redox-balance intact: gosha-jinki-gan but not insulin activates retinal soluble guanylate cyclase in diabetic rats. Ophthalmic Res. 38, 95–104 (2006). [DOI] [PubMed] [Google Scholar]
- Yong P. H. et al. Evidence supporting a role for N-(3-formyl-3,4-dehydropiperidino)lysine accumulation in Müller glia dysfunction and death in diabetic retinopathy. Mol Vis. 16, 2524–2538 (2010). [PMC free article] [PubMed] [Google Scholar]
- Iuchi Y. et al. Rescue of anaemia and autoimmune responses in SOD1-deficient mice by transgenic expression of human SOD1 in erythrocytes. Biochem J 422, 313–320 (2009). [DOI] [PubMed] [Google Scholar]
- Iuchi Y. et al. Implication of oxidative stress as a cause of autoimmune hemolytic anemia in NZB mice. Free Radic Biol Med. 48, 935–944 (2010). [DOI] [PubMed] [Google Scholar]
- Tanaka N., Tajima S., Ishibashi A., Uchida K. & Shigematsu T. Immunohistochemical detection of lipid peroxidation products, protein-bound acrolein and 4-hydroxynonenal protein adducts, in actinic elastosis of photodamaged skin. Arch Dermatol Res. 293, 363–367 (2001). [DOI] [PubMed] [Google Scholar]
- Shibata N. et al. Selective formation of certain advanced glycation end products in spinal cord astrocytes of humans and mice with superoxide dismutase-1 mutation. Acta Neuropathol. 104, 171–178 (2002). [DOI] [PubMed] [Google Scholar]
- Kudo A. et al. Kupffer cells alter organic anion transport through multidrug resistance protein 2 in the post-cold ischemic rat liver. Hepatology 39, 1099–1109 (2004). [DOI] [PubMed] [Google Scholar]
- Luo J., Uchida K. & Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res 30, 291–295 (2005). [DOI] [PubMed] [Google Scholar]
- Aizpurua J. M. et al. Mechanistic insights on the magnesium(II) ion-activated reduction of methyl benzoylformate with chelated NADH peptide beta-lactam models. J Org Chem 74, 6691–6702 (2009). [DOI] [PubMed] [Google Scholar]
- Sharma S., Kumar M., Kumar V. & Kumar N. Metal-free transfer hydrogenation of nitroarenes in water with vasicine: revelation of organocatalytic facet of an abundant alkaloid. J Org Chem 79, 9433–9439 (2014). [DOI] [PubMed] [Google Scholar]
- Fuentes L. et al. Direct chemical method for preparing 2,3-epoxyamides using sodium chlorite. J Org Chem 77, 5515–5524 (2012). [DOI] [PubMed] [Google Scholar]
- Oshima J. et al. Extreme fluorescence sensitivity of some aniline derivatives to aqueous and nonaqueous environments: mechanistic study and its implication as a fluorescent probe. J Phys Chem A 110, 4629–4637, 10.1021/jp0570014 (2006). [DOI] [PubMed] [Google Scholar]
- Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16, 1792–1798 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.