Abstract
The production of testosterone occurs within the Leydig cells of the testes. When production fails at this level from either congenital, acquired, or systemic disorders, the result is primary hypogonadism. While numerous testosterone formulations have been developed, none are yet fully capable of replicating the physiological patterns of testosterone secretion. Multiple stem cell therapies to restore androgenic function of the testes are under investigation. Leydig cells derived from bone marrow, adipose tissue, umbilical cord, and the testes have shown promise for future therapy for primary hypogonadism. In particular, the discovery and utilization of a group of progenitor stem cells within the testes, known as stem Leydig cells (SLCs), has led not only to a better understanding of testicular development, but of treatment as well. When combining this with an understanding of the mechanisms that lead to Leydig cell dysfunction, researchers and physicians will be able to develop stem cell therapies that target the specific step in the steroidogenic process that is deficient. The current preclinical studies highlight the complex nature of regenerating this steroidogenic process and the problems remain unresolved. In summary, there appears to be two current directions for stem cell therapy in male primary hypogonadism. The first method involves differentiating adult Leydig cells from stem cells of various origins from bone marrow, adipose, or embryonic sources. The second method involves isolating, identifying, and transplanting stem Leydig cells into testicular tissue. Theoretically, in-vivo re-activation of SLCs in men with primary hypogonadism due to age would be another alternative method to treat hypogonadism while eliminating the need for transplantation.
Keywords: Stem cell therapy, Leydig cells, Primary hypogonadism, Stem Leydig cells, Testosterone, Bone marrow-derived stem cells, Adipose-derived mesenchymal stem cells
Core tip: Although clinicians are capable of treating primary hypogonadism with exogenous testosterone, there is no therapy that mimics its physiologic release. Two current directions exist for stem cell therapy in male primary hypogonadism. The first method involves differentiating adult Leydig cells from stem cells of various origins from bone marrow, adipose, or embryonic sources. The second method involves isolating, identifying, and transplanting stem Leydig cells (SLCs) into testicular tissue. Re-activation of SLCs in men with primary hypogonadism due to age would also be an alternative method. As researchers are better able to replicate the differentiation process of androgenic tissue, treatments will hopefully follow.
INTRODUCTION
Testosterone is an essential hormone that is required for normal male physiologic development. It not only plays a role in the growth of genital organs in utero, but also initiates spermatogenesis, as well as the development of secondary sexual characteristics during puberty (Figure 1). Low testosterone, otherwise known as hypogonadism, can result from a primary defect within the testes or secondarily from a disruption in the hypothalamic-pituitary-gonadal (HPG) axis.
Figure 1.
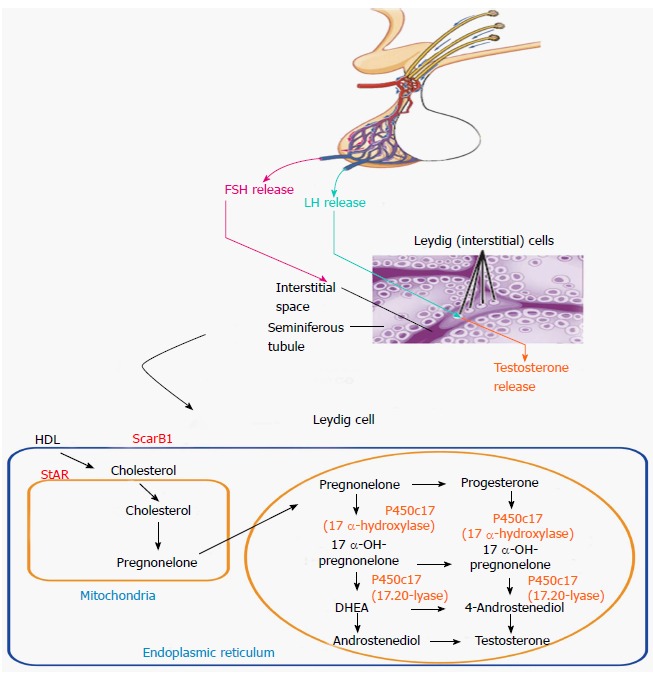
Testosterone biosynthetic pathway. FSH: Follicle stimulating hormone; LH: Luteinizing hormone; HDL: High-density lipoprotein; StAR: Steroidogenic acute regulatory protein.
Primary hypogonadism can result from a number of disorders, the most common of which is Klinefelter’s syndrome, which occurs in one in every 2500 adult males[1]. Other disorders can be separated into those that are congenital, those that are acquired, and those related to systemic conditions (Figure 2). Congenital disorders, that frequently are associated with hypogonadism, include, among others, myotonic dystrophy, Down syndrome, bilateral cryptorchidism, defects in testosterone biosynthetic enzymes, and luteinizing hormone receptor mutations. Acquired disorders include dysfunction related to aging, trauma, orchitis, and testicular failure secondary to radiation or exposure to chemotherapy. Systemic disorders include chronic liver disease, chronic kidney disease, sickle cell, and vasculitides. Age-related dysfunction, in particular, has become an intensely debated subject as physicians continue to discuss how to properly diagnose and treat hypogonadal men. This debate has led researchers to better understand the role of testosterone in the aging male, and to appreciate just how common this deficiency is in the general population. For example, in the Hypogonadism in Males study, researchers found that when using a testosterone threshold of 300 ng/dL to define hypogonadism, the overall prevalence of androgen deficiency in men over 45 years of age was 38.7%[2].
Figure 2.
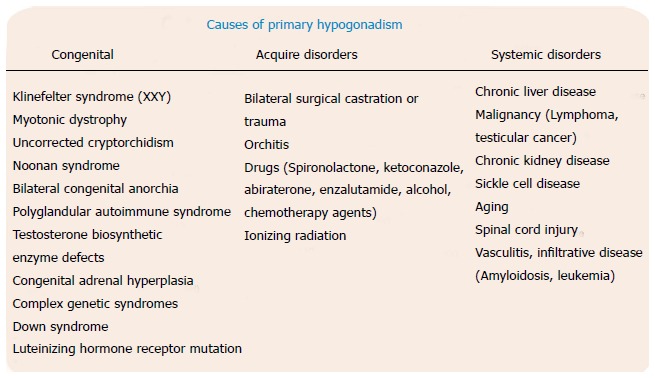
Various possible causes of primary hypogondism.
The detrimental effects that hypogonadism can have on patients are multiple. Symptomatically, patients can experience fatigue, depressed mood, decreased libido, erectile dysfunction, infertility, alterations in body composition, and decreased cognitive function. Furthermore, there is evidence to suggest that hypogonadal patients are at an increased risk for coronary artery disease, cerebral vascular disease, and metabolic syndrome[3-5]. Though numerous testosterone formulations have been developed, none are fully capable of replicating the physiological patterns of testosterone secretion from within the testes. With an understanding of Leydig cell development, and an appreciation for the mechanisms that lead to their dysfunction in some of the more commonly encountered etiologies of primary hypogonadism, we can utilize the recent advances in stem cell therapy to provide a long-lasting treatment.
NORMAL LEYDIG CELL DEVELOPMENT
Leydig cells, in the testicle, produce testosterone. They are present in clusters in the interstitium between seminiferous tubules within the testes, totaling 700 million cells. They constitute 2%-4% of testicle volume. The development of Leydig cell function comprises three stages, corresponding to the triphasic development of plasma testosterone levels (Figure 2)[6,7]. It begins in the sixth to seventh week of gestation, when fetal Leydig cells begin producing testosterone[8]. This occurs independent of luteinizing hormone (LH), from the anterior pituitary, and human chorionic gonadotropin (hCG), which is secreted from the placenta[7,9]. After seven weeks, however, hCG and LH are required for Leydig cells to produce enough testosterone for masculinization of the external genitalia[10]. The proliferation and differentiation of Leydig cells continues until 19 wk, at which time cells undergo regression[11]. The second stage begins after birth in the neonatal period, at which time a second testosterone surge occurs that is associated with the development of a second wave of Leydig cells that reach a peak at three months of age[12]. These cells are believed to be a mixture of developing Leydig cells and fetal Leydig cells. Thereafter, regression of fetal Leydig cells occurs, reaching a nadir at one year of age[13]. In addition, immature Leydig cells remain within the interstitium of the testes until activated during the third stage of development that occurs during puberty[13,14]. With the onset of puberty, the immature Leydig cells, visualized surrounding the outer peritubular layer of the seminiferous tubules and vasculature of the interstitial tissue, undergo a cytological transformation that allows for a significant production of testosterone in an LH-dependent manner[15]. This rise in testosterone will then lead to the development of secondary sexual characteristics and sexual reproduction.
STEM LEYDIG CELLS
Despite the well-understood, temporal progression of Leydig cell maturation, the origin of Leydig cells had not been fully elucidated. We now know that stem Leydig cells (SLCs) do exist, and that they are critical to the maturation process. This has been understood through studies that demonstrated the repopulation of adult Leydig cells in rat testes after being depleted by the alkylating agent, ethane dimethanesulfonate (EDS)[16-18]. In further support of this theory, it has been shown that the regeneration of cells does not result from quiescent progenitor cells that have already differentiated into Leydig cell lineage, but rather true stem cells that remain undifferentiated and have the ability to proliferate for extended periods of time without expressing Leydig cell markers[19]. In attempting to isolate these stem cells in rat models, researchers have not clearly identified the location of these cells. Some studies provide evidence to suggest that they exist within the interstitium as the pericytes and vascular smooth muscle cells along the vessel walls[20-22]. Others point towards a peritubular location, lying on the surface of the seminiferous tubules[19,23,24].
To highlight the vascular hypothesis, Davidoff et al[22] demonstrated that after destroying mature Ledyig cells, regeneration was preceded by a proliferation of nestin-expressing vascular smooth muscle cells and pericytes. Expression of nestin, an intermediate filament protein, has not only been observed in stem cells in the nervous system, but in other tissues, including the testes. The proliferating cells in this study were then capable of conversion into steroidogenic Leydig cells. Evidence for the transdifferentiation into Leydig cells was based on the coinciding expression of nestin with steroidogenic genes in newly generated Leydig cells.
In order to elucidate the peritubular hypothesis, it is important to understand that the peritubular compartment contains myofibroblasts, testicular peritubular cells (TPCs), and extracellular matrix[25]. Specifically, the TPCs contribute to testicular function by secreting paracrine factors and components of the extracellular matrix[26]. In Stanley et al[19] researchers isolated cells expressing platelet-derived growth factor receptor-α, but not 3β-hydroxysteroid dehydrogenase (3β-HSDneg) from the testes of EDS-treated adult rats. These were later determined to be the SLC. To localize these cells, the seminiferous tubules and interstitium were physically separated and cultured. During culture, the 3β-HSDneg cells on the tubule surfaces underwent divisions, eventually expressing 3β-HSD and producing testosterone. Removal of these testosterone-producing cells from the tubule surfaces, followed by further culture of the stripped tubules, resulted in their reappearance. In contrast, the interstitial compartment did not develop 3β-HSDpos cells or produce testosterone when cultured. The fact that functional Leydig cells are able to differentiate in the absence of interstitium suggests that macrophages and cells associated with blood vessels in the interstitial compartment (vascular smooth muscle cells, pericytes) may not be critical for the development of new Leydig cells, as suggested in some previous studies. These results were further corroborated in a study using human TPCs[27]. In this study, researchers demonstrated that these cells expressed pluripotency markers, SLC markers, and steroidogenic genes involved in the biosynthesis of sex steroids. Furthermore, these cells were activated to express steroidogenic enzymes that led to the production of pregnenolone and progesterone. Testosterone was not produced, but this may highlight the fact that these progenitor cells have not fully differentiated into a Leydig cell lineage.
It should be noted that the discrepancies in the location of these stem cells might result from differing conditions within the testicular tissue. It may be that SLCs reside in both peritubular and interstitial locales, as suggested by Chen et al[28]. For example, Leydig cell regeneration has been shown to occur more rapidly around regressed tubules than near tubules with normal spermatogenesis[29]. Likewise, in testes with normal spermatogenesis, regeneration appears to occur in proximity to both tubules and the interstitial blood vessels[30]. A third plausible hypothesis that researchers have put forth is that the adult Leydig cells differentiate not from stem cells, but rather from myoid cells, vascular smooth muscle cells, or pericytes that have transdifferentiated[28].
LEYDIG CELL DYSFUNCTION
Understanding the mechanisms that cause Leydig cell dysfunction will ultimately lead researchers and physicians to develop therapies that target the specific step or steps in the steroidogenic process that has been damaged, whether it is at the level of the adult Leydig cell, the Leydig stem cell, or beyond. In an attempt to further elucidate these mechanisms, we will cover those disease states that have been comprehensively studied in the lab, and those that may one day be amenable to stem cell therapy. For example, some congenital and systemic disorders leading to hypogonadism often lead to dysfunction at multiple levels in the HPG axis. Our focus will remain specifically at the level of the testis. Likewise, primary hypogonadism due to genetic mutations or enzymatic deficiencies would not be amenable to autologous stem cell transplant because the stem cells themselves would carry the mutation and/or deficiency.
Despite Klinefelter’s Syndrome being the most common abnormality of sex chromosomes that invariably leads to testicular failure, researchers have not determined what the mechanisms are that underlie the global degeneration of testicular tissue. In a recent study by D’Aurora et al[31] researchers conducted testicular gene expression profiling by a whole genome microarray approach using testicular tissue from patients with Klinefelter’s Syndrome. They found that the genes responsible for increased apoptotic processes were overexpressed. Furthermore, the data suggested that the disregulation of genes involved in the inflammation process were responsible for the high degree of fibrosis that is described in the testicular involution process of patients. They also identified the overexpression of genes central to the steroidogenic activity of the Leydig cells. This finding supports the recently demonstrated increase of intratesticular testosterone concentrations in Klinefelter patients in comparison to control patients[32]. Thus, the low testosterone serum levels commonly seen in these patients could be related to an altered release of the hormone into the bloodstream. Researchers have not yet determined whether the altered release is due to the decreased testicular vasculature commonly seen in these patients, or if it is due to some active transporter that might be involved in testosterone release from Leydig cells[32]. If it is indeed an issue of vasculature, then a cell-based therapy that regenerates not only Leydig cells but also the entire testicular microenvironment may be necessary. However, if an active transporter within the Leydig cell is identified then a more cell-specific approach would be feasible.
Interestingly, primary hypogonadism secondary to aging does not result from a loss of Leydig cells. Instead, studies have indicated that it is Leydig cell function that is lost through a process that is independent of LH secretion[33,34]. Indeed, when LH is administered in vitro to Leydig cells from aged rats, testosterone production remains significantly below that of cells from young rats[35]. Because the steroidogenic process involves a complex interplay of biochemical pathways, researchers have proposed a number of mechanisms responsible for the decreased function[36].
Critical to function is the interaction between LH, its receptor on the Leydig cell, and the subsequent production of 3’,5’-cyclic adenosine monophosphate (cAMP) initiating the steroidogenic process. Researchers have demonstrated a coupling defect of the LH receptor to adenylate cyclase, reducing cAMP production and directly inhibiting testosterone synthesis[37]. There is also evidence to suggest that increased oxidative stress plays a critical role, not only in the above-mentioned uncoupling defect, but also in cell membrane stability. With increasing age, cells experience increased levels of reactive oxygen species (ROS), due in part to the decreased levels of free radical-scavenging proteins[38-41]. With increased ROS, lipid peroxidation within the Leydig cell leads to a destruction of membrane stability[42]. Because steroidogenesis depends on this stability for cholesterol transport, testosterone synthesis is inhibited. Other studies have shown that arachidonic acid positively regulates the effects of LH on steroidogenesis[43,44]. It, however, can be metabolized by cyclooxygenase 2 (COX2). It has been suggested that with increased levels of COX2 in aged Leydig cells, there is a reduction in arachidonic acid, and thus testosterone[45]. Further corroborating the oxidative stress hypothesis, researchers have determined that phosphorylation of p38 mitogen-activated protein kinase (MAPK), may serve as the mediating interaction between increased oxidative stress and decreased steroidogenesis[46]. Relating COX2 inhibition to this theory, it is possible that phosphorylated p38 MAPK increases COX2 synthesis, in turn inhibiting steroidogenic function, although this has not been evaluated in Leydig cells[28,47].
Hypogonadism is frequently found in men who have undergone chemotherapy. While far less evidence explains how Leydig cells are affected, Al-Bader et al[48] studied how bleomycin, etoposide, and cisplatin affected the HPG axis in a rat model. They found that chemotherapy induced both Leydig cell hyperplasia and degenerative changes in Leydig cells after exposure. These degenerative changes persisted after 63 d. The question remains as to whether the observed hyperplasia resulted from activated SLCs. Given that the degenerative changes persisted after recovery, this might suggest that the chemotherapy permanently altered the SLCs. This would stand in contrast to the aging SLCs, which remain quiescent and genomically stable throughout life. Critical to an understanding of these degenerative changes, researchers measured the testicular oxidative stress, which was found to be significantly increased at the end of the chemotherapy, but returned to a normal level after the recovery time. This study went further to evaluate the expression of steroidogenic genes. They found that the two genes critical for completion of the testosterone biosynthesis pathway were downregulated, namely 17β-hydroxysteroid dehydrogenase and 3β-hydroxysteroid dehydrogenase, thus explaining the decreased testosterone levels at the end of chemotherapy. Even after the recovery time, the chemotherapy still had inhibitory effects on the transcription of these genes. However, testosterone levels did not show any significant differences with the control group, most likely due to unaffected steroidogenic acute regulatory protein (StAR) expression in the testis, which actually indicated a trend to increase. The StAR protein mediates transmembrane cholesterol transport in mitochondria, an essential rate-limiting step in testosterone synthesis[49].
Radiation also alters Leydig cell function. Sivakumar et al[50] evaluated the mechanism behind radiation-induced dysfunction by culturing Leydig cells and exposing them to different doses of fractioned gamma radiation. Researchers found that radiation exposure inhibited Leydig cell steroidogenesis in a dose-dependent manner. They found that at higher doses, radiation exposure impaired Leydig cell steroidogenesis by affecting LH signal transduction at the level of both pre- and post-cAMP generation. Just as in the chemotherapy-treated model, radiation seems to directly alter the steroidogenic pathways of the Leydig cells. However, it has not been determined how radiation affects SLCs. To fully design effective therapies, it will be important to understand the pathologic effects of radiation on SLCs. This will determine the point in the process at which time therapy will intervene.
STEM CELL THERAPY
Multiple stem cell therapies to restore androgenic function of the testes are under investigation (Table 1). Leydig cells derived from bone marrow, adipose tissue, umbilical cord, and the testes have shown promise in future therapy for primary hypogonadism. An initial study by Lue et al[51] injected unfractionated bone marrow cells into the seminiferous tubules and testicular interstitium of mice. The results demonstrated that the murine bone marrow cells had the potential to differentiate into germ cells, Sertoli, and Leydig cells in vivo. However, it was unknown which precursor cell from the bone marrow differentiated into each end testicular cell type. Lo et al[52] demonstrated that murine testicular stem cells, isolated from the interstitial space of the testis and transplanted into the interstitial space of LH receptor knockout mice, yielded a time dependent production of testosterone in a hypogonadal murine model. Yet, these cells were derived from a side population and contained stem cells of multiple lineages including spermatogonial stem cells, SLCs, and possibly myoid stem cells. As in the previous study, it was difficult to determine which cell lineage led to the final end testosterone-secreting cell.
Table 1.
Summary of preclinical trials showing successful differentiation of various stem cell lines into steroidogenic Leydig-like cells
| Ref. | Stem cells used | Study type | Design | Results |
| Lo et al[52] | Mouse mixed testicular stem cells (SP) containing spermatogonial, leydig cell, and myoid stem cells | In vivo | SCs were injected into the testes of sterile sertoli-cell only transgenic mice and transgenic mice with a targeted deletion of 4-kb pairs of the LH receptor gene | SP cell transplanted mice had increased time-dependent serum testosterone and spermatogenesis compared to non-SP cell transplanted mice |
| Yazawa et al[53] | Rat BM-MSCs | In vivo | BM-MSCs were injected into the testes of 3-wk old Sprague-Dawley rats | BM-MSCs differentiated into steroidogenic cells similar to Leydig cells |
| Mouse MSCs | MSCs were transfected with Sf-1 followed by treatment with cAMP and cultured in Iscova’s MEM or DMEM with 10% fetal calf serum | Transfected cells differentiated into Leydig cells | ||
| Lue et al[51] | Unfractionated mouse bone marrow stem cells | In vitro | SCs were injected into the testes of busulfan treated mice and c-kit mutant homozygous mice | SCs differentiated into Leydig, Sertoli, and germ cells after 12 wk. Though germ cells were lacking in c-kit mutant mice |
| Gondo et al[63] | Mouse AMCs | In vitro | AMCs and BMCs were transfected with SF-1 and cultured with Medium A | AMCs were more likely to differentiate into adrenal-type steroidogenic cells with increased production of corticosterone |
| Mouse BMCs | BMCs were more likely to differentiate into gonadal-type steroidogenic cells with increased production of testosterone | |||
| Yazawa et al[54] | Human BM-MSCs | In vitro | BM-MSCs were transfected with LRH-1 followed by treatment with cAMP and cultured in DMEM with 10% fetal calf serum | Transfected cells expressed CYP17 and produced testosterone |
| Yazawa et al[55] | UC-MSCs | In vitro | UC-MSCs were transfected with SF-1 followed by treatment with cAMP and cultured in DMEM/Ham’s F-12 supplemented with 0.1% BSA | Transfected cells differentiated into cells with similar characteristics to granulosa-luteal cells |
| Wei et al[62] | Human UC-MSCs | In vitro | UC-MSCs and BM-MSCs were transfected with SF-1 and cultured in the presence of cAMP | Differentiated UC-MSCs had higher expression of steroidogenic mRNAs. They also secreted significantly greater amounts of testosterone and cortisol than BM-MSCs |
| Human BM-MSCs | ||||
| Yazawa et al[64] | Rat BM-MSCs | In vivo | BM-MSCs were transplanted into prepubertal testes | MSCs were able to differentiate into steroidogenic Leydig cells in vivo. SF-1 expression was also detected |
| Yang et al[56] | Rat ADSCs | In vivo | ADSCs were injected into Sprague-dawley rats that had been treated with D-gal (aging model) or saline (control) for 8 wk | ADSCs migrated to damaged areas, reduced the number of apoptotic Leydig cells, and upregulated enzymes to increase testosterone levels in the testis in those treated with D-gal |
| Yang et al[58] | Mouse ESCs | In vivo | ESCs were cultured with cAMP, SF-1, and FSK. These derived Leydig-like cells were then injected into Sprague-dawley rats treated with EDS | FSK enhanced the differentiation of mESCs into Leydig-like cells. Subsequent treatment with these newly differentiated cells led to increased testosterone levels in EDS-treated rats |
| Hou et al[57] | Human BM-MSCs | In vitro | Experimental - BM-MSCs were cultured in conditional medium with different concentrations of HMG/LH Control - BMSCs were cultured in FBS in DMEM medium with normal sodium | Experimental culture medium induced the differentiation of BMSCs into Leydig cells |
| Zhang et al[59] | Rat SLCs | In vitro | SLCs were cultured in a seminiferous tubule model using media containing NGF. The proliferative capacity of SLCs, along with testosterone production, and steroidogenic gene/protein expression was measured | NGF significantly promoted the proliferation of stem Leydig cells and also induced steroidogenic enzyme gene expression and 3β-HSD protein expression |
| Odeh et al[60] | Rat SLCs | In vitro | SLCs were cultured on the surfaces of seminiferous tubules in a media containing PDGF-AA or PDGF-BB for up to 4 wk. SLC proliferation and differentiation were measured | Both PDGF-AA and PDGF-BB stimulated SLC proliferation during the first week of culture. After this first week, PDGF-AA had a stimulatory effect on SLC differentiation. PDGF-BB began inhibiting differentiation after this first week |
| Li et al[61] | Rat SLCs | In vitro | SLCs were cultured on the surface of seminiferous tubules to assess the ability of factors from the seminiferous tubules to regulate their proliferation and their subsequent entry into the Leydig cell lineage | SLC proliferation was stimulated by DHH, FGF2, PDGF, and activin. Differentiation was activated by DHH, lithium-induced signaling, and activin, and inhibited by TGF-β, PDGF-BB, and FGF2 |
BM-MSCs: Bone marrow-derived mesenchymal stem cells; AMCs: Adipose derived mesenchymal cells; BMCs: Bone marrow cells; UC-MSCs: Umbilical cord mesenchymal stem cells; ADSCs: Adipose-derived mesenchymal stem cells; ESCs: Embryonic stem cells; SLCs: Stem leydig cells; LH: Luteinizing hormone; HMG: Human menopausal gonadotropin; FBS: Fetal bovine serum; PDGF-AA: Platelet-derived growth factor alpha; DHH: Desert hedgehog; FGF: Fibroblast growth factor; LRH-1: Liver receptor homolog-1; SF-1: Steroidogenic factor-1; BSA: Bovine serum albumin; cAMP: Cyclic adenosine monophosphate; EDS: Ethane dimethanesulfonate; NGF: Nerve growth factor.
Yazawa et al[53] injected murine bone marrow-derived mesenchymal cells (BMSCs) into murine testis and demonstrated their differentiation into Leydig cells. They also demonstrated that the same murine BMSCs, when cultured in vitro with steroidogenic factor -1 (SF-1) followed by cAMP stimulation, underwent differentiation into Leydig cells. However, when this group cultured human BMSCs with SF-1 followed by cAMP, the cells differentiated into human-derived steroidogenic cells that preferentially produced glucocorticoids, rather than testosterone. Furthermore, when the group injected human BMSCs into murine testis, the cells did not survive long enough for analysis. Researchers hypothesized that the differing steroidogenic products observed in the mouse and human BMSCs were due to heterogeneous populations of stem cells that had different differentiation potentials. Thus, the mouse BMSCs had already committed to the gonadal lineage, whereas the human BMSCs were already committed to the adrenal lineage. This group later demonstrated that human BMSC differentiation into steroidogenic cells was possible with cAMP and liver receptor homolog-1 (LRH-1), rather than cAMP and SF-1, indicating another possible regulator of Leydig stem cell differentiation[54]. Interestingly, when this group used the method of SF-1 and cAMP on umbilical cord blood-derived MSCs, these steroidogenic cells had similar characteristics of granulosa-luteal cells[55]. These studies highlighted the fact that stem cells from multiple species have the potential to differentiate into different types of steroidogenic cells.
Yang et al[56] administered adipose-derived mesenchymal stem cells (ADSCs) into the caudal vein of a D-galactose aging rat model. D-galactose accelerates aging and causes symptoms simulating natural senescence, thus creating an ideal pathophysiological model for evaluating stem cell therapy. This group found that ADSCs migrated to damaged areas of the testes, reduced the number of apoptotic Leydig cells, and increased serum testosterone. The authors suggested that the ADSCs might prevent ROS production and reduce SLC apoptosis. Supporting this line of reasoning, they found that the increased testicular lipid peroxidation in the aged model was reversed by a subsequent increase in antioxidant enzymes after ADSC therapy. As has been observed in other disease states treated by ADSCs, the mechanism of action is more likely due to a secretion of cytokines and growth factors, with little direct effect on stem cell differentiation. As proof, only a few ADSCs differentiated into new Leydig cells based on labeling and 3β-HSD expression, while serum testosterone concentrations increased progressively. The immunohistochemical results of the present study suggest that the treatment effect of ADSCs is mediated, at least in part, by a decrease in intracorporal tissue apoptosis and increase in sinusoidal endothelial cells.
Researchers have also been able to manipulate the hormonal milieu to induce the differentiation of human BMSCs into Leydig cells in vitro. By using a medium containing human menopausal gonadotropin/luteinizing hormone, hCG, platelet-derived growth factor, and interleukin-1α, they were able to promote the differentiation of human BMSCs into Leydig cells. However, the cells exhibited senescence and, thus, androgen decline after three weeks of culture. These results highlight the aforementioned problem that Leydig cells are mitotically inactive and that the primary immature Leydig cells lose their desired characteristics during prolonged cultures[57]. Using murine embryonic stem cells, one study used SF-1, 8-bromoadenosine-3’,5’-cyclic monophosphate (8-Br-cAMP), and forskolin to direct differentiation towards Leydig like cells. In vitro, these cells produced progesterone and testosterone. When injected into EDS-treated rat testes, these cells improved serum testosterone levels. However, this research group was plagued by a difficulty in obtaining a large enough number Leydig-like cells given that they do not proliferate as readily as the undifferentiated cells[58]. Despite using different stem cells reservoirs, bone marrow, and embryonic stem cells, neither group was able to produce mitotically active Leydig cells.
And while there have been numerous studies evaluating the use of stem cells from various tissue origins to regenerate mature Leydig cells, few have attempted to reactivate SLCs. However, there are select studies that in exploring the mechanisms that underlie the SLC maturation process, have found growth factors that lead to reactivation. An initial study explored the role of nerve growth factor (NGF) during SLC differentiation[59]. They found that in an in vitro model, NGF significantly promoted the proliferation of SLCs and also induced steroidogenic enzyme gene expression and 3β-HSD protein expression. Another group evaluated platelet-derived growth factor alpha (PDGF-AA) and beta (PDGF-BB)[60]. They found that both ligands stimulated SLC proliferation during the first week of culture. After this first week, PDGF-AA had a stimulatory effect on SLC differentiation. In contrast PDGF-BB, began inhibiting differentiation after this first week. Corroborating some of the results of this study, another group developed an in vitro system of cultured seminiferous tubules to assess the ability of factors from the seminiferous tubules to regulate the proliferation and differentiation of SLCs[61]. SLC proliferation was stimulated by Desert hedgehog (DHH), basic fibroblast growth factor (FGF2), platelet-derived growth factor (PDGF), and activin. Differentiation of the stem cells was activated by DHH, lithium-induced signaling, and activin, and inhibited by TGF-β, PDGF-BB, and FGF2. Building upon these initial studies, it will be necessary to evaluate these growth factors in an in vivo animal model.
CONCLUSION
There appears to be two current directions for stem cell therapy in male primary hypogonadism. The first method involves differentiating adult Leydig cells from stem cells of various origins from bone marrow, adipose, or embryonic sources. The second method involves isolating, identifying, and transplanting SLCs into testicular tissue. The first method’s shortcomings that should be resolved in future studies include decoding and promoting stem cells to become testosterone-producing steroidogenic cells and improving the mitotic activity of differentiated Leydig cells. One study compared steroidogenic cells from BMSC to those of umbilical cord mesenchymal stem cells and found that umbilical cord mesenchymal stem cells have a greater steroidogenic potential[62]. However, as previously mentioned, the addition of SF-1 and cAMP in vitro to umbilical cord stem cells has been shown to yield cells resembling granulosa-luteal cells, not Leydig-like cells[55]. Another study demonstrated that the addition of SF-1 and cAMP to ADSCs yielded cells that preferentially produced corticosterone, rather than testosterone[63]. Undoubtedly much remains unknown about the cellular environment needed to produce specific steroidogenic cell types[64]. Additionally, this type of therapy may not be durable due to adult Leydig cell senescence and androgen production decline. Younger patients who have undergone premature Leydig cell dysfunction due to chemotherapy and radiation may find long-term success with the transplantation of cells with more regenerative capacity. Alternatively, in the aging population, it might be feasible to differentiate mesenchymal stem cells into SLCs. If this strategy would address the issue of growth arrest, the use of mesenchymal stem cells may be in the best interest of these patients, whose SLCs are likely damaged. Finally, there are also concerns about the delivery of SF-1, which is currently performed episomally or virally. Efforts are underway to determine a method of gene-free delivery of inducing SF-1 and LRH-1 expression[64,65].
The second method for stem cell therapy involves the transplantation of SLCs into hypogonadal testicular tissue, with the idea that this therapy’s regenerative capacity will be self-fulfilling and could be used for younger patients. However, it is currently troubled by the technique for identification and isolation of SLCs, which is in its infancy[66]. Additionally, if the transplant were to be autologous in these men, SLCs could be extracted prior to chemotherapy and radiation, as it is likely that these treatments irreversibly damage SLCs. However, another method that has been proposed includes harvesting SLCs in the hypogonadal male, and then amplifying and differentiating these cells into adult Leydig cells in vitro, then transplanting autologously into the same man[66]. Theoretically, the in vivo re-activation of SLCs in men with primary hypogonadism due to age would be an alternative method to treat hypogonadism, while eliminating the need for transplantation.
These proposed mechanisms all have the advantage of being subject to physiologic cues, standing in contrast to the current option of a lifetime of exogenously administered testosterone. Current and future research collaborations in the field of male hypogonadism and the regeneration of steroidogenic tissue will influence which modalities will become clinical realities for this patient population.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest regarding this manuscript.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: May 3, 2016
First decision: June 13, 2016
Article in press: August 29, 2016
P- Reviewer: Chen LY, Zhang Q, Zou ZM S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88:622–626. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769. doi: 10.1111/j1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94:2353–2359. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 4.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, Milani RV, Sagalowsky AI, Smith MR, Zakai N. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60:194–201. doi: 10.3322/caac.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikova I, Söder O. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93–101. doi: 10.1159/000277141. [DOI] [PubMed] [Google Scholar]
- 7.Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179:47–74. doi: 10.1016/S0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- 8.Tapanainen J, Kellokumpu-Lehtinen P, Pelliniemi L, Huhtaniemi I. Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J Clin Endocrinol Metab. 1981;52:98–102. doi: 10.1210/jcem-52-1-98. [DOI] [PubMed] [Google Scholar]
- 9.Kremer H, Kraaij R, Toledo SP, Post M, Fridman JB, Hayashida CY, van Reen M, Milgrom E, Ropers HH, Mariman E. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet. 1995;9:160–164. doi: 10.1038/ng0295-160. [DOI] [PubMed] [Google Scholar]
- 10.Rabinovici J, Jaffe RB. Development and regulation of growth and differentiated function in human and subhuman primate fetal gonads. Endocr Rev. 1990;11:532–557. doi: 10.1210/edrv-11-4-532. [DOI] [PubMed] [Google Scholar]
- 11.Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- 12.Prince FP. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol. 2001;168:213–216. doi: 10.1677/joe.0.1680213. [DOI] [PubMed] [Google Scholar]
- 13.Nistal M, Paniagua R, Regadera J, Santamarìa L, Amat P. A quantitative morphological study of human Leydig cells from birth to adulthood. Cell Tissue Res. 1986;246:229–236. doi: 10.1007/BF00215884. [DOI] [PubMed] [Google Scholar]
- 14.Prince FP. Ultrastructural evidence of mature Leydig cells and Leydig cell regression in the neonatal human testis. Anat Rec. 1990;228:405–417. doi: 10.1002/ar.1092280406. [DOI] [PubMed] [Google Scholar]
- 15.Chemes HE, Gottlieb SE, Pasqualini T, Domenichini E, Rivarola MA, Bergadá C. Response to acute hCG stimulation and steroidogenic potential of Leydig cell fibroblastic precursors in humans. J Androl. 1985;6:102–112. doi: 10.1002/j.1939-4640.1985.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 16.Molenaar R, de Rooij DG, Rommerts FF, Reuvers PJ, van der Molen HJ. Specific destruction of Leydig cells in mature rats after in vivo administration of ethane dimethyl sulfonate. Biol Reprod. 1985;33:1213–1222. doi: 10.1095/biolreprod33.5.1213. [DOI] [PubMed] [Google Scholar]
- 17.Kerr JB, Donachie K, Rommerts FF. Selective destruction and regeneration of rat Leydig cells in vivo. A new method for the study of seminiferous tubular-interstitial tissue interaction. Cell Tissue Res. 1985;242:145–156. doi: 10.1007/BF00225571. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- 19.Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Chen H. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153:5002–5010. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidoff MS, Middendorff R, Müller D, Holstein AF. The neuroendocrine Leydig cells and their stem cell progenitors, the pericytes. Adv Anat Embryol Cell Biol. 2009;205:1–107. [PubMed] [Google Scholar]
- 21.Kerr JB, Bartlett JM, Donachie K, Sharpe RM. Origin of regenerating Leydig cells in the testis of the adult rat. An ultrastructural, morphometric and hormonal assay study. Cell Tissue Res. 1987;249:367–377. doi: 10.1007/BF00215521. [DOI] [PubMed] [Google Scholar]
- 22.Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci USA. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landreh L, Stukenborg JB, Söder O, Svechnikov K. Phenotype and steroidogenic potential of PDGFRα-positive rat neonatal peritubular cells. Mol Cell Endocrinol. 2013;372:96–104. doi: 10.1016/j.mce.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Cigorraga SB, Chemes H, Pellizzari E. Steroidogenic and morphogenic characteristics of human peritubular cells in culture. Biol Reprod. 1994;51:1193–1205. doi: 10.1095/biolreprod51.6.1193. [DOI] [PubMed] [Google Scholar]
- 26.Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB, Mayerhofer A. Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-alpha. Endocrinology. 2008;149:1678–1686. doi: 10.1210/en.2007-1064. [DOI] [PubMed] [Google Scholar]
- 27.Landreh L, Spinnler K, Schubert K, Häkkinen MR, Auriola S, Poutanen M, Söder O, Svechnikov K, Mayerhofer A. Human testicular peritubular cells host putative stem Leydig cells with steroidogenic capacity. J Clin Endocrinol Metab. 2014;99:E1227–E1235. doi: 10.1210/jc.2013-4199. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Stanley E, Jin S, Zirkin BR. Stem Leydig cells: from fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90:272–283. doi: 10.1002/bdrc.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Shaughnessy PJ, Morris ID, Baker PJ. Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction. 2008;135:851–858. doi: 10.1530/REP-07-0529. [DOI] [PubMed] [Google Scholar]
- 30.Yan W, Kero J, Huhtaniemi I, Toppari J. Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev Biol. 2000;227:169–182. doi: 10.1006/dbio.2000.9885. [DOI] [PubMed] [Google Scholar]
- 31.D’Aurora M, Ferlin A, Di Nicola M, Garolla A, De Toni L, Franchi S, Palka G, Foresta C, Stuppia L, Gatta V. Deregulation of sertoli and leydig cells function in patients with Klinefelter syndrome as evidenced by testis transcriptome analysis. BMC Genomics. 2015;16:156. doi: 10.1186/s12864-015-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuttelmann F, Damm OS, Luetjens CM, Baldi M, Zitzmann M, Kliesch S, Nieschlag E, Gromoll J, Wistuba J, Simoni M. Intratesticular testosterone is increased in men with Klinefelter syndrome and may not be released into the bloodstream owing to altered testicular vascularization- a preliminary report. Andrology. 2014;2:275–281. doi: 10.1111/j.2047-927.2014.00190.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15:551–557. doi: 10.1002/j.1939-4640.1994.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 34.Sokanovic SJ, Janjic MM, Stojkov NJ, Baburski AZ, Bjelic MM, Andric SA, Kostic TS. Age related changes of cAMP and MAPK signaling in Leydig cells of Wistar rats. Exp Gerontol. 2014;58:19–29. doi: 10.1016/j.exger.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Sinha Hikim AP, Lue YH, Leung A, Baravarian S, Swerdloff RS. Reproductive aging in the Brown Norway rat is characterized by accelerated germ cell apoptosis and is not altered by luteinizing hormone replacement. J Androl. 1999;20:509–518. doi: 10.1002/j.1939-4640.1999.tb02550.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Ge RS, Zirkin BR. Leydig cells: From stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Liu J, Luo L, Zirkin BR. Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology. 2004;145:4441–4446. doi: 10.1210/en.2004-0639. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36:1361–1373. doi: 10.1016/S0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Irizarry RA, Luo L, Zirkin BR. Leydig cell gene expression: effects of age and caloric restriction. Exp Gerontol. 2004;39:31–43. doi: 10.1016/j.exger.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Duan T, Fan K, Chen S, Yao Q, Zeng R, Hong Z, Peng L, Shao Y, Yao B. Role of peroxiredoxin 2 in H2O2induced oxidative stress of primary Leydig cells. Mol Med Rep. 2016;13:4807–4813. doi: 10.3892/mmr.2016.5147. [DOI] [PubMed] [Google Scholar]
- 41.Matzkin ME, Miquet JG, Fang Y, Hill CM, Turyn D, Calandra RS, Bartke A, Frungieri MB. Alterations in oxidative, inflammatory and apoptotic events in short-lived and long-lived mice testes. Aging (Albany NY) 2016;8:95–110. doi: 10.18632/aging.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Ronco AM, Moraga PF, Llanos MN. Arachidonic acid release from rat Leydig cells: the involvement of G protein, phospholipase A2 and regulation of cAMP production. J Endocrinol. 2002;172:95–104. doi: 10.1677/joe.0.1720095. [DOI] [PubMed] [Google Scholar]
- 44.Wang XJ, Dyson MT, Mondillo C, Patrignani Z, Pignataro O, Stocco DM. Interaction between arachidonic acid and cAMP signaling pathways enhances steroidogenesis and StAR gene expression in MA-10 Leydig tumor cells. Mol Cell Endocrinol. 2002;188:55–63. doi: 10.1016/S0303-7207(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Luo L, Liu J, Zirkin BR. Cyclooxygenases in rat Leydig cells: effects of luteinizing hormone and aging. Endocrinology. 2007;148:735–742. doi: 10.1210/en.2006-0925. [DOI] [PubMed] [Google Scholar]
- 46.Abidi P, Zhang H, Zaidi SM, Shen WJ, Leers-Sucheta S, Cortez Y, Han J, Azhar S. Oxidative stress-induced inhibition of adrenal steroidogenesis requires participation of p38 mitogen-activated protein kinase signaling pathway. J Endocrinol. 2008;198:193–207. doi: 10.1677/JOE-07-0570. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Sakamoto K. Reactive oxygen species up-regulates cyclooxygenase-2, p53, and Bax mRNA expression in bovine luteal cells. Biochem Biophys Res Commun. 2001;284:203–210. doi: 10.1006/bbrc.2001.4927. [DOI] [PubMed] [Google Scholar]
- 48.Al-Bader M, Kilarkaje N. Effects of bleomycin, etoposide and cisplatin treatment on Leydig cell structure and transcription of steroidogenic enzymes in rat testis. Eur J Pharmacol. 2015;747:150–159. doi: 10.1016/j.ejphar.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sivakumar R, Sivaraman PB, Mohan-Babu N, Jainul-Abideen IM, Kalliyappan P, Balasubramanian K. Radiation exposure impairs luteinizing hormone signal transduction and steroidogenesis in cultured human leydig cells. Toxicol Sci. 2006;91:550–556. doi: 10.1093/toxsci/kfj178. [DOI] [PubMed] [Google Scholar]
- 51.Lue Y, Erkkila K, Liu PY, Ma K, Wang C, Hikim AS, Swerdloff RS. Fate of bone marrow stem cells transplanted into the testis: potential implication for men with testicular failure. Am J Pathol. 2007;170:899–908. doi: 10.2353/ajpath.2007.060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo KC, Lei Z, Rao ChV, Beck J, Lamb DJ. De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of leydig stem cells. Endocrinology. 2004;145:4011–4015. doi: 10.1210/en.2003-1729. [DOI] [PubMed] [Google Scholar]
- 53.Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–4111. doi: 10.1210/en.2006-0162. [DOI] [PubMed] [Google Scholar]
- 54.Yazawa T, Inanoka Y, Mizutani T, Kuribayashi M, Umezawa A, Miyamoto K. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology. 2009;150:3885–3893. doi: 10.1210/en.2008-1310. [DOI] [PubMed] [Google Scholar]
- 55.Yazawa T, Inaoka Y, Okada R, Mizutani T, Yamazaki Y, Usami Y, Kuribayashi M, Orisaka M, Umezawa A, Miyamoto K. PPAR-gamma coactivator-1alpha regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endocrinol. 2010;24:485–496. doi: 10.1210/me.2009-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang C, Du YK, Wang J, Luan P, Yang QL, Huang WH, Yuan L. Transplanted Adipose-Derived Stem Cells Ameliorate Testicular Dysfunction In A D-Galactose-Induced Aging Rat Model. J Cell Physiol. 2015;230:2403–2414. doi: 10.1002/jcp.24970. [DOI] [PubMed] [Google Scholar]
- 57.Hou L, Dong Q, Wu YJ, Sun YX, Guo YY, Huo YH. Gonadotropins facilitate potential differentiation of human bone marrow mesenchymal stem cells into Leydig cells in vitro. Kaohsiung J Med Sci. 2016;32:1–9. doi: 10.1016/j.kjms.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Su Z, Xu W, Luo J, Liang R, Xiang Q, Zhang Q, Ge RS, Huang Y. Directed mouse embryonic stem cells into leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cells Dev. 2015;24:459–470. doi: 10.1089/scd.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, Wang H, Yang Y, Liu H, Zhang Q, Xiang Q, Ge R, Su Z, Huang Y. NGF induces adult stem Leydig cells to proliferate and differentiate during Leydig cell regeneration. Biochem Biophys Res Commun. 2013;436:300–305. doi: 10.1016/j.bbrc.2013.05.098. [DOI] [PubMed] [Google Scholar]
- 60.Odeh HM, Kleinguetl C, Ge R, Zirkin BR, Chen H. Regulation of the proliferation and differentiation of Leydig stem cells in the adult testis. Biol Reprod. 2014;90:123. doi: 10.1095/biolreprod.114.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Wang Z, Jiang Z, Guo J, Zhang Y, Li C, Chung J, Folmer J, Liu J, Lian Q, et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci USA. 2016;113:2666–2671. doi: 10.1073/pnas.1519395113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei X, Peng G, Zheng S, Wu X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012;45:101–110. doi: 10.1111/j.1365-2184.2012.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gondo S, Okabe T, Tanaka T, Morinaga H, Nomura M, Takayanagi R, Nawata H, Yanase T. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149:4717–4725. doi: 10.1210/en.2007-1808. [DOI] [PubMed] [Google Scholar]
- 64.Yazawa T, Imamichi Y, Miyamoto K, Umezawa A, Taniguchi T. Differentiation of mesenchymal stem cells into gonad and adrenal steroidogenic cells. World J Stem Cells. 2014;6:203–212. doi: 10.4252/wjsc.v6.i2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruiz-Babot G, Hadjidemetriou I, King PJ, Guasti L. New directions for the treatment of adrenal insufficiency. Front Endocrinol (Lausanne) 2015;6:70. doi: 10.3389/fendo.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Ge R, Hardy MP. Androgen-forming stem Leydig cells: identification, function and therapeutic potential. Dis Markers. 2008;24:277–286. doi: 10.1155/2008/905025. [DOI] [PMC free article] [PubMed] [Google Scholar]


