Abstract
Trihelix genes play important roles in plant growth and development and responses to biotic and abiotic stresses. Here, we identified 56 full-length trihelix genes in Populus trichocarpa and classified them into five groups. Most genes within a given group had similar gene structures and conserved motifs. The trihelix genes were unequally distributed across 19 different linkage groups. Fifteen paralogous pairs were identified, 14 of which have undergone segmental duplication events. Promoter cis-element analysis indicated that most trihelix genes contain stress- or phytohormone-related cis-elements. The expression profiles of the trihelix genes suggest that they are primarily expressed in leaves and roots. Quantitative real-time reverse transcription polymerase chain reaction analysis indicated that members of the trihelix gene family are significantly induced in response to osmotic, abscisic acid, salicylic acid, methyl jasmonate and pathogen infection. PtrGT10 was identified as a target gene of miR172d, which is involved in the osmotic response. Repression of PtrGT10 could increase reactive oxygen species scavenging ability and decrease cell death. This study provides novel insights into the phylogenetic relationships and functions of the P. trichocarpa trihelix genes, which will aid future functional studies investigating the divergent roles of trihelix genes belonging to other species.
Trihelix proteins comprise one of the first families of transcription factors discovered in plants and are classified as GT factors due to their binding specificity for GT elements1. GT elements are highly degenerated, and the deduced consensus core sequence is 5′-G-Pu-(T/A)-A-A-(T/A)3′2. To date, 30 trihelix proteins have been identified in Arabidopsis, and 31 have been identified in rice. The Arabidopsis trihelix proteins are grouped into five classes, namely, GT-1, GT-2, SH-4, GTγ and SIP1. Each class is named after the corresponding founding member3. The DNA-binding domain of GT factors features a typical trihelix (helix-loop-helix-loop-helix) structure. Although each trihelix subfamily has at least one trihelix structure, small differences still exist. For example, in the GT-2 and GTγ subfamilies, a conserved tryptophan is replaced by phenylalanine (F), while in the SIP1 subfamily, it is replaced by isoleucine (I).
Trihelix transcription factors play important roles in the regulation of developmental processes involving flowers4, trichomes, stomata, seed abscission layers and late embryogenesis and in responses to biotic and abiotic stresses5,6,7 or to treatments with phytohormones such as abscisic acid (ABA) or salicylic acid (SA)8. The Arabidopsis gene PETAL LOSS (PTL), which belongs to the GT-2 group, regulates petal and sepal growth as well as sepal fusion9. A pair of Arabidopsis genes in the GT-1 clade, GT-3a and GT-3b, have been shown to respond to salt and pathogen stress10. A GT-factor in rice, OsRML1, was reported to be induced in response to the rice pathogen Magnaporthe grisea11. The Arabidopsis GT-2 Like 1 (ATGTL1) gene negatively regulates water use efficiency by modulating stomatal density, with mutations leading to increased plant tolerance to drought stress12. Two genes encoding putative GT-2-type proteins in soybean (GmGT-2A and GmGT-2B) were shown to stimulate enhanced tolerance to salt, drought and freezing stresses upon overexpression in transgenic Arabidopsis plants13.
Poplar (Populus trichocarpa) trees are perennial woody deciduous plants with significant commercial and ecological value14. Studies of poplars have become prevalent in recent years because of their economic significance for pulp and biofuel production. Poplars are frequently threatened by environmental stresses such as drought, cold and salt. Although trihelix genes have been investigated in Arabidopsis, rice, and soybean, studies in poplar are still limited. To date, only one trihelix gene has been studied functionally in poplars, and the results suggest that trihelix genes may play important roles in the drought response15. Therefore, there is a need for a comprehensive analysis of this protein family in poplars.
In this study, we report the genome-wide identification, phylogenetic analysis, gene structure and promoter cis-elements of 56 novel trihelix genes in P. trichocarpa. We measured gene expression during biotic and abiotic stresses and phytohormone treatments. We also identified a target gene of miR172d, PtrGT10, which may function during osmotic stress. Our preliminary results provide novel insights into the roles of trihelix genes in the poplar’s responses to biotic and abiotic stresses and phytohormone treatments that will aid future studies.
Results
Identification of trihelix genes in Populus
The Phytozome V10.3 and NCBI databases were used to identify trihelix genes in the P. trichocarpa genome. In total, 80 candidate trihelix genes were identified, all of which were examined for the presence of the trihelix domain (PF13837, SM00717) using the Pfam 28.0 and SMART databases. These genes were then compared with the trihelix gene family in PlnTFDB V3.0, which contained 78 candidate trihelix genes. By removing repetitive and redundant genes, we finally identified 56 putative trihelix genes in P. trichocarpa. The number of trihelix genes in P. trichocarpa is much higher than those in Arabidopsis and rice (30 and 31, respectively)3. According to the nomenclature of trihelix genes in Arabidopsis16 and soybean13, we named the 56 Populus trihelix genes PtrGT1 to PtrGT56. Of the 56 trihelix genes, 39 had only one gene product, while the remaining 17 genes (30%) had multiple gene products, which could be attributed to alternative splicing according to the Phytozome V10.3 database. This percentage was similar to that of the C2H2 gene family (26%) in P. trichocarpa17. Among these 17 genes, 15 encoded two possible cDNAs, while PtrGT24 and PtrGT37 each encoded three possible transcripts (Supplementary Table S1).
The proteins encoded by these 56 trihelix genes ranged from 251 to 994 amino acids (aa) in length, with an average length of 453 aa. The trihelix protein sequences showed large variations in isoelectric point (pI) values (ranging from 4.29 to 10.36) and molecular weight (ranging from 51.475 kDa to 111.294 kDa). Localization predictions made with the Wolf PSORT database classified 50 of the trihelix proteins as nuclear proteins, five as cytoplasmic or chloroplast proteins and only one as a peroxisomal protein (PtrGT53). Additional information about the 56 trihelix genes in P. trichocarpa is provided in Supplementary Table S2 and Supplementary Data S1.
Phylogenetic analysis and gene structure of the trihelix gene family
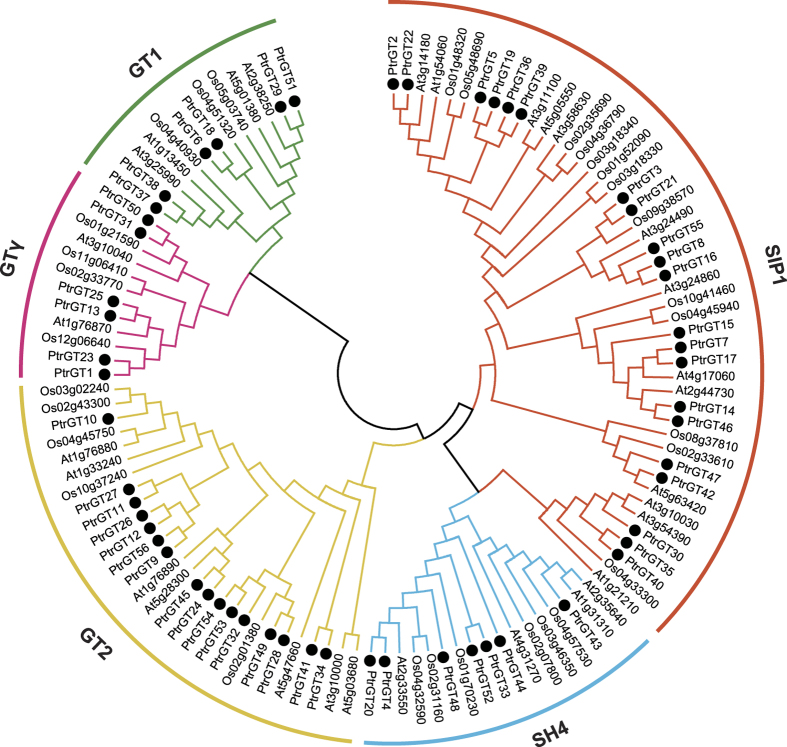
An unrooted phylogenetic tree was constructed using the full-length protein sequences of P. trichocarpa, Arabidopsis and rice (Fig. 1). The P. trichocarpa trihelix genes grouped into five subgroups (GT-1, GTγ, GT-2, SH4, and SIP1) according to the previous study in Arabidopsis18, with six GT-1, six GTγ, 16 GT-2, seven SH4, and 21 SIP1 members. Based on this analysis, 15 pairs of paralogous genes were identified, all of which had strong bootstrap support (>90%) (Table 1). A multiple sequence alignment is shown in Supplementary Fig. S1 and shows similar characteristics as previously observed3.
Figure 1. Phylogenetic tree of trihelix proteins from Populus, Arabidopsis and rice.
Full-length amino acid sequences were aligned by ClustalX 1.83, and the NJ tree was constructed by MEGA5.0 with 1000 bootstrap replicates. Each trihelix subfamily is indicated in a specific color. Populus trihelix proteins are marked with solid dots.
Table 1. Divergence between trihelix gene pairs.
| Paralogous pairs | Ks | Ka | Ka/Ks | Duplicate type |
|---|---|---|---|---|
| PtrGT1-PtrGT23 | 0.2596 | 0.0912 | 0.3512 | Segmental |
| PtrGT2-PtrGT22 | 0.5016 | 0.1202 | 0.2395 | Segmental |
| PtrGT3-PtrGT21 | 0.3401 | 0.0834 | 0.2454 | Segmental |
| PtrGT4-PtrGT20 | 0.2943 | 0.0496 | 0.1684 | Segmental |
| PtrGT5-PtrGT19 | 0.3053 | 0.0447 | 0.1463 | Segmental |
| PtrGT6-PtrGT18 | 0.2970 | 0.0524 | 0.1763 | Segmental |
| PtrGT8-PtrGT16 | 0.3541 | 0.0777 | 0.2195 | Segmental |
| PtrGT11-PtrGT27 | 0.1915 | 0.0935 | 0.4882 | Segmental |
| PtrGT12-PtrGT26 | 0.3171 | 0.0863 | 0.2721 | Segmental |
| PtrGT13-PtrGT25 | 0.2668 | 0.0475 | 0.1782 | Segmental |
| PtrGT14-PtrGT46 | 0.2906 | 0.0492 | 0.1695 | Segmental |
| PtrGT31-PtrGT50 | 0.2912 | 0.0671 | 0.2303 | Segmental |
| PtrGT36-PtrGT39 | 0.3374 | 0.1374 | 0.4073 | Segmental |
| PtrGT37-PtrGT38 | 0.2574 | 0.0434 | 0.1685 | Segmental |
| PtrGT53-PtrGT54 | 0.1104 | 0.0776 | 0.7029 | tandem |
Gene pairs were identified at the terminal nodes (>90% identical) of the phylogenetic tree. Synonymous (Ks) and nonsynonymous substitution (Ka) rates are presented for each pair. Gene pairs created by tandem duplication or segmental duplication are indicated in the table.
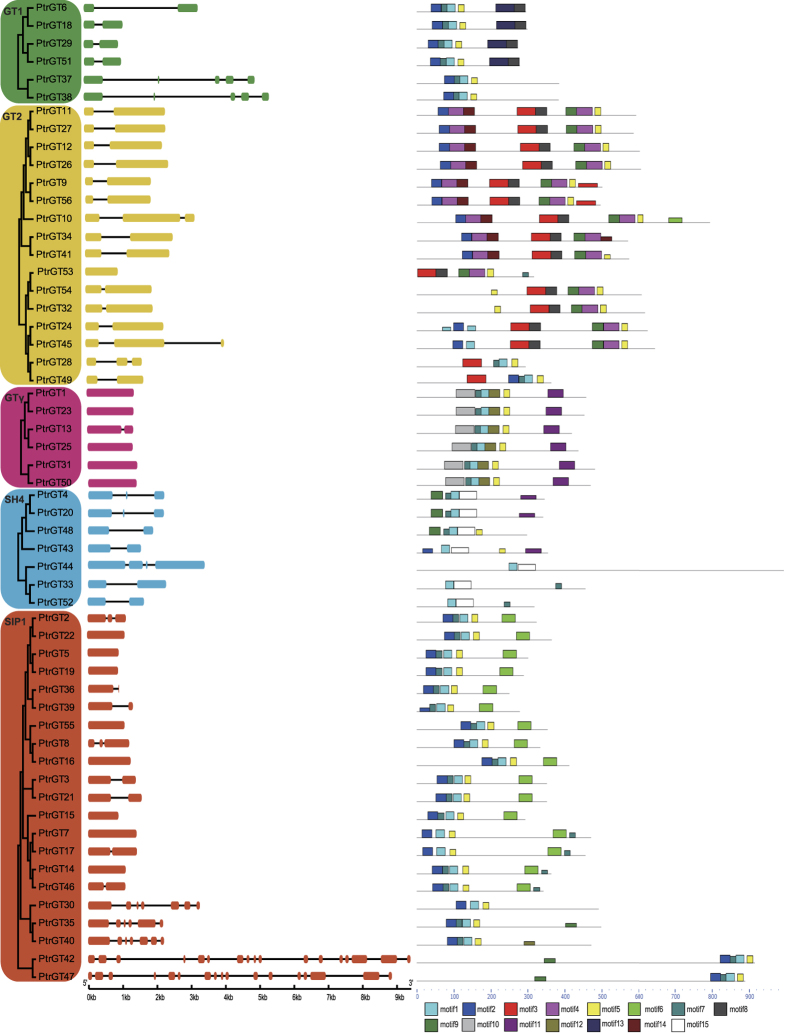
Figure 2b shows that the most closely related trihelix members within the same subfamilies shared similar gene structures in terms of intron numbers or exon lengths. The similarity in gene structures was consistent with the phylogenetic analysis. Interestingly, most genes had only one or two exons, except PtrGT30, 35, 40, 42 and 47 in the SIP1 subfamily.
Figure 2. Phylogenetic relationships, gene structures and motif compositions of Populus trihelix genes.
(a) An unrooted phylogenetic tree generated with the MEGA5.0 program using the full-length amino acid sequences with 1000 bootstrap replicates. The five subfamilies are marked with different colors. (b) Exon/intron structures of Populus trihelix genes. Exons and introns of each subfamily are represented by colored boxes and black lines, respectively. (c) Schematic representation of the conserved motifs in Populus trihelix proteins by MEME. Each colored box represents a motif, and black lines represent non-conserved sequences.
Putative conserved motifs predicted by MEME show the diversity of the P. trichocarpa trihelix genes (Fig. 2c). In this prediction, 15 distinct motifs were identified. The best possible match and domain are shown in Supplementary Table S3. As expected, most of the closely related members had common motif compositions, suggesting functional similarities among the trihelix proteins in the same subgroup.
Chromosomal locations and duplications analysis
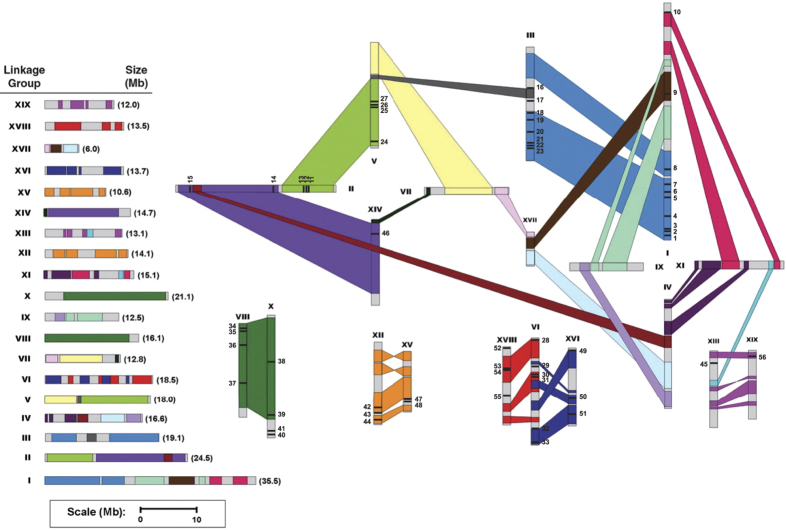
Trihelix genes were physically mapped on 19 linkage groups (LGs). LGI contains 10 genes, which was the highest number, followed by eight genes on LGIII. In contrast, no genes were located on LGIV, LGVII, LGIX, LGXI or LGXVII (Fig. 3).
Figure 3. Chromosomal locations of Populus trihelix genes.
Segmental duplicated homologous blocks are indicated by the same color. The scale represents mega bases (Mb). The LG numbers are indicated above each bar.
According to a previous study, at least three rounds of genome-wide duplications occurred in the Populus genome, followed by multiple segmental duplications, tandem duplications and transposition events19. In our study, we mapped the P. trichocarpa trihelix genes to the duplicated blocks identified in the previous study19. Forty-four genes were located in duplicated regions, and 30 of those were present in both duplicates, while the others were only present in one of the blocks (Fig. 3). By contrast, 12 trihelix genes were located outside the duplicated blocks, suggesting that the loss of some genes is caused by dynamic changes after segmental duplication.
Promoter cis-element analysis
The promoter regions of P. trichocarpa trihelix genes contained numerous abiotic stress response- and phytohormone-related cis-elements (Supplementary Table S4). These trihelix promoters also contained cis-elements related to biotic stresses, such as EIRE and ELI-box3. Most trihelix promoters (46) contained HSE elements, followed by G-Box and TC-rich repeats (43 genes). Of all of the genes, the PtrGT14 and PtrGT26 promoters contained the most cis-elements (11), while PtrGT4 only had two cis-elements (Supplementary Table S5).
Gene Ontology (GO) annotation analysis
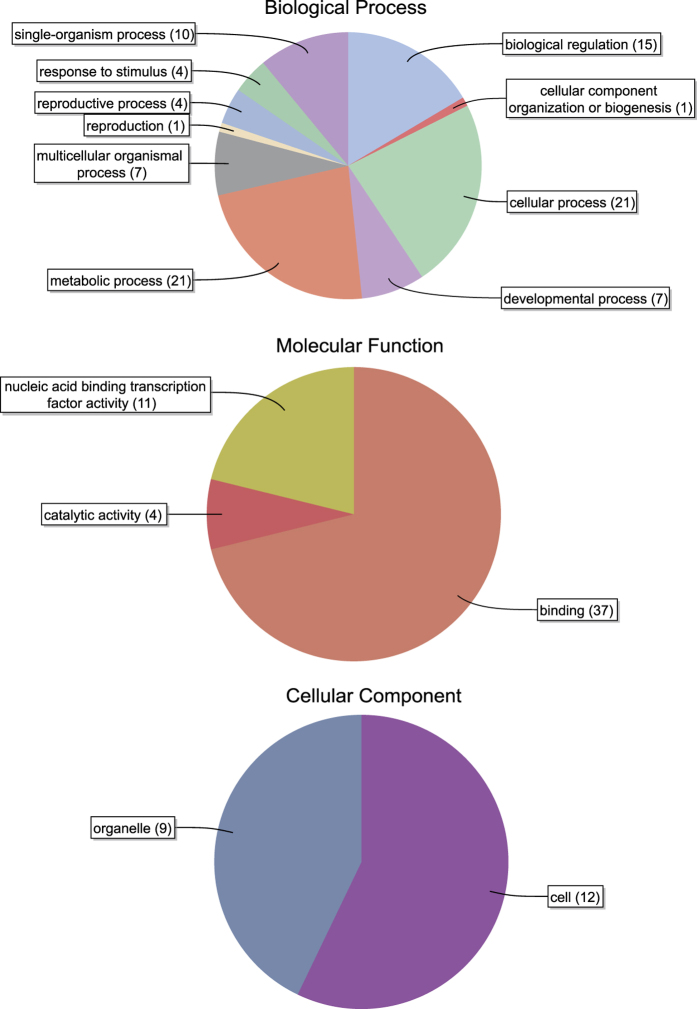
GO annotation analysis of the 56 trihelix genes associated them with different biological processes. Of the 10 GO terms associated with trihelix genes, metabolic process and cellular process were the categories with the largest numbers of genes (21), followed by biological regulation (15). PtrGT31 and PtrGT50 are related to stress responses (Supplementary Table S6). Only one gene was predicted to function in reproduction and cellular component organization or biogenesis. As for genes in the molecular function category, most were annotated with binding ability (37), while only four genes were annotated to have catalytic activity. Under the cellular component term, 12 genes were located in the cell part, while nine genes were associated with organelles (Fig. 4).
Figure 4. Gene Ontology (GO) results for Populus trihelix proteins.
Expression profiles of trihelix genes in P. trichocarpa
The exImage tool in the PopGenIE v3.0 database compiled gene expression patterns in different Populus tissues using public microarray datasets (accession number: GSE6422)20. In Fig. 5, 20 trihelix genes were co-enriched in young leaves, inter nodes and mature leaves, 24 trihelix genes were enriched in nodes, 29 were enriched in roots, and 15 genes were co-expressed in internodes and nodes. Similar numbers of genes were expressed in the other three tissues (nine in roots and 10 in both young leaves and mature leaves).
Figure 5. ExImage analysis of Populus trihelix genes in different tissues.

YL, IN, N, ML and R refer to young leaves, internodes, nodes, mature leaves and roots, respectively. The exImage was visualized by the exImage tool in the PopGenIE v3.0 database. Red and green indicate high and low levels of transcript abundances, respectively.
Trihelix genes have previously been shown to play crucial roles in the drought stress response13. The expression profiles of trihelix genes in response to drought stress were thus investigated using the publicly available explot data in the exPlot tool of the PopGenIE v3.0 database. As shown in Supplementary Figs S2 and 23 trihelix genes were upregulated in leaves and roots, while 16 genes were downregulated in leaves, and 27 genes were downregulated in roots. Of these genes responding to drought, 11 were upregulated in both leaves and roots, while seven were downregulated in both leaves and roots.
Examination of trihelix gene expression by qRT-PCR
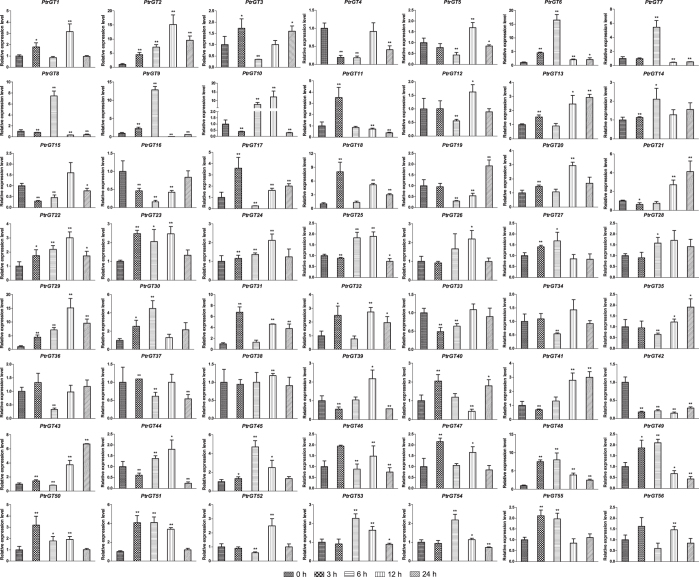
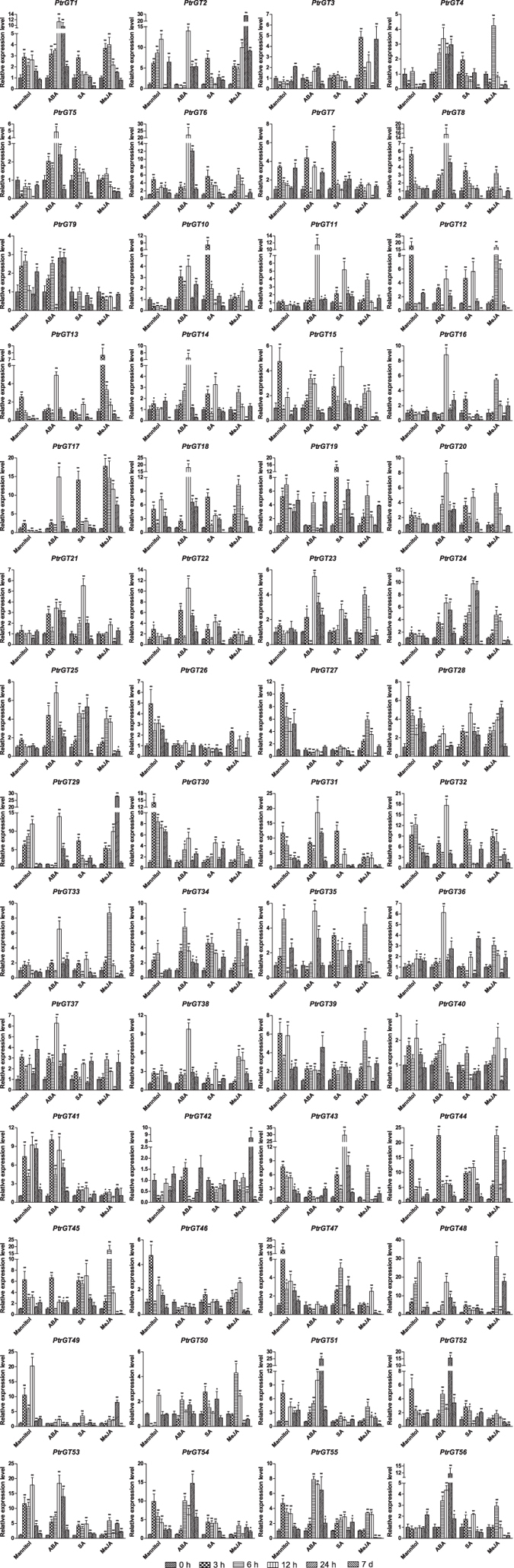
To measure individual gene expression of P. trichocarpa trihelix genes, qRT-PCR was conducted under osmotic stress or ABA, SA, methyl jasmonate (MeJA) or pathogen treatment for 0 h, 3 h, 6 h, 12 h, 24 h or 7 d (Fig. 6). To verify that the treatments were working well, four marker genes (RD29A, RAB18, PR1 and PR4) were used as positive controls under these conditions. As expected, the expressions of these marker genes were all significantly induced after treatments (Supplementary Fig. S3). Genes up- or downregulated by more than 2.0-fold were considered significantly differentially expressed21. For osmotic stress, 42 trihelix genes were induced, eight trihelix genes were suppressed, and six trihelix genes showed no change. Notably, PtrGT12, 27, 29, 30, 31, 32, 44, 47, 48, 49, 53 and 54 were significantly upregulated (>10.0-fold relative to the control). Moreover, PtrGT2, 6, 19, 20, 25, 27, 28, 30, 40, 41, 43, 49 and 55 were upregulated at 3 h, 6 h, 12 h and 24 h in leaves, whereas only PtrGT3, PtrGT5 and PtrGT10 were downregulated at 3 h, 6 h, 12 h and 24 h. The expression trends were generally consistent with the exPlot analysis. Most trihelix genes were downregulated at day 7 of mannitol treatment, except for PtrGT3, 10, 37, 42 and 56.
Figure 6. Expression analysis of trihelix genes in leaves under osmotic, ABA, SA and MeJA by qRT-PCR.
The x-axis represents time after the onset of stress treatments. Error bars represent the standard deviations of three biological replicates. Asterisks indicate stress treatment groups that showed a significant difference in transcript abundance compared with the control group (*P < 0.05, **P < 0.01).
Under ABA stress, 50 trihelix genes were upregulated, and five genes (PtrGT26, 27, 42, 46 and 47) were downregulated. Thirteen trihelix genes (PtrGT1, 8, 17, 22, 31, 32, 41, 44, 51, 52, 53, 54 and 56) were significantly upregulated at 3 h, 6 h, 12 h and 24 h, while only three genes (PtrGT27, 46 and 47) were downregulated. Under SA stress, 47 genes were upregulated, and eight genes were downregulated; six genes (PtrGT17, 24, 31, 32, 43 and 44) were significantly upregulated, whereas only PtrGT9 was downregulated. Under MeJA stress, 49 trihelix genes were upregulated, and seven were downregulated, similar to the SA stress results. Only eight genes (PtrGT2, 12, 17, 18, 29, 44, 45 and 48) were significantly upregulated, while PtrGT9 was significantly downregulated. Most trihelix genes were downregulated under ABA, SA and MeJA treatments, whereas PtrGT9, 19, 39, 42and 43 were upregulated in ABA treatment and PtrGT36, 37 and PtrGT3 were upregulated in SA and MeJA treatments, respectively.
According to a previous study, GT factors play a role in pathogen-induced SCaM-4 gene expression in both soybean and Arabidopsis10. In our study, because some genes have cis-elements related to fungal infection, we also carried out qRT-PCR during Alternaria alternate (pathogen of leaf blight) infection (Fig. 7). We observed that 35 genes were upregulated, 15 were downregulated, and six showed no obvious change. PtrGT2, 6, 9, 10 and 29 were significantly upregulated at all time points, whereas PtrGT42 was downregulated at all time points.
Figure 7. Expression analysis of trihelix genes in leaves under pathogen infection treatments by qRT-PCR.
The x-axis represents time after the onset of stress treatments. Error bars represent the standard deviations of three biological replicates. Asterisks indicate stress treatment groups that showed a significant difference in transcript abundance compared with the control group (*P < 0.05, **P < 0.01).
MicroRNA target sites and expression analysis
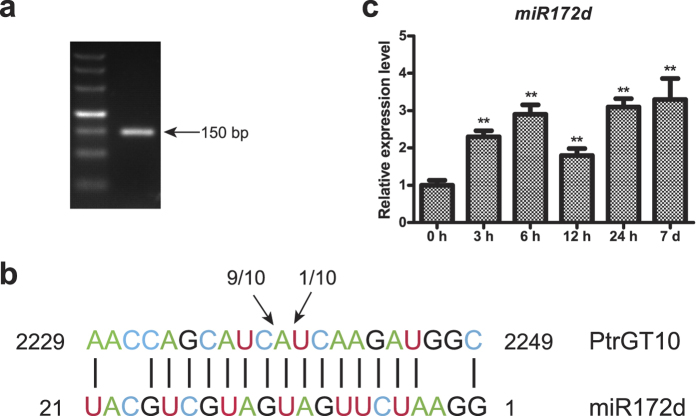
To study the relationship between trihelix genes and miRNAs, the psRNATarget online tool was used to find putative trihelix gene targets and found none. We then searched the flower bud target database during early stages (pollen at tetrad) obtained from our previous study using high-throughput sequencing. Here, we found a putative target of miR172d. Validation of miRNA cleavage was conducted using 5′ RLM RACE. Figure 8a shows the agarose gel electrophoresis of the 5′ RACE products of PtrGT10. The identified cleavage validation at the 10th base of miR172d is shown in Fig. 8b, while the original sequencing peaks were shown in Supplementary Fig. S4. These results demonstrate that PtrGT10 is a target gene of miR172d.
Figure 8. The cleavage site of miR172d on PtrGT10.
(a) Agarose gel electrophoresis of the modified 5′RACE experiment on PtrGT10. (b) Alignment of the target site in PtrGT10 with miR172d. The arrow and the numbers above the sequences indicate the cleavage site and the number of sequenced clones that revealed cleavage in that position, respectively. (c) The relative expression of miR172d under osmotic stress. The bars indicate standard deviation. The asterisks indicate statistically significant differences between the relative expression of control and treatment groups (*P < 0.05, **P < 0.01).
We then measured miR172d expression levels by qRT-PCR. The results showed that miR172d was upregulated under osmotic stress (Fig. 8c), opposite to the observed pattern for of PtrGT10 expression. This result is consistent with the typical relationship between miRNAs and their target genes.
Generation of transiently transformed P. ussuriensis plants with repression of PtrGT10
Agrobacterium-mediated transient transformation systems are powerful tools for analyzing the function of genes and the generation of gene products22. In this study, two types of transiently transformed P. ussuriensis plants were generated, i.e., plants transform with 35S::PtrGT10SRDX for dominant repressing PtrGT10 (DR) and control (transformed with empty pBI121 vector). To determine the expression of PtrGT10 in DR and control plants, qRT-PCR was performed. At 48 h after transformation, the plants were grown under normal condition or treated with 200 mM mannitol for 24 h and 48 h, and the expression of PtrGT10 in whole plants of DR and control plants were investigated. The expression levels of PtrGT10 in DR plants were normalized by using that in control plants at time point of 24 h. Compared with in control plants, the expression of PtrGT10 was significantly decreased in DR plants under both normal and osmotic stress conditions (Supplementary Fig. S5). These results indicated that the transient transformation system is suitable for function studies of PtrGT10.
Biochemical staining and physiological measurement of 35S::PtrGT10SRDX transformed plants
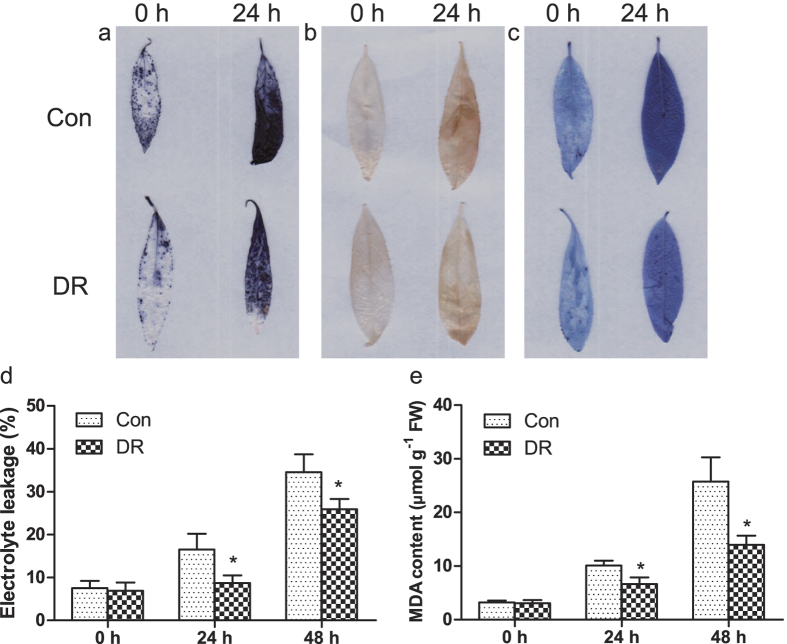
To study reactive oxygen species (ROS) accumulation, nitroblue tetrazolium (NBT) and 3,3-diamin-obenzidine (DAB) in situ staining on Populus ussuriensis were performed, which can stain two main ROS species, H2O2 and O2−. Both NBT and DAB staining showed that the DR plants (plants transform with 35S::PtrGT10SRDX for dominant repressing PtrGT10) had lower H2O2 and O2− levels compared with control plants under osmotic stress (Fig. 9a,b). These results suggested that the repression of PtrGT10 could improve the ROS scavenging ability. Evans blue in situ staining on P. ussuriensis showed that cell death decreased in the DR plants compared with control plants under osmotic stress (Fig. 9c). An electrolyte leakage assay further confirmed these results. It showed that the electrolyte leakage rate was lower in DR plants than in control plants under osmotic stress (Fig. 9d). Under normal conditions, there was no difference in MDA levels between control and DR plants. However, under osmotic stress, the DR plants displayed the lower MDA content (Fig. 9e). These results indicated that the repression of PtrGT10 could reduce cell death and MDA accumulation under osmotic stress.
Figure 9. Histochemical staining and related physiological changes analyses of transformed P. ussuriensis plants.
(a,b) The plants were stained with NBT (a) and DAB (b) to reveal the accumulation of O2− and H2O2, respectively. (c) Analysis of cell death by Evans Blue staining. (d) Analysis of electrolyte leakage rate. (e) Analysis of MDA level. The asterisks indicate statistically significant differences between the relative expression of control and DR plants (*P < 0.05).
Discussion
Trihelix genes play a key role in plant physical development and in responses to environmental stimuli. In this study, we identified 56 trihelix genes in P. trichocarpa, each of which had at least one trihelix domain. The number of identified trihelix genes is much higher than that found in Arabidopsis and rice (30 and 31, respectively), reflecting differences between herbaceous and woody plants. Gene duplications are known to play a crucial role in genome expansions and realignments, which include tandem and segmental duplications23. According to our phylogenetic analysis, 15 paralogous pairs were identified, of which only one pair (PtrGT53/54) has undergone tandem duplication; the other 14 paralogous pairs were all segmental duplications, suggesting a high rate of segmental repetition, which is helpful for gene evolution.
Gene structural variation plays an important role in gene evolution, as integrations and realignments of gene fragments can cause exon/intron increases or decreases24. In this study, we identified such variation in PtrGT42 and PtrGT47 of the SIP1 subfamily, which each contained 17 exons, whereas most of the other genes in this subfamily contained only two or three exons. PtrGT30, 35 and 40 also each contain six or seven exons. These results indicate that these specific genes have undergone a series of gene evolution events leading to exon increases and thus may be functionally different. Structures and conserved motifs were similar across most genes within a given subfamily, indicative of similar functions and stable evolution. However, there were also some special genes whose motifs differed from others in the same subgroup, such as PtrGT33, 44 and 52, which lacked motifs 6, 7 and 11. This result suggests that these genes have divergent functions compared with the other genes.
Promoter cis-elements are known to play important roles in response to biotic and abiotic stresses25. In this report, many biotic and abiotic stress-related and phytohormone-related cis-elements were identified in the promoters of trihelix family genes26, including ABRE, W-Box, DRE, ERE, G-Box and EIRE elements. PtrGT14 and PtrGT26 had 11 cis-elements, suggesting important functions under different stresses. In particular, PtrGT14 was the only gene with a dehydration-responsive DRE cis-element. However, no obvious expression changes under osmotic stress were detected by qRT-PCR. This may be attributable to later gene upregulation after 24 h. PtrGT4 had two SA- and stress-related cis-elements (TCA-element and TC-rich repeats). The qRT-PCR results showed that PtrGT4 was induced not only by SA but also by ABA and MeJA, suggesting a close relationship between ABA and MeJA with SA. ELI-box3 and EIRE are fungal stress-related cis-elements that were present in six trihelix genes (PtrGT13, 14, 15, 27, 32 and 34). Our qRT-PCR results showed that three of these genes (PtrGT13, 14, 32) were upregulated during pathogen infection, while the other three genes showed no obvious change. This result indicates that some trihelix genes are involved in a different pathogen expression network.
From our study of exImage data, we found trihelix genes expressed in different tissues of Populus; however, there are obvious differences. PtrGT9, 32, 45 and 47 were highly expressed in young leaves, though the expression of all but PtrGT32 was less remarkable in mature leaves. Current information suggests that trihelix transcription factors regulate light-response genes1,27 and that loss of AtGTL1 gene can negatively regulate water use efficiency by modulating stomatal density, thus leading to increased plant tolerance to water deficits12. These previous studies suggested relationships between trihelix genes and leaf stoma or light responses. Therefore, PtrGT32, which is highly expressed in both young leaves and mature leaves, may be related to stoma or photosynthesis through cooperation with other photosynthesis-related genes. In addition under drought conditions, roots can sense soil changes and send a series of signals to the shoots and leaves to reduce root damage28. In our study, PtrGT4, 20, 31, 41 and 52 were highly expressed in roots, suggesting that these genes might enhance the ability of tissues to absorb water to adapt to the drought environment. The distinct expression patterns of the trihelix family members suggest a diversity of functions during plant growth and development.
Plants are frequently threatened by abiotic and biotic stresses that influence stress resistance or even cause fatal damage in trees29. However, many genes help plants adapt to these stresses via gene expression changes. Under osmotic conditions, 42 trihelix genes were induced, eight genes were downregulated, and six genes showed no obvious change. This result was broadly consistent with the exPlot data, which indicated that trihelix genes act synergistically and have important functions in drought stress. A soybean homolog (Glyma10g34520) of one of the upregulated genes (PtrGT40) was similarly upregulated under drought conditions. This finding suggests that the homologous genes have similar functions in different species. PtrGT10 was suppressed by osmotic, consistent with a previous study15 that showed that poplar GT1 (PtrGT10 in our study) functioned in drought tolerance. ABA is an important regulatory factor during drought stress30. SA and MeJA are well-known naturally occurring signaling molecules that play key roles in biotic stresses24,31. In our study, most of the trihelix genes were induced under ABA, SA and MeJA treatment, indicating that phytohormones regulate expression of trihelix genes in P. trichocarpa. However, different genes were induced by different phytohormones. For example, PtrGT3 and PtrGT13 were induced by ABA and MeJA, but suppressed by SA. PtrGT9 was induced by ABA, but suppressed by SA and MeJA. These variations indicate that a broad set of hormonal signals exist in the trihelix gene family. OsGTγ-1 (LOC_Os02g33770) was specifically upregulated by an ABA and SA stimulus8. However, the homologous gene in P. trichocarpa (PtrGT13) was only induced by ABA, indicating different expression patterns between woody plants and crops. Notably, GmGT2A and GmGT2B, which are orthologs of PtrGT9 and PtrGT24, were induced by drought and ABA in soybean, suggesting that these two genes have similar responses to drought and ABA treatment in different species13. In our study, expression of most trihelix genes was induced by mannitol and phytohormone treatments within a short term (24 h), but only a few genes were induced long term (7 d). Among these long-time response genes, PtrGT3, Ptrgt37 and PtrGT42 were simultaneously upregulated under mannitol, ABA, SA and MeJA treatment, respectively. This result further suggests that these three genes are involved in biotic and abiotic stresses as well as long-term response genes to biotic and abiotic stresses.
Alternaria alternate causes poplar leaf blight, one of the most common diseases in Northeast China, and can cause serious economic impacts32. As little research has investigated the expression levels of trihelix genes during fungal infection of Populus, we carried out such an analysis based on the presence of fungi-related cis-elements in trihelix gene promoters and a related study in soybean10. Most of the genes with fungus-related cis-elements in their promoters were upregulated by A. alternate infection, except for PtrGT15, PtrGT35 and PtrGT44, which were downregulated. Conversely, 24 genes that were upregulated by SA and MeJA stresses were also upregulated by A. alternate infection. This may indicate a relationship among SA, MeJA and fungi, where SA and MeJA have a regulatory function under this type of biotic stress (fungal infection), consistent with previous studies24,31. Based on expression profile analysis and our qRT-PCR study, 22 genes responded to all five stresses, 19 genes responded to four stresses, eight genes responded to three stresses, six genes responded to two stresses, and only one gene responded to one stress. These results indicate that a given trihelix gene may not be associated with a single type of stress. Follow-up studies should establish interactions among the particular pathways activated by biotic stress, abiotic stress and phytohormones.
Transient transformation system provides a powerful and convenient tool to investigate gene functions in vivo, avoiding difficult drawbacks that typically affect the stable transformation protocols, such as transformation efficiency, transformants selection, and regeneration33. A prior research has already demonstrated that the transient transformation system used in this study is reliable for plants stress tolerance studies34. In our study, the decreased expression levels in transient transformed plants (DR plants) also confirmed the reliability of gene function analysis. A previous study showed that overexpression of PtrGT10 suppressed water use efficiency and drought tolerance by increasing stomatal density15. In this study, repression of PtrGT10 in P. ussuriensis was related to reduce ROS accumulation, indicating that repression of PtrGT10 is involved in ROS scavenging. The lower electrolyte leakage rate of DR plants indicated the decreased cell death under osmotic stress. Meanwhile, repression of PtrGT10 was related to greatly decreased MDA content under mannitol condition, suggesting that lipid peroxidation in cell membrance was also decreased. Increased ROS scavenging is a common mechanism to induce stress tolerance in plants. Therefore, these results suggested that repression of PtrGT10 could decrease cell death by increasing ROS scavenging ability and decreasing cell damage, thus increasing the osmotic stress tolerance.
MiRNAs have important functions in plant growth, development and abiotic stress responses. MiR172 is a key miRNA in flower development, regulating calyx and petal formation and developmental timing35. The targets of miR172 were identified in a previous study as AP2-like transcription factor genes36. Previous studies reported that AP2 regulates flower and seed development37. In recent years, some studies have shown that miR172 is also involved in abiotic stress responses38. For example, it was significantly induced by salt in wheat39, but decreased under drought stress in rice40. Some studies have identified targets of miR172 during abiotic stress responses. Overexpression of soybean gma-miR172c in Arabidopsis resulted in reduced leaf water loss and an increased survival rate. Moreover, the target of gma-miR172c, Glyma01g39520, an AP2-like gene, can reduce tolerance to drought stress in Arabidopsis mutants38. MiR172 downregulates AP2 through transcript cleavage and translational repression in A. thaliana41. However, to date, no studies have reported interactions between miR172d and abiotic stress-related targets except for AP2 genes. Our study provides a new target gene, PtrGT10, which is an osmotic-related gene in P. trichocarpa. In this study, PtrGT10 may negatively regulate the osmotic stress response. Therefore, miR172d may regulate this new target gene, PtrGT10, as part of the osmotic response. However, whether the regulatory mechanism of miR172d involves translational repression of PtrGT10 needs further study.
In this study, 56 trihelix genes were identified in the Populus genome and classified into five groups. The exon/intron structures and motif compositions of most trihelix genes were conserved in each subfamily, indicating their likely functional conservation. The genes were non-randomly distributed on 19 LGs and were predicted to be expressed in different tissues (mostly in leaves and roots) and under different stresses. Some genes were significantly induced under biotic or abiotic stresses and phytohormone treatments, which will be helpful for future functional studies aiming to reveal their divergent roles. Additionally, a new target of miR172d, PtrGT10, was identified, with the expression levels of miR172d and PtrGT10 showing inverse trends under osmotic stress. Repression of PtrGT10 could increase ROS scavenging ability and decrease cell death. These important discoveries may lay foundations for follow-up studies.
Materials and Methods
Identification of the trihelix gene family in Populus
The P. trichocarpa genome database Phytozome V10.3 and the NCBI database were searched to compile a cDNA library and a protein database. All of the obtained sequences were examined for the presence of trihelix domains (PF13837, SM00717) using Pfam 28.019 and the SMART database42. The identified genes were compared in PlnTFDB V3.043. Related trihelix genes in Arabidopsis and rice were downloaded from the Arabidopsis Information Resource (TAIR) and the Rice Information Resource, respectively. WOLF PSORT was used to predict subcellular localizations44. Physicochemical parameters were calculated using ExPASy.
Phylogenetic analysis
Further multiple sequence alignments were performed by ClustalX 1.8345 and manually corrected using BioEdit 7.146. Phylogenetic trees were constructed using MEGA 5.0 with the neighbor-joining (NJ) method, and the bootstrap test was conducted using 1,000 replicates7. Gene clusters of the homologs in the three species (P. trichocarpa, A. thaliana and O. sativa) were identified based on the NCBI database (http://www.ncbi.nlm.nih.gov/).
Gene structure and conserved motif analysis
The trihelix gene exon/intron organization was determined using the GSDS 2.0 program47. The MEME system (v4.10.2)48 was used to identify conserved motifs. MEME was run locally with the following parameters: number of repetitions, any; maximum number of motifs, 15; and the optimum motif widths, between 6 and 200 residues. In addition, structural motif annotation was performed with Pfam and the SMART database.
Chromosomal location analysis
The chromosomal locations were identified and plotted with the PopGenIE v3.0 database49. Tandem gene duplications in Populus were identified based on the same criteria described in rice (http://compbio.dfci.harvard.edu/tgi/). Genes separated by ≤5 gene loci in a range of 100 kb physical distance were considered tandem duplicates50. The synonymous (Ks) and nonsynonymous substitution (Ka) rates were calculated based on a previous study51.
Promoter cis-element identification
Promoter sequences located 2 kb upstream of the translation start site were obtained from the Phytozome v10.3 database and analyzed with PlantCARE52.
Gene Ontology (GO) annotation
Functional annotation of trihelix sequences and the associated analyses were performed using Blast2GO v3.053. The protein sequences were imported into the Blast2GO program to associate GO terms. The GO categories were placed in a hierarchical classification with three independent classes, namely, biological processes, cellular components and molecular functions.
exImage and exPlot analysis
The exImage tool was used to analyze expression levels across different tissues of P. trichocarpa using the NCBI GEO number GSE642220 as a control. The exPlot tool in PopGenIE v3.0 was used to visualize the explot of genes in different tissues. The data can be directly obtained using the accession numbers.
Plant materials and stress treatments
In-vitro plantlets of P. trichocarpa (genotype Nisqually-1) were cultured in half-strength Murashige and Skoog (1/2 MS) medium54 under long-day conditions (16 h light/8 h dark) at a temperature of 25 °C. Plants were grown in 250-ml erlenmeyer flasks containing 100 ml medium and were subcultured at 4-week intervals. In a preliminary experiment, we evaluated the effect of different concentrations of mannitol on growth of in vitro plants. The results showed that 150 mM mannitol could reduce but not abolish growth of in vitro plants. Thus, 150 mM mannitol was use as moderate osmotic stress treatment in this study. For phytohormone treatments, the plants were cultured in 1/2 MS medium with 200 μM ABA55, 100 μM SA56 or 100 μM MeJA57, according to a previous study. Prior to this, we also confirmed the ABA, SA and MeJA concentrations mentioned above, which can reduce growth but not induce wilting or necrosis within 7 d. All media were adjusted to pH 5.8 before addition of 0.6% plant agar (Biotopped, Beijing, China), and then sterilized by autoclaving at 121 °C for 15 min. Mannitol was added to medium before autoclaved sterilization. The ABA, SA and MeJA stock solutions were filter sterilized and added to the medium after autoclaving, respectively. To induce fungal infection, mycelial plugs of Alternaria alternate (the pathogen of Populus leaf blight) were placed on excised leaves, which were then cultured in medium, according to a previous study32. The 3, 6, 12 and 24 h time points were chosen to obtain early responsive genes, and the 7 d point for late responsive genes. Fungal infections lasted for 0 h, 3 h, 6 h, 12 h and 24 h. Three different plants were collected from each treatment time point. Three independent biological replicates were performed for each treatment. All samples were subsequently frozen and stored at −80 °C for RNA isolation.
RNA isolation and qRT-PCR analysis
Total RNA was extracted as previously described58. cDNA was synthesized based on the manufacturer’s instructions for the ReverTra Ace qPCR RT Master Mix with a gDNA Remover Kit (TOYOBO, Osaka, Japan). Primer Premier 5 was used to design gene-specific primers. Each primer was checked by BLAST for specificity, and a melting curve analysis was performed. The actin gene (XM_002298674) of P. trichocarpa was used as the reference gene59. To verify that the treatments were working well, the homologous genes in poplars of RD29A (POPTR_0012s13180), RAB18 (POPTR_0002s07510), PR1 (POPTR_0009s08670) and PR4 (POPTR_0013s03890) were used as marker genes for mannitol, ABA, SA and MeJA treatments, respectively. Primer details are in Supplementary Table S7. qRT-PCR was performed in a volume of 20 μL with 10 μL of 2 × SYBR Premix, 6 μL of ddH2O, 2 μL of template and 1 μL of each specific primer (final concentration 10 μM). The PCR conditions and relative expression levels were calculated as previously described60. Three biological replicates were used for each sample.
Cleavage validation of miRNA targets and qRT-PCR analysis
The psRNATarget online tool61 and previous results62 were used to search for the presence of microRNA targets. The modified RNA ligase-mediated rapid amplification of 5′ cDNAs method (5′RLM-RACE) was performed using the GeneRacer kit (Invitrogen, Carlsbad, CA, USA). Total RNA was isolated from P. trichocarpa leaves under osmotic stress. A poly(A) tail was added to the 3′ end prior to transcription. A SYBR Green miRNA qPCR Detection Kit (Bioteck, Beijing, China) was used to detect miRNA expression levels, with 5.8S rRNA used as the endogenous reference. All reactions were run in triplicate. All primers mentioned above are listed in Supplementary Table S7.
Cloning of the PtrGT10 gene and plant transformation
To verify the function of trihelix genes, we constructed repression vectors of PtrGT10 using the method described in previous study63. The full-length PtrGT10 was amplified with gene-specific primers (see in Supplementary Table S7) by RT-PCR with 2 μL cDNA from P. trichocarpa leaves. The PCR reaction was performed with LA Taq DNA polymerase (TaKaRa, Dalian, China) in a total volume of 50 μL with an initial denaturing step at 94 °C for 3 min, 35 cycles of 94 °C for 30s, 60 °C for 30s and 72 °C for 3 min, and a final extension step at 72 °C for 7 min. The PtrGT10 dominant repression constructs were created by fusing the full-length PtrGT10 cDNA in frame with the SRDX repression sequence, which was ligated downstream of the 35S promoter in pBI12163. Agrobacterium-mediated transient transformation of whole P. ussuriensis plantlets, as well as the osmotic treatment, were performed using a previous method34. The 4-week-old WT plantlets with similar size were used for transformation. Two kinds of transiently transfected plantlets were generated, i.e., plants transform with 35S::PtrGT10SRDX for dominant repressing PtrGT10 (DR) and control (transformed with empty pBI121 vector).
Biochemical staining and physiological measurement of 35S::PtrGT10SRDX transformed plants
After culture on agar medium, the plantlets were harvested for biochemical staining. P. ussuriensis repressing plants and control plants subjected to the 200 mM mannitol were infiltrated with DAB solution NBT solution following the procedure described by ref. 64. Cell death was examined by Evans blue staining as described by ref. 65. In each biochemical staining experiment, at least ten plantlets were used.
After being cultured on agar medium, the plants were moved to new medium with 200 mM mannitol for stress induction for 24 h and 48 h. Then, the plants were harvested for physiological analyses. MDA measurement was conducted as in ref. 66, while the electrolyte leakage measurement was performed as described by ref. 34. Biological replicates were performed in triplicate.
Additional Information
How to cite this article: Wang, Z. et al. Comprehensive analysis of trihelix genes and their expression under biotic and abiotic stresses in Populus trichocarpa. Sci. Rep. 6, 36274; doi: 10.1038/srep36274 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the Innovation Project of State Key Laboratory of Tree Genetics and Breeding (Northeast Forestry University; Grant number 2013A04) and the Key Project of Chinese National Programs for Fundamental Research and Development (973 Program: 2009CB119100).
Footnotes
Author Contributions C.L. and C.Y. conceived and designed the experiments. Z.W., Q.L., H.W. and H.Z. performed the experiments. Z.W. and Q.L. analyzed the data and wrote the paper. C.L. revised the paper. All authors read and approved the final version of manuscript.
References
- Green P. J., Kay S. A. & Chua N. H. Sequence-specific interactions of a pea nuclear factor with light-responsive elements upstream of the rbcS-3A gene. EMBO J. 6, 2543–2549 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. X. Regulatory mechanism of plant gene transcription by GT-elements and GT-factors. Trends Plant Sci. 4, 210–214 (1999). [DOI] [PubMed] [Google Scholar]
- Kaplan-Levy R. N., Brewer P. B., Quon T. & Smyth D. R. The trihelix family of transcription factors–light, stress and development. Trends Plant Sci. 17, 163–171 (2012). [DOI] [PubMed] [Google Scholar]
- Li X., Qin G., Chen Z., Gu H. & Qu L. J. A gain-of-function mutation of transcriptional factor PTL results in curly leaves, dwarfism and male sterility by affecting auxin homeostasis. Plant Mol Biol. 66, 315–327 (2008). [DOI] [PubMed] [Google Scholar]
- Xi J., Qiu Y., Du L. & Poovaiah B. W. Plant-specific trihelix transcription factor AtGT2L interacts with calcium/calmodulin and responds to cold and salt stresses. Plant Sci. 185-186, 274–280 (2012). [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A. et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39, D225–D229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M. & Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Xie K., Hou X., Hu H. & Xiong L. Systematic analysis of GT factor family of rice reveals a novel subfamily involved in stress responses. Mol Genet Genomics. 283, 157–169 (2010). [DOI] [PubMed] [Google Scholar]
- Brewer P. B. et al. PETAL LOSS, a trihelix transcription factor gene, regulates perianth architecture in the Arabidopsis flower. Development 131, 4035–4045 (2004). [DOI] [PubMed] [Google Scholar]
- Park H. C. et al. Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol. 135, 2150–2161 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Hong G. & Han B. Transcript abundance of rml1, encoding a putative GT1-like factor in rice, is up-regulated by Magnaporthe grisea and down-regulated by light. Gene 324, 105–115 (2004). [DOI] [PubMed] [Google Scholar]
- Yoo C. Y. et al. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22, 4128–4141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z. M. et al. Soybean Trihelix transcription factors GmGT-2A and GmGT-2B improve plant tolerance to abiotic stresses in transgenic Arabidopsis. PloS One 4, e6898 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Taylor G. Populus: arabidopsis for forestry. Do we need a model tree? Ann Bot 90, 681–689 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H., Yoo C. Y., Gosney M. J., Hasegawa P. M. & Mickelbart M. V. Poplar GTL1 is a Ca2+/Calmodulin-Binding transcription factor that functions in plant water use efficiency and drought tolerance. PloS One 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman J., Whitcraft J., Johnson C. & Arias J. Abiotic and biotic stress differentially stimulate as-1 element activity in Arabidopsis. Plant Cell Rep. 21, 180–185 (2002). [Google Scholar]
- Liu Q., Wang Z., Xu X., Zhang H. & Li C. Genome-Wide analysis of C2H2 zinc-finger family transcription factors and their responses to abiotic stresses in Poplar (Populus trichocarpa). PloS One 10, e0134753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M. J. et al. Repression of seed maturation genes by a trihelix transcriptional repressor in Arabidopsis seedlings. Plant Cell 21, 54–71 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R. D. et al. Pfam: clans, web tools and services. Nucleic Acids Res. 34, D247–D251 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. et al. The F-box gene family is expanded in herbaceous annual plants relative to woody perennial plants. Plant Physiol 148, 1189–1200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Tang N., Du H., Ye H. & Xiong L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol 148, 1938–1952 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. et al. A high-throughput transient gene expression system for switchgrass (Panicum virgatum L.) seedling. Biotechnol Biofuels 3, 9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Tyagi A. K. & Sharma A. K. Genome-wide analysis of auxin response factor (ARF) gene family from tomato and analysis of their role in flower and fruit development. Mol Genet Genomics 285, 245–260 (2011). [DOI] [PubMed] [Google Scholar]
- Xu G., Guo C., Shan H. & Kong H. Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci USA 109, 1187–1192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 9, 436–442 (2006). [DOI] [PubMed] [Google Scholar]
- Narusaka Y. et al. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 34, 137–148 (2003). [DOI] [PubMed] [Google Scholar]
- Green P. J. et al. Binding site requirements for pea nuclear protein factor GT-1 correlate with sequences required for light-dependent transcriptional activation of the rbcS-3A gene. EMBO J. 7, 4035–4044 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. Root Architecture and Plant Productivity. Plant Physiol 109, 7–13 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvertsen J. P. & Garcia-Sanchez F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ Exp Bot. 103, 128–137 (2014). [Google Scholar]
- Yamaguchi-Shinozaki K. & Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 57, 781–803 (2006). [DOI] [PubMed] [Google Scholar]
- Huang D., Wu W., Abrams S. R. & Cutler A. J. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J Exp Bot. 59, 2991–3007 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Liu H., Jia Z., Fang Q. & Luo K. Combined expression of antimicrobial genes (Bbchit1 and LJAMP2) in transgenic poplar enhances resistance to fungal pathogens. Tree Physiol. 32, 1313–1320 (2012). [DOI] [PubMed] [Google Scholar]
- Guidarelli M. & Baraldi E. Transient transformation meets gene function discovery: the strawberry fruit case. Front Plant Sci. 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X. et al. A Transient Transformation System for the Functional Characterization of Genes Involved in Stress Response. Plant Mol Biol Rep 32, 732–739 (2014). [Google Scholar]
- Wu G. et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W., Li J., Song R., Messing J. & Chen X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Current Biol. 12, 1484–1495 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku K. D., Omidyar P. K., Gee Z. & Okamuro J. K. Control of seed mass and seed yield by the floral homeotic gene APETALA2. P Natl Acad Sci USA 102, 3117–3122 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. B., Wang T., Zhang Y. H. & Li Y. G. Overexpression of soybean miR172c confers tolerance to water deficit and salt stress, but increases ABA sensitivity in transgenic Arabidopsis thaliana. J Exp Bot. 67, 175–194 (2016). [DOI] [PubMed] [Google Scholar]
- Zhou L. G. et al. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot. 61, 4157–4168 (2010). [DOI] [PubMed] [Google Scholar]
- Gupta O. P., Meena N. L., Sharma I. & Sharma P. Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol Biol Rep. 41, 4623–4629 (2014). [DOI] [PubMed] [Google Scholar]
- Chen X. M. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303, 2022–2025 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I. et al. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32, D142–D144 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riano-Pachon D. M., Ruzicic S., Dreyer I. & Mueller-Roeber B. PlnTFDB: an integrative plant transcription factor database. BMC Bioinformatics 8, 42 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P. et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res., 35, W585–W587 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F. & Higgins D. G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic acids symposium series 41, 95–98 (1999). [Google Scholar]
- Hu B. et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31, 1296–1297 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T. L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A., Street N. R., Sandberg G., Gustafsson P. & Jansson S. The Populus genome integrative explorer (PopGenIE): a new resource for exploring the Populus genome. New Phytol. 182, 1013–1025 (2009). [DOI] [PubMed] [Google Scholar]
- Hu R. et al. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol. 10, 145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P. L. et al. A novel drought-inducible gene, ATAF1, encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis. Plant Mol Biol. 63, 289–305 (2007). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. The Arabidopsis ATAF1, a NAC transcription factor, is a negative regulator of defense responses against necrotrophic fungal and bacterial pathogens. Mol Plant Microbe In. 22, 1227–1238 (2009). [DOI] [PubMed] [Google Scholar]
- Conesa A. & Gotz S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics. 619832 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T. & Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 15, 473–497 (1962). [Google Scholar]
- Jia X. et al. Differential and dynamic regulation of miR398 in response to ABA and salt stress in Populus tremula and Arabidopsis thaliana. Plant mol biol. 71, 51–59 (2009). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 84, 136–142 (2011). [DOI] [PubMed] [Google Scholar]
- Chen Z. et al. The lipoxygenase gene family in poplar: Identification, classification, and expression in response to MeJA treatment. PloS One 10, e0125526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola L., Pirttila A. M., Halonen M. & Hohtola A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol. 19, 201–203 (2001). [DOI] [PubMed] [Google Scholar]
- An X. et al. Ectopic expression of a poplar APETALA3-like gene in tobacco causes early flowering and fast growth. Biotechnol lett. 33, 1239–1247 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Z. et al. Phylogenetic, expression, and bioinformatic analysis of the ABC1 gene family in Populus trichocarpa. The Scientific World J. 785070 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X. B. & Zhao P. X. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 39, W155–W159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. C. et al. Identification of microRNAs associated with male flower bud development of Populus simonii x Populus nigra. Trees. 29, 1329–1339 (2015). [Google Scholar]
- Hiratsu K., Matsui K., Koyama T. & Ohme-Takagi M. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J. 34, 733–739 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol Biol. 75, 365–378 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. et al. Activation of the programmed cell death pathway by inhibition of proteasome function in plants. J Biol Chem. 278, 19406–19415 (2003). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. A novel bZIP gene from Tamarix hispida mediates physiological responses to salt stress in tobacco plants. J Plant Physiol. 167, 222–230 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.